Abstract
Background: Whether African Americans (AA) are more susceptible to COPD than non-Hispanic Whites (NHW) and whether racial differences in disease phenotype exist is controversial. The objective is to determine racial differences in the extent of emphysema and airway remodeling in COPD. Methods: First, 2,500 subjects enrolled in the COPDGene study were used to evaluate racial differences in quantitative CT (QCT) parameters of% emphysema, air trapping and airway wall thickness. Independent variables studied included race, age, gender, education, BMI, pack-years, smoking status, age at smoking initiation, asthma, previous work in dusty job, CT scanner and center of recruitment. Results: Of the 1,063 subjects with GOLD Stage II-IV COPD, 200 self-reported as AA. AAs had a lower mean% emphysema (13.1% vs. 16.1%, p = 0.005) than NHW and proportionately less emphysema in the lower lung zones. After adjustment for covariates, there was no statistical difference by race in air trapping or airway wall thickness. Measured QCT parameters were more predictive of poor functional status in NHWs compared to AAs. Conclusions: AAs have less emphysema than NHWs but the same degree of airway disease. Additional factors not easily assessed by current QCT techniques may account for the poor functional status in AAs.
Introduction
Chronic obstructive pulmonary disease (COPD), encompassing both emphysema and chronic bronchitis, is characterized by incompletely reversible airflow obstruction. COPD affects over 5% of U.S. adults and is the only major cause of death with increasing mortality (Citation1). Current trends indicate COPD mortality may be leveling off among non-Hispanic Whites (NHWs), but continues to rise among African Americans (AAs) (Citation2–4). Despite this increasing impact among AAs, COPD continues to be understudied in this population.
AA individuals metabolize nicotine more slowly than NHW (Citation5); however, whether AAs are more susceptible to developing COPD remains controversial (Citation6–8). Although most studies have evaluated COPD severity using lung function measures, it is increasingly recognized that quantitative CT (QCT) measures of emphysema and airway wall thickness capture important components of COPD and can predict symptoms, clinical course of disease, and treatment response (Citation9). Few studies, however, have examined whether racial differences are evident in QCT measures. QCT scan measurements of 34 AA patients from the National Emphysema Treatment Trial (NETT) showed less severe emphysema based on whole-lung percentage of emphysema, despite similar impairments in lung function, compared to Whites (Citation6). Although suggesting racial differences in CT emphysema, this study was limited by the small number of AA subjects, the highly selected patient population, and the absence of examination of airway wall thickness.
The Genetic Epidemiology of COPD (COPDGene) Study is a large multi-center observational cohort study designed to identify genetic factors associated with COPD. Subjects span the spectrum of disease severity and include a large number of AAs, in addition to NHW (Citation10). All subjects undergo volumetric chest CT scanning with quantitative analysis. We hypothesized that AAs with COPD would have less emphysema but more airway wall thickness compared to NHWs.
Methods
Study Population
The COPDGene study includes self-reported NHW and AA smokers with ≥ 10 pack-years of cigarette smoking, age 45–80 years, from 21 U.S. clinical centers (Citation10). Based on a priori plan of the COPDGene® Steering Committee to analyze data for the first 2,500 enrolled subjects, the population for this analysis includes 1,063 subjects with COPD, including post-bronchodilator FEV1/FVC < 70%, GOLD Stage II-IV disease (FEV1<80% predicted) and verified data including QCT analysis. The COPDGene study was approved by the institutional review board at participating centers (Clinical Trials Registration # NCT00608764).
Physiologic testing
Patients underwent spirometry before and after albuterol administration (Citation11). Spirometric reference values were calculated using NHANES III equations for the general U.S. population (Citation12). The 6-minute walk test (6MWT) was performed according to ATS criteria (Citation13). Quality of life was assessed with the St. Georgeís Respiratory Questionnaire (SGRQ) and body –mass index (BMI), airflow obstruction, dyspnea and exercise capacity (BODE) index was calculated as published (Citation14, 15).
Computed Tomography
CT scans were acquired using multi-detector helical CT scanners with 16 or more detectors (Citation10, Citation16). Specific CT protocols for each scanner type have been published previously (Citation17). The severity and distribution of emphysema and airway disease were obtained from the inspiratory CT acquisition, and air trapping from the expiratory acquisition. Total percent (%) emphysema and total percent (%) air trapping were calculated using SLICER software (www.slicer.org) and defined as the percent of total lung voxels with an attenuation of less than –950 and –856 Hounsfield units, respectively (Citation18–20). Regional measures of emphysema were obtained by dividing the lung in 3 equal volumes from apex to base (Citation21).
Airway disease was measured by airway wall area percent (WA% = wall area/total cross-sectional area) of sub-segmental airways using the Pulmonary Workstation 2.0 (VIDA Diagnostics, Coralville, Iowa, http://www.vidadiagnostics.com). Sub-segmental airways were measured in the following pathways: right upper apical (RB1); right middle lateral (RB4); right lower posterior basal (RB10); left upper apicoposterior (LB1); left superior lingual (LB4); and left lower posterior basal (LB10). The mean value across all six lobes was used for analysis as previously described (Citation9). Results were not substantially different when analyzing either the segmental or sub-sub-segmental generation airway walls (data not shown).
Statistical analysis
The patient sample was characterized using proportions or means with standard deviations where appropriate. Means of continuous variables were compared using Student's t-test. Differences for categorical variables were determined by Pearson's Chi-square (χ2).
Initial analyses evaluated the effect of each independent variable on QCT parameters adjusting only for the CT scanner of acquisition. Independent variables studied in general linear models included race, age, gender, education (less than high school (HS), HS graduate or some college, college graduate or higher), BMI, pack-years, smoking status (current or former), age at smoking initiation, asthma diagnosis, previous exposure in dusty job for one or more years, CT scanner type, and center of recruitment. Variables associated with at least one CT parameter with p-value < 0.1 in bivariate analyses were included in the final multivariate analysis models.
Although both CT scanner type and center were significant, only scanner type was included in the final model because of the high level of collinearity between the two, and because adjusting for center did not significantly affect results. Secondary analyses were conducted including FEV1% predicted in the multivariate models, in order to determine whether the differences in radiological phenotype between races were independent of achieved lung function. Interaction terms, with and without adjustment for age, gender, BMI, education and center, were used to determine whether the association between radiological parameters and health outcomes (6MWT, BODE or SGRQ) differed by race.
All analyses were performed with SAS software (version 9.2, SAS Institute, Cary, NC). Two-tailed tests were used and p-values < 0.05 were considered statistically significant.
Results
Patient characteristics
Of 1,063 COPD subjects with GOLD Stage II-IV disease, 200 self-reported as AA. Similar to comparisons presented between the AA and NHW participants with GOLD Stage I-IV (Citation22), AA participants were younger, more likely to be current smokers, smoked less than NHWs, but had similar levels of lung function impairment. AAs were also more likely to carry a diagnosis of asthma and to have held a dusty job (). After adjusting for age, gender, pack-years smoked, education, BMI, and asthma diagnosis, AAs tended to have lower lung function (FEV1% predicted) than NHWs; however, this difference was not statistically significant (β = –2.3, p = 0.13).
Table 1. Patient characteristics.
Severity of emphysema, air-trapping, and airway wall thickness by race
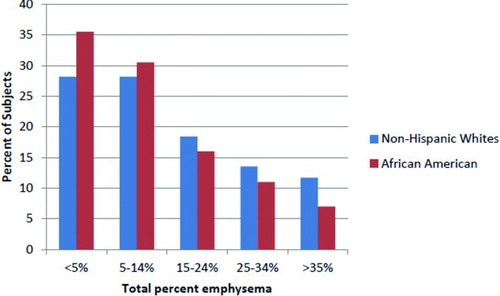
AAs had less total% emphysema (13% vs. 16%, p = 0.005) than NHWs and were more likely to have less than 15% total lung emphysema (). In unadjusted analyses, AAs had higher WA% (66.1% vs. 65.6%, p = 0.02), but tended to have lower% air-trapping (40.0% vs. 42.8%, p = 0.09), compared to NHWs (). After adjustment for potential confounders, there was no statistically significant difference by race in air-trapping or airway wall thickness measures; however, AAs had 2.2% less total% emphysema compared to NHWs (p = 0.03) (). Results were similar when excluding subjects with minimal smoking history (<20 pack-years) or those with a reported asthma history (data not shown). Differences in emphysema were greater in the lower lung zones than the upper and mid lung zones.
Table 2. Association of race with QCT phenotypes: Bivariate analysis.
Table 3. Association of race with QCT phenotypes: Multivariate models
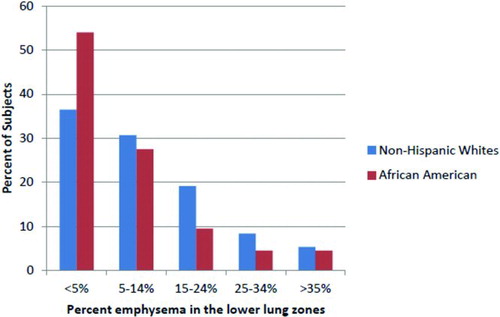
About 54% of AAs had little if any emphysema (<5%) in the lower lung zones, compared to 37% of NHWs (). AAs had a higher Upper zone: Lower zone (U:L) ratio as compared to NHWs (mean U:L was 3.3 vs. 2.5, respectively), which remained statistically significant after adjustment for total% emphysema (β = 0.82, p = 0.047), suggesting that the regional differences between race is not explained by the overall lower total% emphysema seen in AAs.
To confirm racial differences in QCT phenotypes at a given level of lung function and degree of COPD severity, FEV1% predicted was included in sensitivity analyses: the tendency towards lower total% emphysema (β = –1.53, p = 0.07) in AAs remained, but was no longer statistically significant, and there was no significant change in the relationship between racial differences and WA% (β = 0.32, p = 0.13) or% air-trapping (β = –0.99, p = 0.41).
Association between radiological measures and health outcomes
Given the differences in emphysema scores between AAs and NHWs, additional analyses were performed to determine if the association between radiologic parameters and health outcomes (6MWT, BODE, SGRQ or FEV1% predicted) differed by race. Interactions between race and total% emphysema and% upper zone emphysema were statistically significant (p < 0.05) in predicting 6MWT, but not BODE, SGRQ or lung function. Using the COPDGene cohort, it was previously shown that distance walked in the 6MWT was higher in NHWs compared to AAs (Citation23); however, we demonstrate that 6MWT was more strongly associated with total% emphysema (β = –9.4 for NHW, –2.7 for AA) and% upper lung zone emphysema (β = –73.1 for NHW, -22.1 for AA) in NHWs than AAs.
Degree of air trapping was a stronger predictor of 6MWT, BODE score and lung function in NHWs compared to AAs (6MWT: β = –6.8 and –2.1, respectively, p = 0.002 for interaction; BODE: β = 0.06 and 0.04, respectively, p < 0.001 for interaction; FEV1% predicted: β = –0.66 and –0.52, respectively, p = 0.006 for interaction). Similarly, airway wall thickness was a stronger predictor of BODE score and lung function in NHWs compared to AAs (BODE score: β = 0.3 and 0.09, respectively, p = 0.003 for interaction; FEV1% predicted: β = –2.78 and –1.10, respectively, p = 0.009 for interaction). This effect modification remains statistically significant when adjusting for potential confounders. There was no interaction between race and airway wall thickness in predicting 6MWT or SGRQ.
Discussion
COPD is a multi-component disease resulting in airflow limitation caused by a combination of emphysema-induced loss of elastic recoil and small and large airway remodeling. Some studies, including ours, suggest that AAs may be more susceptible to cigarette smoke, as they present with similar impairment in lung function as Whites but typically report fewer pack-years of smoking (Citation22). Spirometry alone does not adequately capture variations of COPD disease expression, and quantitative CT (QCT) has been proposed for identifying relevant COPD imaging phenotypes. QCT measures of emphysema and airway wall thickness independently predict airflow obstruction (Citation9), symptoms, and clinical course, and likely represent different pathobiological pathways. Our results suggest that AAs have less emphysema but similar amounts of air-trapping and airway wall thickness compared to NHWs after adjusting for smoking history and other explanatory variables. Furthermore, our results suggest that AAs display different patterns of emphysema, with less lower-lung disease, compared to NHWs.
Emphysema is defined histologically as the enlargement of airspaces distal to the terminal bronchioles and destruction of alveolar walls. In vivo, the extent and severity of emphysema can be measured globally and regionally using CT densitometry. Despite presenting with similar lung function, AAs had less emphysema on QCT, even when adjusting for potential confounders. After adjusting for FEV1% predicted, AAs tended to have less emphysema for a given lung function level, although these results were not statistically significant. Overall, our findings are consistent with those of Chatila et al., who found that AA patients (n = 38) demonstrated less severe emphysema on QCT (Citation6).
Our results expand on these findings by including a larger sample size and subjects spanning the breadth of COPD severity. Furthermore, our results support the hypothesis that racial differences in disease patterns may exist, as AAs had proportionately less emphysema in the lower lung zones than in the upper lung zones, compared to NHWs. Accurate characterization of regional emphysema is associated with differences in clinical outcomes (Citation24, 25) and severity of airflow obstruction (Citation26). Recently, studies have suggested that emphysema distribution is independently associated with COPD progression. For instance, in one study upper-lobe predominant CT quantified emphysema was associated with more rapid decrease in lung function than lower-lobe predominant disease (Citation27); and in another recent study, more homogenous emphysema distribution was associated with accelerated decline in lung function as compared to heterogenous disease (Citation28). AAs may have less lower-lung emphysema because of genetic differences, such as α1 anti-trypsin deficiency, which occurs at higher frequency among Whites of European descent (Citation29). However, subjects with known α1 anti-trypsin deficiency were excluded from this study, and reasons for this possible heterogeneity in emphysema distribution are unclear.
Bronchial wall thickening is associated with frequent exacerbations and symptoms of chronic bronchitis and has been assessed by multiple metrics including WA%, airway wall thickness, and estimates of theoretical airway dimensions (Citation9, Citation30). Quantitative assessment of small airways disease (airways <2 mm in diameter and the major site of airflow obstruction in COPD (Citation31)) is currently measured indirectly by determining the degree of air-trapping (Citation32). Although expiratory air trapping has been used as a measure of small airway disease in asthma (Citation18, 19), for COPD the contribution of airway disease or emphysema in the etiology of regional differences in this measure on the expiratory CT scan cannot be readily discerned. Furthermore, it is unclear which parameter best defines the “airway-predominant phenotype,” although our results suggest that AAs and NHWs have similar degrees of airway wall thickness and air-trapping, after adjustment for multiple covariates.
Whether the noted differences in QCT measures lead to differences in health outcomes remains unknown. In NHWs, upper lung zone emphysema and total% emphysema were more strongly predictive of poor 6MWT compared to AAs. Similarly, air trapping was more predictive of poor 6MWT and BODE, while airway wall thickness was more predictive of BODE score in NHWs than AAs. Taken together, the lower amount of total% emphysema found in AAs and stronger correlation of QCT measures with health outcomes in NHWs suggest that other factors, not measured by current QCT techniques, must contribute to the lower functional status seen in AAs in our cohort, including the lower 6MWD (Citation23) and worse quality of life in those with exacerbations (Citation22).
One possible explanation is a greater degree of small airway disease, not captured by air-trapping measures, such as the presence of centrilobular nodules or tree-in-bud opacities, in AA smokers. Given the absence of a standard approach to measuring these changes and the significant sample size, a visual assessment of these changes was not performed. Furthermore, several studies suggest that AAs with COPD may have lower mortality rates for COPD hospitalizations (Citation33) and lower health care utilization than Whites (Citation34). Longitudinal follow-up will be critical to determine whether variations in QCT parameters differentially predict disease progression, health care utilization, or mortality in AAs compared to Whites.
Although our study has several strengths, including a large number of AA subjects, it has several limitations. First, the clinical importance of the modest absolute differences in percent emphysema is unclear. A previous study showed, however, that total% emphysema was associated with lung function decline, and the effect of 5% difference in emphysema was similar to that of 10 additional pack-years of smoking (Citation35). Similarly, degree of emphysema detected on QCT is known to be associated with mortality in COPD patients (Citation36, 37), and in α1-antitrypsin deficiency patients, a 1% increase in upper zone emphysema was associated with a 6% increased rate of respiratory mortality (Citation38). Thus, even small differences may have significant clinical impact.
Second, although cigarette smoke is the major cause of COPD in the developed world, other noxious exposures, including biomass fuels and occupational dusts and chemicals, significantly contribute to the COPD disease burden worldwide, and our findings may not be generalizable to COPD induced by these exposures. For instance, AA coal miners have been shown to have greater emphysema severity than Whites at any given cumulative dust exposure (Citation39). Additionally, although we adjust for previous dusty job exposures, we do not account for other risk factors that may lead to developing COPD, including early respiratory infections, diet, and air pollution. After adjusting for FEV1% predicted, AAs continued to have a trend towards less emphysema for a given level of lung function; however, these results were no longer statistically significant. Last, in our cross-sectional study, we cannot evaluate the rate of change in QCT metrics over time, which may be clinically relevant.
Our results suggest that, as in previous studies, African Americans with COPD compared to Non-Hispanic Whites present with similar lung function impairment despite smoking less, but have less total% emphysema. In particular, they have less lower-zone emphysema, but similar airway wall thickness and air-trapping. Our results derive from a large-scale study, allowing for more thorough investigation of racial differences in radiological subphenotypes of COPD across a range of disease severity than in prior studies. Furthermore, emphysema, air trapping, and sub-segmental airway wall thickness were more predictive of poor functional status in NHWs compared to AAs, even though AAs had worse functional status overall. Factors likely accounting for poor functional status in AAs may include a greater degree of small airway disease such as respiratory bronchiolitis in AA smokers, which is not easily assessed by quantitative CT.
The members of the COPDGene® study group as of June 2010:
Ann Arbor VA: Jeffrey Curtis, MD (PI), Ella Kazerooni, MD (RAD)
Baylor College of Medicine, Houston, TX: Nicola Hanania, MD, MS (PI), Philip Alapat, MD, Venkata Bandi, MD, Kalpalatha Guntupalli, MD, Elizabeth Guy, MD, Antara Mallampalli, MD, Charles Trinh, MD (RAD), Mustafa Atik, MD
Brigham and Women's Hospital, Boston, MA: Dawn DeMeo, MD, MPH (Co-PI), Craig Hersh, MD, MPH (Co-PI), George Washko, MD, Francine Jacobson, MD, MPH (RAD)
Columbia University, New York, NY: R. Graham Barr, MD, DrPH (PI), Byron Thomashow, MD, John Austin, MD (RAD)
Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., MD (PI), Lacey Washington, MD (RAD), H Page McAdams, MD (RAD)
Fallon Clinic, Worcester, MA: Richard Rosiello, MD (PI), Timothy Bresnahan, MD (RAD)
Health Partners Research Foundation, Minneapolis, MN: Charlene McEvoy, MD, MPH (PI), Joseph Tashjian, MD (RAD)
Johns Hopkins University, Baltimore, MD: Robert Wise, MD (PI), Nadia Hansel, MD, MPH, Robert Brown, MD (RAD), Gregory Diette, MD
Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Los Angeles, CA: Richard Casaburi, MD (PI), Janos Porszasz, MD, PhD, Hans Fischer, MD, PhD (RAD), Matt Budoff, MD
Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, MD (PI), Charles Trinh, MD (RAD), Hirani Kamal, MD, Roham Darvishi, MD
Minneapolis VA: Dennis Niewoehner, MD (PI), Tadashi Allen, MD (RAD), Quentin Anderson, MD (RAD), Kathryn Rice, MD
Morehouse School of Medicine, Atlanta, GA: Marilyn Foreman, MD, MS (PI), Gloria Westney, MD, MS, Eugene Berkowitz, MD, PhD (RAD)
National Jewish Health, Denver, CO: Russell Bowler, MD, PhD (PI), Adam Friedlander, MD, David Lynch, MB (RAD), Joyce Schroeder, MD (RAD), John Newell, Jr., MD (RAD)
Temple University, Philadelphia, PA: Gerard Criner, MD (PI), Victor Kim, MD, Nathaniel Marchetti, DO, Aditi Satti, MD, A. James Mamary, MD, Robert Steiner, MD (RAD), Chandra Dass, MD (RAD)
University of Alabama, Birmingham, AL: William Bailey, MD (PI), Mark Dransfield, MD (Co-PI), Hrudaya Nath, MD (RAD)
University of California, San Diego, CA: Joe Ramsdell, MD (PI), Paul Friedman, MD (RAD)
University of Iowa, Iowa City, IA: Geoffrey McLennan, MD, PhD (PI), Edwin JR van Beek, MD, PhD (RAD), Brad Thompson, MD (RAD), Dwight Look, MD
University of Michigan, Ann Arbor, MI: Fernando Martinez, MD (PI), MeiLan Han, MD, Ella Kazerooni, MD (RAD)
University of Minnesota, Minneapolis, MN: Christine Wendt, MD (PI), Tadashi Allen, MD (RAD)
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD (PI), Joel Weissfeld, MD, MPH, Carl Fuhrman, MD (RAD), Jessica Bon, MD
University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, MD (PI), Sandra Adams, MD, Carlos Orozco, MD, Mario Ruiz, MD (RAD)
Administrative Core: James Crapo, MD (PI), Edwin Silverman, MD, PhD (PI), Barry Make, MD, Elizabeth Regan, MD, Sarah Moyle, MS, Douglas Stinson
Genetic Analysis Core: Terri Beaty, PhD, Barbara Klanderman, PhD, Nan Laird, PhD, Christoph Lange, PhD, Michael Cho, MD, Stephanie Santorico, PhD, John Hokanson, MPH, PhD, Dawn DeMeo, MD, MPH, Nadia Hansel, MD, MPH, Craig Hersh, MD, MPH, Jacqueline Hetmanski, MS, Tanda Murray
Imaging Core: David Lynch, MB, Joyce Schroeder, MD, John Newell, Jr., MD, John Reilly, MD, Harvey Coxson, PhD, Philip Judy, PhD, Eric Hoffman, PhD, George Washko, MD, Raul San Jose Estepar, PhD, James Ross, MSc, Rebecca Leek, Jordan Zach, Alex Kluiber, Jered Sieren, Heather Baumhauer, Verity McArthur, Dzimitry Kazlouski, Andrew Allen, Tanya Mann, Anastasia Rodionova
PFT QA Core, LDS Hospital, Salt Lake City, UT: Robert Jensen, PhD
Biological Repository, Johns Hopkins University, Baltimore, MD: Homayoon Farzadegan, PhD, Stacey Meyerer, Shivam Chandan, Samantha Bragan
Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: James Murphy, PhD, Douglas Everett, PhD, Carla Wilson, MS, Ruthie Knowles, Amber Powell, Joe Piccoli, Maura Robinson, Margaret Forbes, Martina Wamboldt
Epidemiology Core, University of Colorado School of Public Health, Denver, CO: John Hokanson, MPH, PhD, Marci Sontag, PhD, Jennifer Black-Shinn, MPH, Gregory Kinney, MPH
Declaration of Interest
Conflict of Interests: MKH has performed consulting for Nycomed and Novartis; participated on advisory boards for Novartis, Genentech, GlaxoSmithKline, Pfizer, Boehringer Ingelheim and MedImmune; and, participated on speaker bureaus for GlaxoSmithKline, Boehringer Ingelheim and Pfizer. BM has participated in advisory boards, speaker bureaus, consultations and multi-center clinical trials related to COPD with funding from the National Heart Lung and Blood Institute, Abbott, Astellas, AstraZeneca, Boerhinger-Ingelheim, Dey, Embryon, Forest, GlaxoSmithKline, NABI, NyComed, Novartis, Pfizer, Respironics, Schering, Sequal and Talecris. He has no direct conflicts with the topic of this manuscript. EKS received grant support and consulting fees from GlaxoSmithKline for studies of COPD genetics; and received honoraria and consulting fees from AstraZeneca. All other authors –none. All authors are responsible for the content and writing of this paper.
Acknowledgments
COPDGene® Investigators
References
- Coultas DB, Mapel D, Gagnon R, Lydick E. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med 2001; 164(3):372–377.
- Kirkpatrick P, Dransfield MT. Racial and sex differences in chronic obstructive pulmonary disease susceptibility, diagnosis, and treatment. Curr Opin Pulm Med 2009; 15(2):100–104.
- Wise RA. Changing smoking patterns and mortality from chronic obstructive pulmonary disease. Prev Med 1997; 26(4):418–421.
- Gillum RF. Chronic obstructive pulmonary disease in blacks and whites: pulmonary function norms and risk factors. J Natl Med Assoc 1991; 83(5):393–401.
- Berg JZ, Mason J, Boettcher AJ, Hatsukami DK, Murphy SE. Nicotine metabolism in African Americans and European Americans: variation in glucuronidation by ethnicity and UGT2B10 haplotype. J Pharmacol Exp Ther 2010; 332(1):202–209.
- Chatila WM, Hoffman EA, Gaughan J, Robinswood GB, Criner GJ. Advanced emphysema in African-American and white patients: do differences exist? Chest 2006; 130(1):108–118.
- Dransfield MT, Bailey WC. COPD: Racial disparities in susceptibility, treatment, and outcomes. Clin Chest Med 2006; 27(3):463–471, vii.
- Dransfield MT, Bailey WC. Studying COPD in African-Americans: Better late than never. COPD 2008; 5(1):1–3.
- Han MK, Bartholmai B, Liu LX, Clinical significance of radiologic characterizations in COPD. COPD 2009; 6(6):459–467.
- Regan EA, Hokanson JE, Murphy JR, Genetic epidemiology of COPD (COPDGene) study design. COPD 2010; 7(1):32–43.
- American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 1995; 152(3):1107–1136.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159(1):179–187.
- ATS statement. Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1):111–117.
- Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther 2000; 22(9):1121–1145.
- Celli BR, Cote CG, Marin JM, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10):1005–1012.
- Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3(6):519–532.
- Han MK, Kazerooni EA, Lynch DA, Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology 2011; 261(1):274–282.
- Busacker A, Newell JD, Jr., Keefe T, A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 2009; 135(1):48–56.
- Jain N, Covar RA, Gleason MC, Newell JD, Jr., Gelfand EW, Spahn JD. Quantitative computed tomography detects peripheral airway disease in asthmatic children. Pediatr Pulmonol 2005; 40(3):211–218.
- Kim WJ, Silverman EK, Hoffman E, CT metrics of airway disease and emphysema in severe COPD. Chest 2009; 136(2):396–404.
- Hersh CP, Washko GR, Jacobson FL, Interobserver variability in the determination of upper lobe-predominant emphysema. Chest 2007; 131(2):424–431.
- Han MK, Curran-Everett D, Dransfield MT, Racial differences in quality of life in patients with COPD. Chest 2011; 140(5):1169–1176.
- Rambod M, Porszasz J, Make BJ, Crapo JD, Casaburi R. Six minute walk distance predictors, including computed tomography measures, in the COPDGene(R) Cohort. Chest 2012; 141(4):867–875.
- Flaherty KR, Kazerooni EA, Curtis JL, Short-term and long-term outcomes after bilateral lung volume reduction surgery : Prediction by quantitative CT. Chest 2001; 119(5):1337–1346.
- Chae EJ, Seo JB, Song JW, Slope of emphysema index: an objective descriptor of regional heterogeneity of emphysema and an independent determinant of pulmonary function. AJR Am J Roentgenol 2010; 194(3):W248–W255.
- Gietema HA, Zanen P, Schilham A, Distribution of emphysema in heavy smokers: impact on pulmonary function. Respir Med 2010; 104(1):76–82.
- Mohamed Hoesein FA, van Rikxoort E, van Ginneken B, CT-quantified emphysema distribution is associated with lung function decline. Eur Respir J 2012, in press.
- Tanabe N, Muro S, Tanaka S, Emphysema distribution and annual changes in pulmonary function in male patients with chronic obstructive pulmonary disease. Respir Res 2012; 13(1):31.
- de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest 2002; 122(5):1818–1829.
- Mair G, Maclay J, Miller JJ, Airway dimensions in COPD: Relationships with clinical variables. Respir Med 2010; 104(11):1683–1690.
- Hogg JC, Chu F, Utokaparch S, The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(26):2645–2653.
- Matsuoka S, Yamashiro T, Washko GR, Kurihara Y, Nakajima Y, Hatabu H. Quantitative CT assessment of chronic obstructive pulmonary disease. Radiographics 2010; 30(1):55–66.
- Sarrazin MV, Cannon KT, Rosenthal GE, Kaldjian LC. Racial differences in mortality among veterans hospitalized for exacerbation of chronic obstructive pulmonary disease. J Natl Med Assoc 2009; 101(7):656–662.
- Shaya FT, Maneval MS, Gbarayor CM, Burden of COPD, asthma, and concomitant COPD and asthma among adults: racial disparities in a medicaid population. Chest 2009; 136(2):405–411.
- Mohamed Hoesein FA, de HB, Zanen P, CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax 2011; 66(9):782–787.
- Haruna A, Muro S, Nakano Y, CT scan findings of emphysema predict mortality in COPD. Chest 2010; 138(3):635–640.
- Martinez FJ, Foster G, Curtis JL, Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173(12):1326–1334.
- Dawkins PA, Dowson LJ, Guest PJ, Stockley RA. Predictors of mortality in alpha1-antitrypsin deficiency. Thorax 2003; 58(12):1020–1026.
- Kuempel ED, Wheeler MW, Smith RJ, Vallyathan V, Green FH. Contributions of dust exposure and cigarette smoking to emphysema severity in coal miners in the United States. Am J Respir Crit Care Med 2009; 180(3):257–264.