Abstract
The mechanism for the association between diabetes mellitus and lung function impairment is unknown, as are any respiratory effects of antidiabetic agents. We aimed to assess whether treatment with metformin, an oral insulin-sensitising agent, improved lung function or symptoms in individuals with COPD and glucose intolerance. A prospective open-label observational study was conducted. Participants with moderate or severe COPD, BMI > 25 kg/m2, and type 2 diabetes mellitus or impaired glucose tolerance took metformin twice daily for 6 months. Clinical outcomes included St George's Respiratory Questionnaire (SGRQ), transition dyspnoea index, and incremental shuttle walk test. Physiological outcomes including pulmonary function tests, exhaled nitric oxide, respiratory mouth pressures and handgrip strength. In total, 17 participants completed the study. SGRQ score improved by a median of 5 points, and TDI scores improved by 2 points. Inspiratory mouth pressures increased by 7.5 cmH2O. There were trends to improvements in hyperinflation, gas trapping and shuttle walk distance. Spirometry and exhaled nitric oxide were unchanged. In this proof-of-concept study, metformin was associated with improved dyspnoea and health status in COPD, possibly related to increased inspiratory muscle strength. These and other endpoints should be examined in a definitive study.
Introduction
Perhaps the most important consequence of the epidemic of diabetes mellitus (DM) is the burden of disease and disability caused by end-organ damage. Chronic hyperglycaemia damages blood vessels and connective tissue, leading to well-known complications of coronary artery disease, stroke, peripheral vascular disease, retinopathy, nephropathy, peripheral neuropathy, and joint contractures (Citation1). DM also adversely affects respiratory function (Citation2), although the mechanism remains unclear. Confounding by obesity is unlikely, since respiratory impairment is seen in type 1 DM, which is characterised by a slim body habitus (Citation3). Chronic glycosylation of pulmonary connective and vascular tissue is supported by some histopathological evidence (Citation4, 5), but there is also extensive evidence of respiratory dysfunction in prediabetic individuals (Citation6), and lung function abnormality is unrelated to diabetes duration or control (Citation2). The spirometric pattern is characteristically restrictive rather than obstructive (Citation7), arguing against airways disease as the cause. Insulin resistance is associated with skeletal muscle dysfunction (Citation8), but its effects on respiratory muscle are mostly unknown.
The lung function impairment that accompanies DM is typically mild, but could become clinically significant when patients also suffer from chronic cardiorespiratory conditions. In particular, DM is a common co-morbidity in COPD (Citation9) and baseline COPD is associated with increased risk of incident DM (Citation10).
Despite the evidence linking DM with respiratory impairment, there is almost no literature examining the respiratory effects of insulin (Citation11, 12) or other antidiabetic medications (Citation13, 14). Metformin is a widely available oral antidiabetic medication which acts to both control hyperglycaemia and directly increase end-organ insulin sensitivity. Metformin is well-tolerated by nondiabetic individuals. The effects of metformin and thiazolidinediones on respiratory function among patients with COPD and T2DM were recently examined in a retrospective study (Citation13); mean FVC was 132 mL higher among patients taking metformin or thiazolidinediones than among controls. Though allocation bias is a concern in the study design, the observed effect on FVC was impressive.
We conducted a prospective open-label observational study assessing the effects of metformin on respiratory symptoms, quality of life and lung function. We wished to assess first whether any effect was detectable; second, what the mechanism for such an effect might be; and third, the amount of time taken for the effect to appear. We therefore included endpoints that were both clinically relevant, and informative with regard to how glucose intolerance influences lung function.
Methods
Study design and participants
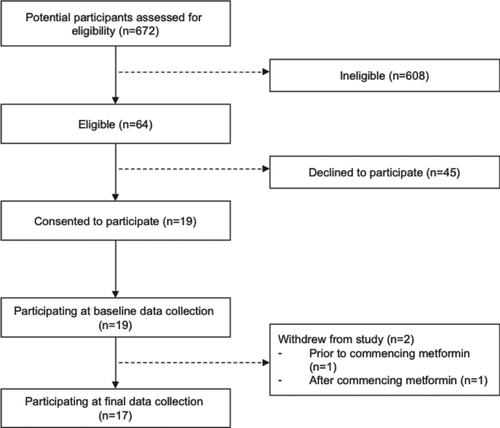
This was a prospective open-label observational study. Adults aged 18–75 who were overweight or obese (BMI >25 kg/m2) with symptomatic COPD (GOLD stage 2 or higher on post-bronchodilator spirometry) and either impaired glucose tolerance (IGT) or diet-controlled type 2 diabetes mellitus (T2DM) were invited to participate. Most participants were recruited from specialist respiratory outpatient clinics; some were recruited from primary care using advertisements. Participant recruitment and study design are depicted in .
Participants were ineligible if they had a history of congestive heart failure, chronic respiratory failure, other active respiratory disease, hepatic cirrhosis, chronic liver failure, weekly alcohol intake above 21 units (males) or 14 units (females), or previous documented episode of lactic acidosis; if estimated GFR was < 45 mL/min; or if they were were taking metformin, other hypoglycaemic agents, or regular oral corticosteroids. All participants provided written consent. Ethical approval was obtained from the Auckland Ethics Committees.
Study procedures
Subjects attended an initial screening visit where written consent was obtained. Spirometry was measured according to ATS/ERS guidelines (Citation15) using an electronic spirometer (Micro Plus CE 0120, CareFusion 232 Limited, Chatham Maritime, Kent, UK), 15 minutes after inhalation of 400 mcg of salbutamol via spacer. Blood was drawn to measure renal function, liver function and coagulation.
Eligible participants then attended a baseline visit where several procedures were performed. Exercise capacity was assessed using an incremental shuttle walk test. Health-related quality of life was measured using the St George's Respiratory Questionnaire (SGRQ) (Citation16). Dyspnoea was assessed using the Baseline Dyspnoea Index (BDI) (Citation17). Habitual physical activity was assessed using the CHAMPS questionnaire (Citation18).
Dynamic and static lung volumes, gas transfer (DLCO), respiratory mouth pressures and exhaled nitric oxide were measured in a pulmonary function laboratory according to ATS/ERS guidelines (Citation15). Handgrip strength was measured using a Jamar Plus digital dynamometer (Patterson Medical, Bolingbrook, Illinois, USA). Body fat content and distribution were measured using a Tanita BC-418 body composition analyser (Tanita Corporation, Arlington Heights, Illinois, USA).
Following this visit, all participants began taking metformin at an initial dose of 500 mg twice daily, increasing to 850 mg twice daily after 4 weeks. Subjects attended three interim followup visits, scheduled every 6 weeks. At these visits body weight, waist and hip circumference, handgrip strength, and spirometry were remeasured, venesection was repeated, and the transition dyspnoea index (TDI) questionnaire was administered.
Criteria for withdrawal included participant choice, unacceptable side effects, significant changes in regularly prescribed respiratory medications, or at the discretion of the responsible physician following hospitalisation with a severe illness associated with risk of lactic acidosis. Participants who experienced significant gastrointestinal side effects were offered a dose reduction (to 500 mg twice daily) as an alternative to withdrawal. After 6 months, subjects attended a final visit where the procedures of the initial visit were repeated.
Statistical analysis
Analysis was performed using the statistical software package R (http://www.r-project.org). Nonparametric methods were used for hypothesis testing. Paired Wilcoxon signed-rank tests were used to detect changes in continuous variables between the study beginning and end. Spearman's test was used to test for correlations between each outcome variable and potential confounders. The significance limit was set at 0.05. In all tables, median and range are given for measurements at baseline and final visits, and for the difference between these measurements.
Results
Baseline characteristics
A total of 17 participants completed the study. Participant baseline characteristics are shown in . Most subjects (76%) were of European ethnicity, and 59% were current or former smokers.
Table 1. Baseline characteristics of study participants
Adverse events
No severe metformin-related adverse events occurred during the study. One participant dropped out after approximately 6 weeks, owing to gastrointestinal adverse effects of metformin. Another four participants experienced moderately troublesome gastrointestinal side-effects which resolved after lowering their metformin dose to 1 g daily. Thus, 5 participants (28%) experienced gastrointestinal side-effects sufficient to alter management, but only 1 (6%) exited the study.
Health status and symptoms
The SGRQ total score improved by a median of 5 points (p = 0.005), exceeding the minimal clinically important difference of 4 points (Citation16) (, ). The impacts subscore fell by 4 points and and there was a trend to a reduction in the symptoms subscore. Dyspnoea also improved: at the final visit, total TDI and the “task magnitude” and “effort magnitude” subscores showed statistically and clinically significant (Citation17) improvements.
Figure 2. Change in St George's Respiratory Questionnaire total score. The dashed line shows change in the median score.
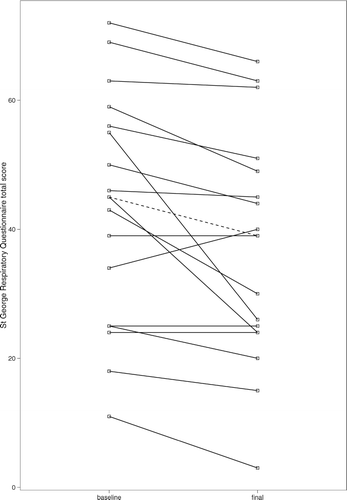
Table 2. Changes in health status and symptoms
Exercise capacity
There was a trend to a small improvement in the median incremental shuttle walk test (ISWT) (). The Borg score at test end improved by a median of 1 point.
Lung function
Baseline severity of AFO was moderate for most participants (), and gas trapping was common (). No significant changes in spirometry or gas transfer were seen during the study. There were trends towards reductions in TLC, RV and the RV/TLC ratio (p < 0.15).
Table 3. Changes in lung function, gas transfer and exhaled nitric oxide
Table 4. Changes in general and respiratory muscle strength
Muscle function
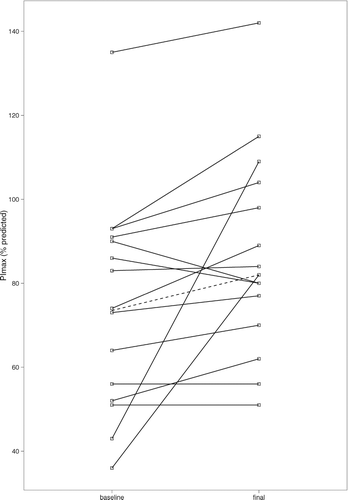
Inspiratory mouth pressures (PImax) increased during the study ( and ). PImax increased among all but two participants, by a median of 7.5 cm H2O. No significant changes were observed in expiratory mouth pressures, and grip strength decreased slightly (median loss of 1.4 kg).
Airway and systemic inflammation
There were trends to reductions in CRP and total and LDL cholesterol (not shown). No significant changes were seen in other lipid markers, or ENO ().
Changes in other variables
Participants lost a median 2.4 kg during the study, with corresponding small decreases in fat mass, BMI (median 0.9 kg/m2) and WC. Metformin produced a median 3 mmol/mol decrease in HbA1c. Fasting insulin and HOMA insulin resistance scores were stable. Reported daily physical activity was unchanged over the study, apart from small increases in frequency of moderate exercise (without changes in moderate-intensity caloric expenditure). None of the observed endpoint changes discussed above was statistically related to changes in body weight, BMI, or central obesity.
Discussion
This prospective observational study demonstrated beneficial effects of 6 months of oral metformin on health status, dyspnoea and physiological parameters in a group of adults with COPD and abnormal glucose homoeostasis.
Metformin was considered an ideal investigational agent for studying antidiabetic therapy in respiratory disease. It is an oral medication which acts through several mechanisms to control hyperglycaemia and increase end-organ insulin sensitivity. Metformin acts through AMP-associated protein kinase (AMPK), an energy-sensing enzyme present in all cells (Citation19), to mimic the effects of caloric restriction, activate antioxidant defenses, and inhibit target of rapamycin. Metformin has effects beyond glucose metabolism: it has been associated with reduced incidence of, and mortality from, several cancers in epidemiological studies (Citation20). Metformin has a well-established safety record, and the most serious biguanide-associated adverse event (lactic acidosis) appears to be extremely rare (Citation21). As an oral agent, administration is more convenient than insulin. Finally, it is off-patent and inexpensive.
Clinical respiratory effects of metformin
The first aim of this study was to assess whether treatment with metformin was associated with clinical benefit in patients with COPD. To this end, respiratory health status, dyspnoea, and exercise capacity were assessed.
Health status and symptoms
The St George's Respiratory Questionnaire (SGRQ) is a well-established, self-administered health status questionnaire developed for use among patients with chronic airways disease (Citation16). In this study, the SGRQ total score showed highly clinically and statistically significant improvements over the followup period, comparable in magnitude to the improvements seen in randomised trials of long-acting bronchodilators for COPD (Citation16). The transition dyspnoea index (TDI) also showed clinically significant improvements (Citation17). Taken together, the changes in SGRQ and TDI suggest that metformin produces meaningful symptomatic benefits in COPD.
Exercise capacity
The ISWT is a measure of maximal exercise capacity that has low intra-subject variability and close correlation with maximal oxygen uptake among patients with COPD (Citation22). Although the increase in shuttle walk distance was statistically nonsignificant, it was consistent with the statistically significant increase in Borg dyspnoea scores at test end. A definitive study should reassess the effect of metformin on exercise, either using the ISWT or the 6-minute walk test.
Pathophysiological insights
The other aim of the present study was to gain insight into the mechanisms underlying any benefit. The most plausible mechanisms include reduced tissue glycosylation, reduced airway inflammation, and improved skeletal muscle function.
Tissue glycosylation
The hypothesis that chronic glycosylation of pulmonary tissue accounts for the adverse effects of DM on respiratory function is supported by histopathological studies showing microangiopathy (Citation4) with basal laminar thickening, increased synthesis and deposition of collagen and elastin, and unusual patterns of fibrosis (Citation5).
However, an expected corollary is that DM–associated respiratory abnormalities should be slight at first, worsening with increased DM duration or with worse blood sugar control. In fact, lung function impairment is detectable before DM develops (Citation6), and seems unrelated to control or duration of diabetes (Citation2).
The present study was able to assess glycosylation only indirectly, by using changes in DLCO to reflect changes in severity of pulmonary microangiopathy. The lack of improvement in DLCO suggests that the pulmonary microvasculature did not respond to metformin within the followup period. However DLCO must be interpreted cautiously in small studies given its high intra-subject variability.
Airways disease
Despite the frequency with which DM coexists with COPD and asthma, little evidence specifically supports a causative role for DM in airways disease. The lung function impairment associated with insulin resistance is almost always restrictive rather than the expected obstructive pattern (Citation7). The literature on airway inflammatory markers in DM is nonexistent for ENO, and minimal for other exhaled substances (Citation23). Despite in vitro evidence that hyperinsulinaemia causes proliferation and hypercontractility of smooth muscle (Citation24), the diabetic response to bronchoconstrictor challenge appears to be normal or blunted (Citation25).
In the present study, ENO did not change significantly with metformin treatment, suggesting that insulin sensitisation does not affect allergic airway inflammation. However, ENO was generally within normal limits at baseline, and most participants were taking inhaled corticosteroids, which are known to suppress ENO (Citation26). No effects were seen on spirometry, but there were nonsignificant trends to reductions in TLC, RV, and RV/TLC ratio, possibly reflecting reduced hyperinflation and gas trapping. Overall, the evidence for airway-specific effects is weak, but the hints of improvements in static lung volumes are worthy of further study.
Muscle function
General skeletal muscle function is abnormal in DM (Citation27), and handgrip strength correlates negatively with insulin resistance (Citation8). Inspiratory muscle strength is abnormal in diabetic subjects (Citation28) and metformin has improved diaphragmatic function in animal studies (Citation29). There is no published literature on the effects of antidiabetic treatment on respiratory muscle function in humans.
In this study, the observed 11% increase in inspiratory muscle strength (PImax) was highly statistically significant. The change was independent of changes in daily caloric expenditure, and was not accompanied by improved grip strength. The clinical significance of this result is unclear as literature on minimum clinically important differences in respiratory muscle strength is lacking. In a small trial of inspiratory muscle training, a 29% increase in PImax was associated with clinically significant improvements in dyspnoea scores (Citation30).
Hyperinflation can impair respiratory muscle function by altering diaphragmatic force-length relationships (Citation31). However, we found no correlation between change in PImax and change in RV (Spearman p > 0.Citation5). Perhaps insulin resistance affects the diaphragm differently from other skeletal muscle. The results of the present study should prompt further examination of respiratory muscle function in glucose-intolerant subjects.
Adverse events and participant withdrawal
One participant discontinued the study (due to constipation); another 4 participants reported moderately troublesome gastrointestinal side effects which resolved after their metformin dose was reduced to 1g daily. However, the risk of attenuating any treatment effect must be considered before allowing dose reductions in any future study. If the 4 dose-reduced participants are counted as dropouts, the dropout rate is comparable to larger randomised trials of metformin (Citation32). Other than gastrointestinal effects, no serious metformin-related adverse effects were recorded.
Limitations
The study lacked a control group. The ideal study design would have been a randomised placebo-controlled trial, which would have allowed us to measure the influence of placebo effects on SGRQ results, and of learning effects on PImax. However we felt that available information was insufficient to allow confident selection of a primary endpoint. The single-arm design also maximised the number of participants exposed to metformin, maximising the chance of observing any treatment effect.
A longer follow-up period may have identified responses which were slower to appear. On the other hand, a longer study duration increases costs and dropout rates. Six months of followup was felt to be reasonable as evidence exists from other studies that diabetic complications begin appearing within this time frame (Citation33).
The reduced dose of metformin taken by some participants may have been subtherapeutic. A larger randomised placebo-controlled study should require participants to withdraw if they cannot tolerate the full dose.
Data were not collected on COPD exacerbations, which are an important clinical outcome measure. More detailed assessment of airway function (bronchodilator reversibility, respiratory mechanics) and inflammation (exhaled breath condensate, sputum examination) may have produced additional pathophysiological insights.
Conclusions
In this prospective study of the effects of six months of metformin therapy in COPD, statistically and clinically significant improvements were observed in symptoms, health status, and inspiratory muscle function. The changes remained significant after correction for small changes in BMI, arguing against confounding by obesity. These observations are sufficiently encouraging to require confirmation in a larger, definitive study.
Such a study should be powered to observe a clinically significant increase in SGRQ score (not enough is known about the clinical effects of changes in PImax to use it as a primary outcome). It should also aim to reexamine the other outcomes examined by the present study as secondary endpoints. A 6-minute walk test should be considered as a more directly clinically relevant alternative to the ISWT.
A longer study duration would be ideal. Diabetes or glucose intolerance may affect lung function through multiple mechanisms, some of which might take longer than 6 months to respond to normalisation of glucose metabolism. Continued observation might also reveal larger degrees of improvement in those endpoints which began to change in the present study.
Declaration of Interest Statement
This study was funded by a grant from the Greenlane Research and Educational Fund. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care 2010; 33 Suppl 1:S11–S61.
- van den Borst B, Gosker HR, Zeegers MP, Schols AMWJ. Pulmonary function in diabetes: a meta-analysis. Chest 2010; 138:393–406.
- Innocenti F, Fabbri A, Anichini R, Tuci S, Pettina G, Vannucci F, De Giorgio LA, Seghieri G. Indications of reduced pulmonary function in type 1 (insulin-dependent) diabetes mellitus. Diabetes Res Clin Pract 1994; 25:161–168.
- Kodolova IM, Lysenko LV, Saltykov BB. (Changes in the lungs in diabetes mellitus). Arkh Patol 1982; 44:35–40.
- Fariña J, Furió V, Fernandez-Aceñero MJ, Muzas MA. Nodular fibrosis of the lung in diabetes mellitus. Virchows Arch 1995; 427:61–63.
- McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutritio n Examination Survey. Am J Epidemiol 2005; 161:546–556.
- Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, Selvin E, Brancati FL. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2008; 31:741–746.
- Lazarus R, Sparrow D, Weiss ST. Handgrip strength and insulin levels: cross-sectional and prospective associations in the Normative Aging Study. Metabolism 1997; 46:1266–1269.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32:962–969.
- Song Y, Klevak A, Manson JE, Buring JE, Liu S. Asthma, chronic obstructive pulmonary disease, and type 2 diabetes in the Women's Health Study. Diabetes Res Clin Pract 2010; 90:365–371.
- Niranjan V, McBrayer DG, Ramirez LC, Raskin P, Hsia CC. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am J Med 1997; 103:504–513.
- Terzano C, Morano S, Ceccarelli D, Conti V, Paone G, Petroianni A, Graziani E, Carnovale A, Fallarino M, Gatti A, Effect of insulin on airway responsiveness in patients with type 2 diabetes mellitus: a cohort study. J Asthma 2009; 46:703–707.
- Kim HJ, Lee JY, Jung HS, Kim D-K, Lee S-M, Yim J-J, Yang S-C, Yoo C-G, Chung HS, Kim YW, The impact of insulin sensitisers on lung function in patients with chronic obstructive pulmonary disease and diabetes. Int J Tuberc Lung Dis 2010; 14:362–367.
- Spears M, Donnelly I, Jolly L, Brannigan M, Ito K, McSharry C, Lafferty J, Chaudhuri R, Braganza G, Bareille P, Bronchodilatory effect of the PPAR-gamma agonist rosiglitazone in smokers with asthma. Clin Pharmacol Ther 2009; 86:49–53.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Standardisation of spirometry. Eur Resp J 2005; 26:319–338.
- Jones PW: St. George's Respiratory Questionnaire: MCID. COPD 2005; 2:75–79.
- Mahler DA, Witek TJ: The MCID of the transition dyspnea index is a total score of one unit. COPD 2005; 2:99–103.
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001; 33:1126–1141.
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108:1167–1174.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009; 32:1620–1625.
- Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No.: CD002967. DOI: 10.1002/14651858.CD002967.pub4.
- Eiser N, Willsher D, Doré CJ. Reliability, repeatability and sensitivity to change of externally and self-paced walking tests in COPD patients. Respir Med 2003; 97:407–414.
- Gartner M. Exhaled Breath Analysis of Emergency Room Patients with Diabetes and Respiratory Diseases. University of California, 2011 PhD thesis.
- Gosens R, Nelemans SA, Hiemstra M, Grootte Bromhaar MM, Meurs H, Zaagsma J. Insulin induces a hypercontractile airway smooth muscle phenotype. Eur J Pharmacol 2003; 481: 125–131.
- Piccioni M, Manfrini S. Bronchial responsiveness to methacholine in insulin-dependent diabetic patients with autonomic neuropathy. Chest 1994; 105:644–645.
- Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med 1996; 153:454–457.
- Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 1997; 83:166–171.
- Wanke T, Formanek D, Auinger M, Popp W, Zwick H, Irsigler K. Inspiratory muscle performance and pulmonary function changes in insulin-dependent diabetes mellitus. Am Rev Respir Dis 1991; 143:97–100.
- Frayn KN, Adnitt PI. Effects of metformin on glucose uptake by isolated diaphragm from normal and diabetic rats. Biochem Pharmacol 1972; 21:3153–3162.
- Hill K, Jenkins SC, Philippe DL, Cecins N, Shepherd KL, Green DJ, Hillman DR, Eastwood PR. High-intensity inspiratory muscle training in COPD. Eur Respir J 2006; 27: 1119–1128.
- De Troyer A. Effect of hyperinflation on the diaphragm. Eur Respir J 1997; 10:708–713.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Group DPPR. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TME, Moffitt MS, Taskinen M-R, Simes RJ, Tse D, Williamson E, Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007; 370:1687–1697.