Abstract
Background: It was reported that Cathepsin E (Cat E) plays a critical role in antigen processing and in the development of pulmonary emphysema. The aim of this study was to investigate the role of Cat E and airflow limitation in the pathogenesis of COPD. Methods: Sixty-five patients with COPD, 20 smoking control subjects without COPD and 15 non-smoking healthy control subjects were enrolled. Cat E and EIC (Elastase inhibitory capacity) expressions were measured by ELISA in sputum and serum samples and compared according to different subgroups. Results: Cat E concentrations were significantly higher in patients with COPD than smoking control and non-smoking control subjects (P < 0.01). The levels of CatE were inversely correlated with FEV1% predicted in COPD patients (r = −0.95, P < 0.01). The levels of EIC were inversely positively correlated with FEV1% predicted in COPD patients (r = 0.926, P < 0.01). Levels of Cat E were also inversely correlated with the levels of EIC (r = −0.922, P < 0.01). Conclusions: Cat E contributes to the severity of airflow limitation during progression of COPD.
Introduction
COPD, a leading cause of morbidity and mortality worldwide (Citation1), is characterized by persistent airflow limitation that is not fully reversible and chronic inflammation of the lungs (Citation2). Globally, total deaths from COPD are projected to increase by more than 30% in the next decade without interventions to cut risks, particularly exposure to tobacco smoke (Citation3). COPD arises due to an abnormal inflammatory response to inhalation of a myriad of noxious particles or gases. Inflammation induced structural alterations in the small airways and lung parenchyma is the most important factor contributing to airflow limitation. However, the relationship between chronic inflammation and airway remodeling is still not fully elucidated (Citation4). Because COPD is a preventable and treatable disease, identifying the predictive inflammatory cytokines in patients with COPD is urgent to prevent its further development.
Cat E, a tremendously multi-functional protein, is a major intracellular aspartic protease which is predominantly present in the immune cells and is frequently implicated in antigen processing via the MHC class II pathway (Citation5). Unlike the highly homologous aspartic proteinase cathepsin D, Cat E possesses only few notable properties (Citation6), including limited distribution, cell-specific localization, cell-specific processing, intracellular trafficking, and gene regulation (Citation7). Several research groups have associated Cat E with various physiological functions and pathological conditions. Cat E knockout mice, which do not exhibit any obvious phenotypes, are associated with different conditions, such as atopic dermatitis (Citation8), accumulation of mast-cell carboxypeptidase A (Citation9) and increased bacterial infections (Citation10). Conversely, the conditions in which over-expression of Cat E is observed are pancreatic ductal adenocarcinoma (Citation11), cervical adenocarcinoma (Citation12) and lung carcinoma (Citation13). Otherwise, Cat E also plays a substantial role in preventing Tumor Growth and Metastasis by Catalyzing the Proteolytic Release of Soluble TRAIL from Tumor Cell Surface (Citation14). Hence, Cat E with respect to cancer has been studied as a diagnostic marker (Citation15) as well as an anti-tumor factor produced by the host defense system (Citation16). Surprisingly, the previous studies focusing on the relationship between Cat E and lung disease, particularly COPD, have been poorly published. So far, only one study showed that the expression of cathepsin E increased obviously in lung epithelial cells of patients with COPD, and COPD lung sections and inducible Cat E transgenic mice also confirmed the high expression of cathepsin E lung epithelial cell could cause emphysema (Citation17).
We hypothesized that the excessive expression of Cat E in human lungs is insufficiently counterbalanced by a rise in antiproteolytic molecules, which results in the destruction of lung parenchyma and then contributes to the severity of airflow limitation during progression of COPD. For further clarification of the role of Cat E in the endolysosomal system, we performed this study to investigate the association of Cat E expression with the severity of airflow limitation in patients with COPD.
Methods
Study subjects
Sixty-five patients with COPD, 20 smoking and 15 non-smoking control subjects were recruited for this study from Shanghai Pulmonary Hospital, Tongji University School of Medicine (). Non-smoker was defined as people who had smoked less than 100 cigarettes in their whole life. The inclusion criteria for the study were: (Citation1) current or ex-smokers with a history of more than 20 pack-years before enrollment; (Citation2) no history suggestive of asthma, bronchiectasis, carcinoma of the bronchus or other cardiopulmonary disease; or, (Citation3) post-bronchodilator FEV1/FVC < 70%.
Table 1. Demographic and lung function data for the study subjects
The percentage of predicted values suitable for equations formulated for Asian populations were used in spirometry values; and (Citation4) subjects in stable status without evidence of exacerbation for at least 6 weeks. The exclusion criteria were: (Citation1) therapy with corticosteroids, anticholinergics, long-acting beta(2)-agonists, theophylline and non-steroidal anti-inflammatory drugs in the last 6 weeks; (Citation2) coexistence with the heart, liver or kidney disease; and, (Citation3) other pulmonary diseases. The COPD patients were divided into four groups (stages I–IV) in accordance with the criteria of the Global Initiative for Chronic Obstructive Lung Disease (Citation18). The smoking control subjects without history of respiratory disease had a more than 20 pack-years history of smoking for at least 10 years and normal pulmonary function tests. The 15 non-smoking control subjects were age-matched healthy volunteers. The non-smoking control subjects without a history of lung disease or smoking had normal pulmonary function tests. This study was approved by the Institutional Review Board of Shanghai Pulmonary Hospital, Tongji University School of Medicine and written informed consent was obtained from each subject before the initiation of this study
Sputum induction and processing for cell counts
Subjects were required to expectorate an adequate sputum sample that was induced and processed using a validated technique as described previously (Citation19, Citation20). The patients, pretreated with an inhaled short-acting beta(2)-agonist (salbutamol, 200 μg) to inhibit airway constriction, inhaled 3% hypertonic sodium chloride aerosols for three periods of 5 min each. Finally we set the quantitative sputum as 3 mL. An equal volume of 0.1% dithiothreitol (Sigma Aldrich Co, St Louis, MO, USA) was added to the sputum. The sputum was vortex mixed for 30 sec and then incubated on a shaker for 15 min at room temperature.
An equal volume of Dulbecco's PBS was mixed with an equal volume of dithiothreitol to minimize the effects of dithiothreitol on the cell suspension and the sample was further incubated for 5 min. The total cell count, manually determined using a standard haemocytometer, was expressed as the percentage of intact round nucleated cells, excluding squamous epithelial cells. The sample was deemed to be sufficient if squamous epithelial cells were less than 5% in the slide. For the entire experiment period, a blinded fashion was performed by two investigators. After centrifugation, the sputum supernatant was stored at −80°C for measurement and assessment of the concentrations of Cat E expression.
Cat E expression measurement
The concentrations of Cat E in sputum from lung epithelial cells of all subjects were measured in triplicate by ELISA. The Capture antibody was mouse monoclonal antihuman Cat E antibody (Santa Cruz) and second antibody was a polyclonal rabbit anti-mouse Cat E. Alkaline phosphatase-conjugated anti-rabbit IgG was used for detection in this assay and the recombinant human Cat E was used to create the standard curve. Briefly, 96-well microplates were coated with the capture antibody (100 μL of a 500-fold dilution in phosphated-buffered saline (PBS) at 4°C overnight.
Plates were washed with PBS and blocked with 2% bovine serum albumin (BSA) at 4°C for 2 h. 50 μL of sputum supernatants were added to the plates and incubated at room temperature for 2 h. Plates were washed with wash buffer and the second antibody (100 μL of a 500-fold dilution in PBS) was added and allowed to sit at room temperature for 2 h. The bound antibodies were detected via sequential incubation with alkaline phosphatase-conjugated goat anti-rabbit IgG and enzyme substrate solution. Plates were read on an ELISA reader at 405 nm. Cat E could be detected at concentrations as low as 1.75 ng/mL.
EIC testing
First, 2 ml of fasting blood samples were obtained in the morning from the patients to measure serum EIC levels. Blood samples, stored at 4°C overnight, were centrifuged for serum. EIC was measured in serum by the percentage of inhibition that these samples exerted on the in vitro elastase-induced Suc-Ala-Ala-Ala-p-nitroanilide hydrolysis test, according to Bieth and Wermuth (Citation21). Additionally, 50 ul of a serum dilution (1:150) was used in the assay. The samples of p-nitroanilide released at 25°C were followed for 10 to 60 min at 405 nm using a microplate spectrophotometer reader (Citation22, Citation23).
Pulmonary function examination
All pulmonary function tests were performed in a dedicated pulmonary function laboratory under the supervision of a certified pulmonary technologist, and were conducted using a spirometry (Jaeger/Toennies, Germany) that met the criteria of American Thoracic Society (Citation24, Citation25). The following indexes were estimated: FEV1; the ratio of the forced expiratory volume in 1 sec to the forced vital capacity (FEV1/FVC); maximal mid-expiratory flow (MMEF) and peak expiratory flow (PEF). FEV1, FEV1/FVC and PEF were expressed as the percentages of predicted values (percentage of predicted).
CT Data Acquisition
Emphysema is characterized by permanent expansion of airspaces distal to the terminal bronchioles and can be characterized by CT of the chest (Citation26). All the subjects underwent imaging on a Philips Brilliance 64-slice spiral CT. Breath-hold training was performed before each examination. All subjects were asked to hold their breath at the end of inspiration as long as possible. Emphysema was evaluated by CT according to the method reported previously (Citation27), CT findings were evaluated at 3 anatomic levels in both lungs: the superior margin of the aorta 1 cm (level of the upper lung field), subcarinal 1 cm (level of the middle lung field), and above the top of the diaphragm 3 cm approximately (level of the lower lung field).
Areas of vascular disruption and low attenuation value were scored for the upper, middle and lower lung field in both lungs. Additionally, the extent of emphysema was estimated using the Goddard score (Citation28): 0, no emphysema; 1, up to 25% of lung parenchyma involved; 2, between 26% and 50% of lung parenchyma involved; 3, between 51% and 75% of lung parenchyma involved; and 4, between 76% and 100% of lung parenchyma involved. Scanned CT data were stored in DICOM format, which is the international standard for interconnecting medical imaging devices on standard networks.
Statistical analysis
Data were analyzed using a software package (SPSS 20.0). Continuous data were expressed as means ± SD. Differences between control subjects and each of the subgroups of COPD patients were assessed using one-way analysis of variance with the P values, followed by multiple comparisons performed using the Bonferroni correction. The Tamhane test was applied to compare pulmonary function indices between the control subjects and each subgroup of the COPD patients. The relationships of FEV1% with the concentrations of both Cat E and EIC in the sputum of COPD patients were analyzed using Pearson's correlation. A P-value < 0.0 accepted to be significant.
Results
Lung function results for all study subjects
The pulmonary function data of all study subjects are analyzed and presented in . As for age and gender, it was comparable between the four subgroups of COPD and the two control subjects. Statistically significant differences were not noticed in FEV1%, FEV1/FVC%, MMEF, or PEF between two control subjects (P < 0.01). As might be expected, FEV1%, FEV1/FVC%, MMEF and PEF were remarkably lower in each of the COPD subgroups than in the two control subjects (P < 0.01), except no evident difference existed in PEF and FEV1% between the two control subjects and the stage I patients (P < 0.05).
Characteristics of sputum cells
Total and differential cell counts of sputum specimen were shown in . The total cell count and the numbers of macrophages and neutrophils in specimen were markedly higher in the patients with COPD and smoking control patients than in the non-smoking control patients (P < 0.01). Additionally, the percentage of macrophages was markedly lower overall in the COPD patients than in the two control subjects (P < 0.01). The percentage of neutrophils was prominently lower in the stage I and two control subjects than in the stage II through stage IV COPD (P < 0.01).
Table 2. Cellular analysis of sputum samples
Concentrations of Cat E in sputum
The concentrations of Cat E in sputum are shown in . Cat E levels were prominently higher in the COPD patients than in the non-smoking controls and smoking control subjects (P < 0.01). Among the patients with COPD and smoking control subjects, the levels of Cat E tended to vary with the severity of COPD. More specifically, Cat E levels were markedly higher in COPD patients with stage III and stage IV than in stage I and smoking control subjects (P < 0.01). In addition, Cat E levels were dramatically lower in patients with stage III compared with stage IV COPD (P < 0.01). Statistically significant differences were not noticed in Cat E levels between the smoking control subjects (P < 0.01).
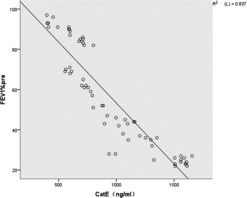
Correlation of Cat E with airflow limitation in patients with COPD
The relationships between Cat E in the sputum and the severity of airflow limitation in all the patients with COPD are illustrated in . Cat E was inversely correlated with FEV1% in patients with COPD (r =−0.915, P < 0.01).
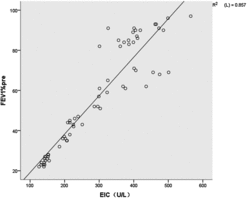
Expression of EIC in serum
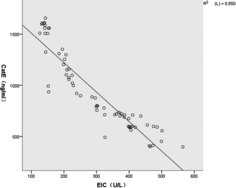
The expression of EIC was assessed in all subjects (). There was basal expression of EIC in the non-smoking controls. The expression of EIC was decreased in COPD patients compared with that in the non-smoking controls. EIC was positively correlated with FEV1% in patients with COPD (: r = 0.926, P < 0.01). In addition, there was a significant inverse correlation between EIC and Cat E in the COPD patients (:r = −0.922, P < 0.01).
CT data analysis
In the present study, the emphysema data for the study subjects are presented in . Among the COPD patients and smoking controls, the degree of emphysema tended to vary depending on the severity of COPD. Our combined analysis did find that significant differences in the degree of emphysema between the non-smoking controls and the stage I–IV patients and smoking control subjects (P < 0.01) were detected. Moreover, the degree of emphysema was inversely correlated with FEV1% in patients with COPD, and was significantly higher in all patients with COPD and smoking control subjects than in the non-smoking control subjects (P < 0.01).
Discussion
As far as we know, this study is the first one to investigate the association of Cat E expression with the severity of airflow limitation in patients with COPD. We found that the concentrations of Cat E in sputum were not only remarkably increased in both COPD patients compared with non-smoking control subjects and the smoking control subjects, but also increased gradually with the development of airflow limitation in COPD patients. Moreover, the concentrations of Cat E were inversely associated with FEV1% in patients with COPD. The results were consistent with expectations.
Cat E, an endopeptidase with substrate specificity similar to that of cathepsin D, belongs to a member of the cathepsin superfamily. The earlier study by Bosi and her colleagues described the occurrence of Cat E in bronchiolar Clara cells and their enhanced expression in several types of non-neoplastic lung disease characterized by hyperplasia and/or hypertrophy of bronchiolo-alveolar epithelium (Citation29). Cat E is highly secreted by many cell types including activated phagocytes and in cells of the immune system (Citation30).
Additionally, it also plays a predominant role in parenchymal remodeling through collagen and elastin degradation that occurs in fibrosing lung disease (Citation29) and the execution of neuronal death pathways (Citation31). Bosi et al. reported that Cat E expression was increased with the magnitude of inflammation (Citation29). Moreover, the present study showed that expression of Cat E was significantly increased in patients with COPD, suggesting that Cat E may be closely associated with chronic inflammation in COPD. Furthermore, the concentration of serum Cat E not only increased gradually with the clinical severity of COPD, but also correlated inversely with the degree of FEV1% in COPD. These observations indicate that Cat E might be closely involved in the pathogenesis of both inflammation and airflow limitation in the progression of COPD.
It is noteworthy that obstructive emphysema, anatomically defined as the destruction of the distal lung parenchyma and enlargement of the air spaces (Citation17), is the primary pathological symptom of COPD. A little known fact is that many non-smokers develop emphysema-like changes as they age, with associated loss of lung function. The molecular mechanisms of emphysema remain poorly defined. Established sources of potentially damaging endopeptidases in the lung are neutrophils, mast cells and macrophages (Citation32). Indeed, the alveolar wall destruction observed in emphysema is generally accepted to be the result of imbalanced actions of neutrophil-derived elastase and serum or bronchial antiproteases (Citation33).
On the other hand, the balance between alveolar cell repair and apoptosis would account for the disappearance of alveolar structures in emphysema. Moreover, there is evidence of ongoing cell proliferation in emphysematous lungs in association with apoptosis (Citation34). Cat E in vivo might exert its anticancer activity via the proteolytic release of soluble TRAIL from not only cancer cells but also these immune system cells, as well as the reduction of Ki-67-positive cells in xenografts treated with Cat E suggest that this enzyme might regulate cellular proliferation rates (Citation14). Using genetic mouse models and human COPD lung specimens, researchers discovered that Cat E promoted emphysema through mitochondrial fission-induced cell death (Citation17).
However, the correlation of this work to clinical practice is unclear in that the applicability of animal models of COPD to human disease remains unexplored. We therefore hypothesized that some causative factors for the development of COPD, such as cigarette smoking, may stimulate Cat E expression in epithelial cells of the airway and initiate the cascade of molecular changes which result in emphysema. In fact, the present study also showed that the clinical severity of emphysema and the concentration of sputum Cat E increased gradually with the degree of COPD with stage I through stage IV, suggesting that emphysema might be potentially related with Cat E. Moreover, most of the above-mentioned reports suggested that Cat E might be an important mediator of the mechanisms leading to emphysema in COPD.
Additionally, previous studies have shown that the activity of proteolytic enzymes plays an important pathogenetic role in the development of respiratory conditions like emphysema and COPD (Citation35, Citation36). In this regard, the emphysema is thought to result in part from a combination of increased elastinolytic activity and the loss of EIC, namely protease-antiprotease imbalance. Serum EIC levels represent the functional status of anti-enzyme system in the human body. Researches showed that CatE possesses significant proteolytic activity at the physiological pH with an apparently rather restricted cleavage specificity (Citation37, Citation38).
Consequently, we wanted to identify whether there was a relationship between Cath E and EIC, which might support the hypothesis that Cat E played a major role in the pathogenesis of COPD. In the present study, there was a significantly inverse correlation between EIC and Cat E in the COPD patients (r = −0.922, P < 0.01), which suggested that Cat E might have a potential relationship with the pathogenesis of emphysema. Moreover, the correlation of FEV1% with EIC was not only negative, but also provides additional evidence supporting our findings that deterioration in FEV1% was inversely related with Cat E in the sputum.
It is well known that inflammation plays a pivotal role in the pathogenesis of COPD patients in both human and mouse lungs. Several studies have indicated that the numbers of macrophages, T lymphocytes and neutrophils are increased in the lung tissue of patients with COPD (Citation39, Citation40). Snoeck-Stroband et al. have also shown that the increased inflammatory cell counts obtained from the induced sputum of COPD patients were closely correlated with the declining health status (Citation41). Additionally, both neutrophils (Citation42) and alveolar macrophages (AMs) (Citation43) in the sputum play a substantial role in systemic inflammation and the pathogenesis of small airway dysfunction in COPD patients. In line with these results, we found that both types of cells contribute to the chronic inflammatory process in the airways of stable COPD patients. Although lung function did not show a distinctive difference between healthy smokers and non-smokers, the number of inflammatory cells did differ dramatically between the two groups.
In conclusion, we found that Cat E might be a novel mediator of the pathogenesis of COPD involved in Cat E-induced emphysema, and the increasing of Cat E expression were closely relevant to the severity of airflow limitation in COPD. Taken together, our findings might provide further clarification of the pathogenesis of COPD at the molecular level, and indicate that Cat E may be one promising biomarker and potentially effective treatment strategy for inhibiting the onset and development of COPD.
Funding
This work was supported by the National Science Foundation of China [NSFC81170003, 81370109] and the Science and Technology Commission of Shanghai Municipality [12PJD004, 134119a6400, 12JC1402300].
Declaration of Interest Statement
We declare that there is no potential conflict of interest associated with this manuscript. The funders did not play any role in the study design, study performance, collection and analysis of data, interpretation of results, or drafting of the manuscript.
The authors alone are responsible for the content and writing of the paper.
References
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380(9859):2163–2196.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2014. Available from: http://www.goldcopd.org/uploads/users/files/ ( accessed 2 November 2014).
- World Health Organization, Reviewed October 2013: Chronic obstructive pulmonary disease (COPD). 2013. Available from: URL: http://www.who.int/mediacentre/factsheets/fs315/en/( accessed 2 November 2014).
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 2001; 164:S28–S38.
- Zaidi N, Kalbacher H. Cathepsin E: A mini review. Biochem Biophys Res Commun 2008; 367(3):517–522.
- Overall CM, Kleifeld O. Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 2006; 6(3):227–239.
- Kawakubo T, Okamoto K, Iwata J. Cathepsin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer Res 2007; 67(22):10869–10878.
- Tsukuba T, Okamoto K, Okamoto Y. Association of cathepsin E deficiency with development of atopic dermatitis. J Biochem 2003; 134(6):893–902.
- Henningsson F, Yamamoto K, Saftig P. A role for cathepsin E in the processing of mast-cell carboxypeptidase A. J Cell Sci 2005; 118(Pt 9):2035–2042.
- Tsukuba T, Yamamoto, S, Yanagawa, M, Yamamoto. Cathepsin E-deficient mice show increased susceptibility to bacterial infection associated with the decreased expression of multiple cell surface Toll-like receptors. J Biochem 2006; 140(1):57–66.
- Sessa F, Bonato M, Frigerio B. Ductal cancers of the pancreas frequently express markers of gastrointestinal epithelial cells. Gastroenterology 1990; 98(6):1655–1665.
- Tenti P, Romagnoli S, Silini E. Cervical adenocarcinomas express markers common to gastric, intestinal, and pancreatobiliary epithelial cells. Pathol Res Pract 1994; 190(4):342–349.
- Ullmann R, Morbini P, Halbwedl I. Protein expression profiles in adenocarcinomas and squamous cell carcinomas of the lung generated using tissue microarrays. J Pathol 2004; 203(3):798–807.
- Yasukochi A, Kawakubo T, Nakamura S. Cathepsin E enhances anticancer activity of doxorubicin on human prostate cancer cells showing resistance to TRAIL-mediated apoptosis. Biol Chem 2010; 391(8):947–958.
- Uno K, Azuma T, Nakajima M. Clinical significance of cathepsin E in pancreatic juice in the diagnosis of pancreatic ductal adenocarcinoma. J Gastroenterol Hepatol 2000; 15(11):1333–1338.
- Kawakubo T, Okamoto K, Iwata J. Cathepsin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer Res 2007; 67(22):10869–10878.
- Zhang X, Shan P, Homer R, et al. Cathepsin E promotes pulmonary emphysema via mitochondrial fission. Am J Pathol 2014; 184(10):2730–2741.
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- Pizzichini E, Pizzichini MM, Leigh R, et al. Safety of sputum induction. Eur Respir J Suppl 2002; 37:9s–18s.
- Demedts IK, Morel-Montero A, Lebecque S, et al. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax 2006; 61(3):196–201.
- Bieth J, Wermuth CG. The action of elastase on p-nitroanilide substrates. Biochem Biophys Res Commun 1973; 53(2):383–390.
- Ying QL, Simon SR. DNA from bronchial secretions modulates elastase inhibition by alpha(1)-proteinase inhibitor and oxidized secretory leukoprotease inhibitor. Am J Respir Cell Mol Biol 2000; 23(4):506–513.
- Borzone G, Liberona L, Olmos P. Rat and hamster species differences in susceptibility to elastase-induced pulmonary emphysema relate to differences in elastase inhibitory capacity. Am J Physiol Regul Integr Comp Physiol 2007; 293(3):R1342–1349.
- Society AT. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152(3):1107–1136.
- Ritz T, Dahme B, Dubois AB. Guidelines for mechanical lung function measurements in psychophysiology. Psychophysiology 2002; 39(5):546–567.
- Cheng DT, Kim DK, Cockayne DA, et al. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188(8):948–957.
- Mori K, Shirai T, Mikamo M, et al. Respiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysema. Respir Physiol Neurobiol 2013; 185(2):235–240.
- Goddard PR, Nicholson EM, Laszlo G. Computed tomography in pulmonary emphysema. Clin Radiol 1982; 33(4):379–387.
- Bosi F, Silini E, Luisetti M. Aspartic proteinases in normal lung and interstitial pulmonary diseases. Am J Respir Cell Mol Biol 1993; 8(6):626–632.
- Nishioku T, Hashimoto K, Yamashita K. Involvement of cathepsin E in exogenous antigen processing in primary cultured murine microglia. J Biol Chem 2002; 277(7):4816–4822.
- Tsukuba T, Okamoto K, Yasuda Y. New functional aspects of cathepsin D and cathepsin E. Mol Cells 2000; 10(6):601–611.
- Senior RM, Connolly NL, Cury JD. Elastin degradation by human alveolar macrophages. A prominent role of metalloproteinase activity. Am Rev Respir Dis 1989; 139(5):1251–1256.
- De Water R, Willems LN, Van Muijen GN. Ultrastructural localization of bronchial antileukoprotease in central and peripheral human airways by a gold-labeling technique using monoclonal antibodies. Am Rev Respir Dis 1986; 133(5):882–890.
- Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest 2004; 125(2):626–632.
- McElvaney NG, Birrer P, Chang-Stroman LM, Crystal RG. Neutrophil elastase and the fragile lung: the pathogenesis and therapeutic strategies relating to lung derangement in the common hereditary lung disorders. In: Grassi C, Travis J, Casali L, Luisetti M, eds. Biochemistry of Pulmonary Emphysema. 1st ed. London, UK: Springer; 1992:169–187.
- Fujita J, Nelson NL, Daughton DM, et al. Evaluation of elastase and antielastase balance in patients with chronic bronchitis and pulmonary emphysema. Am Rev Respir 1990; 142(1):57–62.
- Abd-Elgaliel WR, Tung CH. Selective detection of Cathepsin E proteolytic activity. Biochim Biophys Acta 2010; 1800(9):1002–1008.
- Athauda SB, Takahashi T, Inoue H, et al. Proteolytic activity and cleavage specificity of Cathepsin E at the physiological pH as examined towards the B chain of oxidized insulin. FEBS Lett 1991; 292(1–2):53–56.
- Liao SX, Ding T, Rao XM, et al. Cigarette smoke affects dendritic cell maturation in the small airways of patients with chronic obstructive pulmonary disease. Mol Med Rep 2015; 11(1):219–225.
- Wielgat P, Mroz RM, Stasiak-Barmuta A, et al. Inhaled corticosteroids increase Siglec-5/14 expression in sputum cells of COPD patients. Adv Exp Med Biol 2015; 839:1–5.
- Snoeck-Stroband JB, Postma DS, Lapperre TS. Airway inflammation contributes to health status in COPD: a cross-sectional study. Respir Res 2006; 7:140.
- Lapperre TS, Willems LN, Timens W. Small airways dysfunction and neutrophilic inflammation in bronchial biopsies and BAL in COPD. Chest 2007; 131(1):53–59.
- Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol 2014; 5:435.