Abstract
The authors’ laboratory has reported potent block of Pannexin1 (Panx1) currents by the antimalarial quinine derivative mefloquine. However, other laboratories have found little or no mefloquine sensitivity of Panx1 currents or processes attributable to these channels. In order to resolve this issue, the authors have performed extensive dose-response studies on Panx1-transfected neuroblastoma (Neuro2A) and rat insulinoma (Rin) cells, comparing mefloquine obtained from three suppliers and also comparing the sensitivity to diastereomers. Results indicate a 20-fold difference in sensitivity to the (−)-threo-(11R/2R) diastereomer compared to the erythro enatiomers and much lower potency of (±)-erythro-(R*/S*)-mefloquine obtained from one of the commercial sources. This markedly lower efficacy presumably accounts for the disparity in results from different laboratories who have applied it in Panx1 studies.
INTRODUCTION
The vertebrate pannexin gene family consists of three members, Pannexin1 (Panx1), -2, and -3. Although pannexins are weakly homologous to the invertebrate gap junction proteins, the innexins, they do not share sequence homology with the vertebrate gap junction proteins, the connexins (CitationBarbe et al. 2006; CitationPanchin 2005). Panx1, which is widely expressed in both neural and noneural tissues (Bruzzone et al. 2003), forms high-conductance (about 500 pS) channels in the plasma membrane that are opened by depolarization (Locovei et al. 2007; Pellegrin and Surprenant 2006), strong elevation of intracellular calcium (CitationLocovei et al. 2006), membrane stretch (CitationBao et al. 2004), high extracellular potassium (CitationSilverman et al. 2009), and prolonged activation of the purinergic P2X7 receptor (Locovei et al. 2007; Pellegrin and Surprenant 2006). These last two modes of activation are likely mediators of the inflammasome protein complex in both neural and immune cells and of cell death (CitationKanneganti et al. 2007; CitationSilverman et al. 2009; Locovei et al. 2007), and such activation is proposed to amplify erythrocyte hemolysis in response to alpha-hemolysin (CitationSkals et al. 2009). Moreover, Panx1 channel opening under hypoxic conditions likely contributes to neuronal cell death in ischemia through efflux of glucose, glutamate, and ATP and influx of Ca2+ (CitationBargiotas et al. 2009; CitationThompson et al. 2006).
In addition to Panx1 channels, several types of outwardly rectifying currents have been proposed to participate in the release and uptake of moderately large molecules, including the volume-regulated anion channels, calcium-activated chloride channels (VRACs and CaCCs; see CitationKimelberg 2005; CitationNilius and Droogmans 2003), and the nonjunctional, half gap junction channels or “hemichannels” (see CitationSpray et al. 2006; CitationContreras et al. 2004). Although a number of pharmacological treatments are known to affect these various channel types, drug effects overlap, and high-affinity blockers specific for Panx1 are unknown. We have suggested that the antimalarial drug mefloquine, which we discovered to be highly effective in blocking gap junctions formed of certain connexins but not others (CitationCruikshank et al. 2004), might be such a compound when used at very low concentrations. Although IC50 values for blockade of gap junction channels were generally above 10 μM, nanomolar mefloquine concentrations were shown to block dye uptake evoked by P2X7 receptor stimulation in astrocytes (CitationSuadicani et al. 2006) and to inhibit both depolarization- and BzATP-evoked Panx1 currents in astrocytes and in the J774 macrophage cell line (CitationIglesias et al. 2008, Citation2009). However, this high sensitivity of Panx1-related phenomena to mefloquine has not been observed in studies by another group (CitationMa et al. 2009; CitationPelegrin et al. 2008), and prevention of HIyA-induced hemolysis in human and murine erythrocytes required concentration >10 μM (Skas et al. 2009).
We have attempted to resolve this issue through quantitative evaluation of the effects of one diastereomer of this compound as well as samples of a racemic mixture of two enatiomers obtained from three sources. We tested the race-mate (±)-erythro-(R*/S*)-mefloquine originally developed at Walter Reed Army Institute for Research in the 1970s, and those obtained from Sigma Chemical Corporation and from Bioblocks, as well as the diastereomer (−)-threo-(R/R)-mefloquine from Bioblocks. Our inhibition-response curves for the effects of mefloquine on depolarization-induced Panx1 currents in transfected Neuro2A cells indicate high and virtually identical potencies of the Walter Reed and Bioblocks (±)-erythro-(R*/S*)-mefloquine and 10-fold difference in sensitivity to the (±)-erythro and (−)-threo diastereomers of mefloquine from Bioblocks. The much lower potency of the mefloquine obtained from the other supplier presumably explains the discrepant results reported by different laboratories.
MATERIALS AND METHODS
Cell Cultures
The mouse neuroblastoma cell line Neuro2A and the rat beta cell insulinoma line Rin-m were originally obtained from American Tissue Type Collection (Rockville MD). Neuro2A cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% fetal calf serum (Gibco) and 1% penicillin/streptomycin. Rin-m cells were cultured in RMPI medium (Gibco), supplemented with 5% fetal calf serum and 1% penicillin/streptomycin. Both cell lines were maintained in a humidified incubator with 5% CO2 at 37°C and generally passaged twice a week and not used beyond the 20th passage from our original stock.
Transfection with Panx1 cDNA
Neuro2A and Rin-m cells were transfected with 2 μg of mouse (m)Panx1-GFP (green fluorescent protein) in mGFP-N1 vector using 6 μl lipofectamine reagent (Invitrogen) in 1.5 ml Optimen (Gibco). In some experiments untagged mPanx1 constructs were also employed. The mPanx1 construct was originally obtained from Dr. Georg Zoidl (Rurh University, Bochum, Germany) in the pEYFP vector, from which the Panx1 sequence was polymerase chain reaction (PCR) amplified and inserted into the monomeric GFP vector (a gift from Dr. Erik Snapp, Albert Einstein College of Medicine) to generate a GFP-tagged Panx1 in a pcDNA3 vector. After overnight exposure, transfection reagents were removed and cells replated on coverslips for an additional 24–36-h incubation in DMEM or RPMI medium.
Electrophysiology
Neuro2A and Rin-m cells were plated on coverslips 12–24 h at low confluence prior to recordings. The single-whole-cell and inside-out patch clamp recording configurations were performed at room temperature on brightly fluorescent cells bathed in external solution containing (mM): NaCl 147, Hepes 10, glucose 13, CaCl2 2, MgCl2 1, and KCl 2, pH 7.4. Patch pipettes (resistance 4–6 MOhms) were filled with solution containing (mM): CsCl 130, EGTA 10, Hepes 10, CaCl2 0.5, and 1 mM ATP, and connected to an Axopatch 1D amplifier (Molecular Devices). Membrane potential was routinely held at −60 mV. Voltage activation of Panx1 channels was achieved using 10-s voltage ramps from −60 to +100 mV (16 mV/s) with 20-s intervals between ramps.
For pharmacological analysis, mefloquine was obtained from several sources as indicated in . These blockers were superfused while applying voltage ramps. Immediately after the effect of the drug reached a stable plateau, reagents were washed with external solution and new series of voltage ramps were applied to evaluate reversibility of the blockers. Data were acquired with Clampex 6.0 or 8.2 software, digitized using an Axon Instruments Digitizer and analyzed with Clampfit 9.0 software (Molecular Devices).
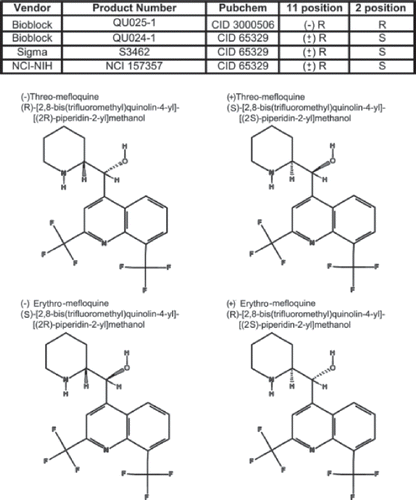
Figure 1. Molecular structures and sources of mefloquine isomers used in this study. The table indicates the sources, catalogue number, PubChem number, and the position of carbon asymmetry of mefloquine isomers employed in the present study. The molecular structures of the four diastereomers of mefloquine shown were obtained from PubChem.
RESULTS
Mefloquine has two asymmetric carbon centers, and therefore has four different diastereomers. Mefloquine is usually sold as a racemic mixture of the (±)-erythro-(R*/S*) isomers by several companies, including Sigma and Bioblocks, and the latter also provides one of the mefloquine diastereomers, the (−)-threo-(11R/2’R), but not the other, (+)-threo(11S/2’S).
In the present study, we evaluated whether Panx1 currents induced in transfected Neuro2A cells were equally sensitive to the racemic mixture of the (±)-erythro-(R*/S*)-mefloquine from three different sources (NCI-NIH, Sigma, Bioblocks) and to the mefloquine diastereomer (−)-threo-(11R/2’R) from Bioblocks. We chose a wide range of drug concentrations (1 nM to 1 mM) in order to completely characterize the sensitivity of Panx1 currents to each drug. Mefloquine was applied only on cells that displayed substantial voltage activation of Panx1 currents upon depolarization beyond 0 mV.
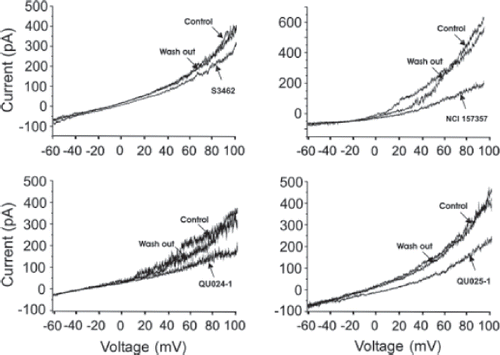
All mefloquines obtained from different sources reduced the magnitude of the Panx1-GFP currents evoked by depolarizing voltage ramps (). However, the effective concentrations for the different mefloquines were substantially different (). The race-mate (±)-erythro-(R*/S*)-mefloquine from Sigma only minimally blocked the currents at 100 μM, whereas the same racemic mixture from Bioblocks and from NCINIH substantially reduced currents when applied at 100 nM. The mefloquine diastereomer (−)-threo-(11R/2’R) from Bioblocks prevented Panx1-GFP currents when applied at an intermediate concentration (1 μM).
Figure 2. Pannexin voltage–activated current is differently inhibited by mefloquine from different sources and different diastereomers. (A–D) Representative traces of 10-s voltage ramps from the holding potential of −60 to +100 mV applied in Neuro2A cells in whole-cell patch clamp configuration. (A) 100 μM of (±)-erythro-(R*/S*)-mefloquine (Sigma S3462) was perfused during the voltage protocol and washed out after maximal inhibition was achieved. (B) 100 nM (±)-erythro-(R*/S*)-mefloquine (NSC 157357) was perfused and then washed out. (C) 100 nM of (±)-erythro-(R*/S*)-mefloquine (Bioblocks QU024-1) was perfused during the voltage protocol and washed out. (D) 1 μM (−)-threo-(11R/2R)-mefloquine (Bioblocks QU025-1) was perfused and washed out. Note that the drug concentration necessary to inhibit Panx1-GFP currents is higher for QU025-1 and S3462 compared with NSC157357 and QU024-1.
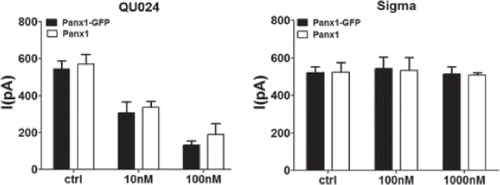
To evaluate whether distinct sensitivities to the different mefloquines could be related to GFP tag, we performed similar experiments on untagged Panx1. As shown in , both Panx1-GFP–tagged and untagged Panx1 currents were similarly reduced by 10 and 100 nM mefloquine from Bioblocks and were not affected by 100 nM and 1 μM mefloquine from Sigma. Given that no significant differences were observed between tagged and untagged Panx1 in terms of their sensitivity to distinct mefloquines, all subsequent experiments were performed on cells expressing Panx1-GFP.
Figure 3. Similar mefloquine sensitivities of untagged and GFP-tagged Panx1. Bar histograms showing the mean ± SE current values of Panx1-GFP (black bars) and untagged Panx1 (white bars) to different concentrations of mefloquine from Bioblocks (QU024) and from Sigma. Note that both tagged and untagged Panx1 currents were similarly reduced by 10 nM and by 100 nM mefloquine from Bioblocks (QU024) and were not affected by 100 and 1000 nM mefloquine from Sigma. Experiments were performed on three to eight N2A cells transfected with mPanx1 and mPanx1-GFP.
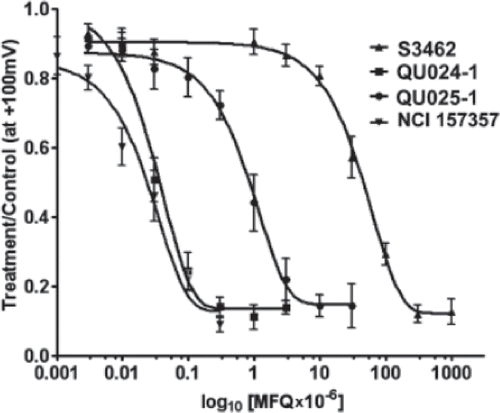
Concentration-response curves for the four drugs () demonstrate that erythro-mefloquine race-mate from Bioblocks and from NCI-NIH displayed very similar IC50 values (52.7 ± 2.2 and 47.3 ±1.2 nM, n = 9 and 11, respectively; p > .05, t test), whereas that from Sigma was 10,000 times less potent than from the two other sources (IC50 483.8 ± 37.5 μM, n = 7). Compared to the race-mate (±)-erythro-(R*/S*)-mefloquine from Bioblocks, the EC50 value of the mefloquine diastereomer (−)-threo-(11R/2’R) was shifted to the right by about 20-fold (EC50 = 0.8 ± 0.25 μM, n = 7).
Figure 4. Dose-response curves for the mefloquine isomers obtained from different sources. Dose response curves for mefloquines obtained from Bioblocks (QU024-1 and QU025-1), Sigma (S3462) and from National Cancer Institute (NCI 157357). Note that both QUO24 and NSC157357 (±)-erythro-(R*/S*)-mefloquine have very similar dose response curves. Number of cells for each point ranged from 4 to 17.
As illustrated in , Panx1-GFP currents were recovered following washout of each compound, showing complete reversibility for all mefloquines. The time required for 50% blockade of Panx1 currents by (±)-erythro-(R*/S*)-mefloquine from Bioblocks and NCI-NIH when applied at 100 nM was 0.5 min after the application (, ; analysis of variance [ANOVA] followed by Dunnett’s multiple comparison test); longer time (1.5 min) for 50% blockade was observed when using higher concentrations of (±)-erythro-(R*/S*)-mefloquine (1 mM) from Sigma and of (−)-threo-(11R/2’R)-mefloquine from Bioblocks (10 μM) (, ; ANOVA followed by Dunnett’s multiple comparison test).
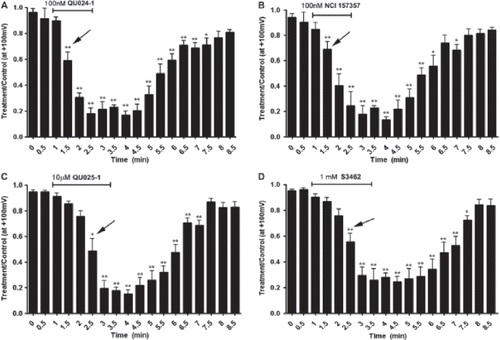
Figure 5. Kinetics of mefloquine action. (A–D) Plots showing currents at +100 mV during voltage ramps delivered every 30 s for 8.5 min, during which cells were perfused with different mefloquines. Effects of mefloquine concentrations illustrated are 100 nM for QU024-1 (A), 100 nM for NSC157357 (B), 10 μm for QU025-1 (C), and 1 mM for S3462 (D). Arrows indicate the time in which current amplitudes were significantly reduced compared to control (time 0 min) by the tested compounds. Note that for both QU024-1 and NSC157357 current amplitudes were significantly reduced at time 1.5 min, whereas for the other two compounds (QU025-1 and S3462) current reduction occurred at time 2.5 min. *p < .01; **p < .001 (ANOVA followed by Dunnett’s multiple comparison test).
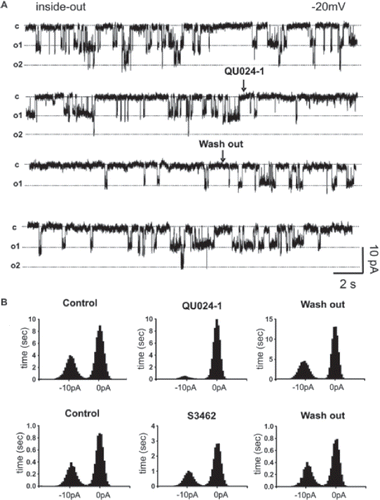
In order to further investigate the effect of the mefloquine compounds on individual Panx1 channels, inside-out patch clamp recordings were performed on the insulinoma cell line, Rin-m transfected with Panx1-GFP. These cells have been demonstrated to not express connexins in the membrane (Citationdel Corsso et al 2006), therefore eliminating the possible presence of connexin hemichannels in our recordings. Single channels with unitary conductance ∼450 pS, as reported for Panx1-GFP channels (CitationBao et al. 2004) were found in four of six Panx1-transfected cells and in zero of seven recordings from parental Rin-m cells. Perfusion of 100 nM (±)-erythro-(R*/S*)-mefloquine from Bioblocks for 20 s decreased the open time of Panx1-GFP channel (, ). After washout, the mean open time of Panx1 channel was restored. By contrast, 1 μM (±)-erythro-(R*/S*)-mefloquine from Sigma did not appreciably affect Panx1-GFP open time ().
Figure 6. Open time of Panx1 channels is reduced by (±)-erythro-(R*/S*)-mefloquine from Bioblocks (QUO24-1) but not by that from Sigma (S3462). Single-channel inside-out recordings were performed on mPanx1-GFP–transfected Rin-m cells. (A) Single channels were recorded while holding the pipette potential at −20 mV; arrows indicate the moments of drug application and washout. (B) Bar histograms showing the frequency distribution of time that Panx1 channel spent at two conductance states (closed: 0 pA; open: −10 pA). Note that QU024 but not S3462 mefloquine greatly reduced the time that Panx1 spent on the open state. Analyses were performed on 60–90-s single-channel recordings digitized at 2000 Hz.
DISCUSSION
In the present study, we evaluated the pharmacological properties of the racemic mixture (±)-erythro-(R*/S*)-mefloquine (obtained from three sources) and of the diastereomer (−)-threo-(11R/2’R)-mefloquine on exogenously expressed Panx1. Our findings indicate that two sources of the racemic mixture of erythro-mefloquine from two sources were more effective for the inhibition of Panx1 currents than the other and that the erythro-(R/R) mefloquine had intermediate potency.
Mefloquine is a chiral compound that contains two centers of asymmetry at positions 11 and 2' and has four diastereomers, (−)-threo-(11S/2’S)-, (+)-threo-(11R/2’R)-, (+)-erythro-(11R/2’S)-, and (−)-erythro-(11S/2’R)-mefloquine (see ). Mefloquine (Lariam) is administered clinically as racemic mixture of the (±)-erythro-(R*/S*) diastereomer (review in CitationBrocks and Mehvar 2003) for the prevention of chloroquine-resistant malaria. Interestingly, the (−)-erythro enantiomer was reported to bind to adenosine receptors (CitationWeiss et al., 2003), which supposedly causes neuropsychiatric reactions and convulsions, one of the associated neurotoxic side effects of this antimalarial drug (Bern, et al 1995; review by Borcks, 2003). Unfortunately, because the individual isomers are not commercially available for most of the racemic antimalarial drugs, it was not possible to evaluate the concentration-effect relationships for all (±)-erythro enantiomers.
A deficiency that is limiting progress in studies of both connexons and pannexons is that of specific and efficacious inhibitors. A comparison of sensitivity of Cx46 hemichannels and Panx1 channels in oocytes led to the recognition that there might be a 5–10-fold difference in potency for blockade by carbenoxolone and flufenamic acid (CitationBruzzone et al. 2006), although comparable studies have not been performed on Cx43, which does not form nonjunctional channels in oocytes (CitationWhite et al. 1999). A drug that has been shown to have marked specificity for inhibition of gap junction channels formed by certain connexins is (±)-erythro-mefloquine, with an IC50 of 0.3 μM for Cx36 and 12 μM for Cx43 (Cruikshank et al. 2007). We have previously shown that (±)-erythro-mefloquine, at nM concentrations, blocks dye uptake evoked by P2X7 receptor stimulation in astrocytes (CitationSuadicani et al. 2006) and blocks both depolarization and BzATP-evoked currents in the J774 macrophage cell line and in astrocytes (CitationIglesias et al. 2008, Citation2009). Furthermore, mefloquine was shown to prevent hemolysis in murine erythrocytes but at >10 μM (CitationSkals et al. 2009). Our data in this study provide further evidence that (±)-erythro-melfoquine from BioBlocks and NCI-NHI but not from Sigma can be used to differentiate Panx1 currents from those through connexin channels. Although the nature of differences in potency between commercial suppliers of mefloquine among sources is unknown, our results likely explain the reports of lower sensitivity to this compound by other groups. Moreover, our findings indicate a remarkable specificity of action of mefloquine diastereomers, which may be of utility in studies designed to limit inhibition to certain targets while sparing others.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Bao L, Locovei S, Dahl G (2004). Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572: 65–68.
- Barbe MT, Monyer H, Bruzzone R (2006). Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda). 21: 103–114.
- Bargiotas P, Monyer H, Schwaninger M (2009). Hemichannels in cerebral ischemia. Curr Mol Med. 9: 186–194.
- Brocks DR, Mehvar R (2003). Stereoselectivity in the pharmacodynamics and pharmacokinetics of the chiralantimalarial drugs. Clin Pharmacokinet. 42: 1359–1382.
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H (2005). Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 92: 1033–1043.
- Contreras JE, Sánchez HA, Véliz LP, Bukauskas FF, Bennett MV, Sáez JC (2004). Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev. 47: 290–303.
- Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M (2004). Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci U S A. 101: 12364–12369.
- Del Corsso C, Srinivas M, Urban-Maldonado M, Moreno AP, Fort AG, Fishman GI, Spray DC (2006). Transfection of mammalian cells with connexins and measurement of voltagesensitivity of their gap junctions. Nat Protoc. 4: 1799–809.
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E (2009). Pannexin 1: The molecular substrate of astrocyte “hemichannels.” J Neurosci. 29: 7092–7097.
- Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E (2008). P2X7 receptor-Pannexin1 complex: Pharmacology and signaling. Am J Physiol Cell Physiol. 295: C752–C760.
- Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Núñez G (2007). Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 26: 433–443.
- Kimelberg HK (2005). Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy [review]. Glia. 50: 389–397.
- Locovei S, Wang J, Dahl G (2006). Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580: 239–244.
- Ma W, Hui H, Pelegrin P, Surprenant A (2009). Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 328: 409–418.
- Nilius B, Droogmans G. (2003). Amazing chloride channels: An overview. Acta Physiol. Scand. 177: 119–147.
- Panchin YV (2005). Evolution of gap junction proteins—The pannexin alternative. J Exp Biol. 208(Pt 8): 1415–1419.
- Pelegrin P, Barroso-Gutierrez C, Surprenant A (2008). P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 180: 7147–7157.
- Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G (2009). The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 284: 18143–18151.
- Skals M, Jorgensen NR, Leipziger J, Praetorius HÁ (2009). Alpha-hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci U S A. 106: 4030–4035.
- Spray DC, Ye ZC, Ransom BR (2006). Functional connexin “hemichannels”: A critical appraisal. Glia. 54: 758–773.
- Suadicani SO, Brosnan CF, Scemes E (2006). P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 26: 1378–1385.
- Thompson RJ, Zhou N, MacVicar BA (2006). Ischemia opens neuronal gap junction hemichannels. Science. 312: 924–927.
- White TW, Deans MR, O’Brien J, Al-Ubaidi MR, Goodenough DA, Ripps H, Bruzzone R (1999). Functional characteristics of skate connexin35, a member of the gamma subfamily of connexins expressed in the vertebrate retina. Eur J Neurosci. 11: 1883–1890.
- Weiss SM, Benwell K, Cliffe A, Gillespie RJ, Knight AR, Lerpiniere J, Misra A, Pratt RM, Revell D, Upton R, Dourish CT (2003). Discovery of nonxanthine adenosine A2A receptor antagonists for the treatment of Parkinson’s disease. Neurology. 61: S101–S106.