Abstract
Areca quid (AQ) chewing is a popular oral habit, especially in Southeast Asia cultures, in which children may be engaged in the addictive habit early in their lives. Extracts of areca nuts, the main component of AQ, have been shown to affect the functionality of T-cells. However, the potential influence of ANE on the development of T-cells is unknown. This study, therefore, investigated the impact of areca nut extracts (ANE) on thymocytes and the potential mechanisms of action. Mice administered intraperitoneally with ANE at 1, 5, or 25 mg/kg daily for 5 days showed significant dose-dependent reductions in thymocyte viability. A marked decrease in the total number of thymocytes and the proportion of thymic CD4+CD8+ cells was observed in the 25 mg ANE/kg-treated mice, whereas the proportion of CD4 and CD8 single positive and CD4−CD8− cells was significantly increased. Further examination on the functionality of thymocytes showed that ANE suppress IL-2 production both ex vivo and in vitro. These results suggest that ANE may attenuate the development and functionality of thymic T-cells. ANE also directly induced apoptosis in thymic T-cells through activation of casapase-3 and apoptosis inducing factor (AIF). Collectively, the data suggested that the thymus is a sensitive target to ANE. Early exposure to ANE may interfere with the development and functionality of thymic T-cells.
Introduction
Areca quid (AQ) chewing is one of the most addictive oral habits in the world (Wollina et al., Citation2004). There are >600 million AQ chewers in the world, mostly in Southeast Asia (Gupta & Warnakulasuriya, Citation2002). AQ chewing is a major risk factor for oral cancers and pre-cancer lesions (Coppola & Mondola, Citation2012), whose pathophysiology has been shown to be associated with immune deterioration, including alterations of T-cell immunity (Chang et al., Citation2005). Epidemiological evidence suggests that, in certain cultures, children may start AQ chewing before age 10 and pre-natal exposure to areca ingredients may exist due to the continuing chewing habit in the pregnant female population (Farrand et al., Citation2001; Shah et al., Citation2002). Exposure to environment toxicants during early stages of immunological development has been demonstrated to compromise immunity and increase susceptibility to infections (Dietert & Dewitt, Citation2010). Clinical findings have linked areca chewing and the deregulation of T-cell-mediated immune responses in AQ chewers and oral cancer patients (Changrani & Gany, Citation2005; Haque et al., Citation2000). However, compared to the rich data on the influence of chewing areca nuts to the oral mucosa and matured T-cells, little is known about the impact of areca nut ingredients on the immune system under development.
We previously reported that the ingredients of areca nuts, the main component of AQ, directly attenuated T-cell functionality (Wang et al., Citation2007). T-cells are key effector cells in the acquired immunity, which participate in a wide range of immune responses through a complicated cytokine network and interactions with other cells (Doherty, Citation1993). Early T-cell progenitors are generated in the bone marrow and migrate to the thymus, where multi-stage lineage commitment and differentiation steps take place to yield mature T-cells (Ciofani & Zuniga-Pflucker, Citation2007). Atrophy in the thymus has been recognized as one of the key events that may lead to inefficient functioning of the immune system and is, therefore, used as a sensitive index to detect the adverse effects of toxicants on lymphocyte development. Thymic atrophy has been reported in bacterial and viral infections, tumor development, and exposure to immunosuppressive chemicals (Frawley et al., Citation2011; Haynes et al., Citation2000; Taub & Longo, Citation2005), accompanied by a severe depletion of immature thymocytes (CD4+CD8+ cells) and an increase in the percentage of CD4+CD8−, CD4−CD8+, and CD4−CD8− thymocytes.
ANE was shown to be toxic to various immune cells. For example, the functionality of bone marrow cells, neutrophils, and dendritic cells has been reported attenuated by exposure to areca ingredients in vitro (Hung et al., Citation2006; Kumpawat et al., Citation2003; Wang et al., Citation2012). In addition, we previously reported that ANE directly inhibited proliferation of T-cells and impaired T-cell-mediated immune responses in vitro (Wang et al., Citation2007). Early studies demonstrated that subcutaneously injection of sub-toxic dosage of arecoline, the major areca-derived alkaloid, induced thymus atrophy in vivo, demonstrating the potential immunotoxic effects of areca ingredients on the thymus (Selvan et al., Citation1989). However, whether exposure to areca nut extracts may deteriorate thymocyte functions remains mostly unknown.
ANE-mediated splenocyte dysfunction was suggested through induction of cell apoptosis (Wang et al., Citation2009b). Apoptosis is a key process for maintaining physiological homeostasis, including maintenance of immune homeostasis. Generally, apoptosis plays an important role in the termination of the acute phase immune responses and the deletion of self-reacting T-cells in the thymus during negative selection (Palmer, Citation2003). However, the exceeding induction of apoptosis in immunocompetent cells may lead to immunosuppression. Inhibition of T-cell development or induction of thymocyte apoptosis have been demonstrated in chemical-induced immunotoxicity (Fine et al., Citation1990; Kamath et al., Citation1997; Lundberg et al., Citation1990). The presented study was, therefore, designed to investigate the effect of ANE on the viability, cellularity, and functionality of thymocytes in vivo and to study the role of apoptosis in ANE-mediated effects on thymocytes in vitro.
Materials and methods
Reagents and areca nut extract (ANE) preparation
Fetal bovine serum (FBS) and RPMI 1640 medium were purchased from Hyclone (Logan, UT). Reagents for an IL-2 ELISA were purchased from BD Biosciences (San Jose, CA). All other reagents were from Sigma (St. Louis, MO). Areca nut extracts (ANE) were prepared and freeze-dried as previously described (Liu et al., Citation1996). In brief, nuts were sliced thinly and extracted three times with water at 35 °C; all the pooled liquids were then filtered through cellulose filter paper. The filtrate was then freeze-dried to obtain ANE; extraction yields routinely were ≈26%. The level of arecoline in the ANE was measured at 8.4 mg/g ANE (using reverse-phase high-performance liquid chromatography). ANE was confirmed endotoxin-free using a Limulus amebocyte lysate assay kit (Kinetic-QCL®; Lonza Walkersville Inc., Walkersville, MD).
Animals and thymocytes cultures
Male BALB/c mice (4-weeks-of-age) were purchased from BioLASCO Taiwan Co. Ltd. (Taipei, Taiwan). On arrival, the mice were randomized and transferred to plastic cages containing sawdust bedding (five/cage) and quarantined at least for 1 week. The mice were housed in a temperature (24 ± 2 °C), humidity (60 ± 20%), and light-controlled environment (12 h light/dark cycle), and each had ad libitum access to standard laboratory food and water. The Institutional Animal Care and Use Committee at the Kaohsiung Medical University approved all the studies performed here.
For in vitro studies investigating the direct effect of ANE exposure on thymocytes, a set of naive mice had their thymus isolated aseptically and made into single cell suspensions as described previously (Lee et al., Citation2008; Wang et al., Citation2007). The thymocytes were then cultured in RPMI 1640 medium supplemented with 100 U penicillin/ml, 100 µg streptomycin/ml, and 5% heat-inactivated FBS. In all cases, cells were cultured at 37 °C in a 5% CO2 incubator.
For in vivo studies here, mice (five per group) were either left untreated (naïve; NA), or administered ANE (1–25 mg/kg) or vehicle (VH; saline) by intraperitoneal (IP) injection once a day on 5 consecutive days. The mice were then euthanized by CO2 inhalation 24 h after the final dosing. The weight of each mouse was taken and then the thymus of each was harvested for measurements of cellularity, metabolic activity, and interleukin (IL)-2 production.
Analysis of thymocyte cellularity
Thymocytes obtained from each group in the in vivo studies were stained with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD4 and PE-Cy5-conjugated rat anti-mouse CD8 antibodies (BioLegend, San Diego, CA). In brief, 1 × 106 thymocytes were incubated with the antibodies (2 μg/ml for CD4 and 1 μg/ml for CD8) in the dark on ice for 30 min. After washing to remove unbound antibody, the single cell fluorescence of 5000 cells in each sample was measured by a flow cytometer (BD FACSCalibur, San Jose, CA). All data were analyzed using Flowjo 8.0 software (Tree Star Inc., Ashland, OR).
Measurement of thymocyte metabolic activity
Thymocytes (5 × 106 cells/ml) were cultured (in quadruplicate) in 96-well plates (at 100 μl/well). For the ex vivo studies, the same number of viable thymocytes isolated from NA-, VH-, and ANE-treated mice were cultured (5 × 105 live cells/well) and stimulated with phorbol-12-myristate-13-acetate plus ionomycin (PI, 80 nM/1 μM) for 24 h. For the in vitro studies, naïve mice thymocytes were left untreated or stimulated with PI (80 nM/1 μM) in the absence or presence of ANE (1–40 μg/ml) for 24 h. The range of ANE concentrations employed was based on previous reports examining the efficacy of ANE to suppress cytokine production by splenocytes (Wang et al., Citation2007). The metabolic activity of the thymocytes was measured using the 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. In brief, at the end of the 24 h culture period, the cells were treated MTT reagent (50 μg/ml) and then cultured a further 4 h before being lysed with 10% SDS in N.N-demethylformamide overnight in the dark. The optical densities of the samples in each well were then measured using a microplate reader (Molecular Devices, Inc., Sunnyvale, CA) at 570 nm, using 630 nm as background reference.
IL-2 quantification
For the ex vivo studies, the same number of viable thymocytes from NA, VH-, and ANE-treated mice were cultured (in quadruplicate; 1.25 × 106 live cells/well) in 48-well plates, followed by PMA/Io stimulation for 24 h. For the in vitro studies, thymocytes were cultured (in quadruplicate; 1.25 × 106 cells/ml) for 24 h in 48-well plates in the absence or presence of ANE (1–40 μg/ml). Supernatants of thymocytes stimulated with PMA/Io (as described above) were harvested, and IL-2 content quantified using a commercial sandwich ELISA kit (BD Biosciences; 3.1 pg/ml sensitivity) as previously described (Wang et al., Citation2007).
Cell cycle analysis
Flow cytometric analysis of cell cycle distribution of thymocytes was conducted as previously described (Wang et al., Citation2009b). Briefly, thymocytes were fixed with 70% ethanol and then incubated with RNase (0.1 mg/ml) and propidium iodide (50 μg/ml) for 30 min at 37 °C in the dark. The single-cell DNA fluorescence in each sample (5000 cells/sample) was measured in the FL2 channel (using an emission filter of 575 nm) in the BD FACS Calibur flow cytometer. All data were analyzed using Flowjo 8.0 software. Apoptotic cells were defined as those in sub-G0/G1 phase with hypodiploid DNA content (Pagliacci et al., Citation1991).
Terminal dUTP nick-end labeling (TUNEL) assay
Thymocytes were exposed to ANE, and fixed with ethanol as described above. The DNA single-strand breaks in apoptotic cells were measured using a commercial TUNEL assay kit (Roche Diagnostics GmbH, Penzgerg, Germany) as previously described (Wang et al., Citation2009b). Briefly, the fixed cells were stained with terminal deoxynucleotidyl transferase and fluorescein-labeled dUTP for 1 h at 37 °C. After washing, the single-cell fluorescence of 5000 cells in each sample was measured by flow cytometry at a 525 nm emission (FL1). All data were then analyzed using Flowjo 8.0 software.
Western blotting analysis for caspase-3 and apoptosis inducing factor
Thymocytes were left untreated (NA) or treated with ANE (10–40 µg/ml) for 3 h. After washing, the cells were lysed using a buffer containing 250 mM sucrose, 10 mM HEPES (pH 7.5), 50 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol, and protease inhibitors. The cell lysates were then electrophoresed using SDS-PAGE and the resolved proteins transferred to a PDVF membrane. After pre-incubation in blocking buffer (1% skimmed milk in phosphate-buffered saline [PBS, pH 7.4]) to minimize non-specific binding, the blots were incubated at 4 °C overnight with anti-mouse primary polyclonal antibody (Sigma) against caspase-3 (1:2500 dilution), AIF (1:4000 dilution), or actin (1:20 000 dilution; used as loading control). After washing, the blots were then incubated with horseradish peroxidase-conjugated secondary antibody (1:20 000 dilution; Sigma) against the primary forms. The bound enzyme was, in turn, detected using an enhanced chemiluminescence system (Pierce, Rockford, IL), and the intensity of binding quantitatively analyzed using an imaging system (Alpha Innotech Corp, San Leandro, CA).
Statistical analysis
The mean ± SE was determined for each treatment group in the individual experiments. Normality and homoscedasticity of data were tested by the Shapiro-Wilk test and the Bartlett’s test, respectively. Analysis of variance was performed by one-way ANOVA. Dunnett’s two-tailed t-test was used to compare treatment groups to the control group. A p value <0.05 was defined as indicative of statistical significance.
Results
ANE administration decreased thymic cellularity and suppressed IL-2 production
We first examined whether in vivo ANE administration influenced the body weight, as well as thymus cellularity and thymocyte functionality. Mice administered with the highest dose (25 mg/kg) of ANE for five doses demonstrated an 11% decrease in body weight compared to the VH control group (17.20 versus 19.42 g), while lower doses of ANE had no effect (). The thymus weight in ANE (5 and 25 mg/kg)-treated groups was 11% and 40% lighter than that in the VH group, respectively (). Concordantly, total numbers of thymocytes decreased by 11% and 49% in mice administered, respectively, 5 or 25 mg ANE/kg (). Cellularity analyses revealed that the 25 mg ANE/kg treatment increased the proportions of CD4+CD8−, CD8+CD4−, and CD4−CD8− cells, but decreased that of CD4+CD8+ cells and absolute numbers of each population. Levels of immature CD4+CD8+ and CD4−CD8− cells were dramatically decreased by 56% and 36%, respectively, in the 25 mg ANE/kg-treated mice (). Results from the MTT assays showed that ANE administration did not affect the metabolic activity of thymocytes stimulated ex vivo with phorbol-12-myristate-13-acetate plus ionomycin for 24 h (PI; 80 nM/1 μM) (). However, the capability of thymocytes to produce IL-2, a pivotal T-cell growth factor regulating maturation of T-cells in the thymus (Tentori et al., Citation1988), was attenuated by 63% at a high ANE dose (25 mg/kg; ).
Figure 1. Administration of ANE impaired thymocyte IL-2 production. Thymocytes (5 × 106 cells/ml) isolated from different groups treated with or without ANE in vivo were cultured ex vivo and stimulated with PMA + ionomycin for 24 h. (a) Metabolic activity of the thymic cells was measured by MTT assay. (b) Concentrations of IL-2 in culture supernatants were measured by ELISA. Data are expressed as mean (±SE) of quadruplicate cultures. Results are representative of three independent experiments. *Value significantly different compared to VH group at p < 0.05.
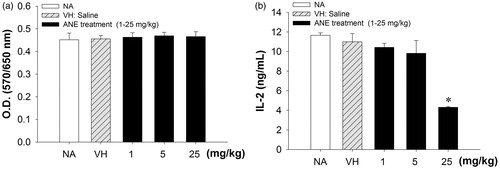
Table 1. The in vivo effect of ANE on body weight and on thymus weight and cellularity.
ANE directly suppressed IL-2 production by thymocytes
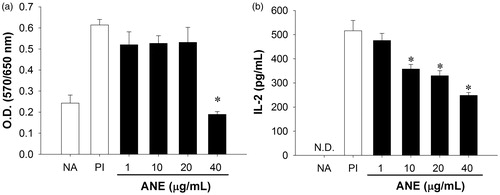
We next investigated whether ANE directly affected the functionality and viability of thymocytes. Naïve thymocytes were left untreated or stimulated with PI (80 nM/1 μM) in the absence or presence of ANE (1–40 μg/ml) for 24 h. As measured via MTT assay, the metabolic activity of the stimulated thymocytes was suppressed by 60% at the highest ANE concentration tested (40 μg/ml; ). In addition, direct exposure to ANE (at 10–40 μg/ml) suppressed PI-induced IL-2 production in a concentration-dependent manner ().
Figure 2. ANE directly suppressed IL-2 production by thymocytes. Thymocytes (5 × 106 cells/ml) were left untreated (NA) or treated with ANE (1–40 µg/ml) for 30 min and then stimulated with PMA + ionomycin for 24 h. (a) Metabolic activity of the thymic cells was measured by MTT assay. (b) Production of IL-2 as measured by ELISA. Data are expressed as mean (±SE) of quadruplicate cultures. Results are representative of three independent experiments. *Value significantly different compared to VH group at p < 0.05.
ANE-induced thymocyte apoptosis
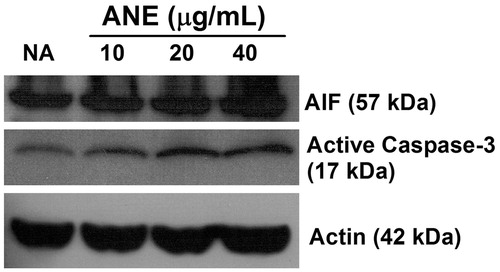
Because ANE was previously shown to induce splenocyte apoptosis (Wang et al., Citation2009b), the effect of ANE on apoptosis of thymocytes was characterized in vitro. Thymocytes were left untreated (NA) or were treated with ANE (0.1–40 μg/ml) for 3 h and then cell apoptosis was examined. Direct exposure to ANE (10–40 μg/ml) markedly enhanced hypodiploid apoptosis in a concentration-related manner (). Hypodiploidity was increased 30% among cells treated with 40 μg ANE/ml as compared to among cells in the NA group (). Results from TUNEL assays confirmed that ANE, at concentrations >10 μg/ml, significantly induced apoptosis (increased from 10–50%; ). We further examined potential underlying mechanisms for ANE-mediated thymocyte apoptosis. Results of the Western blot analyses revealed that apoptosis-inducing factor (AIF), a protein that triggers chromatin condensation and DNA degradation, was increased in ANE-treated thymocytes (). In addition, ANE also increased the levels of cleaved casapase-3 () in these cells.
Figure 3. ANE-induced thymocyte apoptosis. Thymocytes were left untreated (NA) or treated with ANE (1–40 µg/ml) for 12 h, and then fixed with ethanol. The single cell fluorescence of 5000 cells per sample was then measured by flow cytometry. (a) Data presented as a proportion of apoptotic cells, defined as sub-G0/G1-phase cells with hypodiploid DNA content. (b) Portion of TUNEL+ cells. (c) Representative histograms of TUNEL analyses on NA and ANE (20–40 μg/ml)-treated cells. Data are expressed as mean (±SE) of quadruplicate cultures. Results are representative of three independent experiments. *Value significantly different compared to VH group at p < 0.05.
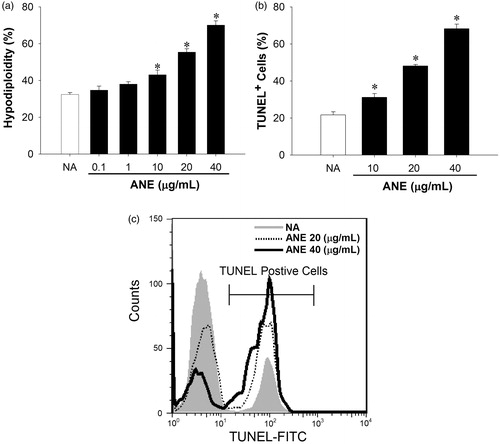
Figure 4. Induction of apoptosis-inducing factor (AIF) and active caspase-3 by ANE. Thymocytes were left untreated (NA) or treated with ANE (10–40 μg/ml) for 3 h. The cells were then lysed and amounts of apoptosis-inducing factor (AIF) and the active form of caspase-3 in the cytosolic extracts were measured using Western blotting. The level of actin was used as a loading control. The data shown are representative of three independent experiments.
Discussion
Epidemiological evidence suggests that female areca nut chewers are likely to continue the chewing habit during pregnancy and breastfeeding (Senn et al., Citation2009), and children under the influence of Southeast Asian cultures may start areca quid (AQ) chewing before the age of 10 (Farrand et al., Citation2001; Senn et al., Citation2009; Shah et al., Citation2002). Clinical findings have linked AQ chewing and the deregulation of T-cell-mediated immune responses in AQ chewers and oral cancer patients (Changrani & Gany, Citation2005; Haque et al., Citation2000). T-cells play a pivotal role in adaptive immunity. We previously reported that areca nut ingredients directly suppressed splenic T-cell functions in vitro and interfered with antigen-specific T-cell immunity in vivo (Wang et al., Citation2007, Citation2011). However, little is known pertaining to the influence of areca nut extracts (ANE) on thymocytes. The thymus provides an essential microenvironment for T-cell development and maturation. Thymus atrophy is one of the key events associated with immune dysfunction. In this study, we demonstrated that exposure to ANE decreased thymic cellularity (a signature of thymic atrophy) in vivo and suppressed IL-2 production by thymocytes, highlighting the potential immunotoxicity of ANE on thymocytes.
Thymic involution has been considered to initiate at puberty; however, functional thymic tissue remains in humans for at least 60 years and plays a pivotal role to generate T-cells under certain circumstances such as human immunodeficiency virus infection (Jamieson et al., Citation1999). Early T-cell progenitors are generated in the bone marrow and migrate to the thymus, where they undergo multi-stage lineage commitment and differentiation steps to yield mature T-cells. Immature thymocytes in the early stages of T-cell maturation are classified as double-negative thymocytes, owing to their lack of CD4 and CD8 expression. Immature thymocytes comprise 1–3% of the total thymus cellularity and are essential for the maintenance of naïve T-cell homeostasis in the periphery (Drela, Citation2006). These thymocytes then develop into CD4CD8 double positive cells that, in turn, then undergo further positive/negative selection to generate major histocompatibility complex-restricted and self-tolerant CD4 or CD8 single positive T-cells.
Thymic atrophy, a key event that may lead to inefficient functioning of the immune system, has been reported in bacterial and viral infections, graft versus host-disease, tumor development, and following exposure to immunosuppressive chemicals (Frawley et al., Citation2011; Haynes et al., Citation2000; Holladay & Luster, Citation1996; Taub & Longo, Citation2005). In the present study, ANE (25 mg/kg) administration reduced the number of both immature thymic cells and mature CD4+ and CD8+ lymphocytes. In addition, the immature thymocytes appeared to be more sensitive than the CD4 and CD8 single-positive cells to ANE-induced toxic effects. These results indicated that ANE might cause thymus dysfunction in vivo. Whether in vivo exposure to ANE suppresses responsiveness of thymocytes to mitogenic stimulation is an intriguing issue that needs more investigation. AQ chewing has been linked to adverse pregnancy outcomes including low birth weight, pre-term labor, and miscarriage (Chue et al., Citation2012; Yang et al., Citation2001). Our results suggested that exposure to areca nut ingredients may cause a deteriorating effect on T-cell development and maturation, which may contribute to the reported adverse pregnancy outcomes.
The major bioactive components in ANE are areca alkaloids and polyphenols (Ranadive et al., Citation1976). Intraperitoneal administration of arecoline (20 mg/kg) has been shown to reduce thymus weight and total number of thymocytes (Selvan et al., Citation1989). In the present study, a significant change in thymus weight was noted in mice administered with 5 or 25 mg ANE/kg. The amount of arecoline in our ANE preparation has been determined to be 6.65 μg/mg (unpublished data). Hence, the dose of arecoline in 5 mg ANE/kg was equivalent to 0.03 mg/kg, a level far lower than the effective dose of arecoline reported previously (Selvan et al., Citation1989). We thus speculate that alkaloids other than arecoline and polyphenols present in areca nuts cannot be ruled out as potential factors contributing to the ANE-induced thymic dysfunction seen here.
A variety of chemicals and environmental toxicants have been reported to induce thymus atrophy that has been used as a sensitive index of immunotoxicity on lymphocytes (Ashwell et al., Citation2000). For example, mice exposed to halogenated and polycyclic aromatic hydrocarbons demonstrate impaired thymocyte maturation and increased susceptibility to infections (Drela, Citation2006; Lutz et al., Citation1998). There are diverse mechanisms involved in chemical-induced thymus atrophy, such as alterations in bone marrow-derived thymocyte precursors, direct toxic effects on thymocytes or thymic epithelial cells, and/or induction of adrenal glucocorticoids (Comment et al., Citation1992; Holladay & Luster, Citation1996; Lundberg, 1991). It has been demonstrated that glucocorticoids inhibit thymocyte functions and induce thymocyte apoptosis in vivo (Young et al., Citation1981). A number of studies reported that chemical-induced thymic atrophy was also associated with increased levels of glucocorticoids (Pruett et al., Citation1993). Other studies showed that elevation of corticosterone was a mechanism contributing to arecoline-induced thymus atrophy (Selvan et al., Citation1989). It is currently unclear whether ANE exposure affects the hypothalamic-pituitary-adrenal axis under the present experimental conditions. This warrants further investigation.
The influence of areca ingredients on the immune system has been intensively investigated. Several in vitro studies demonstrated the toxic effects of ANE on immune cells, including neutrophils, dendritic cells, bone marrow cells, and splenocytes (Panigrahi & Rao, Citation1986; Wang et al., Citation2009a,Citationb; Citation2012). We previously demonstrated that ANE dramatically suppressed IL-2 and interferon (IFN)-γ production in splenocytes via induction of oxidative stress and apoptosis; these may be the underlying mechanisms for ANE-mediated T-cell dysfunction in mature T-cells (Wang et al., Citation2007, Citation2009b). To the best of our knowledge, the effects of ANE on thymocytes have not been reported. In the present study, ANE administration decreased thymic cellularity and decreased total thymocyte numbers. Double-negative thymocytes typically have high proliferation rates and undergo discrete stages of proliferative expansion to generate a large pool of double-positive thymocytes. IL-2, a pivotal cytokine, regulates T-cell growth, proliferation, and differentiation, as well as the maturation of different sub-sets of T-cells in the thymus. Abnormal IL-2 levels are associated with immunodeficiency syndromes and several T-cell lymphoproliferative disorders (Tentori et al., Citation1988). Our data showed that ANE suppressed thymocyte IL-2 production both ex vivo and in vitro, confirming the deteriorating impact of ANE on T-cell functionality.
During the development of T-cells in the thymus, self-reactive T-cells are deleted by apoptosis, a process of programmed cell death important for maintaining physiological homeostasis (Cohen et al., Citation1992). Induction of apoptosis has been reported to be the significant mechanism of copper- and 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced thymus damage (Kamath et al., Citation1997; Mitra et al., Citation2012). In the present study, we also provide one possible mechanism, i.e., an induction of apoptosis, to explain ANE-mediated inhibition of thymocyte proliferation and IL-2 production. Caspase-3 is a cysteine protease that participates in both intrinsic and extrinsic apoptotic pathways that function as central executioners of apoptosis. Apoptosis-inducing factor (AIF), released by mitochondria, is also involved in a caspase-independent cell death pathway (Susin et al., Citation2000). Our data showed that there was activation of caspase-3 and AIF in ANE-treated thymocytes, suggesting that both caspase-dependent and -independent pathways were affected by the product.
Conclusions
The present study demonstrated that ANE decreased thymus cellularity and induced the dysfunction of thymocytes. Thymocytes were a sensitive target to an ANE-mediated immunotoxicity in which induction of apoptosis may play a critical role. Suppression of IL-2 production by ANE in thymocytes may further deteriorate T-cell development. Cellular events triggered by ANE included activation of both caspase-dependent and -independent apoptosis pathways. The toxic effects of ANE on the thymus should raise concerns regarding exposure to areca (and associated) ingredients during pregnancy or by young children.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by grants SKH-TMU-99-10 from Shin Kong Wu Ho Su Memorial Hospital Research Foundation (Taipei, Taiwan), KMU-Q110004 and KMU-ER013 from Kaohsiung Medical University Research Foundation (Kaohsiung City, Taiwan), and NSC101-2321-B-002-053 from National Science Council, Executive Yuan, Taiwan.
References
- Ashwell, J., Lu, F., and Vacchio, M. 2000. Glucocorticoids in T-cell development and function. Annu. Rev. Immunol. 18:309–345
- Chang, M. C., Chiang, C. P., Lin, C. L., et al. 2005. Cell-mediated immunity and head and neck cancer with special emphasis on betel quid chewing habit. Oral Oncol. 41:757–775
- Changrani, J., and Gany, F. 2005. Paan and Gutka in the United States: An emerging threat. J. Immigr. Health 7:103–108
- Chue, A. L., Carrara, V. I., Paw, M. K., et al. 2012. Is areca innocent? The effect of areca (betel) nut chewing in a population of pregnant women on the Thai-Myanmar border. Int. Health 172:204–209
- Ciofani, M., and Zuniga-Pflucker, J. C. 2007. The thymus as an inductive site for T-lymphopoiesis. Annu. Rev. Cell Dev. Biol. 23:463–493
- Cohen, J. J., Duke, R. C., Fadok, V. A., and Sellins, K. S. 1992. Apoptosis and programmed cell death in immunity. Annu. Rev. Immunol. 10:267–293
- Comment, C., Blaylock, B., Germolec, D., et al. 1992. Thymocyte injury after in vitro chemical exposure: Potential mechanisms for thymic atrophy. J. Pharmacol. Exp. Ther. 262:1267–1273
- Coppola, M., and Mondola, R. 2012. Potential action of betel alkaloids on positive and negative symptoms of schizophrenia: A review. Nord. J. Psychiatry 66:73–78
- Dietert, R., and Dewitt, J., (Eds.). 2010. Developmental immunotoxicity (DIT): The why, when, and how of DIT testing. In: Methods in Molecular Biology. Clifton, NJ: Humana Press, pp. 598:17–25
- Doherty, P. C. 1993. Immune exhaustion: Driving virus-specific CD8+ T-cells to death. Trends Microbiol. 1:207–209
- Drela, N. 2006. Xenobiotic-induced alterations in thymocyte development. APMIS 114:399–419
- Farrand, P., Rowe, R. M., Johnston, A., and Murdoch, H. 2001. Prevalence, age of onset and demographic relationships of different areca nut habits amongst children in Tower Hamlets, London. Br. Dent. J. 190:150–154
- Fine, J. S., Silverstone, A. E., and Gasiewicz, T. A. 1990. Impairment of prothymocyte activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Immunol. 144:1169–1176
- Frawley, R., White, K. Jr, Brown, R., et al. 2011. Gene expression alterations in immune system pathways in the thymus after exposure to immunosuppressive chemicals. Environ. Health Perspect. 119:371–376
- Gupta, P. C., and Warnakulasuriya, S. 2002. Global epidemiology of areca nut usage. Addict. Biol. 7:77–83
- Haque, M. F., Meghji, S., Khitab, U., and Harris, M. 2000. Oral submucous fibrosis patients have altered levels of cytokine production. J. Oral Pathol. Med. 29:123–128
- Haynes, B. F., Markert, M. L., Sempowski, G. D., et al. 2000. The role of thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 18:529–560
- Holladay, S. D., and Luster, M. I. 1996. Alterations in fetal thymic and liver hematopoietic cells as indicators of exposure to developmental immunotoxicants. Environ. Health Perspect. 104:809–813
- Hung, S. L., Lee, Y. Y., Liu, T. Y., et al. 2006. Modulation of phagocytosis, chemotaxis, and adhesion of neutrophils by areca nut extracts. J. Periodontol. 77:579–585
- Jamieson, B., Douek, D., Killian, S., et al. 1999. Generation of functional thymocytes in the human adult. Immunity 10:569–575
- Kamath, A. B., Xu, H., Nagarkatti, P. S., and Nagarkatti, M. 1997. Evidence for the induction of apoptosis in thymocytes by 2,3,7,8-tetrachlorodibenzo-p-dioxin in vivo. Toxicol. Appl. Pharmacol. 142:367–377
- Kumpawat, K., Deb, S., Ray, S., and Chatterjee, A. 2003. Genotoxic effect of raw betel nut extract in relation to endogenous glutathione levels and its mechanism of action in mammalian cells. Mutat. Res. 538:1–12
- Lee, C. Y., Wey, S. P., Liao, M. H., et al. 2008. A comparative study on cannabidiol-induced apoptosis in murine thymocytes and EL-4 thymoma cells. Int. Immunopharmacol. 8:732–740
- Liu, T. Y., Chen, C. L., and Chi, C. W. 1996. Oxidative damage to DNA induced by areca nut extract. Mutat. Res. 367:25–31
- Lundberg, K. 1991. Dexamethasone and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) can induce thymic atrophy by different mechanisms in mice. Biochem. Biophys. Res. Commun. 178:16–23
- Lundberg, K., Gronvik, K. O., Goldschmidt, T. J., et al. 1990. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters intrathymic T-cell development in mice. Chem.-Biol. Interact. 74:179–193
- Lutz, C., Browne, G., and Petzold, C. 1998. Methylcholanthrene causes increased thymocyte apoptosis. Toxicology 128:151–167
- Mitra, S., Keswani, T., Dey, M., et al. 2012. Copper-induced immunotoxicity involves cell cycle arrest and cell death in the spleen and thymus. Toxicology 293:78–88
- Pagliacci, M. C., Tognellini, R., Grignani, F., and Nicoletti, I. 1991. Inhibition of human breast cancer cell (MCF-7) growth in vitro by the somatostatin analog SMS 201-995: Effects on cell cycle parameters and apoptotic cell death. Endocrinology 129:2555–2562
- Palmer, E. 2003. Negative selection - clearing out the bad apples from the T-cell repertoire. Nat. Rev. Immunol. 3:383–391
- Panigrahi, G., and Rao, A. 1986. Study of the genotoxicity of the total aqueous extract of betel nut and its tannin. Carcinogenesis 7:37–39
- Pruett, S. B., Ensley, D. K., and Criltenden, P. L. 1993. The role of chemical-induced stress responses in immunosuppression: A review of quantitative associations and cause-effect relationships between chemical-induced stress responses and immunosuppression. J. Toxicol. Environ. Health 39:163–192
- Ranadive, K. J., Gothoskar, S. V., Rao, A. R., et al. 1976. Experimental studies on betel nut and tobacco carcinogenicity. Int. J. Cancer 17:469–476
- Selvan, R. S., Venkateswaran, K. S., and Rao, A. R. 1989. Influence of arecoline on immune system: I. Short-term effects on general parameters and on the adrenal and lymphoid organs. Immunopharmacol. Immunotoxicol. 11:347–377
- Senn, M., Baiwog, F., Winmai, J., et al. 2009. Betel nut chewing during pregnancy, Madang province, Papua New Guinea. Drug Alcohol Depend. 105:126–131
- Shah, S. M., Merchant, A. T., Luby, S. P., and Chotani, R. A. 2002. Addicted schoolchildren: prevalence and characteristics of areca nut chewers among primary school children in Karachi, Pakistan. J. Paediatr. Child Health 38:507–510
- Susin, S., Daugas, E., Ravagnan, L., et al. 2000. Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 192:571–580
- Taub, D. D., and Longo, D. L. 2005. Insights into thymic aging and regeneration. Immunol. Rev. 205:72–93
- Tentori, L., Longo, D. L., Zuniga-Pflucker, J. C., et al. 1988. Essential role of the IL 2-IL 2 receptor pathway in thymocyte maturation in vivo. J. Exp. Med. 168:1741–1747
- Wang, C. C., Chen, T. Y., Wu, H. Y., et al. 2012. Areca nut extracts suppress the differentiation and functionality of human monocyte-derived dendritic cells. J. Periodontal. Res. 47:198–203
- Wang, C. C., Huang, P. L., Liu, T. Y., and Jan, T. R. 2009a. Highly oligomeric procyanidins from areca nut induce lymphocyte apoptosis via the depletion of intracellular thiols. Toxicol. In Vitro 23:1234–1241
- Wang, C. C., Lin, H. L., Wey, S. P., and Jan, T. R. 2011. Areca-nut extract modulates antigen-specific immunity and augments inflammation in ovalbumin-sensitized mice. Immunopharmacol. Immunotoxicol. 33:315–322
- Wang, C. C., Liu, T. Y., Cheng, C. H., and Jan, T. R. 2009b. Involvement of the mitochondrion-dependent pathway and oxidative stress in the apoptosis of murine splenocytes induced by areca nut extract. Toxicol. In Vitro 23:840–847
- Wang, C. C., Liu, T. Y., Wey, S. P., et al. 2007. Areca nut extract suppresses T-cell activation and IFNγ production via the induction of oxidative stress. Food Chem. Toxicol. 45:1410–1418
- Wollina, U., Verma, S., Parikh, A., and Parikh, D. 2004. Oral disease caused by the chewing of betel nut and concoctions containing betel nut. J. Eur. Acad. Dermatol. Venereol. 18:233–235
- Yang, M. J., Chung, T. C., Hsu, T. Y., and Ko, Y. C. 2001. Betel quid chewing and risk of adverse birth outcomes among aborigines in eastern Taiwan. J. Toxicol. Environ. Health 64:465–472
- Young, D. A., Voris, B. P., and Nicholson, M. L. 1981. Cellular and biochemical actions of adrenal glucocorticoid hormones on rat thymic lymphocytes. Environ. Health Perspect. 38:89–97
