| Abbreviations | ||
| BP | = | Back pain |
| CI | = | Confidence interval |
| CSS | = | Central spinal stenosis |
| CT | = | Computed tomography |
| D | = | Decompression |
| DF | = | Decompression and fusion |
| DOS | = | Duration of symptoms |
| DS | = | Degenerative spondylolisthesis |
| EQ-5D | = | The 5-dimensional scale of the EuroQol |
| FS | = | Foraminal stenosis |
| HR | = | Hazard ratio |
| HRQoL | = | Health related quality of life |
| MCS | = | Mental component summary |
| MRI | = | Magnetic resonance imaging |
| LP | = | Leg pain |
| LRS | = | Lateral recess stenosis |
| LSS | = | Lumbar spinal stenosis |
| ODI | = | Oswestry disability index |
| PCS | = | Physical component summary |
| PLF | = | Posterolateral fusion |
| PROM | = | Patient reported outcome measures |
| RCT | = | Randomized controlled trial |
| SD | = | Standard deviation |
| SEWD | = | Self estimated walking distance |
| SF-36 | = | Medical outcomes study short form survey, 36 items |
| SPORT | = | The spine outcomes research trial |
| VAS | = | Visual analogue scale |
Acknowledgements
Björn Strömqvist, head supervisor, for initiating this work, for his time spent on reading and reviewing manuscripts included in this thesis as well as for generously sharing with me his immense clinical knowledge highly relevant for this thesis on a day to day basis.
Bo Jönsson, co-supervisor, for allowing me to explore many of his ideas about spinal stenosis surgery and for many clinical discussions relevant to this thesis.
Xiao Peng Kang, for his meticulous measurements of magnetic resonance images.
Jonas Ranstam, for his expert statistical advice.
Erik and Angelica Sparres stiftelse, Maggie Stephens stiftelse and Greta and Johan Kocks stiftelse, for economic support.
The Swedish Association of Spinal Surgeons, for the research grant and allowing me to use the Register for research and all the patients for participating.
Introduction
The current situation
Lumbar spinal stenosis (LSS) is a common affliction of the elderly as the prevalence of absolute and relative stenosis reaches 20% and 47% respectively in the 60–69 age category (Kalichman et al. Citation2009). The prevalence of symptomatic LSS is however not known (Andreisek et al. Citation2011). Decompressive surgery for LSS is in Sweden, as in many other developed countries, the most common spine operation today (Deyo et al. Citation2005; Strömqvist et al. Citation2009, 2013a; Bae et al. Citation2013). A decade ago the average annual rate of surgery for spinal stenosis in Sweden was estimated to be 10–15 per 100,000 inhabitants but has now increased to 30–35 per 100,000 inhabitants (Jansson et al. Citation2003; Strömqvist et al. Citation2013a). The surgical procedure of decompression, most often laminectomy or laminotomy with “undercutting” of the roof of the recesses is well described and fairly standardized (Malmivaara et al. Citation2007).
Even if the procedure has been used for decades, it is only recently that randomized controlled studies (RCT) have showed superior outcome of surgery compared to conservative treatment (Malmivaara et al. Citation2007; Weinstein et al. Citation2007, 2008). Despite the superiority of surgical treatment there is considerable inconsistency in the type of surgery offered to the patients as well as lack of consensus as to which treatments are appropriate for different degenerative pathologies (Katz et al. Citation1997; Irwin et al. Citation2005; Weinstein et al. Citation2006). Furthermore, there are different subtypes of LSS, but little is known about what characterizes these subtypes in terms of pain, function and HRQoL. The effect of a concomitant spinal fusion in LSS surgery is debated since data is conflicting (Herkowitz and Kurz Citation1991; Bridwell et al. Citation1993; Mardjetko et al. Citation1994; Grob et al. Citation1995; Katz et al. Citation1997; Ghogawala et al. Citation2004; Matsudaira et al. Citation2005; Martin et al. Citation2007; Försth et al. Citation2013).
The main arguments for fusion is that it alleviates back pain by stabilizing the degenerative segment as well as prevents further mechanical instability sometimes associated with decompression, thereby minimizing the risk for residual pain or development of new symptoms. Many advocate a concomitant fusion for LSS with DS based on data in the literature (Herkowitz and Kurz Citation1991; Bridwell et al. Citation1993; Mardjetko et al. Citation1994; Ghogawala et al. Citation2004; Martin et al. Citation2007). The arguments against fusion are that adding fusion has not been shown to influence the outcome when DS is not present (Grob et al. Citation1995). Fusion increases costs, morbidity and the risk for complications in addition to conferring an increased risk of developing adjacent segment degeneration (Deyo et al. Citation2010; CitationMunting et al. 2014; Mannion et al. Citation2014b). The outcome of surgery for LSS has consistently shown patients, in spite of surgical treatment, to have residual leg and back symptoms and lower HRQoL compared to the background population (Cornefjord et al. Citation2000; Jansson et al. Citation2009; Hara et al. Citation2010). Furthermore, the satisfaction rate after surgery is no more than 60–70% and in patients with predominant back pain, the satisfaction rate seems even lower (Katz et al. Citation1995b; Weinstein et al. Citation2010; Strömqvist et al. Citation2013a).
Presently, MRI is most often used to confirm the clinical diagnosis of spinal stenosis and to plan for surgery. Still, there is lack of consensus on how to state the diagnosis in radiological terms and to what degree the core symptoms of spinal stenosis correlate to radiological finding considered consistent with spinal stenosis (Haig and Tomkins Citation2010; Andreisek et al. Citation2011; Mattei Citation2013).
Therefore, focus should be given to improving the outcome for this large group of patients by searching for prognostic factors and/or elaborating on the surgical technique. By identifying patients encompassing positive (or negative) prognostic factors we could hopefully better target individuals suitable for surgical intervention and subsequently improve the surgical results. To reach this goal experts in this field presently advocate shared decision making underlining the importance of a thorough discussion with the patient, particularly regarding their expectations with regards to a probable outcome of surgery (Kurd et al. Citation2012; Pearson et al. Citation2012). Furthermore, as RCT’s are difficult and cumbersome to perform and their validty is undermined by crossover, experts recommend creation of patient registries to allow for prospective study of surgical outcome in lumbar degenerative disorders (Resnick et al. Citation2014a; Resnick et al. Citation2014b).
The purpose of this thesis is to investigate the different subtypes of LSS and to study factors determining the outcome of surgery for spinal stenosis. Particularly, this thesis focuses on the influence of back and leg pain on the outcome and whether spinal fusion improves outcomes in patients with either predominant leg or back pain.
Historical aspects
Symptoms attributable to LSS were described already in the achondroplastic Greek God Hephaestus who as a result of a trauma to a narrow spinal canal developed a limp with radiating symptoms. Because of his pain and limp Hephaestus was mocked by the Olympians (Nixon Citation1991). In 1803, fifty years before Charcots description of claudicatio intermittens of vascular origin, another French physician, Antoine Portal, described weakness, numbness and paralysis of the lower extremities due to narrowing of the spinal canal (Nixon Citation1991). Dejerine described intermittent claudication of the spinal cord in 1894 and postulated that its cause was syphilitic vasculitis (Nixon Citation1991). Oppenheim and Kause described the cauda equina syndrome in 1909 and further reports more thoroughly described the symptoms attributable to compression of the cauda equina (Nixon Citation1991). In 1913 Thomson measured the anterior to posterior diameter of the vertebral foramen (Nixon Citation1991) and the same year Elsberg decompressed a lumbar nerve root that was trapped after a trauma and also described enlarged ligamenta flava (Nixon Citation1991). In 1925 Donath and Vogl described the morphological characteristics of the achondroplastic spine (Donath and Vogl Citation1925). Few years later, Junghanns (Citation1931) described pseudo-spondylolisthesis, a forward slip without a defect in the pars interarticularis. Love and Walsh (1940) highlighted the importance of the ligamentum flavum and Sarpyener (Citation1945) described congenital stenosis of the spinal canal. In 1950 Macnab extended the knowledge within this field when reporting the typical clinical symptoms associated with pseudo-spondylolisthesis in detail. The term degenerative spondylolisthesis (DS) was finally launched by Newman (Citation1955), attributing the vertebral body slip to degenerative changes in the lumbar spine. Wiltse, Newman and Macnab classified spondylolisthesis according to etiology and morphology in 1976.
In 1949 Henk Verbiest, a neurosurgeon from the Netherlands, first coined the term spinal stenosis in a paper published in French (). Verbiest later regretted giving the disease this name and explained that he preferred the name narrow vertebral canal as there had been a lack of consensus on the term stenosis (Verbiest Citation1992). Verbiest submitted his paper in English to neurosurgical and neurological journals but was repeatedly rejected (Verbiest Citation1992), but finally the Journal of Bone and Joint Surgery (Br) accepted his paper in which Verbiest (Citation1954) in detail described the clinical manifestations, the radiographic appearance, including myelographic block of the dural sac, in spinal stenosis. In the subsequent years the diagnosis gradually became accepted and surgical treatment started to emerge as one treatment strategy although there for a long time was and still is controversy as regards on how to establish the diagnosis, decide on treatment and, if selecting surgery, the operative methods of choice (Deyo et al. Citation2004; Deyo Citation2007, 2010).
Figure 1. Portrait of Henk Verbiest (1909–1997), professor of neurosurgery 1963–1980. Oil on canvas, 144 x 79 cm. Painted in 1982 by E.T.H. Visser (1919–2007). The portrait was offered to Verbiest in 1983 by his co-workers at his farewell as professor. Now in Collection of the Utrecht University Museum, inv. no. UG-5027. The Utrecht University Medical Center. By permission.

Spinal fusion is today an integral part of the treatment of spinal stenosis with concomitant DS. Although attempts at spinal stabilization were already performed by Hadra in 1891 and Lange in 1910 in patients diagnosed with a cervical spine fracture and spondylitis, the era of spinal fusion is considered to begin first in 1911 when Albee and Hibbs independently presented different methods for posterior spinal fusion. The method of posterolateral fusion was initiated by Cleveland et al. (Citation1948) when they described placing autologous iliac bone strips between the transverse processes in patients with pseudarthrosis of the spine. Pseudarthrosis of the fusion has always been a concern for spinal surgeons and evidence suggests that instrumentation increases likelihood for achieving a solid fusion which may lead to better long term outcome (Fischgrund et al. Citation1997; Kornblum et al. Citation2004; Martin et al. Citation2007). Spinal instrumentation has evolved and improved continuously since Harrington developed a system of hooks and rods in the late 1950s (1962). Today the most common concept includes pedicle screws, a method usually attributed to Roy-Camille et al. (Citation1970).
History of the Swedish Spine Register
To enable scientific evaluation of outcome of LSS surgery a Register was established at the Department of Orthopedic Surgery in Lund in 1986. A standardized protocol for outcome evaluation was constructed and used at predefined follow-up intervals. The initiative was later supported by the Federation of County Councils and the Swedish National Board of Health and Welfare, aiming for identical follow-up protocol at every hospital in Sweden. The protocol was modified before and after a State of the Art conference in Lund in 1992, to enhance its scientific utility (Strömqvist and Jönsson Citation1993). Computerized follow-up was subsequently implemented with modifications of the original protocol as time went by. In the early 1990s only 4–6 department performing spinal surgery participated but when the ownership for the register was transferred to the Swedish Society of Spinal Surgeons, secretaries were recruited and the data was stored on an independent computer server, increased participation of departments performing spinal surgery in Sweden resulted. Presently, the Register covers about 90% of clinics performing spinal surgery in Sweden (Strömqvist et al. Citation2013a).
Lumbar spinal stenosis – clinical and radiological characteristics
Spinal stenosis literally means narrowing of the spinal canal but many definitions of spinal stenosis in anatomical, clinical or radiological terms can be found. The North American Spine Society has defined spinal stenosis as:
“a clinical syndrome of buttock or lower extremity pain, which may occur with or without back pain, associated with diminished space available for the neural and perivascular elements in the lumbar spine” (Watters et al. Citation2008).
However, the term spinal stenosis implies that normative values of spinal canal dimensions exist but this is not the case. The MRI findings in can undoubtedly be classified as spinal stenosis but the individual imaged may be anything from asymptomatic to unable to walk.
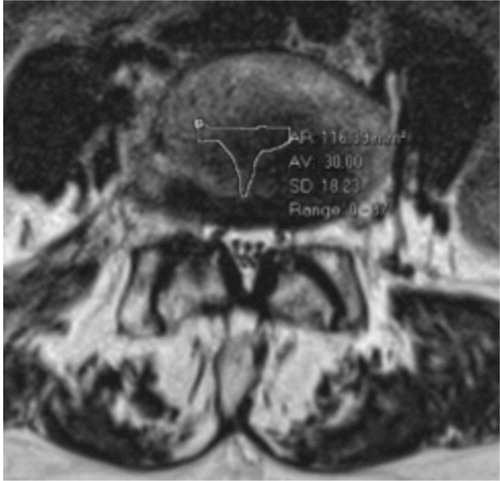
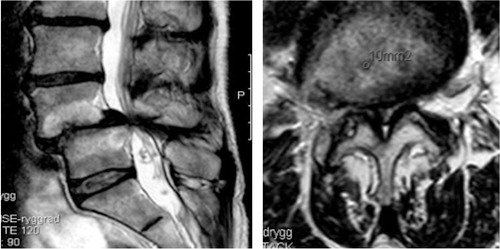
Figure 2. Sagittal (left) and axial (right) MR images showing severe spinal stenosis (10 mm2) with concomitant degenerative spondylolisthesis.
In morphological terms, spinal stenosis can be central, lateral recess or foraminal with or without spondylolisthesis (Arnoldi et al. Citation1976) (). Combined types often exist, both at the same spinal level as well as on adjacent levels in the severely degenerated spines (Tomkins-Lane et al. Citation2014) (). Spinal stenosis with a forward slip (with intact neural arc) of the superior vertebral body on the inferior one is termed degenerative spondylolisthesis (DS) (Newman Citation1955)(). Spondylolisthesis is often graded according to Meyerding (Citation1932). In this grading system the slip is graded as I (1–25% slip), II (26–50% slip), III (51–75% slip) and IV (76–100% slip). Slip in DS is most often of grade I. Stenosis at more than one level is common but correlation between symptoms and radiologic findings is often poor making treatment decisions complex (Boden et al. Citation1990; Jensen et al. Citation1994). Furthermore, other common diseases in the elderly, such as degenerative hip and knee disease, polyneuropathy and arterial occlusive disease (sometime giving vascular claudication) act as masqueraders of spinal stenosis and can coexist with a radiological stenosis further complicating treatment decisions (Offierski and MacNab Citation1983; Haig and Tomkins Citation2010). Subsequently, ruling out these masqueraders can be a concern for the spinal surgeon, as well as evaluating to what extent the lumbar spinal stenosis contributes to the clinical symptoms (Haig and Tomkins Citation2010). The clinical examination aims at discriminating the different pathologies but the ability to discriminate by an examination is only modest (Deyo Citation2010).
Figure 3. Lateral recess stenosis (left), foraminal stenosis (center) and central spinal stenosis (right).
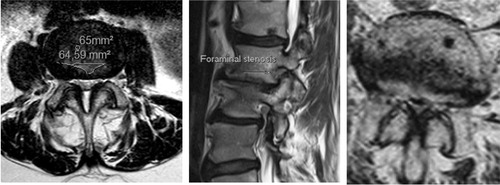
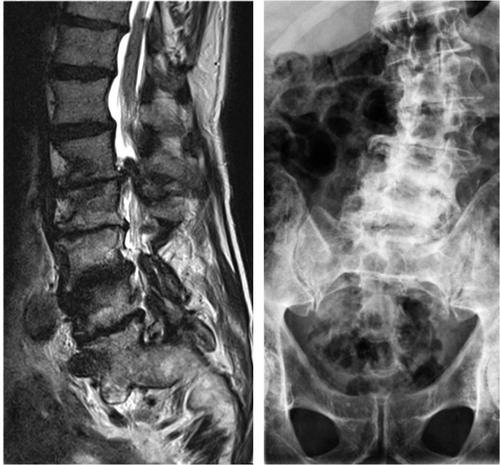
Figure 4. Sagittal MR image (left) and a radiograph (right) showing severely degenerated lumbar spine. Observe severe disc degenerations, facet joint hypertrophy with lateral recess stenosis and degenerative scoliosis in addition to the ankylotic and spondylolisthetic L5–S1 segment.
The clinical symptoms of LSS are most often neurogenic claudication (pseudoclaudication), radicular leg pain as well as back and buttock pain. Balance problems and numbness of the legs is also frequent (Katz et al. Citation1995a). The clinical presentation is highly variable but the occurrence of neurogenic claudication is considered to be a reliable clinical construct (Haig et al. Citation2013; Nadeau et al. Citation2013). Some patients with LSS present with predominant leg symptoms, other with predominant back symptoms while yet others describe negligible pain but numbness or that the legs become heavy when they are walking (Pearson et al. Citation2011). Some patients have a dynamic clinical picture, experiencing neurogenic claudication that is relieved in a stooped forward posture while others predominantly experience worsening when standing. Other patients may have jabbing pain in the back or legs indicating segmental pain (Sengupta and Herkowitz Citation2005). Furthermore, symptoms can vary considerably over time (Johnsson et al. Citation1992). Currently there is lack of consensus about what constitutes the clinical syndrome of LSS and what diagnostic tests should be utilized to confirm the diagnosis, most are however in agreement that a cross-sectional imaging study is necessary for planning surgery (Haig and Tomkins Citation2010; Kreiner et al. Citation2013; Haig Citation2014). All this makes outcome research in LSS fraught with difficulties.
Classification and pathophysiology
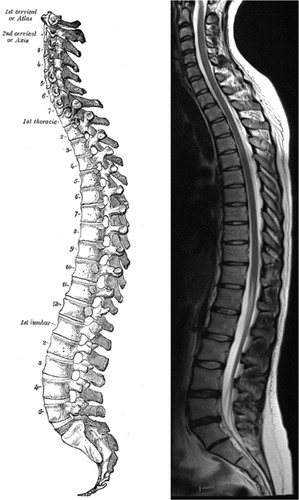
The spine protects the spinal cord and the cauda equina. The spine usually consists of 33 vertebrae in 4 regions (). The regions are called the cervical spine (7 vertebrae), the thoracic spine (12 vertebrae), the lumbar spine (5 vertebrae) and the sacrum and the coccyx (usually including 9 fused vertebrae). The spine has 4 curves in the sagittal plane, a cervical lordosis, a thoracic kyphosis, a lumbar lordosis and a sacral kyphosis (). A normal spine is straight in the coronal plane. The most mobile parts of the spine are the cervical and lumbar spine where degenerative changes most often are seen.
Figure 5. Sagittal view of the spine. A drawing from 20th US edition of Gray`s Anatomy of the Human Body (left). This edition was originally published in 1918 and is now in the public domain. For comparison is a MR image of the spine showing mild degenerative changes in the lower lumbar spine (right).
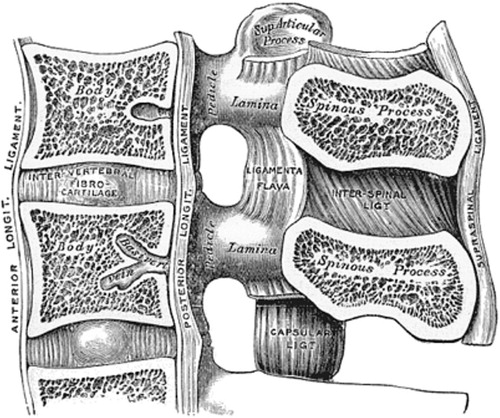
The spinal canal is delineated anteriorly by the vertebrae and discs as well as the posterior longitudinal ligament, laterally by the pedicles, the ligamentum flavum and the neuroforaminae and posteriorly by the ligamentum flavum, the laminae and the facet joints (). The cross-section of the spinal canal is considered to present three different morphological forms; (i) the circular and (ii) the oval forms in which there is ample room centrally and in the lateral recesses and the (iii) trefoil form, which has the smallest cross-sectional area and can predispose to lateral recess stenosis (Hilibrand and Rand Citation1999) ().
Figure 6. A sagittal view of the spinal canal exposing the structures that delineate the spinal canal and in the degenerative process can impinge on the dural sac and nerve roots. A drawing from 20th US edition of Gray`s Anatomy of the Human Body. This edition was originally published in 1918 and is now in the public domain.
The degenerative cascade in the spinal segment leading to symptomatic stenosis is considered to begin with disc degeneration (Kirkaldy-Willis et al. Citation1978). With disc degeneration the disc bulges into the canal and the segment loses height, leading to buckling of the ligamentum flavum and settling of the facet joints (Yong-Hing and Kirkaldy-Willis Citation1983). With time the facet joints degenerate and form osteophytes further narrowing the spinal canal. These changes lead to altered alignment (slight subluxations) in all planes as well as pathologic biomechanics further propagating the degenerative process (Yong-Hing and Kirkaldy-Willis Citation1983). The instability of the spinal segment is considered to be associated with the lower degenerative grades but as the segment stabilizes with increased degeneration of the disc and facets, the pathological movement is postulated to decrease (Kirkaldy-Willis and Farfan Citation1982). The degenerative cascade may be regarded as a “working model” and it is not evident that all patients with LSS pass through all these stages (Axelsson and Karlsson Citation2004). Supporting the degenerative cascade hypothesis, a study has shown increased translational and angular movement to characterize normal or mildly degenerated disks but not the more severely degenerated disks (Murata et al. Citation1994). A study designed to test the validity of the degenerative cascade hypothesis using radiostereometric analysis, showed that a stage of relative stabilization is achieved when disc height is reduced by 50% (high degenerative grade). However, a preceeding stage of increased instability could not be revealed throughout the earlier stages of the degenerative cascade in that study (Axelsson and Karlsson Citation2004). More recently, the results from a study employing intraoperative measurements of mobility support the degenerative cascade theory (Hasegawa et al. Citation2014).
The classification of LSS is based on the region afflicted with neurological compression. Central spinal stenosis (CSS) denotes impingement of the dural sac from all directions at the level of the disc, making a transverse section of the spinal canal small (). The clinical appearance of neurogenic claudication, with alleviation of symptoms in a forward stooped position is considered typical for CSS at more than one spinal level (Porter Citation1996). Lateral recess stenosis (LRS) denotes impingement of a nerve root in the lateral recess, mainly due to disc protrusion in combination with hypertrophy of the ligamentum flavum and/or the articular facet joint. Radiculopathy, often with a more insidious onset than disc herniation, is considered typical for LRS (Porter et al. Citation1984; Vanderlinden Citation1984; Kunogi and Hasue Citation1991).
Foraminal stenosis (FS) implies compression of the nerve root in the neuroforamen. Leg pain that is relieved by flexion, but also well-defined radiculopathies, are consistent with FS. In FS the exiting nerve root can be compressed because of spondylolisthesis, osteophytes from the endplates or facet joints and disc herniations. Narrowing of foraminae invariably results from disc degeneration, especially in conjunction with spondylolisthesis, but its role in the development of radicular symptoms is not self-evident.
In 1976 Arnoldi and colleagues published a classification for LSS and lumbar nerve entrapment syndromes (Arnoldi et al. Citation1976). The etiologic classification of Arnoldi describes two main types of spinal stenosis; congenital or acquired ().
Table 1. The etiologic classification of spinal stenosis according to Arnoldi et al. (Citation1976)
The degenerative changes described above can lead to instability as the degenerative process affects the anatomy and alignment of the spinal segment. With further degeneration slip can occur in all planes and a rotational abnormality may occasionally develop. DS is about 4 times more frequent in women than in men (Rosenberg Citation1975), possibly due to more pronounced ligamentous laxity (Bird et al. Citation1980; Matsunaga et al. Citation1990). Also, increased facet joint angles (sagittal orientation) appears to predispose the development of DS (Grobler et al. Citation1993; Imada et al. Citation1995; Boden et al. Citation1996; Cinotti et al. Citation1997; Berlemann et al. Citation1999; Dai Citation2001). The development of DS leads to CSS and LRS and often even FS. As a result compression of the cauda equina and/or nerve roots may occur so that the neurophysiology of the neural elements become affected (Rydevik Citation1993), leading to both motor and sensory deficits (Delamarter et al. Citation1990; Pedowitz et al. Citation1992). Why individual adaptive mechanisms to neural compression lead to the great variation in symptoms between patients with similar nerve impingement and similar radiological appearance is poorly understood but one factor is the usual slow progression over time.
Imaging in the diagnosis of spinal stenosis
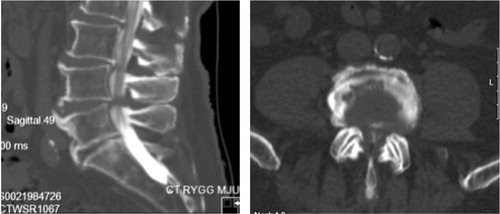
During the First World War initial experiences with the use of contrast media and spinal radiography were obtained. In the beginning radiopaque contrast was injected into facet joints. However, incidental injection into the dural sac raised the possibility of conducting a myelography (Siccard and Forestier Citation1921) and the initial radiological definition of LSS by Verbiest (Citation1954) was actually based on myelographic studies (). The measure used to define absolute LSS by myelography was set to an anterio-posterior sagittal distance of the dural sac <10 mm and a relative LSS 10–12 mm (Verbiest Citation1977). The invention of the computed tomography (CT) scan made cross-sectional imaging of the spine possible (Schellinger et al. Citation1975) and with injection of radiopaque contrast provided excellent delineation of neural anatomy (Di Chiro and Schellinger Citation1976; Verbiest Citation1979; Bolender et al. Citation1985). Magnetic resonance imaging (MRI) revolutionized spine imaging when the first commercial scanner was introduced in 1980 and the first superconducting magnet was introduced to clinical practice in 1981 (Hoeffner et al. Citation2012). MRI offers direct visualization of the spine and spinal cord pathology and gives immensely more detailed information compared to CT myelography, which with help of contrast only allows the margins of neural structures to be visualized but not the neural structures per se (Hoeffner et al. Citation2012) ().
Cross-sectional imaging
Although myelographic block was associated with the symptoms of LSS it was not uniformly observed in patients with symptoms of LSS (de Graaf et al. Citation2006). With the introduction of CT and subsequently MRI, cross-sectional visualization and evaluation of the spine became possible. The cross-sectional area was the measurement that showed the strongest correlation to symptoms. Schönström coined the term “critical size”, which he found in his experiments to be 75 mm2 ± 13 mm2, referring to the minimum space necessary for the cauda equina and the dural sac (Schönström et al. Citation1984; Schönström and Hansson Citation1988). Furthermore, the cross-sectional experimental range producing increased pressure was found to correspond well to the cross-sectional measurements obtained from patients with symptoms of spinal stenosis (Schönström et al. Citation1985). The dynamic (posture dependent) symptoms of LSS were explained by obtaining measurements under axial loading and in flexion and extension (Schönström et al. Citation1989). The studies by Schönström and later others and the advances in technology created greater knowledge, but still there is uncertainty in the relationship between the clinical symptoms and imaging techniques, including the measured area of the dural sac (Geisser et al. Citation2007; Andreisek et al. Citation2011; Mattei Citation2013). In spite of this, some evidence indicates that there is a critical threshold of a cross-sectional area of 70 mm2 with lower values generally yielding inferior clinical functional status (Athiviraham et al. Citation2007; Ogikubo et al. Citation2007). However, there is an ongoing search for improved diagnostic methods for LSS (Andreisek 2011; Mattei Citation2013). One such method is analyzing the MRI scans for the “nerve root sedimentation sign”, defined as absence of sedimenting nerve roots and when positive appears to be quite consistently associated with symptomatic LSS (Barz et al. Citation2010; Fazal et al. Citation2013). However, this method has still not been accepted as a standard clinical measurement.
While some spinal surgeons use measurements of dural sac area to confirm the diagnosis and plan for surgery others may use morphological grading systems (Schizas et al. Citation2010). A grading system takes into account the amount of cerebral spinal fluid at the stenotic level, the appearance of the rootlets in the dural sac and amount of epidural fat. A recent study has shown a good inter- and intraobserver agreement between measurements of dural sac area and morphological grading and this study suggests that both methods may be used in the MRI evaluation of LSS (Lønne et al. Citation2014).
Other radiological characteristic of spinal stenosis include the so-called redundant roots implying that on the CT-myelography or the MRI the nerve roots appear large, tortous or serpentine, and elongated (Cressman and Pawl Citation1968; Suzuki et al. Citation1989). Redundant roots may develop secondary to mechanical trapping of the roots at the site of the stenosis. The repeated streatch of the nerve roots leads to elongation of the nerve roots but when the streatch is relaxed the nerves pile-up at the level of the stenosis.
The natural history of spinal stenosis
As LSS is most often acquired through degenerative changes, symptoms are usually not experienced until the patients are in their 60’s, with the exception of congenital spinal stenosis were patients typically have a much earlier onset of symptoms (Singh et al. Citation2005). The level between the fourth and the fifth vertebral body (L4–L5) is most often affected. Women are more frequently affected and the gender differences are even more pronounced for DS (Hall et al. Citation1985). Although recent studies have shown the superiority of surgical treatment in spinal stenosis (Malmivaara et al. Citation2007; Weinstein et al. Citation2008) the natural history is in no way abysmal albeit highly variable (Johnsson et al. Citation1992). Many patients respond favourably to non-operative treatment modalities (Atlas et al. Citation2000; Malmivaara et al. Citation2007; Weinstein et al. Citation2008) and even without operative treatment disastrous deterioration is seldom observed (Porter et al. Citation1984; Johnsson et al. Citation1991, 1992; Amundsen et al. Citation2000; Malmivaara et al. Citation2007; Miyamoto et al. Citation2008; Weinstein et al. Citation2008). Patients with severe symptoms at baseline, block stenosis on MRI or myelography as well as DS are however more likely to require surgery (Benoist Citation2002).
The natural course of untreated DS has been studied by Matsunaga et al. (Citation2000). With a follow-up of 10 years they found that DS progressed in 34% of the cases but there was no correlation between progression of the slip and clinical symptoms (Matsunaga et al. Citation2000). In addition, no further progression of the slip was observed in the segments with collapsed discs and the back pain improved over time as the discs collapsed (Matsunaga et al. Citation2000). Most of the patients (85%) who displayed a neurological deficit at the initiation of the study deteriorated further during the follow-up but most of the patients (76%) who did not have any neurological deficit at the beginning of the study remained intact at the 10-year follow-up (Matsunaga et al. Citation2000).
Treating lumbar spinal stenosis
Operative and non-operative (conservative) treatment options exist for spinal stenosis. The conservative treatment modalities are heterogeneous, most often encompassing physiotherapy and analgesics but more invasive conservative modalities include epidural injections. The essential feature of operative treatment is decompression of the neural elements. This can be achieved directly by laminectomy or laminotomy or indirectly with interspinous spacers. Laminectomy or laminotomy can be combined with spinal fusion when indicated.
Conservative versus surgical treatment of lumbar spinal stenosis
Recent randomized controlled studies have shown greater improvements in patients after surgery than after conservative treatment (Malmivaara et al. Citation2007; Weinstein et al. Citation2007, 2008). A recent systematic review comparing surgery to conservative treatment in LSS suggested that for patients with radicular pain caused by LSS, in whom a trial of 3–6 months of conservative treatment had failed, surgery did not improve walking ability but improved pain, function, and HRQoL to a higher degree than continuing conservative treatment (Kovacs et al. Citation2011). Small improvements are generally reported by patients treated conservatively and serious complications or deterioration are rare with conservative treatment (Atlas et al. Citation1996; Malmivaara et al. Citation2007; Weinstein et al. Citation2008). Comparing conservative and surgical treatment for spinal stenosis is not straightforward as treatment in both arms is heterogeneous. The surgical treatment varies as patients receive different types of decompression with or without different types of fusion (Kovacs et al. Citation2011). The conservative treatment is even more heterogeneous, including spinal orthosis, rehabilitation and physical therapy, exercise, analgesics and anti-inflammatory medication, calcitonin, education, ultrasound, epidural steroids, heat and cold and transcutaneous electrical nerve stimulation (Kovacs et al. Citation2011). At present there is no significant evidence that favors conservative treatment over surgery in spinal stenosis (May and Comer Citation2013).
Outcome of surgery for lumbar spinal stenosis
Two recent RCT’s show decompressive laminectomy to result in significant improvements (Malmivaara et al. Citation2007; Weinstein et al. Citation2008). These studies however, include patients also receiving fusion but in the Spine Outcomes Research Trial (SPORT) the majority received decompression only (Malmivaara et al. Citation2007; Weinstein et al. Citation2008). Decompressive surgery for CSS is the most frequently performed spinal operation in Sweden (Strömqvist et al. Citation2013a). Although the basic operative technique, decompressive laminectomy or laminotomy/multiple laminotomies is well established, it is debated who will benefit most from surgery (Pearson et al. Citation2012). It is also discussed what type of surgery should be recommended for different constellations of symptoms and types of stenosis, i.e. if the decompression should be accompanied by fusion or not (Eisenstein Citation2002; Pearson et al. Citation2012) and if new indirect decompressive surgical methods, such as interspinous spacers (i.e. X-stop) have a role in the treatment (Strömqvist et al. Citation2013b). Different kinds of LSS also present with different clinical appearance. Patients with LSS are often elderly and often afflicted with comorbidities that add to disability, reduced function and reduced HRQoL. Patients with LSS often have very low preoperative HRQoL compared to an age matched population (Zanoli et al. Citation2006a; Jansson et al. Citation2009). Although significant clinical improvements are associated with surgery for LSS with and without DS on a group level it remains difficult to predict prognosis in terms of function, pain, and HRQoL on an individual basis. In general, 60–80% of all surgically treated patients report a satisfactory outcome (Strömqvist et al. Citation2013a). On the other hand, as many as 20–40% of all operated patients report unsatisfactory outcome due to remaining leg and/or back pain and/or remaining paresis of the lower extremity (Jönsson and Strömqvist Citation1995; Hara et al. Citation2010).
The strongest evidence for surgery in LSS comes from three studies (Atlas et al. Citation1996; Malmivaara et al. Citation2007; Weinstein et al. Citation2008). The Maine Lumbar Spine Study was an observational cohort study comparing non-operative and operative treatment for spinal stenosis (Atlas et al. Citation1996). The predominant symptom, be it back or leg pain, improved in 55% of the surgically treated patients compared with 28% of the nonsurgically treated patients at the one-year follow-up (Atlas et al. Citation1996). The outcomes remained superior for surgery at the four-year follow-up, albeit with some decline in the advantage of surgery (Atlas et al. Citation2000). In this study the outcome of decompression only was mostly studied as only 4% of patients had fusion (Atlas et al. Citation1996).
The Finnish Spinal Stenosis study was the first RCT published comparing non-operative and operative treatment for spinal stenosis. Four university hospitals in Finland participated in randomizing 50 patients to surgery and 44 to non-operative treatment. Unfortunately, the patients were heterogeneous in terms of diagnosis and treatment as 41% and 44% of the operated and non-operated patients had DS and 10 patients had fusion (9 of which had DS and 1 of which had scoliosis). The conservative treatment consisted of physiotherapy, analgesics and education. Patients with surgery displayed more pronounced improvements in ODI, leg and back pain but walking ability did not improve significantly compared to non-operative treatment (Malmivaara et al. Citation2007).
SPORT was an RCT supplemented with a prospective observational cohort arm. The RCT part included 289 patients and the observational cohort included 365 patients. Patients with DS were excluded and instrumented fusion was performed in only 6% of the patients. The primary outcomes were the SF-36 bodily pain (BP) and physical functioning (PF) measures as well as ODI. The results from the RCT were biased by crossover in both directions (from non-operative to the operative arm and vice versa). At the two-year follow-up 67% of patients assigned to surgery and 43% of patients assigned to non-operative treatment had undergone surgery. The results were analyzed as intention to treat and showed only a significant benefit for surgery in the SF-36 BP. The lack of treatment effect was attributed to the high level of crossover. In the as treated analysis the results of all outcome measures favoured surgery, evident as early as 6 weeks after the operation (Weinstein et al. Citation2008).
The most significant evidence for surgical treatment for LSS with DS also derives from SPORT (Weinstein et al. Citation2007). This study was however essentially a study of decompression and fusion for DS as 6% were decompressed only and 21% had uninstrumented fusion while 73% had instrumented fusion. As described above this was also a RCT with an observational cohort. In the study, 304 patients accepted to be randomized while 303 accepted to take part in the observational cohort. The primary outcomes were the SF-36 BP and PF as well as ODI. The validity of the RCT was undermined by a high rate of crossover between assigned treatment groups, 64% of patients assigned to surgery and 44% of patients assigned to non-operative treatment had undergone surgery at the two-year follow-up. The intention to treat analysis showed no significant difference in the outcome for the operative versus non-operative groups at two-year follow-up. The as treated analysis however, demonstrated significant differences in favor of surgery for all the primary and secondary variables at the two-year follow-up. The advantage in favor of surgery was maintained at the four-year follow-up (Weinstein et al. Citation2009).
Current evidence shows constitutional patient characteristics to be of importance for the outcome of surgery in LSS (Airaksinen et al. Citation1997; Jönsson et al. Citation1997; Hurri et al. Citation1998; Iguchi et al. Citation2000; Mariconda et al. Citation2000; Spratt et al. Citation2004; Kleinstück et al. Citation2009). In SPORT, diabetes (Freedman et al. Citation2011), number of stenotic levels (Park et al. Citation2010) and predominant pain location (Pearson et al. Citation2011) were found to be predictors of the surgical outcome (Pearson et al. Citation2012). Park et al. found patients with one level DS to do better than patients with multilevel stenosis and DS but in the spinal stenosis group, the number of spinal levels operated had no impact on the outcome (Park et al. Citation2010). A variety of studies have studied predictors of surgical outcome. These studies infer, that although specific disease characteristics pertaining to LSS may have a predictive value, psychosocial and demographic parameters are usually even more associated with the outcome of surgery (Sinikallio et al. Citation2009; Atlas et al. Citation2010; Cobo Soriano et al. Citation2010; Pearson et al. Citation2012). Additional factors of importance, as shown by data from the Swedish Spine Register, are smoking and obesity (Sandén et al. Citation2011; Knutsson et al. Citation2013).
There has also been a systematic review published that includes only prospective studies and RCT’s evaluating prognostic factors in LSS (Aalto et al. Citation2006). This review found only 21 out of 885 scrutinized publications to be of adequate quality to merit inclusion (Aalto et al. Citation2006). The conclusion of this review was that two high quality publications found low preoperative walking capacity and depression to predict inferior walking capacity and depression to predict poor outcome (Iversen et al. Citation1998; Katz et al. Citation1999). In addition, cardiovascular comorbidity, disorders influencing walking capacity and scoliosis have also been identified as predictors of inferior outcome (Katz et al. Citation1995b, 1999; Jönsson et al. Citation1997; Aalto et al. Citation2006). On the contrary, good preoperative walking ability, high self-rated health, high income, low overall comorbidity and pronounced central stenosis of the dural sac (Jönsson et al. Citation1997; Yukawa et al. Citation2002) were all factors associated with a superior subjective outcome (Aalto et al. Citation2006).
Among reasons for unsatisfactory outcome after surgery probably range: unrealistic patient expectations, insufficient decompression and recurrent or adjacent stenosis. It is important to realize that LSS in most cases is due to a congenitally narrow spinal canal, further narrowed by ligamentous hypertrophy and intraspinal osteophytes. Patients with neurogenic claudication, buttock and leg pain can be offered surgery (Watters et al. Citation2008) while symptoms such as balance problems / lack of coordination and muscular atrophy are less likely to be reversed by surgery, especially in the older patients with LSS. While neurogenic claudication is an established indication for surgery for spinal stenosis the evidence for superiority of surgery compared to other treatment modalities in improving walking ability is low to very low (Ammendolia et al. Citation2014).
Spinal instability or painful mobility – the rationale for fusion
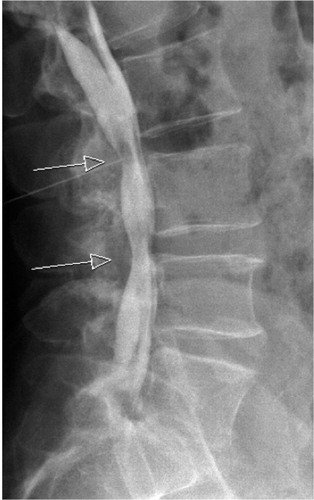
Most patients with LSS complain of back pain (Pearson et al. Citation2011) but the role of back pain in LSS is considered controversial and back pain in LSS requires detailed clinical analysis (Suri et al. Citation2010). The patient can very well interpret radicular buttock pain as back pain and the back pain can also be related to the neurologic compression of the cauda equina or nerve roots. Improvements in back pain after surgery for spinal stenosis with decompression only confirms this (Strömqvist et al. Citation2013a). The back pain generators can also be the degenerated discs and facet joints and the associated painful mobility as the degeneration of the spinal segment progresses (Kirkaldy-Willis and Farfan Citation1982). These degenerations are not addressed when decompression only is performed but when fusion is added to the procedure the possibly painful mobility of these degenerated structures is hindered. However, despite fusion for back pain the results are highly unpredictable and significant residual pain is common at follow-up (Fritzell et al. Citation2001). Excessive movement of the spinal segment (segmental instability) has served as a rationale for spinal fusion because of back pain even though the relationship between abnormal movement of the spinal segment and back pain has remained elusive and poorly defined (Mulholland Citation2008). Spinal instability has for a long time been regarded to be associated with disk degeneration (Knutsson Citation1944). However, the disadvantage of conventional flexion-extension radiographs when evaluating sagittal movement is poor accuracy (Axelsson and Karlsson Citation2004). A minimum 20% difference between two examinations is considered to represent a true progressive slip (Danielson et al. Citation1988, 1989) (). Many studies use sagittal movement and angulation on flexion and extension radiographs to define instability and pseudoarthrosis (Posner et al. Citation1982; Fischgrund et al. Citation1997; Yone and Sakou Citation1999; Birkmeyer et al. Citation2002). Degree of sagittal slip is however, not the only factor determining instability. A recent study incorporating validated PROM’s and intraoperative biomechanical data shows the likelihood of instability to be 92% if the lumbar segment showed DS, intermediate MRI grade, facet opening and the absence of subchondral sclerosis of the facet joints (Hasegawa et al. Citation2011). However, if the segment did not show DS, high MRI grade, absence of facet opening and subchondral sclerosis the probability of instability was only 4% (Hasegawa et al. Citation2011). Despite the coexistence of scoliosis and DS as well as facet joint opening the outcome is not conditionally poor with decompression only as can been observed in .
Figure 10. On the supine sagittal MRI, the degenerative spondylolisthesis is subtle but on the standing lateral radiograph the slip increases markedly and the disk height decreases simultaneously. Note the fluid signal in the facet joints on the axial MRI indicating potential for pathological segmental movement. The patient had left sided radiating pain, corresponding to the L5 nerve root and reported negligible back pain.
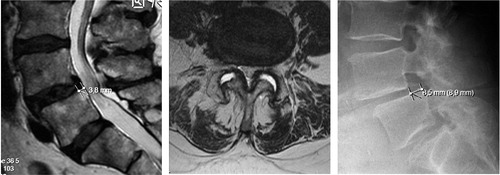
Figure 11. 72-year old woman with DS and scoliosis as well as fluid in the facet joints before she was operated with decompression only in 2002, preoperative MRI (left). She reported excellent clinical outcome. In 2014 she had an MRI of the whole spine as she had pain in the thoracic spine and pancreatic cancer. The DS and the scoliosis had not progressed despite the lack of stabilizing surgery (right).
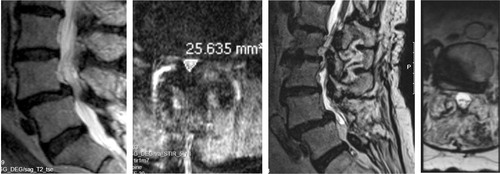
In the SPORT trial radiographic factors commonaly associated with instability, such as DS grade, disc height and disk mobility were not associated with outcome or baseline PROM values. Surprisingly, increased mobility of the DS segment was in the non-operative cohort associated with better outcomes (Pearson et al. Citation2008).
The unpredictable results of rigid spinal fusion, even in the light of successful radiological fusion or lack thereof, have cast doubt on the concept of painful segmental instability (Fischgrund et al. Citation1997; Mulholland Citation2008). When initially described, spinal instability did not imply hypermobility of the segment as it was well-established that anatomic hypermobility of the spinal segment was not consistently symptomatic (Harmon Citation1964). Instability meant low back, gluteal or thigh pain often coupled to regional weakness or pain (Harmon Citation1964). As pointed out by Mulholland (Citation2007, 2008), this clinical definition of instability was ignored and the concept of mechanical instability was accepted although the evidence for biomechanical instability associated with symptoms remained questionable. In addition to relief of mechanical pain, the rationale for fusion in spinal stenosis has been to prevent further nerve root irritation, re-stenosis and development of iatrogenic spondylolisthesis (Sengupta and Herkowitz Citation2005).
Spinal fusion and outcome
In the absence of DS or spinal deformity there is no evidence that the addition of a spinal fusion would lead to better outcome than a decompressive procedure only (Grob et al. Citation1995). For DS however, there are indications that an additional spinal fusion could improve outcome (Herkowitz and Kurz Citation1991; Bridwell et al. Citation1993), a question addressed by Martin et al. (Citation2007) in a systematic review. In this review, 8 studies were included, all considered to have a low level of evidence in the evidence based system (Martin et al. Citation2007). The main objective of this review was to analyze the benefit of concomitant fusion in decompressive surgery for DS (Martin et al. Citation2007). Based on two RCT’s (Herkowitz and Kurz Citation1991; Bridwell et al. Citation1993) and 5 observational studies (Feffer et al. Citation1985; Lombardi et al. Citation1985; Satomi et al. Citation1992; Yone et al. Citation1996; Ghogawala et al. Citation2004) it was concluded that a concomitant fusion in DS surgery conferred a better outcome than decompression without fusion. The two cited RCT’s (Herkowitz and Kurz Citation1991; Bridwell et al. Citation1993) were however noticeably flawed in their design in terms of randomization (pseudo-randomized or not described adequately), blinding (not described) and the lack of validated general or specific patient related outcome measures as end point variables (Martin et al. Citation2007). The observational studies were also flawed in their design and the data reporting (Martin et al. Citation2007). In one of these studies, the treatment groups (D versus DF) were similar at baseline in terms of demographic factors, duration and severity of symptoms, and preoperative outcome measure scores. In that study, adding spinal fusion did not lead to significantly better outcome (Matsudaira et al. Citation2005). This notion was actually supported by a recent large register study from Sweden (Försth et al. Citation2013) and a recent systematic review on DS stated there to be insufficient evidence to draw conclusions regarding indications for specific types of surgical treatment (Steiger et al. Citation2014).
Back pain and spinal stenosis surgery
There is lack of well founded evidence that a decompression only in patients with predominant back pain in LSS will have satisfactory outcome (Watters Citation2011). Patients with LSS and concomitant DS have been postulated to have more back pain due to the observed radiologic sagittal abnormality, therefore requiring concomitant spinal fusion (Sengupta and Herkowitz Citation2005). A fusion could hypothetically stabilize the unstable/olisthetic segment, thereby reducing back pain. However, it has not even been possible to show that patients with LSS and DS consistently have significantly higher back pain scores than patients with LSS without DS (Pearson et al. Citation2011).
Thus, there are many factors to consider when planning surgery in LSS. Some of these factors relate to morphology and biomechanics, while others pertain to the individual general health, bone quality as well as pain characteristics, behavior and comorbidity (Knaub et al. Citation2005; Pearson et al. Citation2012). The main indication for surgery in LSS is generally considered to be leg pain and or neurogenic claudication (Kreiner et al. Citation2013) while the indication regarding type of surgery in patients with predominance of back pain is more controversial (Eisenstein Citation2002; Kleinstück et al. Citation2009; Watters Citation2011). Also, the surgical outcome after a decompression of LSS with predominance of BP has been shown to be inferior to that for predominant LP (Kleinstück et al. Citation2009; Pearson et al. Citation2011). Kleinstück et al. (Citation2009) showed preoperative higher BP levels compared to LP levels to be associated with inferior outcome in decompressive surgery for LSS both in terms of the multidimensional patient-oriented Core Outcome Measure Index (COMI) and in terms of global assessment of outcome. This study highlighted the importance of addressing back pain when planning surgical treatment for LSS. Pearson et al. (Citation2011) also showed that predominance of leg pain was associated with superior outcome when patients from SPORT were dichotomized into groups of pain predominance. In that study about one third had predominant leg pain and the remainder equal pain in the back and legs or predominance of back pain (Pearson et al. Citation2011). This applied to patients with LSS as well as patients with concomitant DS (Pearson et al. Citation2011). Unfortunately the sample size was too small for further subdivision of the patients according to treatment provided (Pearson et al. Citation2011).
The surgical paradigm for addressing back pain is spinal fusion/arthrodesis (Sengupta and Herkowitz Citation2005). When significant back pain coexists with documented biomechanical instability and spondylolisthesis or scoliosis, adding fusion to the decompressive procedure is not controversial (Herkowitz and Kurz Citation1991; Bridwell et al. Citation1993; Knaub et al. Citation2005; Sengupta and Herkowitz Citation2005). Without evident segmental instability, adding fusion to the decompression in LSS is considered highly controversial (Deyo et al. Citation2004).
Theses on lumbar spinal stenosis at Lund University, Sweden
Two PhD theses on LSS have been published at the Faculty of Medicine at Lund University. The first one was by Dr. Karl-Erik Johnsson in 1987, a thesis that included 7 publications within the field of LSS called “Lumbar Spinal Stenosis – a clinical, radiological and neurophysiological investigations” (Johnsson Citation1987). The other PhD thesis was presented by Dr. Bo Jönsson in 1995, a thesis that included nine papers within the field of LSS and disc herniation called “Lumbar Nerve Root Compression Syndromes – symptoms, signs and surgical results”, in which four concentrated on disc herniations while the remaining five papers mainly focused on spinal stenosis (Jönsson Citation1995).
The main findings from Dr. Johnsson’s thesis was that myelographic stenosis can be observed in asymptomatic patients but the narrower the spinal canal, the greater the likelihood of symptoms. Neurophysiological disturbances occurred in 85% of patients with LSS and were associated with more narrow spinal canals. 30% of patients treated non-operatively improved and 60% were unchanged at follow-up. Progression of neurophysiological abnormalities was observed both in patients treated operatively and non-operatively. The result of operative treatment was similar in patients with complete myelographic block and mild block. With surgery, 60% of patients improved but 25% deteriorated. Patients developing a slip after surgery had inferior outcome but the slip was most often observed at the L4–L5 level. Patients with LSS and DS preoperatively, treated with decompression occasionally developed further slip but despite this, the outcomes were not inferior to those of LSS without DS. The thesis also showed that patients operated with a more radical decompression more often developed iatrogenic slip and had worse outcome but facetectomy was often performed during this time period.
Dr. Jönsson showed symptoms and signs of nerve root compression syndromes to vary according to the morphological diagnosis and this could aid in establishing the diagnosis. CSS was found to be characterized by high patient age, long preoperative duration of symptoms as well as pronounced reduction in walking ability. Lateral spinal stenosis was also characterized by long duration of symptoms, often a negative straight leg raising test but otherwise with similar neurological disturbances as disc herniations. In patients > 70 years old, CSS was the most common nerve root entrapment type (80%) but surgical outcome was similar for these patients compared with the younger cohort (< 70 years old).
The work of Johnsson and Jönsson has been frequently cited but their studies were performed when surgery for LSS was in its childhood and generally lacked general and organ specific PROM’s frequently used to day as well as elaborate statistical analysis. The work of Dr. Jönsson and Dr. Strömqvist has subsequently generated many research questions, many of which are put to test in the current thesis.
Aims of the studies
General aims
This thesis has three general aims. The first was to provide information about what uniquely characterizes the three different subtypes of LSS in terms of pain, function and HRQoL. The second aim was to search for preoperative factors impacting the oftcome of surgery for LSS. The third aim was to analyze the outcome of decompressive surgery according to pain predominance and subsequently explore the role of added spinal fusion in patients with predominant back or leg pain.
Specific aims
To study if morphology of the degenerative spine focused, on the degree of stenosis, multilevel stenosis, and DS show a correlation to preoperative leg and back pain, HRQoL, and function, Study I.
To study if morphology of the degenerative spine including degree of stenosis, multilevel stenosis, and DS correlate to outcome in terms of leg and back pain, HRQoL, and function one year after surgical intervention, Study II.
To study if duration of leg and back pain, preoperative function and HRQoL show correlation to the outcome one year after a surgical intervention, Study II.
To study if predominance of leg or back pain influences the preoperative function and HRQoL in patients with LSS and to study if there is a relationship between preoperative level of pain, function and HRQoL in the three different subtypes of spinal stenosis, central spinal stenosis, lateral recess stenosis and spinal stenosis with concomitant degenerative spondylolisthesis, Study III.
To study the outcome of surgery in patients with LSS, in relation to leg or back pain predominance, Study IV.
To study the outcome of surgery in patients with LSS with and without DS according to pain predominance and treatment, decompression or decompression and fusion, Study IV and V.
Patients and methods
Studies I and II
The cohort of patients in Studies I–II includes patients operated on for LSS in Lund during the period 2001–2008. The study population belonged to the primary catchment area of Lund with MRI scans stored at a local server at the Lund University Hospital. To be included in the database the patients had to have an MRI of the whole lumbar spine, including axial slices at all lumbar levels. The cohort initially included 148 patients operated for degenerative disorders, including LSS with and without concomitant DS. Some of these patients did not have CSS but instead segmental instability and were fused by various methods. These patients were excluded from our analysis as well as patients with LRS. By these exclusion criteria 39 patients were removed from the database. We then conducted systematic analysis of the MRI scans that included measurements of central dural sac area (mm2) and degree of vertebral body slip (mm). The patients also answered the preoperative as well as the one-year follow-up part of the Swespine protocol.
In Study I, we investigated the relationship between preoperative MRI findings, such as the minimal dural sac area, multilevel stenosis, in degenerative spondylolisthesis the degree of slip and the preoperative pain level estimated by VAS leg and back pain, functional status (ODI), self-estimated walking distance and HRQoL (SF-36 and EQ-5D).
In Study II, we examined the correlation between the MRI parameters described in Study I and the outcome in terms of pain, function, and HRQoL one year after surgical intervention. We also evaluated the predictive value of the preoperative function, duration of leg and back pain as well as preoperative usage of analgesics in relation to the outcome one year after surgery.
Studies III, IV and V
In Studies III–V, we analyzed data from Swespine. The database consisted of 15,495 patients operated for CSS, LRS and LSS with concomitant DS during the period January 2003 to June 2010.
In Study III, we examined the preoperative levels of pain, function and HRQoL in the different morphological forms of LSS ((i) the CSS, (ii) the LRS and (iii) LSS with DS), and if function and HRQoL varied between three different back and leg pain constellations, BP > LP, BP < LP and BP = LP.
In Study IV, we examined the surgical outcome according to preoperative pain predominance (BP ≥ LP or BP < LP) in CSS without concomitant DS. In the outcome analysis, we included the VAS for leg and back pain, the ODI, SEWD, the SF-36 and EQ-5D as well as subjective satisfaction rate one year after surgery (Study IV). Outcome was analyzed in 4 groups of patients one and two years after surgery in the following way: (i) preoperative BP equal to or worse than LP and decompression, (ii) preoperative BP equal to or worse than LP and decompression and fusion, (iii) preoperative BP less than LP and decompression, (iv) preoperative BP less than LP and decompression and fusion. When evaluating the outcome in terms of preoperative pain predominance (Study IV) all treatment modalities were included, i.e. the evaluation was done irrespective of which type of operation was performed. When comparing outcome in terms of preoperative pain predominance and surgical treatment provided (Study IV), we only included patients operated with decompression (D) or decompression and instrumented posterolateral fusion (DF).
In Study V, only patients with DS at the L4–L5 level operated on with either decompression only or decompression and instrumented posterolateral fusion were compared according to pain predominance.
Surgical techniques
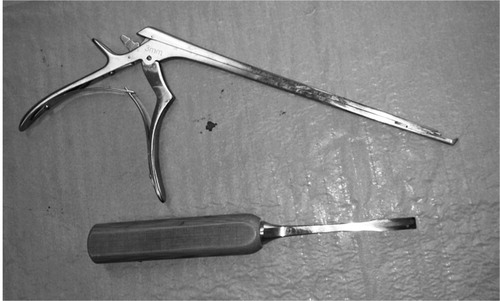
Conventional decompression of the lumbar spinal canal consists of exposure of the posterior spine, then subsequently performing a laminectomy or partial laminectomy. In this process the spinous process and the lamina are removed where after the exposed ligamenta flava and medial parts of the facet joints are removed with a chisel or a Kerrison rongeur (). The goal of the procedure is to decompress the central canal as well as the lateral recesses by “undercutting” i.e. by resecting the ligamentum flavum and bone from the anterior and medial parts of the facets, with special care to retain facet joint integrity. When laminotomy is performed the posterior structures are preserved, including the spinous process and inter and intraspinous ligaments.
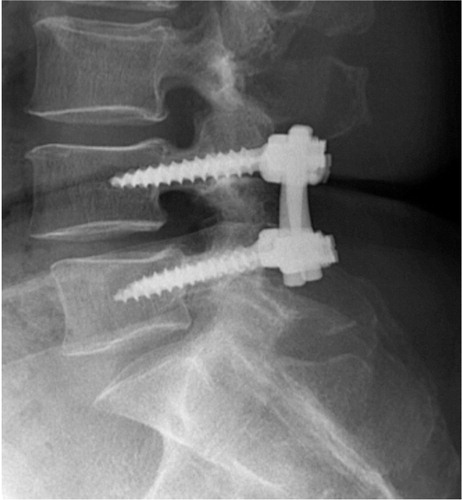
When the decompression is supplemented with instrumented posterolateral fusion (PLF) a more extensive exposure of the posterior spine is required. The procedure includes exposure and decortication of the facet joints as well as the transverse processes of the vertebrae of the level to be fused. Autologous bone is generally used, either from the posterior iliac crest or from the facet joints and/or laminae. Pedicle screws are inserted into the pedicles at the level to be fused and spanned with rods to enhance bony fusion and allow for earlier mobilization ().
Figure 13. Post operative radiograph, of the lumbar spine showing pedicle screw fixation of the L4-L5 segment.
The procedures described above represent standard surgical treatment for LSS and while indirect and mini-invasive methods exist for both the decompression and the fusion these methods are diverse, often lack long-term evaluation and are only applicable in selected patients. Subsequently, we only included patients operated with these two well-established surgical methods.
Analysis of magnetic resonance imaging
The MRI analysis in Studies I and II incorporated measurements of the central dural sac area, and the degree of slip in mm on the MRI scans in patients with DS. Prerequisite for analysis was inclusion of axial slices at all lumbar spinal levels. The central dural sac areal measurements (mm2) were performed on axial slices at the disc level on T2 weighted images (). The region of interest (ROI) function of the Sectra® software (Linköping Sweden) was used for these measurements. In order to calculate intra-observer correlation, three of the authors conducted the same measurements in a subset of 20 random cases.
Statistics
In Study I, normality of data was tested with the Shapiro-Wilk test. We used parametric tests when comparing the SF-36 variables. For the non-normally distributed variables we used the Mann-Whitney U test and the Spearman rho correlation coefficient. The Pearson correlation coefficient was used for analyzing correlation between outcome measures and the minimal dural sac area, adjusting for number of spinal levels involved. For the reliability assessment of measurements of the dural sac area between observers we used the interclass correlation coefficient (ICC).
In Study II, normality of data was tested with the Shapiro-Wilk test. We then used the paired t-test for analysis of all outcome parameters except for the EQ-5D for which Satterthwaite test was used. The 95% confidence interval for difference in medians was calculated with a stratified bootstrap test to estimate accuracy. Spearman’s rank correlation test was used when correlating preoperative EQ-5D value and BP at the one-year follow-up evaluation. Regression analysis was performed for outcome in terms of leg and back pain, EQ-5D, and preoperative walking distance. In these analyses we adjusted for age, preoperative walking distance, duration of leg and back pain, multilevel stenosis and DS.
In Study III, The Oswestry disability index, the physical and mental component summaries of the SF-36 were normally distributed and a parametric test could therefore be used. The EQ-5D, leg and back pain were not normally distributed and non-parametric tests were used such as test for trend and Mann-Whitney U test. A p-value of <0.05 was considered statistically significant.
In Study IV, we used the Mann-Whitney test, when comparing the baseline values for groups of pain predominance. For variables visually expressing normal distribution at the one and two-year follow-up evaluations (the physical component summary, the mental component summary, the Oswestry disability index) we used simple and multivariate linear regression analysis to assess the effect of predominant back pain on the outcome in terms of these PROM’s. We also performed the analysis for fusion separately for patients with either predominant BP or LP to estimate the effect of fusion in these groups. Outcome in terms of back and leg pain in Study IV showed an almost bimodal distribution as there was a significant number of patients reporting almost a painfree outcome. Outcome was therefore dichotomized into two groups (<10 mm VAS versus ≥ 10 mm VAS) () The outcome scores for EQ-5D were also bimodally distributed and EQ-5D outcome data was therefore dichotomized into two groups, < 0.5 or ≥ 0.5 (). For the dichotomous analysis we used Cox proportional hazard model (robust) with constant follow-up time to estimate relative risk directly when calculating the hazard ratio for belonging to each of the two groups (Barros and Hirakata Citation2003). Unadjusted and adjusted analyses were performed where we included preoperative score, age, gender, duration of leg and back pain, comorbidity and smoking as well as previous spine operations in the adjustments. We calculated the risks for patients with predominant back pain to belong to the low or high pain outcome groups and the low or high EQ-5D groups (high score translating superior HRQoL). We also performed the analysis for fusion separately for patients with either predominant back pain or predominant leg pain groups.
Figure 14. Histograms showing the one-year outcome for back pain (top) and leg pain (bottom). Patients more or less pain free (VAS <10mm) are located to the left of the vertical line.
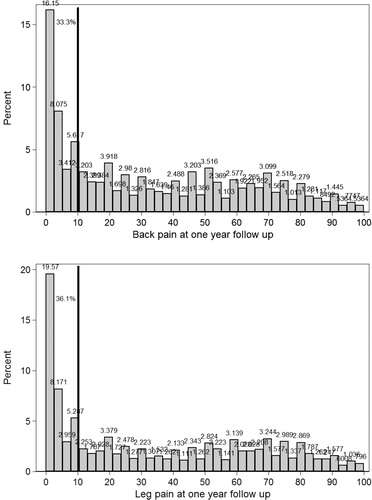
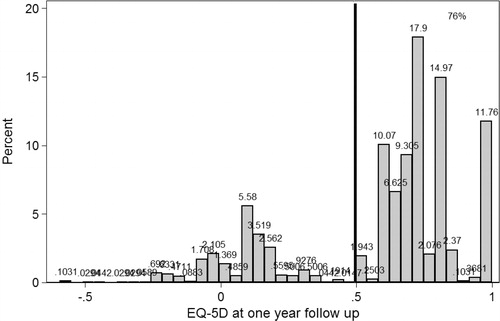
Figure 15. A histogram showing the one-year outcome in terms of the EQ-5D. The majority of patients estimate EQ-5D score > 0.5 (right side of the vertical line).
In Study V, the bimodality of outcome in terms of leg and back pain and the EQ-5D was not as prounounced as in the CSS cohort in Study IV. In Study V, change from baseline values at the one- and two-year follow-up was calculated and the significance of the difference between patients in the PL or PB groups with decompression only versus decompression and fusion was estimated using the Mann-Whitney test. Multivariate regression analysis was also performed for the change in outcome at the one- and two-year follow-ups. In the multivariate analysis we adjusted for factors shown in earlier studies to impact outcome of surgery for degenerative spinal disorders such as; age, gender, duration of leg and back pain, earlier surgery and smoking. When significant in a univariate analysis we subsequently included them as covariates in the multivariate analysis.
Baseline comparisons and missing data
To have a notion of bias introduced by missing values (attrition bias) we performed dropout analysis (). In Study III, the main objective was to present preoperative data for different types of stenosis and dropout analysis was performed but not presented in the published paper but is presented in the results part of this thesis.
Figure 16. Schematic overview of analyses performed to account for selection bias due to missing or drop out patients. * For baseline degenerative spine data, see: http://4s.nu/Kopia_av_patientsida/formular/100301_ver3_LR_Basuppgifter.pdf
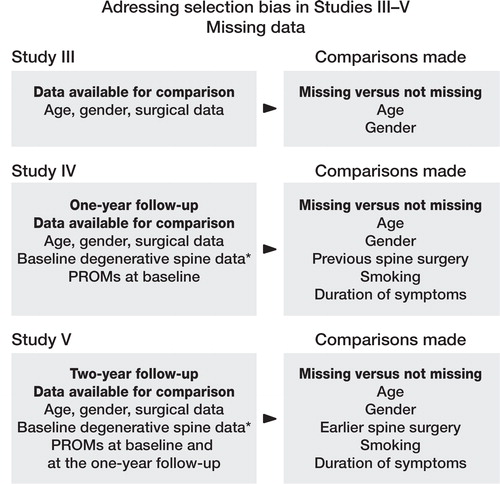
In Study IV, we compared the missing and non-missing populations at the one-year follow-up. These patients had filled out the baseline Register form allowing for baseline comparison between missing and non-missing subjects at the one-year follow-up. Age, gender, treatment, duration leg and back pain, previous surgery, use of analgesics preoperatively, and comorbidity were baseline variables available for comparison between missing and non-missing subjects at the one-year follow-up. Baseline differences in the treatment groups were also estimated to account for selection bias.
In Study V, we similarly compared the baseline characteristics of the patients who attended the two-year follow-up and those who did not. Baseline differences between D and DF treated patients were also analysed. For the analysis we used the χ2 test. The STATA 10 statistical software was used for all calculations in Studies I and II but in Studies III-V the STATA 12 was used (StataCorp, 4905 Lakeway Drive, College Station, Texas 77845 USA).
Outcome measures
Patients reported outcome measures (PROM) were used in all the studies as they are intrinsic to the degenerative Swespine protocol (Strömqvist et al. Citation2013a). The outcome measures focused on pain, HRQoL and function. The measures used in Studies I–V consisted of a 100 mm visual analogue scale for leg and back pain, the EuroQol-5D and Short Form 36 for HRQoL and self-estimated walking distance and the Oswestry disability index for function. Satisfaction with the operation was also estimated (satisfied, undecided or dissatisfied). The degenerative spine protocol from Swespine can be found at: (http://www.4s.nu/Kopia_av_patientsida/ph_resultatmatning_formular.html)
The Oswestry Disability Index (ODI)
The ODI is an organ specific instrument, pertaining to low back pain and function (Fairbank et al. Citation1980). The ODI has been extensively validated and the current version recommended by the original authors is used in the Swespine protocol (Fairbank and Pynsent Citation2000). The ODI takes about 5 minutes to complete and incorporates measures of pain and physical function in 10 dimensions, including; pain intensity, personal care, lifting, walking, sitting, standing, sleeping, sex life, social life and traveling. Because of floor and ceiling effect the ODI is the preferred choice in populations with higher disability levels compared to other back specific measures (Bombardier Citation2000). The results from ODI include scores from 0–100, with 0 being best and 100 being worst. Patients scoring 0–20 on the ODI are considered to experience no or minor disability, those scoring 20–40 moderate disability, those scoring 40-60 severe disability, scoring 60-80 is considered crippled status and 80-100 bedridden status (Fairbank et al. Citation1980). A 10 point difference in ODI is estimated to represent a clinically relevant difference (Birkmeyer et al. Citation2002; Hägg et al. Citation2003).
The Visual Analogue Scale (VAS)
The VAS is a scale for overall pain intensity. The VAS is well evaluated and measures pain with consistency (Englbrecht et al. Citation2012). The VAS scale included in the Swespine protocol is a scale where the patient registers the pain level on a 100 mm line which is then measured with a ruler by the secretary responsible for the registration of data. Hägg et al. (Citation2003) have concluded that an 18-19 point change in VAS is a clinically relevant change in back pain. The VAS is most useful for comparing change over time (for example pre versus post treatment) on an individual level (Zanoli et al. Citation2001).
The EuroQol 5-Dimensions (EQ-5D)
EQ-5D is an instrument designed to measure HRQoL in two parts (EuroQol Group 1990). EQ-5D is regarded as a generic PROM, i.e. it is not being specifically adapted to one disease or one anatomical region. The first part of the EQ-5D contains five dimensions; mobility, self care, daily activities, pain/discomfort and anxiety/depression. Each of the five dimensions has three categories that the patients can register: (i) no problem, (ii) some problem, (iii) extreme problem. This yields 243 (35 + 2) health conditions in addition to registration of death and unconsciousness. EQ-5D as an estimate of health related quality of life has been extensively studied in Sweden in the background population as well as in different diseases and specific socioeconomic sub-groups (Burström et al. Citation2001a, 2001b). The EQ-5D was originally designed to be self-administered and condensed enough to be appropriate to use with other measures (EuroQol Group 1990). The second part of the EQ-5D (not used in our study) is a 20 cm VAS scale. The opposite end-points are labeled best (100) and worst (0) imaginable health states. The patients mark their estimate on the line. Studies on the EQ-5D have shown good reliability and validity (Brazier et al. Citation1993; Hurst et al. Citation1994, 1997; van Agt et al. Citation1994; Coast et al. Citation1998; Dorman et al. Citation1998). Some studies have however shown that the EQ-5D provide more missing data compared to other commonly used PROM’s (Essink-Bot et al. Citation1997). In a spine register, Solberg et al. (Citation2013) recently showed EQ-5D scores to lack specificity and sensitivity and that change corresponding to a level of 0.30 or more, indicates a success of the surgery. Parker et al. (Citation2011) have also shown the minimally clinical important difference in terms of EQ-5D to vary considerably in different conditions, so that no specific general value could be provided.
The Short Form 36 (SF-36)
The Medical Outcomes Study 36-item Short-Form General Health Survey (SF-36) is a generic health measure that includes 8 dimensions and takes about 10 minutes to complete (Ware and Sherbourne Citation1992; Ware Citation2000). The dimensions include; physical function, role physical, bodily pain, general health, vitality, social function, role emotional, and mental health. The 8 dimensions can be aggregated into two summaries, (i) the physical component summary and the (ii) mental component summary. The component summaries have been shown to be valid measures (Ware and Gandek Citation1998). Differences of minimum 3–5 points in the SF-36 are considered clinically relevant and a 3 point increase in PCS is thought to represent a clinically meaningful improvement (Samsa et al. Citation1999; Lauche et al. Citation2013).
Self-Estimated Walking Distance (SEWD)
Taking history in patients with spinal stenosis includes a question on walking ability and performance. Walking ability and performance are important indicators of disability in many diseases. In the Swespine protocol patients are asked to categorize their walking distance in one of four different categories, (1) <100 m, (2) 100–500 m, (3) 500–1000 m, and (4) >1000 m. Severly reduced walking ability is thus assigned the number 1 and good walking ability as the number 4. Despite being an important measure of disability, studies have shown discrepancies between perceived patient and physician estimated walking distances and measured distances (Giantomaso et al. Citation2003; Okoro et al. Citation2010). A study supporting the use of self-reported measures of walking capacity showed subjects who were able to walk their maximum distance tended to underestimate their actual walking capacity (Tomkins-Lane and Battié Citation2010).
The Swedish Spine Register
Data on all the patients was extracted from the Swedish Spine Register (Swespine) (Strömqvist et al. Citation2009, 2013a). The Swespine is a quality register owned and administrated by the Swedish Association of Spinal Surgeons (www.4s.nu), and financed by the Swedish Ministry of Health and Welfare. The Register is useful in monitoring surgical activities within Sweden including surgical trends and implants utilized. More than 90% of departments performing spine surgery in Sweden participate currently (Strömqvist et al. Citation2013a). The degenerative spine patient protocol is self-administered but secretaries at the local level send out follow-up protocols. The operating surgeon is responsible for filling in surgical data. The Register was created in the early 1990s (Strömqvist and Jönsson Citation1993) and has to date published 15 annual reports. The Register protocol includes questions regarding, age, gender, smoking, working ability, working status (including type of work), duration of leg and back pain, use of analgesics, comorbidity, self-estimated walking distance and sport activities. The protocol has been validated showing that the protocol can reliably detect postoperative improvements between large groups of patients (Zanoli et al. Citation2006b). Added to the protocol are also the Oswestry disability index (ODI) (Fairbank et al. Citation1980), the visual analogue scale for pain (VAS), the SF-36 (Ware and Sherbourne Citation1992), and the EQ-5D questionnaires (EuroQol Group 1990) described above. The questionnaries are mailed to all patients one, two and five years after the surgical procedure. Furthermore, there are also inquiries about perceived change in leg and back pain compared to preoperativly as well as patient satisfaction in respect of the surgery. The much used and validated Zurich Claudication Questionnaire (ZCQ) is not included in the Swespine degenerative spine protocol as it is mainly designed and validated for spinal stenosis (Stucki et al. Citation1995).
Ethical considerations
The patients in the studies are a part of the Swedish Spine Registry and have as such given informed consent for participation. The Swespine follows the legislation as set forth in the Personal Data Act from 1989 and is subjected to control by the Swedish Data Inspection Board. The databases used in this study included no personal identifying information.
Results
Study I
The mean dural sac area was 43 mm2. DS was 3.4 times more common in women. The agreement between the raters of MR images was acceptable, the interclass correlation coefficient (ICC) was 0.67 (95%CI: 0.45–0.83, p <0.001). Health related quality of life, functional status, walking distance, leg and back pain showed limited correlation to dural sac area and multilevel stenosis. The patients generally had a very low preoperative HRQoL, low functional status, and high pain levels.
There was a low but significant correlation between multilevel stenosis and increasingly lower levels of leg pain (r: -0.24; p: 0.03). Patients with multilevel stenosis reported better preoperative general health than patients with a single level stenosis (p: 0.04), and less leg pain than patients with single level stenosis although this difference was not statistically significant.
Patients with DS had a smaller mean dural sac area than those without DS, more often multilevel stenosis and were significantly older than those without DS. Despite this, there was no difference in the preoperative score for pain, walking distance, and HRQoL when comparing patients with and without DS.
Study II
Patients reporting preoperative leg pain exceeding two years had inferior outcome in terms of leg and back pain, function and HRQoL compared with patients that reported leg pain for two years and less (). Patients reporting duration of back pain exceeding two years had inferior outcome in terms of the EQ-5D, p: 0.04 compared with patients reporting duration of back pain as two years and less.
Table 2. Duration of leg pain and outcome measures at the one-year follow up. Values are number of cases, mean (SD)
Patients preoperatively using analgesics regularly or intermittently had significantly higher back pain levels at the follow-up, p: 0.02 compared with patients not using analgesics preoperatively. In the multivariate analysis, back pain at follow-up increased 0.5 mm on the VAS for each mm2 in the preoperative dural sac area, (95%CI: 0.06–1.0, p: 0.03). There was also a significant correlation between the preoperative dural sac area and absolute leg pain scores (VAS) at the follow-up as patients with the most pronounced encroachment of the dural sac had the lowest leg pain scores, r: 0.22; p: 0.03.
Patients reporting poor preoperative self-estimated walking ability tended to report lower values for HRQoL in terms of the EQ-5D than those reporting longer preoperative walking ability. Finally, patients who were satisfied with the outcome demonstrated significant improvements in virtually all outcome measures, while those who were undecided or dissatisfied with the outcome in general lacked significant improvements in the PROM’s ().
Table 3. Patient satisfaction at the one-year follow-up in relation to pre- and postoperative HRQoL, functional status, and pain
Study III
Baseline surgical data allowed for some comparisons between missing and non-missing data at baseline (patients with PROM’s registered). Patients with only surgical data and age and gender registered, were older than non-missing 74.2 years old versus 71.7 years old, p < 0.0001. Men were also more likely to be missing at baseline, the ratio of men to women was 0.67 in the missing group but 0.80 in the non-missing group, p: 0.009. There was no difference in the number of missing between the diagnostic groups, p: 0.89.
Women generally had inferior baseline values compared to men (). Patients with LRS were younger (67.4 years) than the patients with CSS (73.2 years) and LSS with DS (72.5 years). High preoperative back and leg pain scores were associated with short SEWD (). The most common pain constellation was LP > BP (49%), followed by BP > LP (39%), while the remaining 12% graded the pain in the back and leg as equal. The type of stenosis with the highest ratio of BP/LP was LSS with DS (ratio: 0.93; 95%CI: 0.92–0.95), followed by central spinal stenosis (ratio: 0.88; 95%CI: 0.88–0.89) with LRS experiencing the lowest burden of back pain (ratio: 0.85; 95%CI: 0.83-0.87).
Table 4. Preoperative values for LSS subtypes according to gender. Mean values are presented with 95% CI in parenthesis
Figure 17. A box plot showing the association between leg and back pain and self-estimated walking distances in LSS. It is evident from this box plot that with increased pain the self-estimated walking distance deteriorates.
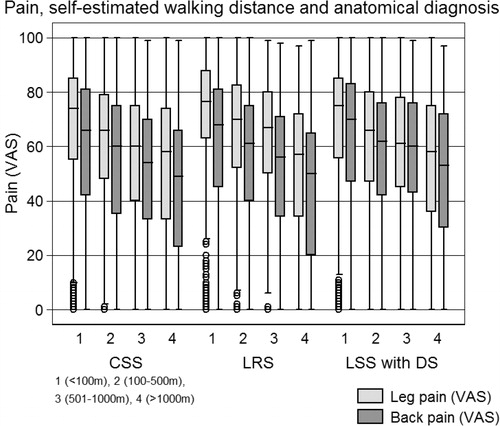
The lowest HRQoL and function was found in LSS with DS in the sub-group with back pain equal to leg pain. In this sub-group 55% (95%CI: 50–59) of the patients reported that they could not walk more than 100 m. Patients with LRS reported better SEWD compared to CSS with or without DS as only 35% of the LRS patients reported SEWD <100 m but the corresponding values for CSS and LSS with DS were 51% and 55%.
Study IV
The patients operated on with decompression and concomitant fusion were observed to be younger, reported higher preoperative back pain and higher preoperative ODI scores as well as lower preoperative EQ-5D than patients with decompression only. In the predominant LP group the patients with fusion had higher baseline BP values (6.8, p: <0.0001), lower baseline HRQoL (EQ-5D, 0.05, p: 0.009) and higher ODI scores (3.4, p: 0.004) compared with the decompressed only patients. These differences were small but statistically significant. In the predominant BP group the patients with fusion had lower baseline HRQoL (EQ-5D, 0.06, p: 0.003) but reported better SEWD (p: 0.007). These baseline differences were very small albeit statistically significant.
Predominant BP was associated with inferior surgical outcome in terms of pain, health-related quality of life and function ( and ). Satisfaction with the operation was associated with the ratio of leg to back pain in an unadjusted analysis ((. The highest proportion of satisfied patients (69%) was seen in the predominant LP group treated with DF. The least satisfied sub-group was patients with predominant BP treated with D (54%). Decompression with instrumented fusion was associated with superior unadjusted EQ-5D values (HR: 1.1, 95%CI: 1–1.3) at the one-year follow-up compared with D in patients with predominant BP. In the unadjusted analysis, DF compared to D was associated with increased leg pain at the two-year follow-up in patients with predominant LP (HR: 1.2, 95%CI: 1–1.4). Also in the unadjusted analysis, patients with predominant BP with DF experienced small gains in the physical component summary of the SF-36 (2.3 points, p: 0.005).
Table 5. Simple and multivariate linear regression analysis of outcome in terms of the Oswestry disability index, the physical component summary and the mental component summary according to pain predominance and treatment, decompression and decompression and fusion. Values are outcome (95%CI) and p-value
Table 6. The hazard ratios for patients having leg or back pain ≥ 10 on the VAS scale (risk for increased pain) and the hazard ratios for estimating a ≥ 0.5 EQ–5D score (risk for better HRQoL) at the one- and two-year follow–ups
Figure 18. The percentage of satisfied patients at the one-year follow-up in 16 different different boxes according to the relationship between preoperative leg and back pain (VAS).
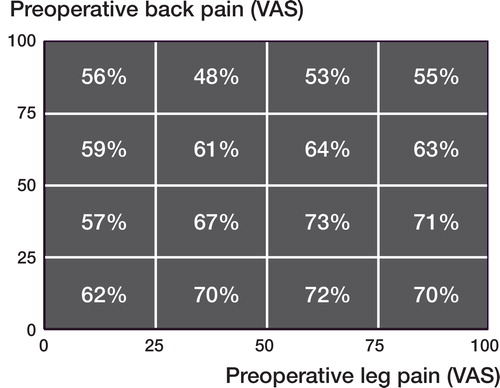
Adding fusion did not result in superior outcome in terms of back pain in the BP predominant group. Adding spinal fusion improved short-term (one-year) unadjusted outcome but the benefit was small and not clinically significant and generally disappeared in the adjusted analysis. At the two-year follow-up no significant benefit was registered in favor of fusion.
Study V
Patients with incomplete follow-up data were slightly younger than those with registered follow-up data, p: 0.02. In the DF group more patients were missing at the two year follow-up compared to the D group (55% versus 41%, p <0.0001).
The female/male ratio was 3:1 in the study but the gender distribution was even between the groups. In the predominant LP group, the patients subsequently fused had higher BP scores at baseline but for other outcome measures no significant baseline differences were found. The D patients were in both the leg and back pain predominant groups older than the DF patients.
The unadjusted outcome for the one- and two-year follow-up is presented in . In the adjusted outcome at the one-year follow-up, patients with predominant LP reported a mean 7.9 mm more pronounced improvement in back pain on the VAS with fusion, compared to decompression only (95%CI: 0.7–15.2), p: 0.03. Despite more change in the fused group, the reported BP levels remained similar in the D and the DF groups at the one-year follow-up, p: 0.77 (). The patients with predominant BP benefited from adding fusion in terms of BP, 7.1 (95%CI: 0.3–13.9), p: 0.04, leg pain 8.8 (95%CI: 2–15.7), p: 0.01, the ODI 5.7 (95%CI: 1.6–9.9), p: 0.006 and the EQ-5D 0.09 (95%CI:1.7–0.02), p: 0.02 at the one-year follow-up as the DF group reported greater change in outcome compared to the D group. At the two-year follow-up no significant differences were found between D and DF in the either the leg or the back pain predominant group in the adjusted analysis.
Figure 19. The outcome in terms of back pain according to pain predominance is depicted. Note that the absolute outcome values for back pain are similar in the DF and D groups but the change (improvement) in the back pain is more pronounced in the DF group compared to the D group. In the predominant leg pain group the baseline values for back pain in the patients decompressed only are significantly lower than that of the DF group.
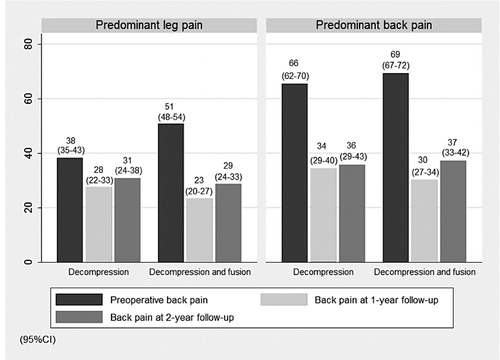
Table 7. Baseline and outcome data for patient reported outcome measures
General discussion
Two of the main reasons for increasing our knowledge about LSS are the facts that surgery for LSS has become the most common spine operation today and that only 60–70% of patients are satisfied with the outcome of surgery.
LSS is an important and understudied research field and as the populations of developed nations get older and want to remain active, research in this field is likely to become prioritized since LSS is a disease of the old individual (Deyo Citation2010). Caring for patients with spinal stenosis and advising them with regards to treatment options will remain challenging and highly dependent on shared decision making (Deyo Citation2007). As greater and costlier surgery has been developed for treating patients with LSS, even in patients without a spine deformity, it is important to remember that the indication for surgery is seldom absolute but that appropriate surgery in the correctly selected patient may result in pain relief and functional improvement (Deyo Citation2007). It’s therefore of great value to collect and analyze evidence for different treatment alternatives, including possible benefits reached by an additional fusion to the generally accepted decompression, because the costs and complications of fusion surgery are higher than those of simple decompression in the elderly population (Deyo et al. Citation2010).
The register study form
The register study form has both advantages and shortcomings. Data in a register is prospectively collected but the research questions or hypotheses may be put forward when all data has been collected. The register study design is often compared to the research with the highest ranking within the evidence based system, the randomized controlled trial (RCT). Through ways of inclusion and exclusion criteria as well as randomization the RCT achieves its internal validity by limiting confounding and selection bias from the onset. However, even with well performed RCT’s questions remain as to their external validity, i.e. whether if the results generated in the rigorously controlled environment of the RCT are applicable to the clinical settings and circumstances most patients are treated within (Black Citation1996; Van Spall et al. Citation2007).
A register is only of value if the accumulated data is of good quality, referring both to the quality and the completeness of the collected data (Levine and Julian Citation2008). Criteria for methodologically appraising the quality of register studies are provided in (Levine and Julian Citation2008).
Table 8. Methodology criteria for critically appraising the quality of a register study
A register accumulates data on many patients but one of the main limitations of register studies is lack of randomization, implying that patients are selected for a specific treatment based on its alleged benefit for that patient (stratification). This stratification can provide a selection bias at baseline so that a treatment effect can be overestimated or underestimated, not based on the treatment per se but due to difference in the groups included at study start (Levine and Julian Citation2008). The results from many high quality observational studies have however yielded similar results as high quality RCT’s with little risk for overestimating the treatment effect (Benson and Hartz Citation2000; Concato et al. Citation2000). Another methodological problem inherent to register studies is the loss to follow-up and missing values (Little et al. Citation2012), which introduce a type of selection bias often referred to as attrition bias. The reason for this might be that the follow-up procedures of the register are not sufficiently vigorous, perhaps even passive and only relying on the spontaneous responsiveness of patients or not sufficiently standardized. Another reason for loss to follow-up might be register tiredness, implying that patients are fatigued by being repeatedly asked to fill out various forms from different registries or polls within our society.
Two main types of error should be accounted for in clinical research. Random error and systematic error, also called bias. Random error is beyond the control of the researchers but the potential for systematic error should be accounted for. Confounding can be regarded as confusion or mixing of effects (Rothman Citation2002). This means that a certain exposure is mixed with the effect of another variable leading to bias. In statistical terms, the confounding factor is a variable that correlates with both the dependent and independent variables. Addressing bias and confounders from the onset of the study is the strength of RCT’s and the influences of clinical preferences, including local/geographical treatment traditions and personal clinical predilections can by this method be minimized or totally eliminated. This is not the case in register studies as patients in the register are participating based on the diagnosis and treatment given by the surgeon, thereby subjected to his or the hospitals clinical paradigm. This implies heterogeneous register populations and introduces the risk of selection bias due to treatment stratification.
Misclassification can also introduce bias. In a register there is always risk for conducting misclassification as the physicians may classify patients differently. In a spine register some surgeons may consider a diagnosis to be central stenosis while others may view it as a lateral recess senosis. The same problem applies to DS as some surgeons may considered patients to have a slip while others do not. Well defined diagnostic criteria should therefore be in place to guide surgeons as to how to classify patients in a register. Measuring interobserver agreement of the classifying surgeons at different locations within a register may increase the validity of a register and with time reduce misclassification.
As pointed out earlier, when analyzing register data, one must always be aware of the risk that there are baseline differences or a case-mix and potential confounders of outcome that could interfere in our conclusions. This means that the results from a study in favor of a given treatment may not be due to the treatment per se but because of baseline differences and/or stratification. Adjusting for baseline differences is one way of reducing a confounding bias, albeit performing adjustments infers risk for conducting overadjustments (Schisterman et al. Citation2009; Cole et al. Citation2010). Overadjustment infers unnecessary control variables obscuring true effect of true relevant variables for outcome. Overadjustment biases results towards the null difference (Rothman and Greenland Citation2005). Overadjustement can by regression adjustment, stratification, and restriction lead to increased net bias or affect precision without affecting bias (Schisterman et al. Citation2009). Registries often include vast amount of information and analyzing such data is important for generating hypotheses, but these hypotheses should then generally be tested in studies with higher impact of evidence, preferably RCT.
Reservations for analysis of pain predominance
Deducing what is the anatomical cause for degenerative BP in various clinical settings is in practice most difficult to conduct (Kapellen and Beall Citation2010). Despite this, many clinicians attempt to assign the pain to an anatomic localization and describe the pain as being discogenic, facetogenic, sacroiliac and/or myofascial (Balagué et al. Citation2012). Surgery for BP is controversial (Balagué et al. Citation2012) and even when acquired structural abnormalities exist, such as spinal stenosis, spondylolisthesis or scoliosis, and these are perceived to be associated with BP but without leg pain or neurogenic claudication, surgery is often disputable (Kleinstück et al. Citation2009).
Katz et al. revealed that patients with predominant BP were less satisfied with surgery for LSS than in those with predominant LP (Katz et al. Citation1995b). However, only recently have studies on LSS confirmed inferior outcome in patients with predominant BP using validated outcome measures (Kleinstück et al. Citation2009; Pearson et al. Citation2011). Both these studies extrapolated predominance of pain from standardized pain scales but in the study by Kleinstück et al. (Citation2009) specific questions were forwarded to define the “main / greatest” pain problem (Pearson et al. Citation2011). Although intuitively simple, assigning predominant pain localization is difficult for most patients with LSS (Wai et al. Citation2009). As there is no specific question on pain predominance in the Swespine protocol, pain predominance was extrapolated from the 100 mm VAS scale. This means that the patients were never asked what constituted their main problem – the leg or the back pain. A similar approach to target the main problem has been used in other studies such as the SPORT study (Pearson et al. Citation2011). We therefore presumed that patients reporting higher VAS BP than VAS LP actually had BP predominance and vice versa. However, projecting pain predominance from a 100 mm VAS scale carries with it a number of methodological problems. The first is if extrapolating pain predominance from the VAS scale legitimately reflects predominance of leg or back pain. Studies on this subject are almost completely lacking as only two studies have analyzed the pain predominance (Wai et al. Citation2009; Mannion et al. Citation2014a). Wai et al. (Citation2009) showed that the ability to identify the leg or back pain was unreliable. The most reliable method was to use the questions (i) how many percent of the total pain is in the back? And (ii) how many percent of the total pain is in the leg? Given the poor reliability of most other questions, they recommended the use of a battery of question when assessing the pain predominance instead of a single question (Wai et al. Citation2009). These results challenge the validity of studies of outcome according to pain predominance, including the present study (Study IV and V).
Recently, Mannion et al. (Citation2014a) studied the validity of using a single item for the “main symptom” in degenerative disorders of the spine. They found good agreement between the differential ratings of a 0–10 pain scale and the expression “main symptom”, leg/buttock pain and low back pain. This supports the validity of our dichotomous analysis according to pain predominance.
The second question pertains to the 100 mm scale. A patient with BP predominance can have a 100 mm rating of the BP and 1 mm rating of the LP, but also a 51 mm rating of BP and 49 mm rating of LP. Both these patients would be classified in the same sub-group. In the former example the patient clearly has predominant BP but in the latter example both leg and back pain afflict the patient to a similar extent. This implies that patients in both the BP and LP predominant groups can be quite heterogeneous in terms of pain distribution. Reporting equal amounts of leg and back pain was common in our analysis of pain as 12% of patients with different forms of LSS actually reported equal amounts of pain. This means that 12% reported the same exact mm value on the 100 mm VAS scale. When we analyzed data before concluding Study IV it was evident that patients in this group were quite similar to the group with predominant BP and therefore all patients with back pain equal to leg pain were assigned to the predominant BP group.
Accounting for bias and confounding
Studies included in this thesis are biased. Selection bias is found in all of the studies included in this thesis. For example, in Study I–II selection bias is introduced as only patients with MRI data present at a local server in Lund were included. This excludes a considerable number of patients with MRI’s located on other hospital servers. Patients of the Lund catchment area may have another sociodemopraphic composition than the general population and factors such as education and income are known to be of prognostic value for surgical outcome (Katz et al. Citation1999; Cobo Soriano et al. Citation2010; Kim et al. Citation2014). Moreover, patients included in the database used in Studies I and II were only included if the MRI’s included axial slices on all spinal levels of the whole lumbar spine. Obtaining axial slices at every level of the lumbar spine is seldom routinely performed as it adds cost and time to the procedure. Axial slices were probably performed at every level as these patients had a global degenerative lumbar spine with suspected stenosis of some type at several different levels. This implies the cohort to have extensive lumbar pathology and then perhaps more likely to have high BP levels and many spinal levels afflicted. This makes our patients more likely to be older and more likely to have multilevel surgery as well as perhaps be more likely to have suboptimal outcome of surgery. As these patients had a significant clinical and radiological disease the risk for surgical selection bias may however be considered negligible.
Baseline differences due to treatment stratification are also evident as the patients in the predominant LP groups with DF generally have worse baseline scores than those with decompression only and this is particularly evident in Study V. Owing to baseline differences in back pain in the predominant leg pain group in Study V, no firm conclusions can be made as to the benefit of adding fusion, despite a greater change for the DF patients in the predominant leg pain group. Although attempts have consistently been made to account for bias in the studies, additional unmeasured bias likely exists. Such a confounding bias is unavoidable and intrinsically affects the treatment decision by the surgeon. The decision to fuse is in each case likely multifacetal, including factors such as age, bone quality, comorbidity, degree of slip, disc height and perhaps the results from further radiological studies. These patients were likely offered fusion not merely because of back pain or only beacause of DS but the surgeon selected to add fusion in these patients based on clinical factors not recorded in the Swespine database. These clinical factors prompting the surgeon to select fusion are physical, mental as well as intuitive and introduce confounding bias (confounding by indication).
Attrition bias can also affect treatment groups unevenly such as in Study V where significantly more patients in the DF groups were lost to the two-year follow-up. If these differences are systematic, thereby excluding particular patients in the cohort the results become biased. Although follow-up within the Register is identical for all patients it is likely that the fused patients had more follow-up visits because of their treatment, for example to assess bony fusion. Perhaps this leads to exhaustion with regard to further follow-up within the Register framework and subsequently more attrition bias in this group.
Limiting the effect of bias
A register study design is methodologically an observational case study. In such studies there may be systematic differences between groups of patients undergoing various forms of surgical treatment. These differences in the groups at baseline can introduce confounding bias and may affect the validity of the results. There are various statistical methods that can be applied in register studies to address this problem (Ranstam et al. Citation2011) We decided to use well-established methods used in the Swedish Arthroplasty Registry with experience in handling statistical data from large registries in Sweden (Ranstam et al. Citation2011). The potential influence of bias was reduced through statistical adjustments, mainly by performing crude and adjusted analyses of linear and Cox regressions. The Cox model can be expanded (adjusted) to include known or assumed confounders (Study IV). By including factors such as age, gender, previous surgery, the hazard ratio will be estimated conditionally on these variables. Adjustments were made for factors previously shown to have prognostic value in surgery for LSS as well as factors that can reasonably affect the outcome of surgery (Aalto et al. Citation2006). There is however always risk for residual confounding factors not adjusted for as well as overadjusting the comparisons (Schisterman et al. Citation2009; Cole et al. Citation2010). As we are bound by our data we are unable to adjust for all factors reasonably affecting outcome, but not included in our database. Such an example is the powerful predictor depression (Sinikallio et al. Citation2009). Introducing multiple potential confounders in the statistical analysis also affects the statistical precision. An example of is found in Study IV where a 2.3 advantage in PCS is observed to be statistically significant. This clinically relevant difference remains essentially unchanged in absolute values when adjustments are made but the difference is no longer statistically significant.
Recently, propensity score matching has gained popularity for analyzing observational data. Propensity score matching is a statistical method for analyzing treatment effect and accounting for bias. The matching attempts at mimicking randomization through sample of units that received the treatment that is comparable on all observed covariates to a sample of units that did not receive the treatment (Rosenbaum and Rubin Citation1983). Although propensity scoring is increasingly being used when analyzing observational data there is limited evidence proving its superiority to traditional regression modeling (Shah et al. Citation2005; Senn et al. Citation2007; Williamson et al. Citation2012) which is one of the reasons why no such analyses has been performed in this thesis.
Bias is introduced if there are significant baseline differences between groups and if there are many dropouts from the follow-up. There are many conceivable reasons for missing values both in terms of the Register structure and follow-up protocols and in terms of patient related reasons, such as death, disease or disinterest. Addressing missing values is important as intrinsic to the missing problem is the selection bias the drop-outs can introduce. In our studies on outcome we have accounted for missing values as described in the method section.
Some group differences were found when comparing missing with non-missing, indicating that there could be some degree of selection bias. We do however not know if these patients would have comparable outcome, were they traced, but a recent analysis from Norwegian Spine Registry has showed this to be likely (Solberg et al. Citation2011). Baseline differences were also analyzed and an example of a relevant baseline difference is found in Study V where the DF group had a significantly more patients with duration of symptoms exceeding two years. Due to this baseline difference there is risk for underestimating benefit of fusion in Study V as patients with long duration of symptoms tend to have inferior outcome inferior outcome (Radcliff et al. Citation2011).
It should also be noted that we did not adjust for all outcome measures, most importantly satisfaction and self-estimated walking distance at the one-year follow-up. What treatment satisfaction means and what propels it is debated (Haldeman Citation2012). Studies from the US have shown satisfied patients to consume more health care in terms of time and costs (Fenton et al. Citation2012). Many spine surgeons have probably experienced satisfied patients despite inadequate treatment effect in terms of pain reduction and functional improvement. This means that satisfaction is not always reflecting treatment effect or clinically important improvement (Yamashita et al. Citation2003, 2006). We could, despite this concern in Study II, show that patients who were satisfied also achieved statistically significant improvements in all outcome measures while undecided (except for EQ-5D and SEWD) and dissatisfied patients did not. These results must however be interpreted carefully as the undecided and dissatisfied groups contained much fewer patients, indicating the possibility of conducting a type II error. As it is difficult to postulate what factors infer satisfaction one year after spinal stenosis surgery we decided not to make adjustment for patient satisfaction in our analysis. In Study IV, it was obvious that lack of satisfaction was more common in patients with high preoperative BP levels as previously shown by Katz et al. (Citation1995b). Thus it’s clear that further studies are needed regarding what determines satisfaction with treatment.
No adjustments were made when analyzing SEWD. Although in Study III, correlation was observed between leg and back pain and groups of predesignated walking distances, , SEWD is known to be notoriously inaccurate (Sharrack and Hughes Citation1997; Watson et al. Citation1997; Giantomaso et al. Citation2003; Okoro et al. Citation2010). The inaccuracy of the SEMD increases with patient age (Okoro et al. Citation2010). The true (measured) walking distance is undoubtedly influenced by a myriad of factors besides the LSS such as age, other comorbidities, weight and general physical ability.
Duration of symptoms
In Study II, patients with duration of LP ≤ 2 years reported better outcome in terms of EQ-5D, ODI, and lower LP, marginally lower BP and longer SEWD compared with patients with DOS > 2 years. Also, the EQ-5D score was higher in patients with duration of BP ≤ 2 years. It seems logical that patients with a long duration of symptoms report inferior outcome as persistent pain and functional impairment leads to inferior HRQoL. Furthermore, the limited physiological reserve in the elderly makes improvements after surgery laborious and time-consuming. Moreover, persistent pressure on nerve roots induces morphological changes in the nerves and influences neuronal blood flow (Parke et al. Citation1981; Olmarker et al. Citation1989; Gupta et al. Citation2004; Chao et al. Citation2008). The influence of duration of symptoms for the final outcome has only sparsely been studied before. In the SPORT study, DOS >12 months was associated with less favorable outcome, both in patients treated operatively and non-operatively (Radcliff et al. Citation2011). Ng et al. (Citation2007) also showed DOS to be correlated with outcome in terms of pain and function (ODI). Patients with symptoms < 33 months in this study had better outcome than those with longer duration of symptoms (Ng et al. Citation2007). Also, Jakola et al. (Citation2010) showed patients with longer duration of leg pain to report less improvement in terms of ODI. A previous study from our department has shown that DOS for more than 4 years is associated with inferior outcome (Jönsson et al. Citation1997). In contrast, other studies refute that DOS is of importance for outcome (Herno et al. Citation1996; Amundsen et al. Citation2000; McGregor and Hughes Citation2002; Spratt et al. Citation2004)
The Swespine protocol has five different groups to assign duration of symptoms (no pain, < 3 months, 3–12 months, 1–2 years, and > 2 years). Due to small numbers in the 3–12 months group in Study II the patients were dichotomized into two groups. When all patients with DOS of ≤ 2 years were pooled, comparable number of patients were presented in the ≤ 2-year and > 2-year groups. Analyzing data in this way has some limitations as the two-year time point can hardly be considered a cut off point for the outcome as functional deterioration should be expected to be linear. Reasons for the often long duration of symptoms before surgery could possibly be the result of insidious development of stenosis symptoms, patient delay and/or doctor delay. Our data highlight the question if early surgery should be advocated in patients with LSS, before functional impairment becomes severe.
Imaging, symptoms and outcome
Evaluating the degree of compression of the dural sac instinctively appears to be a simple task, but in reality this is a matter of confusion and debate (Andreisek et al. Citation2011; Mattei Citation2013). Great variability is found in the literature with respect to which radiological criteria should be used when stating the diagnosis and a myriad of quantitative, semiquantitative and qualitative radiological criteria has been used (Andreisek et al. Citation2011, 2013; Mattei Citation2013). Cross-sectional MRI is currently the modality of choice to confirm the diagnosis of symptomatic LSS and essential for planning the operation. MRI is not of value in asymptomatic elderly people, since a great proportion of healthy elderly have also MRI abnormalities (Haig and Tomkins Citation2010; Haig Citation2014). Furthermore, a considerable part (20%) of people over 60 years old has radiological LSS without symptoms (Boden et al. Citation1990). It is therefore the primary objective of the clinician to correlate the clinical symptoms with the MRI findings, which often expose multiple potentially symptomatic findings. Although imaging findings are evaluated and/or measured in most cases, the robust evidence for distinct connections between imaging findings and symptoms has remained elusive (Geisser et al. Citation2007; Sirvanci et al. Citation2008; Mattei Citation2013). However, some evidence exist for a more prounounced functional disability when the dural sac area is < 70 mm2 (Athiviraham et al. Citation2007; Ogikubo et al. Citation2007).
In Study I, no significant correlation was found between pain, function and HRQoL and dural sac area. This corresponds well to the daily clinical reality where patients with extensive degenerative changes may have few symptoms while those with supposedly trivial or minor degenerative changes may have severe symptoms. The explanations for lack of correlation between imaging findings and clinical symptoms can be numerous. Measurements of the central dural sac area may not be very good at confirming the diagnosis of stenosis (lack of specificity).
It is possible to find patients with clinical symptoms and a dural sac area of 90–100 mm2 but with packed nerve roots (sedimentation sign). This sign might be superior to dural sac area in confirming the clinical diagnosis of LSS (Barz et al. Citation2010). Furthermore, stenosis and slip can be obscured so that the compression of the dural sac may be evident only when dynamic imaging is performed, either by a weight bearing MRI (Danielson and Willén Citation2001; Willén and Danielson Citation2001) or dynamic standing x-rays which can also reveal spondylolisthesis (Chaput et al. Citation2007; Cho et al. Citation2009; Lattig et al. Citation2012a). It may be important to also target patients with “hidden” (revealed only on weight bearing MRI) stenosis as the surgical outcome seems similar to the outcome in LSS where stenosis is only observed on the supine MRI (Willén et al. Citation2008).
Another limitation in Study I is that we only evaluated the dural sac area and the coexistence of DS at one or more levels. By doing so, other potential causes of pain in the lumbar spine, such as disk degeneration, facet joint arthritis, osteoporotic fractures, Modic changes, scoliosis and kyphosis may have been ignored. Most of our patients also had obvious dural sac compression (well under 70 mm2) and it is conceivable that symptoms characterizing LSS become perceptible when the dural sac area approaches 70 mm2, however, it is not known if symptoms develop linearly with increasing degree of stenosis (Athiviraham et al. Citation2007). It is also likely that neuronal adaptation to stenosis occurs when the morphological change develops slowly over time. The results from Study I also indicate that there is a simplification in attributing BP and LP to measurements of the dural sac area or degree of DS, since there is a variety of other pain generators that could explain the clinical picture and supine MRI only tells part of the diagnostic truth. In this context it is also important to highlight the fact that the population studied underwent surgical intervention and therefore could not be regarded as representative for all individuals with LSS as it is well-known that LSS with little or negligible symptoms is common in the elderly population (Kalichman et al. Citation2009; Ishimoto et al. Citation2013). Despite the reservations described above we could in Study II demonstrate a weak correlation between outcome in terms of leg and back pain and the preoperative dural sac area as patients with the more severe constrictions of the dural sac improved more in terms of back pain and had lower absolute leg pain levels. These results may indicate that patients with more severe constrictions have better outcomes.
The role of spinal fusion in lumbar spinal stenosis without DS
In the absence of deformity (spondylolisthesis and/or scoliosis), it is questionable to perform spinal fusion in LSS patients (Deyo et al. Citation2004) as no studies have shown superior outcomes by adding fusion to the decompression in patients without deformity (Grob et al. Citation1995). However, some evidence can be found for superior outcome when fusion is added to the decompression in patients with spondylolisthesis or scoliosis (Herkowitz and Kurz Citation1991; Bridwell et al. Citation1993; Martin et al. Citation2007). Yet, none of these studies have included adequate number of patients to analyze the outcome in terms of pain predominance according to provided treatment (Pearson et al. Citation2011). Although fusion has been suggested to induce better surgical outcome in patients with LSS and predominant BP, no studies support this claim. Moreover, Studies IV and V show that even patients with predominant BP experience improvements in BP with decompression only. These findings suggest that factors other than segmental instability play an important role for back pain in LSS as many of these patients experience improvements in back pain despite the absence of stabilizing surgery.
Study IV shows that patients with CSS without spondylolisthesis with predominant BP experience inferior outcome of surgery than patients with predominant LP, thereby confirming the results of two recent studies (Kleinstück et al. Citation2009; Pearson et al. Citation2011). In Study IV, patients with predominant BP had a small and marginally significant benefit from decompression and fusion in terms of PCS and EQ-5D compared to patients with predominant BP with decompression only. The marginal benefit does not provide a general indication for spinal fusion in patients with predominant BP but may imply that there is a subgroup of patients with LSS who would benefit from fusion. The small benefit for fusion diminished in the adjusted analysis and was not present at the two-year follow-up. Improved identification of patients potentially benefiting from fusion is an important task. Initial steps in that process would be to address how we ask about and identify BP (Wai et al. Citation2009; Mannion et al. Citation2014a) to better distinguish patients with mechanical low back pain coupled to their spinal stenosis. Subsequent steps should address how we identify subtle signs of instability on MRI, such as facet joint effusion (Lattig et al. Citation2012a). Further analysis should include dynamic radiographs and MRI findings such as active Modic changes coupled to back pain at the stenotic segment. In the Register there is no information about these relevant radiological findings.
A substantial number of patients could have more severe instability than the supine preoperative MRI displays (Chaput et al. Citation2007; Cho et al. Citation2009; Lattig et al. Citation2012a). Chaput et al. (Citation2007) showed that 22% of the degenerative olisthesis cases were not detectable on supine MRI but could be revealed on standing x-rays. Their and subsequent studies have also shown facet joint effusions to be associated with DS, not revealed on MRI. Patients with “hidden” instability could hypothetically be expected to have more mechanical back pain and inferior outcome from decompressive surgery only, however this hypothesis has not been proven (Lattig et al. Citation2012b). These patients probably are at an early stage in the degenerative process where the slip occurs only in loaded spine positions but not yet in the supine position in which MRI scans are performed (Chaput et al. Citation2007; Cho et al. Citation2009; Lattig et al. Citation2012a). A decompression only in these patients neglects to address the instability and any effect of a simple decompression might be transient. However, it is also notable that DS is seen frequently in asymptomatics and that hypermobility in the early phase of DS can spontaneously stabilize as degeneration progresses (Matsunaga et al. Citation1990; Hasegawa et al. Citation2014).
Comparing the results of Study IV to the only existing RCT comparing decompression only to decompression and fusion in LSS is not straightforward. The RCT performed by Grob et al. (Citation1995) included 45 patients randomized to 3 treatment alternatives. One group was treated with decompression only, the second with decompression and fusion at the most stenotic level and the third with decompression and fusion at all decompressed levels. They studied the outcome of surgery of LSS without DS but allowed up to 5 mm slip, compared to 3 mm in the Swespine protocol. Follow-up was minimum two years and no significance difference was found between the groups in terms of pain and function at the follow-up. The patients subjected to decompression in the study by Grob et al. were decompressed while maintaining the integrity of the posterior structures thereby perhaps reducing the risk for further slip. This is a significant difference from Study IV where all patients in the D group underwent conventional decompression.
Prospective studies on this subject are lacking, but Yone and Sakou (Citation1999) performed a nuanced analysis of patients with LSS using the Posner criteria for spinal instability. The Posner criteria for instability states that anterior and posterior translation are measured as percentages of vertebral body AP width and the upper limits of translatory and angular motions are 9% for posterior translation and angulation and 8% for anterior translation in the L1–L5 region (Posner et al. Citation1982). They found only 43% of decompressed patients with instability to have good outcome in terms of the Japanese Orthopaedic Association (JOA) score. The group with instability and fusion and the group without instability and decompression both had a good outcome (80%). Comparison with Study IV is difficult as the JOA score was used in Yone and Sakou’s study and they also included few patients and no conclusion can be drawn with regards to superior outcome of fusion in the group without instability as no patients without instability underwent concomitant fusion. When instability was diagnosed, the outcome of decompression and fusion was superior to decompression only. The patient groups were small however, 19 underwent fusion and 14 decompression only. In summary, the most significant results from the above described study are that LSS is occasionally associated with biomechanical instability and/or painful mobility and these patients may benefit from adding fusion. A number of patients in the Register with LSS undoubdedly have these characteristics and would possibly benefit from fusion.
In Study IV, the patients with decompression and fusion were younger and with more preoperative pain and disability. This and the fact that only one in eight patients underwent fusion indicates that these patients were highly selected as the surgeon considered fusion to be indicated. Subsequently, these patients were perhaps more likely to benefit from fusion and there is a risk for overestimating the treatment effect should the results be transferred to all patients with LSS.
The role of spinal fusion in DS
According to systematic reviews (Martin et al. Citation2007; Steiger et al. Citation2014), adding spinal fusion to decompression is considered preferred treatment in spinal stenosis with concomitant DS. In the U.S. 83% of patients with DS undergo fusion when being decompressed (Bae et al. Citation2013). The evidence for generalizing including fusion in DS is inadequate (Herkowitz and Kurz Citation1991; Bridwell et al. Citation1993; Ghogawala et al. Citation2004; Matsudaira et al. Citation2005; Försth et al. Citation2013; Steiger et al. Citation2014). The main evidence for generally performing a concomitant fusion derives from 2 RCT’s, but neither explored the role of pain predominance and outcome (Herkowitz and Kurz Citation1991; Bridwell et al. Citation1993). Comparison between these RCT’s and Study V are problematic as the focus of the studies is different. The seminal study by Herkowitz and Kurz (Citation1991) included 50 patients in two equal groups treated with either laminectomy or laminectomy and unistrumented fusion. The patients were enrolled alternatively into treatment groups and were not actually randomized. Neither the surgeons nor the patients were blinded with regard to treatment. Outcome was evaluated in terms of patient – doctor composite rating and a VAS. No validated general or disease specific outcome measures, such as the SF-36 or the ODI were used. The main result was that patients with DS who were only decompressed had more residual leg and back pain than those decompressed and fused (Herkowitz and Kurz Citation1991).
The other RCT by Bridwell et al. (Citation1993) included 44 patients allocated to 3 treatment groups. Group I included 9 patients who were decompressed, group II included 10 patients who were decompressed with uninstrumented posterolateral fusion and group III included 24 patients who had decompression and instrumented fusion. No validated outcome measures were used in this study and the method of randomization was not described. Outcome was limited to assessment of walking distance and radiological findings, as the patients were asked if their ability to walk distances was better, the same or worse than before the operation. The patients in the instrumented fusion group more often reported they could walk longer distances at follow-up compared with the other groups (Bridwell et al. Citation1993).
The RCT by Malmivaara et al. (Citation2007) comparing surgery to conservative treatment for spinal stenosis also included 20 patients with spondylolisthesis (41% of the operated patients) and 10 of these had fusion. The fused patients generally had better outcomes than decompressed only in terms of pain and ODI but the only statistically significant difference between fused and not fused was in the leg pain improvement. The Finnish Spinal Stenosis study was however not designed to assess the treatment effect of fusion in DS versus decompression only and the low number of patients with DS precludes valid conclusions. Similar results were found in Study V of this thesis where patients with fusion in the back pain predominant group had better outcome in terms of leg pain than those decompressed only.
The observational studies that have compared decompression with decompression and fusion in DS generally support the view that an added fusion to the standard decompression results in better outcome, but no study has analyzed the outcome in terms of pain predominance. There are however two studies that oppose this view. Matsudaira et al. (Citation2005) compared laminoplasty with laminectomy and spinal fusion in patients with DS at the L4–L5 level. The study groups included 18 and 19 patients respectively, and the authors found no significant group difference at the one-year follow-up despite increased slip in the non-fused group. When comparing the study by Matsudaira et al. (Citation2005) with Study V it should be kept in mind that the surgical techniques are different in the two reports. In the laminoplasty group the posterior structures are left intact perhaps reducing the risk for further instability and re-stenosis. Försth et al. (Citation2013), using data from Swespine, compared treatment outcomes for decompression and decompression and fusion in spinal stenosis with and without concomitant DS. This report included data on 5,390 patients collected between 1998 and 2008 compared with 9,051 patients in Study IV and 1,624 patients in Study V collected between 2003 and 2010. They found no significant differences between the two treatment groups at the two-year follow-up (Försth et al. Citation2013). The larger sample size included in this thesis is explained by the increase in LSS surgery in Sweden during the recent years as well as more stringent inclusion criteria for analysis in the study by Försth et al. (Citation2013) compared to Study IV of this thesis, the larger sample size increases the statistical power of our analysis. In addition, Försth et al. (Citation2013) analyzed outcome in relation to diagnosis and treatment, but not according to pain predominance. This is important as it demonstrates an effect of preoperative pain pattern on outcome, although the effect of fusion is still weak in patients with predominant BP. Like Försth et al. (Citation2013) we could not observe any statistical benefit from fusion at the two-year follow-up, despite analyzing the data according to pain predominance. Admittedly, many patients were lost to follow-up at the two-year milestone, thereby increasing the risk of inducing a type II error.
Prospective observational studies that evaluate this issue are also lacking. The results from Matsudaira et al. (Citation2005) have been discussed but there is also a report by Ghogawala et al. (Citation2004) who evaluated the prospective outcome at one year after operation in patients with grade I DS. In this study the decompression group included 20 patients and the decompression and fusion group 14 patients. Although both types of surgery improved the outcome, there was a higher degree of improvement in ODI in the DF group than in the D group. No analysis according to pain predominance was performed in this study and the treatment groups are small.
The few retrospective studies that have analyzed outcome of decompression compared to decompression and fusion in patients with DS include few patients, seldom with consecutive inclusion, patients with and without additional scoliosis, and rarely use validated outcome measures (Feffer et al. Citation1985; Lombardi et al. Citation1985; Satomi et al. Citation1992; Yone et al. Citation1996). In spite of a low level of evidence, it is worth noting that these studies usually favor fusion in DS.
There are most certainly patients who benefit from an additional fusion but fusion is probably not always necessary. Furthermore, biomechanical studies show that not all DS cases fulfill criteria for radiological instability (Hasegewa et al. Citation2009) and the outcome of a decompression with or without fusion in these patients must be evaluated. To date, no studies have explored the outcome of surgery in patients with DS according to pain predominance and treatment (decompression versus decompression and fusion). In Study V, benefit of fusion was mainly experienced in the group with predominant BP. This resonates well with current paradigms on spinal instability, where instability and pain are considered to respond well to stabilizing surgery (Knaub et al. Citation2005; Sengupta and Herkowitz Citation2005).
Kirkaldy-Willis et al. (Citation1978) described three phases of the degenerative process. In the first phase there are dysfunctional discoligamentous structures with minimal structural changes. In the second phase there is a relative instability with disc height reduction, facet capsule and ligament elongation with unspecific articular changes. These changes may lead to abnormal sagittal and rotational motion with pain. Continued degeneration however, leads to restabilization because of increased stiffness, osteophytosis and fibrosis perhaps followed by reduced BP (Matsunaga et al. Citation1990) but continued leg pain because of foraminal stenosis and nerve root compression secondary to the slip. Intuitively, patients in the second phase would gain most benefit from a fusion as they are in a phase of instability associated with BP (Hasegawa et al. Citation2014). Indirectly confirming this theory is the finding that high grade osteoarthritis of the facet joints is less correlated to facet joint effusion than intermediate or low grade osteoarthritis but facet joint effusions have been linked to instability (Chaput et al. Citation2007). Also, Cho et al. (Citation2009) have shown that patients with DS without facet joint effusions on MRI were older than patients with facet joint effusions. This could corroborate with the findings in Study V that the patients with predominant leg pain with decompression only were significantly older and had significantly lower BP compared to DF patients in the LP predominant group. Furthermore, Matsunaga et al. (Citation1990) have shown back pain to decrease with increased disc degeneration (reduced disc height). These patients may find themselves in a biomechanically stable situation with a high degenerative grade, thereby experiencing less BP and subsequently not reaping the benefits of an added fusion in terms of back pain. In Study V, the patients with DF in the PL group were significantly different in terms of back pain at baseline than the aforementioned patients with decompression only. The DF patients in the PL groups had significantly higher baseline back pain levels and therefore had a potential for improvement by DF. The benefit of fusion in terms of back pain in the DF group compared to the D group (PL group) may be due to baseline differences and not the treatment per se.
It can also be argued that the absence of difference in outcomes of decompression with and without fusion at the one- and two-year follow-up may not remain in a longer perspective if the spondylolisthesis progresses in the non-fused group, but this remains to be analyzed. It can also be argued that DS is a self-limiting morphologic entity which does not progress further when the disc space is obliterated (Matsunaga et al. Citation1990). Furthermore, there is the possibility that fusion of one or two levels in a degenerated lumbar spine will create adjacent problems due to altered biomechanics and increased loads on the unfused segments (Park et al. Citation2004; Mannion et al. Citation2014b)
We did not study the reoperation rate related to treatment and pain predominance. Recent studies have however shown that fusion is not associated with significantly lower rates of repeat surgery after the first postoperative year (Deyo et al. Citation2011). Complex fusions were associated with the highest rates of reoperations (Deyo et al. Citation2011).
In conclusion, patients with DS have leg and back pain and DF is considered the treatment of choice for mant but not all patients. Our analysis shows patients with predominant back pain to benefit more from DF compared to D in the short term perspective.
Strength and limitations
The main strengths of our studies are a prospective patient based data collection with many patients included, well-described patient populations and the use of established statistical methods for estimating differences. Also, available data for missing patients at different follow-up time points was compared with data for participating patients to address selection bias. Potential confounding factors are also described and included in the statistical analysis and the discussion. Furthermore, well-known validated general and organ specific patient reported outcome measures are used before and after treatment, a fact that makes it possible to adequately compare the outcome of different studies.
The limitations include the observational study design with well-known flaws that apply to register studies (Concato et al. Citation2000; Diamond Citation2014). These limitations include unknown or unmatched covariates that can be allocated differently in the different groups. In addition, risk stratification is a problem in register studies as some patients are considered to have risk for a certain event, such as the risk for further slip, stratifying them to fusion thereby inferring bias. Also, missing values introduce attrition bias which can change the characteritics of the groups being compared. The Zurich Claudication Questionnaire (ZCQ) was not used in our analyses as the ZCQ, a validated disease specific PROM for LSS, is not a part of the spine protocol which is constructed for lumbar degenerative disorders i general.
Conclusions
Spinal pathomorpholgy, i.e. degree of stenosis, multilevel stenosis and DS, as observed and measured on preoperative MRI’s shows limited correlation to symptoms (leg and back pain), HRQoL (SF-36 and EQ-5D) and function (ODI and SEWD).
Spinal pathomorphology correlates poorly to outcome in terms of pain, function and HRQoL.
Duration of leg and back pain for more than two years, poor preoperative function and regular use of analgesics preoperatively are associated with inferior outcome at the one-year follow-up.
Back pain is frequently experienced in the three morphological forms of stenosis. More than 50% of patients operated on for spinal stenosis in Sweden grade higher or equal back pain levels compared to leg pain levels. The highest ratio of back to leg pain is found in spinal stenosis with DS (0.93) and the lowest in lateral recess stenosis (0.85) while the ratio is intermediate for central spinal stenosis (0.88). The lowest EQ-5D scores and highest ODI scores are observed in patients with DS and equal leg and back pain.
Predominance of back pain in CSS is associated with inferior surgical outcome. Adding spinal fusion to the decompression in patients with predominant BP improves the outcome modestly but the improvement is hardly clinically relevant on an individual level. The improvements seen at the one-year follow-up in the unadjusted analysis generally diminish when adjustments are made for confounders. At the two-year follow-up no benefit in favour of fusion can be registered. Spinal fusion is associated with higher satisfaction score but the reason remains unknown.
Patients with LSS and DS and predominant back pain benefit from fusion in terms of leg and back pain, the ODI and the EQ-5D. The patients with DF experience more improvement in the PROM’s compared with patients with D at the one-year follow-up but the benefit does not persist at the two-year follow-up. The benefits in terms of pain and the ODI are moderate on a group level but may fail to be clinically significant on an individual level. The benefit favouring fusion in terms of EQ-5D is small. In the predominant LP group patients DF have better outcome than D in terms of back pain. However, due to significant baseline differences between DF and D patients in terms of back pain the benefit from fusion may not be due to the added fusion per se.
Implications for further research
There is potential for improvement in the outcome of surgery for lumbar spinal stenosis. Improving the outcome involves selecting the appropriate surgical method to the right patient. The patients with most inferior outcome and residual symptoms after surgery are those with predominant back pain preoperatively. Treating all these patients with decompression and concomitant fusion would be excessive but identifying those benefiting from an additional fusion could potentially improve the outcomes.
First, we must improve our ability to estimate the influence of leg and back pain as well as understand what leg and back pain implies to the patients. Surprisingly little research has been performed on how this should be registered. Focus should be on clinically separating patients with buttock pain from those who predominantly experience mechanical low back pain in addition to their stenosis. Identifying patients with mechanical pain generators and the associated radiological findings of relevance is particularly important. Further studies should analyze the outcome in relation to pain characteristics and defined radiological characteristics. Identifying “hidden” instability in LSS, i.e. patients with degenerative spondylolisthesis (DS) found only when loading the spine is important, since a similar posture dependent deformity might explain the inferior outcome in some individuals.
The fusion is added because of granted instability. However, the theories of the degenerative cascade as well as some recent studies indicate that not all DS fulfill criteria for radiological instability as neglible mobility occurs in the spinal segment. This might obviate the need for adding fusion in selected patients and the outcome of surgery in these patients must be analyzed. Many patients with DS probably do well with fusion but identifying patients who would have little effect from additional fusion with the generally accepted dural sac decompression is another priority. These are probably patients that find themselves in a late stage of the degenerative process.
Studies combining radiological datasets from large centers of spinal surgery coupled with the PROM`s from the Swespine could potentially assist at answering these questions. An alternative is to include detailed information about radiological characteristcs in the Register and combine with the surgical data
Register studies such as those included in this thesis are excellent to create hypotheses. When working with large datasets associations between variables can be identified. These associations should then preferably be tested in RCT’s.
References
- Aalto TJ, Malmivaara A, Kovacs F, Herno A, Alen M, Salmi L, et al. Preoperative predictors for postoperative clinical outcome in lumbar spinal stenosis: systematic review. Spine 2006; 31(18): E648–663.
- Van Agt HM, Essink-Bot ML, Krabbe PF, Bonsel GJ. Test-retest reliability of health state valuations collected with the EuroQol questionnaire. Soc Sci Med 1994; 39(11): 1537–44.
- Airaksinen O, Herno A, Turunen V, Saari T, Suomlainen O. Surgical outcome of 438 patients treated surgically for lumbar spinal stenosis. Spine 1997; 22(19): 2278–82.
- Albee F. Transplantation of portion of the tibia into the spine for Pott´s disease. JAMA 1911(57): 885–6.
- Ammendolia C, Stuber K, Tomkins-Lane C, Schneider M, Rampersaud YR, Furlan AD, et al. What interventions improve walking ability in neurogenic claudication with lumbar spinal stenosis? A systematic review. Eur Spine J 2014; 23(6): 1282-301.
- Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleâs F. Lumbar spinal stenosis: conservative or surgical management?: A prospective 10-year study. Spine 2000; 25(11): 1424–1435.
- Andreisek G, Hodler J, Steurer J. Uncertainties in the diagnosis of lumbar spinal stenosis. Radiology 2011; 261(3): 681–4.
- Andreisek G, Imhof M, Wertli M, Winklhofer S, Pfirrmann CWA, Hodler J, et al. A systematic review of semiquantitative and qualitative radiologic criteria for the diagnosis of lumbar spinal stenosis. Am J Roentgenol 2013; 201(5): W735–746.
- Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop 1976; (115): 4–5.
- Athiviraham A, Yen D, Scott C, Soboleski D. Clinical correlation of radiological spinal stenosis after standardization for vertebral body size. Clin Radiol 2007; 62(8): 776–80.
- Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, et al. The Maine Lumbar Spine Study, Part II. 1-year outcomes of surgical and nonsurgical management lumbar spinal stenosis. Spine 1996; 21(15): 1787–95.
- Atlas SJ, Keller RB, Robson D, Deyo RA, Singer DE. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the maine lumbar spine study. Spine 2000; 25(5): 556–62.
- Atlas SJ, Tosteson TD, Blood EA, Skinner JS, Pransky GS, Weinstein JN. The impact of workers’ compensation on outcomes of surgical and nonoperative therapy for patients with a lumbar disc herniation: SPORT. Spine 2010; 35(1): 89–97.
- Axelsson P, Karlsson BS. Intervertebral mobility in the progressive degenerative process. A radiostereometric analysis. Eur Spine J 2004; 13(6): 567–72.
- Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine 2013; 38(11): 916–26.
- Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non-specific low back pain. The Lancet 2012; 379(9814): 482–91.
- Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003; 3: 21.
- Barz T, Melloh M, Staub LP, Lord SJ, Lange J, Röder CP, et al. Nerve root sedimentation sign: evaluation of a new radiological sign in lumbar spinal stenosis. Spine 2010; 35(8): 892–7.
- Benoist M. The natural history of lumbar degenerative spinal stenosis. Jt Bone Spine Rev Rhum 2002; 69(5): 450–7.
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000; 342(25): 1878–86.
- Berlemann U, Jeszenszky DJ, Bühler DW, Harms J. The role of lumbar lordosis, vertebral end-plate inclination, disc height, and facet orientation in degenerative spondylolisthesis. J Spinal Disord 1999; 12(1): 68–73.
- Bird HA, Eastmond CJ, Hudson A, Wright V. Is generalized joint laxity a factor in spondylolisthesis? Scand J Rheumatol 1980; 9(4): 203–5.
- Birkmeyer NJO, Weinstein JN, Tosteson ANA, Tosteson TD, Skinner JS, Lurie JD, et al. Design of the Spine Patient outcomes Research Trial (SPORT). Spine 2002; 27(12): 1361–72.
- Black N. Why we need observational studies to evaluate the effectiveness of healthcare. BMJ 1996; 312: 1215-8.
- Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990; 72(3): 403–8.
- Boden SD, Riew KD, Yamaguchi K, Branch TP, Schellinger D, Wiesel SW. Orientation of the lumbar facet joints: association with degenerative disc disease. J Bone Joint Surg Am 1996; 78(3): 403–11.
- Bolender NF, Schönström NS, Spengler DM. Role of computed tomography and myelography in the diagnosis of central spinal stenosis. J Bone Joint Surg Am 1985; 67(2): 240–6.
- Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders: summary and general recommendations. Spine 2000; 25(24): 3100–3.
- Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Qual Life Res 1993; 2(3): 169–80.
- Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord 1993; 6(6): 461–72.
- Burström K, Johannesson M, Diderichsen F. Health-related quality of life by disease and socio-economic group in the general population in Sweden. Health Policy 2001a; 55(1): 51–69.
- Burström K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Qual Life Res 2001b; 10(7): 621–35.
- Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia. J Comp Neurol 2008; 506(2): 180–93.
- Chaput C, Padon D, Rush J, Lenehan E, Rahm M. The significance of increased fluid signal on magnetic resonance imaging in lumbar facets in relationship to degenerative spondylolisthesis. Spine 2007; 32(17): 1883–7.
- Di Chiro G, Schellinger D. Computed tomography of spinal cord after lumbar intrathecal introduction of metrizamide (computer-assisted myelography). Radiology 1976; 120(1): 101–4.
- Cho BY, Murovic JA, Park J. Imaging correlation of the degree of degenerative L4-5 spondylolisthesis with the corresponding amount of facet fluid. J Neurosurg Spine 2009; 11(5): 614–9.
- Cinotti G, Postacchini F, Fassari F, Urso S. Predisposing factors in degenerative spondylolisthesis. A radiographic and CT study. Int Orthop 1997; 21(5): 337–42.
- Cleveland M, Bosworth DM, Thompson FR. Pseudarthrosis in the lumbosacral spine. J Bone Joint Surg Am 1948; 30A(2): 302–12.
- Coast J, Peters TJ, Richards SH, Gunnell DJ. Use of the EuroQoL among elderly acute care patients. Qual Life Res 1998; 7(1): 1–10.
- Cobo Soriano J, Sendino Revuelta M, Fabregate Fuente M, Cimarra Díaz I, Martínez Ureña P, Deglané Meneses R. Predictors of outcome after decompressive lumbar surgery and instrumented posterolateral fusion. Eur Spine J 2010; 19(11): 1841–8.
- Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol 2010; 39(2): 417–20.
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000; 342(25): 1887–92.
- Cornefjord M, Byröd G, Brisby H, Rydevik B. A long-term (4- to 12-year) follow-up study of surgical treatment of lumbar spinal stenosis. Eur Spine J 2000; 9(6): 563–70.
- Cressman MR, Pawl RP. Serpentine myelographic defect caused by a redundant nerve root. Case report. J Neurosurg 1968; 28: 391-3.
- Dai LY. Orientation and tropism of lumbar facet joints in degenerative spondylolisthesis. Int Orthop 2001; 25(1): 40–2.
- Danielson B, Frennered K, Irstam L. Roentgenologic assessment of spondylolisthesis. I. A study of measurement variations. Acta Radiol 1988; 29(3): 345–51.
- Danielson B, Frennered K, Selvik G, Irstam L. Roentgenologic assessment of spondylolisthesis. II. An evaluation of progression. Acta Radiol 1989; 30(1): 65–8.
- Danielson B, Willén J. Axially loaded magnetic resonance image of the lumbar spine in asymptomatic individuals. Spine 2001; 26(23): 2601–6.
- Delamarter RB, Bohlman HH, Dodge LD, Biro C. Experimental lumbar spinal stenosis. Analysis of the cortical evoked potentials, microvasculature, and histopathology. J Bone Joint Surg Am 1990; 72(1): 110–20.
- Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery - the case for restraint. N Engl J Med 2004; 350(7): 722–6.
- Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine 2005; 30(12): 1441–1445.
- Deyo RA. Back surgery--who needs it? N Engl J Med 2007; 356(22): 2239–43.
- Deyo RA. Treatment of lumbar spinal stenosis: a balancing act. Spine J 2010; 10(7): 625–7.
- Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010; 303(13): 1259–65.
- Deyo RA, Martin BI, Kreuter W, Jarvik JG, Angier H, Mirza SK. Revision surgery following operations for lumbar stenosis. J Bone Joint Surg Am 2011; 93(21): 1979–86.
- Diamond GA. Randomized Trials, Observational registries, and the foundations of evidence-based medicine. Am J Cardiol 2014; 113(8): 1436-41.
- Donath J, Vogl A. Untersuchungen über den chondrodystrophischen Zwergwuchs Weiner. Wien Arch Intern Med 1925; 10: 1.
- Dorman P, Slattery J, Farrell B, Dennis M, Sandercock P. Qualitative comparison of the reliability of health status assessments with the EuroQol and SF-36 questionnaires after stroke. United Kingdom Collaborators in the International Stroke Trial. Stroke 1998; 29(1): 63–8.
- Eisenstein S. Fusion for spinal stenosis: a personal view. J Bone Joint Surg Br 2002; 84(1): 9–10.
- Englbrecht M, Tarner IH, van der Heijde DM, Manger B, Bombardier C, Muller-Ladner U. Measuring pain and efficacy of pain treatment in inflammatory arthritis: a systematic literature review. J Rheumatol Suppl 2012; 90(0): 3–10.
- Essink-Bot ML, Krabbe PF, Bonsel GJ, Aaronson NK. An empirical comparison of four generic health status measures. The Nottingham Health Profile, the Medical Outcomes Study 36-item Short-Form Health Survey, the COOP/WONCA charts, and the EuroQol instrument. Med Care 1997; 35(5): 522–37.
- EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990; 16(3): 199–208.
- Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980; 66(8): 271–3.
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine 2000; 25(22): 2940–2952.
- Fazal A, Yoo A, Bendo JA. Does the presence of the nerve root sedimentation sign on MRI correlate with the operative level in patients undergoing posterior lumbar decompression for lumbar stenosis? Spine J 2013; 13(8): 837–42.
- Feffer HL, Wiesel SW, Cuckler JM, Rothman RH. Degenerative spondylolisthesis. To fuse or not to fuse. Spine 1985; 10(3): 287–9.
- Fenton JJ, Jerant AF, Bertakis KD, Franks P. The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med 2012; 172(5): 405–11.
- Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine 1997; 22(24): 2807–12.
- Freedman MK, Hilibrand AS, Blood EA, Zhao W, Albert TJ, Vaccaro AR, et al. The impact of diabetes on the outcomes of surgical and nonsurgical treatment of patients in the spine patient outcomes research trial. Spine 2011; 36(4): 290–307.
- Fritzell P, Hägg O, Wessberg P, Nordwall A, Swedish Lumbar Spine Study Group. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine 2001; 26(23): 2521–2532.
- Försth P, Michaëlsson K, Sandén B. Does fusion improve the outcome after decompressive surgery for lumbar spinal stenosis?: A two-year follow-up study involving 5390 patients. Bone Jt J 2013; 95-B(7): 960–5.
- Geisser ME, Haig AJ, Tong HC, Yamakawa KSJ, Quint DJ, Hoff JT, et al. Spinal canal size and clinical symptoms among persons diagnosed with lumbar spinal stenosis. Clin J Pain 2007; 23(9): 780–5.
- Ghogawala Z, Benzel EC, Amin-Hanjani S, Barker FG 2nd, Harrington JF, Magge SN, et al. Prospective outcomes evaluation after decompression with or without instrumented fusion for lumbar stenosis and degenerative Grade I spondylolisthesis. J Neurosurg Spine 2004; 1(3): 267–72.
- Giantomaso T, Makowsky L, Ashworth NL, Sankaran R. The validity of patient and physician estimates of walking distance. Clin Rehabil 2003; 17(4): 394–401.
- De Graaf I, Prak A, Bierma-Zeinstra S, Thomas S, Peul W, Koes B. Diagnosis of lumbar spinal stenosis: a systematic review of the accuracy of diagnostic tests. Spine 2006; 31(10): 1168–76.
- Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am 1995; 77(7): 1036–41.
- Grobler LJ, Robertson PA, Novotny JE, Pope MH. Etiology of spondylolisthesis. Assessment of the role played by lumbar facet joint morphology. Spine 1993; 18(1): 80–91.
- Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol 2004; 187(2): 500–8.
- Hadra BE. Wiring the spinous processes in Pott’s disease. Trans Am Orthop Assoc 1891; (4): 206–10.
- Haig AJ, Tomkins CC. Diagnosis and management of lumbar spinal stenosis. JAMA 2010; 303(1): 71–2.
- Haig AJ, Park P, Henke PK, Yamakawa KSJ, Tomkins-Lane C, Valdivia J, et al. Reliability of the clinical examination in the diagnosis of neurogenic versus vascular claudication. Spine J 2013; 13(12): 1826–34.
- Haig AJ. Diagnostic tests the NASS stenosis guidelines. Spine J 2014; 14(1): 200–1.
- Haldeman S. Commentary: is patient satisfaction a reasonable outcome measure? Spine J 2012; 12(12): 1138–9.
- Hall S, Bartleson JD, Onofrio BM, Baker HL Jr, Okazaki H, O’Duffy JD. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med 1985; 103(2): 271–5.
- Hara N, Oka H, Yamazaki T, Takeshita K, Murakami M, Hoshi K, et al. Predictors of residual symptoms in the lower extremities after decompression surgery on lumbar spinal stenosis. Eur Spine J 2010; 19: 1849-54.
- Harmon PH. Indications for spinal fusion in lumbar diskopathy, instability and arthrosis. I. Anatomic and functional pathology and review of literature. Clin Orthop 1964; 34: 73–91.
- Harrington PR. Treatment of scoliosis. Correction and internal fixation by spine instrumentation. J Bone Joint Surg Am 1962; 44-A: 591–610.
- Hasegewa K, Kitahara K, Hara T, Takano K, Shimoda H. Biomechanical evaluation of segmental instability in degenerative lumbar spondylolisthesis. Eur Spine J 2009; 18(4): 465–70.
- Hasegawa K, Shimoda H, Kitahara K, Sasaki K, Homma T. What are the reliable radiological indicators of lumbar segmental instability? J Bone Joint Surg Br 2011; 93(5): 650–7.
- Hasegawa K, Kitahara K, Shimoda H, Ishii K, Homma T. Lumbar degenerative spondylolisthesis is not always unstable. A clinico-biomechanical evidence. International Society for the Study of the Lumbar Spine; Presented at the annual meeting, Seoul Korea, june 2014.
- Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 1991; 73(6): 802–8.
- Herno A, Airaksinen O, Saari T, Luukkonen M. Lumbar spinal stenosis: a matched-pair study of operated and non-operated patients. Br J Neurosurg 1996; 10(5): 461–5.
- Hibbs R. An operation for progressive spinal deformities. NY Med J 1911: (93): 1013–6.
- Hilibrand AS, Rand N. Degenerative lumbar stenosis: diagnosis and management. J Am Acad Orthop Surg 1999; 7(4): 239–49.
- Hoeffner EG, Mukherji SK, Srinivasan A, Quint DJ. Neuroradiology back to the future: spine imaging. Am J Neuroradiol 2012; 33(6): 999–1006.
- Hurri H, Slätis P, Soini J, Tallroth K, Alaranta H, Laine T, et al. Lumbar spinal stenosis: assessment of long-term outcome 12 years after operative and conservative treatment. J Spinal Disord 1998; 11(2): 110–5.
- Hurst NP, Jobanputra P, Hunter M, Lambert M, Lochhead A, Brown H. Validity of Euroqol--a generic health status instrument--in patients with rheumatoid arthritis. Economic and Health Outcomes Research Group. Br J Rheumatol 1994; 33(7): 655–62.
- Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997; 36(5): 551–9.
- Hägg O, Fritzell P, Nordwall A, Swedish Lumbar Spine Study Group. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J 2003; 12(1): 12–20.
- Iguchi T, Kurihara A, Nakayama J, Sato K, Kurosaka M, Yamasaki K. Minimum 10-year outcome of decompressive laminectomy for degenerative lumbar spinal stenosis. Spine 2000; 25(14): 1754–9.
- Imada K, Matsui H, Tsuji H. Oophorectomy predisposes to degenerative spondylolisthesis. J Bone Joint Surg Br 1995; 77(1): 126–30.
- Irwin ZN, Hilibrand A, Gustavel M, McLain R, Shaffer W, Myers M, et al. Variation in surgical decision making for degenerative spinal disorders. Part I: lumbar spine. Spine 2005; 30(19): 2208–13.
- Ishimoto Y, Yoshimura N, Muraki S, Yamada H, Nagata K, Hashizume H, et al. Associations between radiographic lumbar spinal stenosis and clinical symptoms in the general population: the Wakayama Spine Study. Osteoarthr Cartil 2013; 21(6): 783–8.
- Iversen MD, Daltroy LH, Fossel AH, Katz JN. The prognostic importance of patient pre-operative expectations of surgery for lumbar spinal stenosis. Patient Educ Couns 1998; 34(2): 169–78.
- Jakola AS, Sørlie A, Gulati S, Nygaard OP, Lydersen S, Solberg T. Clinical outcomes and safety assessment in elderly patients undergoing decompressive laminectomy for lumbar spinal stenosis: a prospective study. BMC Surg 2010; 10: 34.
- Jansson K-A, Blomqvist P, Granath F, Németh G. Spinal stenosis surgery in Sweden 1987-1999. Eur Spine J 2003; 12(5): 535–41.
- Jansson K-A, Németh G, Granath F, Jönsson B, Blomqvist P. Health-related quality of life (EQ-5D) before and one year after surgery for lumbar spinal stenosis. J Bone Joint Surg Br 2009; 91(2): 210–6.
- Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994; 331(2): 69–73.
- Johnsson K. Lumbar spinal stenosis. A clinical, radiological and neurophysiological investigation. Malmö: Lund University; 1987.
- Johnsson KE, Udén A, Rosén I. The effect of decompression on the natural course of spinal stenosis. A comparison of surgically treated and untreated patients. Spine 1991; 16(6): 615–9.
- Johnsson KE, Rosén I, Udén A. The natural course of lumbar spinal stenosis. Clin Orthop 1992; (279): 82–6.
- Junghanns H. Spondylolisthesen ohne Spalt im Zwischengelenkstück: Pseudospondylolisthesen. Arch für Orthop Unf-Chir 1931; 29(1): 118–27.
- Jönsson B. Lumbar nerve root compression syndromes. Symptoms, signs and surgical results. Lund: Lund University; 1995.
- Jönsson B, Strömqvist B. Motor affliction of the L5 nerve root in lumbar nerve root compression syndromes. Spine 1995; 20(18): 2012–5.
- Jönsson B, Annertz M, Sjöberg C, Strömqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part II: Five-year follow-up by an independent observer. Spine 1997; 22(24): 2938–44.
- Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J 2009; 9(7): 545–50.
- Kapellen PJ, Beall DP. Imaging Evaluation of Low Back Pain: Important Imaging Features Associated With Clinical Symptoms. Semin Roentgenol 2010; 45(3): 218–25.
- Katz JN, Dalgas M, Stucki G, Katz NP, Bayley J, Fossel AH, et al. Degenerative lumbar spinal stenosis. Diagnostic value of the history and physical examination. Arthritis Rheum 1995a; 38(9): 1236–41.
- Katz JN, Lipson SJ, Brick GW, Grobler LJ, Weinstein JN, Fossel AH, et al. Clinical correlates of patient satisfaction after laminectomy for degenerative lumbar spinal stenosis. Spine 1995b; 20(10): 1155–60.
- Katz JN, Lipson SJ, Lew RA, Grobler LJ, Weinstein JN, Brick GW, et al. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis. Patient selection, costs, and surgical outcomes. Spine 1997; 22(10): 1123–31.
- Katz JN, Stucki G, Lipson SJ, Fossel AH, Grobler LJ, Weinstein JN. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine 1999; 24(21): 2229–33.
- Kim H-J, Kim S-C, Kang K-T, Chang B-S, Lee C-K, Yeom JS. Influence of educational attainment on pain intensity and disability in patients with lumbar spinal stenosis: mediation effect of pain catastrophizing. Spine 2014; 39(10): E637-44.
- Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, Reilly J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine 1978; 3(4): 319–28.
- Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop 1982; (165): 110–23.
- Kleinstück FS, Grob D, Lattig F, Bartanusz V, Porchet F, Jeszenszky D, et al. The influence of preoperative back pain on the outcome of lumbar decompression surgery. Spine 2009; 34(11): 1198–203.
- Knaub MA, Won DS, McGuire R, Herkowitz HN. Lumbar spinal stenosis: indications for arthrodesis and spinal instrumentation. Instr Course Lect 2005; 54: 313–9.
- Knutsson B, Michaëlsson K, Sandén B. Obesity is associated with inferior results after surgery for lumbar spinal stenosis: a study of 2633 patients from the Swedish spine register. Spine 2013; 38(5): 435–41.
- Knutsson F. The instability associated with disk degeneration in the lumbar spine. Acta Radiol. 1944; (25): 593–609.
- Kornblum MB, Fischgrun JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine 2004; 29: 726-33.
- Kovacs FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: a systematic review of randomized controlled trials. Spine 2011; 36(20): E1335–1351.
- Kreiner DS, Shaffer WO, Baisden JL, Gilbert TJ, Summers JT, Toton JF, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J 2013; 13(7): 734–43.
- Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine 1991; 16(11): 1312–20.
- Kurd MF, Lurie JD, Zhao W, Tosteson T, Hilibrand AS, Rihn J, et al. Predictors of treatment choice in lumbar spinal stenosis: a spine patient outcomes research trial study. Spine 2012; 37(19): 1702–7.
- Lange F. Support for the spondylitic spine by means of buried steel bars attached to the vertebrae. Am J Orthop Surg 1910; (8): 344–61.
- Lattig F, Fekete TF, Grob D, Kleinstück FS, Jeszenszky D, Mannion AF. Lumbar facet joint effusion in MRI: a sign of instability in degenerative spondylolisthesis? Eur Spine J 2012a; 21(2): 276–81.
- Lattig F, Fülöp Fekete T, Kleinstück FS, Porchet F, Jeszenszky D, Mannion AF. Lumbar facet joint effusion on MRI as a sign of unstable degenerative spondylolisthesis: should it influence the treatment decision? J Spinal Disord Tech 2012b; published online 24 luly 2012.
- Lauche R, Langhorst J, Dobos GJ, Cramer H. Clinically meaningful differences in pain, disability and quality of life for chronic nonspecific neck pain - a reanalysis of 4 randomized controlled trials of cupping therapy. Complement Ther Med 2013; 21(4): 342–7.
- Levine MN, Julian JA. Registries that show efficacy: good, but not good enough. J Clin Oncol 2008; 26(33): 5316–9.
- Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention of missing data in clinical trails. N Engl J Med 2012; 367: 1355-60.
- Lombardi JS, Wiltse LL, Reynolds J, Widell EH, Spencer C 3rd. Treatment of degenerative spondylolisthesis. Spine 1985; 10(9): 821–7.
- Love JG. Intraspinal protrusion of intervertebral discs. Arch Surg 1940; 40(3): 454.
- Lønne G, Odengard B, Johnsen LG, Solberg TK, Kvistad KA, Nygaard OP. MR evaluation of lumbar spinal stenosis: is rapid visual assessment as good as area measurement? Eur Spine J. 2014; 23: 1320-4.
- Macnab I. Spondylolisthesis with an intact neural arch; the so-called pseudo-spondylolisthesis. J Bone Joint Surg Br 1950; 32(3): 325–33.
- Malmivaara A, Slätis P, Heliövaara M, Sainio P, Kinnunen H, Kankare J, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine 2007; 32(1): 1–8.
- Mannion AF, Mutter UM, Fekete TF, Porchet F, Jeszenszky D, Kleinstück FS. Validity of a single-item measure to assess leg or back pain as the predominant symptom in patients with degenerative disorders of the lumbar spine. Eur Spine J 2014a; 23(4): 882–7.
- Mannion AF, Leivseth G, Brox JI, Fritzell P, Hägg O, Fairbank JC. ISSLS Prize Winner: Long-term follow-up suggests spinal fusion is associated with increased adjacent segment disk degeneration but without influence on clinical outcome: results of a combined follow-up of from 4 randomized controlled trials. Spine 2014b; 39: 1373-83.
- Mardjetko SM, Connolly PJ, Shott S. Degenerative lumbar spondylolisthesis. A meta-analysis of literature 1970-1993. Spine 1994; 19(20 Suppl): 2256S–2265S.
- Mariconda M, Zanforlino G, Celestino GA, Brancaleone S, Fava R, Milano C. Factors influencing the outcome of degenerative lumbar spinal stenosis. J Spinal Disord 2000; 13(2): 131–7.
- Martin CR, Gruszczynski AT, Braunsfurth HA, Fallatah SM, O’Neil J, Wai EK. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine 2007; 32(16): 1791–8.
- Matsudaira K, Yamazaki T, Seichi A, Takeshita K, Hoshi K, Kishimoto J, et al. Spinal stenosis in grade I degenerative lumbar spondylolisthesis: a comparative study of outcomes following laminoplasty and laminectomy with instrumented spinal fusion. J Orthop Sci 2005; 10(3): 270–6.
- Matsunaga S, Sakou T, Morizono Y, Masuda A, Demirtas AM. Natural history of degenerative spondylolisthesis. Pathogenesis and natural course of the slippage. Spine 1990; 15(11): 1204–10.
- Matsunaga S, Ijiri K, Hayashi K. Nonsurgically managed patients with degenerative spondylolisthesis: a 10-to 18-year follow-up study. J Neurosurgery 2000; 93(2 suppl):194-8.
- Mattei TA. A gaze beyond the surface: acknowledging the little we know about radiographic parameters for evaluation of lumbar spinal stenosis. Neurosurgery 2013; 72(1): E135–140.
- May S, Comer C. Is surgery more effective than non-surgical treatment for spinal stenosis, and which non-surgical treatment is more effective? A systematic review. Physiotherapy 2013; 99(1): 12–20.
- McGregor AH, Hughes SPF. The evaluation of the surgical management of nerve root compression in patients with low back pain: Part 2: patient expectations and satisfaction. Spine 2002; 27(13): 1471–1476.
- Meyerding HW. Spondylolisthesis. Surg Gynecol Obstet 1932; 54: 371-7.
- Miyamoto H, Sumi M, Uno K, Tadokoro K, Mizuno K. Clinical outcome of nonoperative treatment for lumbar spinal stenosis, and predictive factors relating to prognosis, in a 5-year minimum follow-up. J Spinal Disord Tech 2008; 21(8): 563–8.
- Mulholland RC. Scientific basis for the treatment of low back pain. Ann R Coll Surg 2007; 89(7): 677–81.
- Mulholland RC. The myth of lumbar instability: the importance of abnormal loading as a cause of low back pain. Eur Spine J 2008; 17(5): 619–25.
- Munting E, Röder C, Sobottke R, Dietrich D, Aghayev E, on behalf of the Spine Tango Contributors. Patient outcomes after laminotomy, hemilaminectomy, laminectomy and laminectomy with instrumented fusion for spinal canal stenosis: a propensity score-based study from the Spine Tango registry. Eur Spine J, published online May 20, 2014.
- Murata M, Morio Y, Kuranobu K. Lumbar disc degeneration and segmental instability: a comparison of magnetic resonance images and plain radiographs of patients with low back pain. Arch Orthop Trauma Surg 1994; 113(6): 297–301.
- Nadeau M, Rosas-Arellano MP, Gurr KR, Bailey SI, Taylor DC, Grewal R, et al. The reliability of differentiating neurogenic claudication from vascular claudication based on symptomatic presentation. Can J Surg 2013; 56(6): 372–7.
- Newman PH. Spondylolisthesis, its cause and effect. Ann R Coll Surg 1955; 16(5): 305–23.
- Ng LCL, Tafazal S, Sell P. The effect of duration of symptoms on standard outcome measures in the surgical treatment of spinal stenosis. Eur Spine J 2007; 16(2): 199–206.
- Nixon JE. Spinal stenosis. London: Edward Arnold; 1991.
- Offierski CM, MacNab I. Hip-Spine syndrome. Spine 1983; 8: 316-21.
- Ogikubo O, Forsberg L, Hansson T. The relationship between the cross-sectional area of the cauda equina and the preoperative symptoms in central lumbar spinal stenosis. Spine 2007; 32(13): 1423–1428.
- Okoro T, Qureshi A, Sell B, Sell P. The accuracy of assessment of walking distance in the elective spinal outpatients setting. Eur Spine J 2010; 19(2): 279–82.
- Olmarker K, Rydevik B, Holm S, Bagge U. Effects of experimental graded compression on blood flow in spinal nerve roots. A vital microscopic study on the porcine cauda equina. J Orthop Res 1989; 7(6): 817–23.
- Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine 2004; 29: 1938-44.
- Park DK, An HS, Lurie JD, Zhao W, Tosteson A, Tosteson TD, et al. Does multilevel lumbar stenosis lead to poorer outcomes?: a subanalysis of the Spine Patient Outcomes Research Trial (SPORT) lumbar stenosis study. Spine 2010; 35(4): 439–46.
- Parke WW, Gammell K, Rothman RH. Arterial vascularization of the cauda equina. J Bone Joint Surg Am 1981; 63(1): 53–62.
- Parker SL, Adogwa O, Paul AR, Anderson WN, Aaronson O, Cheng JS, et al. Utility of minimum clinically important difference in assessing pain, disability, and health state after transforaminal lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Neurosurg Spine 2011; 14(5): 598–604.
- Pearson AM, Lurie JD, Blood EA, Frymoyer JW, Braeutigam H, An H, et al. Spine Patient Outcomes Research Trail: radiographic predictors of clinical outcomes after operative or non-operative treatment of degenerative spondylolisthesis. Spine 2008; 33: 2759-66.
- Pearson A, Blood E, Lurie J, Abdu W, Sengupta D, Frymoyer JW, et al. Predominant leg pain is associated with better surgical outcomes in degenerative spondylolisthesis and spinal stenosis: results from the Spine Patient Outcomes Research Trial (SPORT). Spine 2011; 36(3): 219–29.
- Pearson A, Lurie J, Tosteson T, Zhao W, Abdu W, Weinstein JN. Who should have surgery for spinal stenosis? Treatment effect predictors in SPORT. Spine 2012; 37(21): 1791–802.
- Pedowitz RA, Garfin SR, Massie JB, Hargens AR, Swenson MR, Myers RR, et al. Effects of magnitude and duration of compression on spinal nerve root conduction. Spine 1992; 17(2): 194–9.
- Porter RW. Spinal stenosis and neurogenic claudication. Spine 1996; 21(17): 2046–52.
- Porter RW, Hibbert C, Evans C. The natural history of root entrapment syndrome. Spine 1984; 9(4): 418–21.
- Posner I, White AA 3rd, Edwards WT, Hayes WC. A biomechanical analysis of the clinical stability of the lumbar and lumbosacral spine. Spine 1982; 7(4): 374–89.
- Radcliff KE, Rihn J, Hilibrand A, DiIorio T, Tosteson T, Lurie JD, et al. Does the duration of symptoms in patients with spinal stenosis and degenerative spondylolisthesis affect outcomes?: analysis of the Spine Outcomes Research Trial. Spine 2011; 36(25): 2197–210.
- Ranstam J, Kärrholm J, Pulkkinen P, Mäkelä K, Espehaug B, Pedersen AB, et al. Statistical analysis of arthroplasty data. I. Introduction and background. Acta Orthop 2011; 82(3): 253–7.
- Resnick DK, Watters WC 3rd, Sharan A, Mummaneni P, Dailey AT, Wang JC, et al Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: Lumbar fusion for stenosis with spondylolisthesis. J Neurosurgery Spine 2014a; 21: 54-61.
- Resnick DK, Watters WC 3rd, Mummaneni P, Dailey AT, Choundhri TF, Eck JC, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 10: Lumbar fusion for stenosis without spondylolisthesis. J Neurosurgery Spine 2014b; 21: 62-64.
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70(1): 41–55.
- Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am 1975; 57(4): 467–74.
- Rothman KJ. Epidemiology. An introduction. Oxford University Press, Oxford 2002.
- Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005; 95 Suppl 1: S144–150.
- Roy-Camille R, Roy-Camille M, Demeulenaere C. [Osteosynthesis of dorsal, lumbar, and lumbosacral spine with metallic plates screwed into vertebral pedicles and articular apophyses]. Presse Médicale 1970; 78(32): 1447–8.
- Rydevik B. Neurophysiology of cauda equina compression. Acta Orthop Scand Suppl 1993; 251: 52–5.
- Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. PharmacoEconomics 1999; 15(2): 141–55.
- Sandén B, Försth P, Michaëlsson K. Smokers show less improvement than nonsmokers two years after surgery for lumbar spinal stenosis: a study of 4555 patients from the Swedish spine register. Spine 2011; 36(13): 1059–64.
- Sarpyener M. Congenital stricture of the spinal canal. J Bone Joint Surg Am 1945(29): 70–9.
- Satomi K, Hirabayashi K, Toyama Y, Fujimura Y. A clinical study of degenerative spondylolisthesis. Radiographic analysis and choice of treatment. Spine 1992; 17(11): 1329–36.
- Schellinger D, Di Chiro G, Axelbaum SP, Twigg HL, Ledley RS. Early clinical experience with the ACTA scanner. Radiology 1975; 114(2): 257–61.
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiol 2009; 20(4): 488–95.
- Schizas C, Theumann N, Burn A, Tamsey R, Wardlaw D, Smith FW, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine 2010; 35: 1919–24.
- Schönström N, Bolender NF, Spengler DM, Hansson TH. Pressure changes within the cauda equina following constriction of the dural sac. An in vitro experimental study. Spine 1984; 9(6): 604–7.
- Schönström NS, Bolender NF, Spengler DM. The pathomorphology of spinal stenosis as seen on CT scans of the lumbar spine. Spine 1985; 10(9): 806–11.
- Schönström N. The narrow lumbar spinal canal and the size of the cauda equina in man: a clinical and experimental study. Thesis Göteborg : Gothenburg University; 1988.
- Schönström N, Hansson T. Pressure changes following constriction of the cauda equina. An experimental study in situ. Spine 1988; 13(4): 385–8.
- Schönström N, Lindahl S, Willén J, Hansson T. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res 1989; 7(1): 115–21.
- Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine 2005; 30(6 Suppl): S71–81.
- Senn S, Graf E, Caputo A. Stratification for the propensity score compared with linear regression techniques to assess the effect of treatment or exposure. Stat Med 2007; 26(30): 5529–44.
- Shah BR, Laupacis A, Hux JE, Austin PC. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol 2005; 58(6): 550–9.
- Sharrack B, Hughes RA. Reliability of distance estimation by doctors and patients: cross sectional study. BMJ 1997; 315(7123): 1652–4.
- Siccard J, Forestier J. Methode radiographique d’exploration de la cavite epidurale par le lipiodol. Rev Neurol 1921(37): 1264–6.
- Singh K, Samartzis D, Vaccaro AR, Nassr A, Andersson GB, Yoon ST, et al. Congenital lumbar spinal stenosis: a prospective, control-matched, cohort radiographic analysis. Spine J 2005; 5(6): 615–22.
- Sinikallio S, Aalto T, Airaksinen O, Herno A, Kröger H, Viinamäki H. Depressive burden in the preoperative and early recovery phase predicts poorer surgery outcome among lumbar spinal stenosis patients: a one-year prospective follow-up study. Spine 2009; 34(23): 2573–8.
- Sirvanci M, Bhatia M, Ganiyusufoglu KA, Duran C, Tezer M, Ozturk C, et al. Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J 2008; 17(5): 679–85.
- Solberg T, Johnsen LG, Nygaard ØP, Grotle M. Can we define success criteria for lumbar disc surgery?: estimates for a substantial amount of improvement in core outcome measures. Acta Orthop 2013; 84(2): 196–201.
- Solberg TK, Sørlie A, Sjaavik K, Nygaard ØP, Ingebrigtsen T. Would loss to follow-up bias the outcome evaluation of patients operated for degenerative disorders of the lumbar spine? Acta Orthop 2011; 82(1): 56–63.
- Van Spall HGC, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 2007; 297(11): 1233–40.
- Spratt KF, Keller TS, Szpalski M, Vandeputte K, Gunzburg R. A predictive model for outcome after conservative decompression surgery for lumbar spinal stenosis. Eur Spine J 2004; 13(1): 14–21.
- Steiger F, Becker H-J, Standaert CJ, Balague F, Vader J-P, Porchet F, et al. Surgery in lumbar degenerative spondylolisthesis: indications, outcomes and complications. A systematic review. Eur Spine J 2014; 23(5): 945-73.
- Strömqvist B, Jönsson B. Computerized follow-up after surgery for degenerative lumbar spine diseases. Acta Orthop Scand Suppl 1993; 251: 138–42.
- Strömqvist B, Fritzell P, Hägg O, Jönsson B, Swedish Society of Spinal Surgeons. The Swedish Spine Register: development, design and utility. Eur Spine J 2009; 18 Suppl 3: 294–304.
- Strömqvist B, Fritzell P, Hägg O, Jönsson B, Sandén B, Swedish Society of Spinal Surgeons. Swespine: the Swedish spine register: the 2012 report. Eur Spine J 2013a; 22(4): 953–74.
- Strömqvist B, Berg S, Gerdhem P, Johnsson R, Möller A, Sahlstrand T, et al. X-stop versus decompressive surgery for lumbar neurogenic intermittent claudication: randomized controlled trial with 2-year follow-up. Spine 2013b; 38(17): 1436–42.
- Stucki G, Liang MH, Fossel AH, Katz JN. Relative responsiveness of condition-specific and generic health status measures in degenerative lumbar spinal stenosis. J Clin Epidemiol 1995; 48(11): 1369–78.
- Suri P, Rainville J, Kalichman L, Katz JN. Does this older adult with lower extremity pain have the clinical syndrome of lumbar spinal stenosis? JAMA 2010; 304(23): 2628–36.
- Suzuki K, Ishida Y, Ohmori K, Sakai H, Hashizume Y. Redundant roots of the cauda equina: clinical aspects and consideration of pathogenesis. Neurosurgery 1989; 24: 521-28.
- Tomkins-Lane CC, Battié MC. Validity and reproducibility of self-report measures of walking capacity in lumbar spinal stenosis. Spine 2010; 35: 2097-2102.
- Tomkins-Lane CC, Battié MC, Hu R, Macedo L. Pathoanatomical characteristics of clinical lumbar spinal stenosis. J Back Musculoskelet Rehabil 2014; 27(2): 223-9.
- Vanderlinden RG. Subarticular entrapment of the dorsal root ganglion as a cause of sciatic pain. Spine 1984; 9(1): 19–22.
- Verbiest H. Sur certaines formes rares de compression de la queue de cheval. Les sténoses osseuses du canal vertebral, in Hommage a Clovis Vincent. Maloine Paris 1949: 161–74.
- Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br 1954; 36(2): 230–7.
- Verbiest H. Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal. A review of twenty-seven years’ experience. J Bone Joint Surg Br 1977; 59(2): 181–8.
- Verbiest H. The significance and principles of computerized axial tomography in idiopathic developmental stenosis of the bony lumbar vertebral canal. Spine 1979; 4(4): 369–78.
- Verbiest H. Lumbar spinal stenosis. Andersson G, McNeill TW, Editors. St. Louis: Mosby Year Book; 1992.
- Wai EK, Howse K, Pollock JW, Dornan H, Vexler L, Dagenais S. The reliability of determining ”leg dominant pain”. Spine J 2009; 9(6): 447–53.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473-83.
- Ware JE, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 1998; 51(11): 903–12.
- Ware JE Jr. SF-36 health survey update. Spine 2000; 25(24): 3130–9.
- Watson CJ, Phillips D, Hands L, Collin J. Claudication distance is poorly estimated and inappropriately measured. Br J Surg 1997; 84(8): 1107–9.
- Watters WC. If, When, and How to Fuse When Treating Lumbar Degenerative Stenosis. Semin Spine Surg 2011; 23(4): 218–21.
- Watters WC 3rd, Baisden J, Gilbert TJ, Kreiner S, Resnick DK, Bono CM, et al. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J 2008; 8(2): 305–10.
- Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992-2003. Spine 2006; 31(23): 2707–14.
- Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson ANA, Blood EA, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med 2007; 356(22): 2257–70.
- Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Blood E, Hanscom B, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008; 358(8): 794–810.
- Weinstein JN, Lurie JD, Tosteson TD, Zhao W, Blood EA, Tosteson ANA, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009; 91(6): 1295–304.
- Weinstein JN, Tosteson TD, Lurie JD, Tosteson A, Blood E, Herkowitz H, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine 2010; 35(14): 1329–38.
- Willén J, Danielson B. The diagnostic effect from axial loading of the lumbar spine during computed tomography and magnetic resonance imaging in patients with degenerative disorders. Spine 2001; 26(23): 2607–14.
- Willén J, Wessberg PJ, Danielsson B. Surgical results in hidden lumbar spinal stenosis detected by axial loaded computed tomography and magnetic resonance imaging: an outcome study. Spine 2008; 33(4): E109–115.
- Williamson E, Morley R, Lucas A, Carpenter J. Propensity scores: from naive enthusiasm to intuitive understanding. Stat Methods Med Res 2012; 21(3): 273–93.
- Wiltse LL, Newman PH, Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop 1976; (117): 23–9.
- Yamashita K, Hayashi J, Ohzono K, Hiroshima K. Correlation of patient satisfaction with symptom severity and walking ability after surgical treatment for degenerative lumbar spinal stenosis. Spine 2003; 28(21): 2477–81.
- Yamashita K, Ohzono K, Hiroshima K. Patient satisfaction as an outcome measure after surgical treatment for lumbar spinal stenosis: testing the validity and discriminative ability in terms of symptoms and functional status. Spine 2006; 31(22): 2602–8.
- Yone K, Sakou T, Kawauchi Y, Yamaguchi M, Yanase M. Indication of fusion for lumbar spinal stenosis in elderly patients and its significance. Spine 1996; 21(2): 242–8.
- Yone K, Sakou T. Usefulness of Posner’s definition of spinal instability for selection of surgical treatment for lumbar spinal stenosis. J Spinal Disord 1999; 12(1): 40–4.
- Yong-Hing K, Kirkaldy-Willis WH. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am 1983; 14(3): 491–504.
- Yukawa Y, Lenke LG, Tenhula J, Bridwell KH, Riew KD, Blanke K. A comprehensive study of patients with surgically treated lumbar spinal stenosis with neurogenic claudication. J Bone Joint Surg Am 2002; 84-A(11): 1954–9.
- Zanoli G, Jönsson B, Strömqvist B. SF-36 scores in degenerative lumbar spine disorders: analysis of prospective data from 451 patients. Acta Orthop 2006a; 77(2): 298–306.
- Zanoli G, Nilsson LT, Strömqvist B. Reliability of the prospective data collection protocol of the Swedish Spine Register: test-retest analysis of 119 patients. Acta Orthop 2006b; 77(4): 662–9.
- Zanoli G, Strömqvist B, Jönsson B. Visual analog scales for interpretation of back and leg pain intensity in patients operated for degenerative lumbar spine disorders. Spine 2001; 26(21): 2375–80.