Abstract
Adult-onset idiopathic male hypogonadotropic hypogonadism (IMHH) is a very rare but treatable disease. This study was conducted to examine the efficacy and safety of a combination of human chorionic gonadotropin (hCG) and recombinant human follicle-stimulating hormone (rhFSH) for inducing spermatogenesis in men with adult-onset IMHH. Seven men (34–45 years of age) with azoospermia and/or sexual dysfunction, with a low serum testosterone concentration, and apulsatile secretion of luteinizing hormone, were referred to our hospital for infertility. All had normal secondary sexual characteristics. Thorough endocrinologic examination and magnetic resonance imaging revealed no identifiable cause of hypogonadotropic hypogonadism. Adult-onset IMHH was diagnosed in all cases and treatment was started with 150 IU rhFSH and 5,000 IU hCG, both administered two times per week. Spermatogenesis was restored in five of the seven patients. During treatment one patient achieved spontaneous pregnancy with his wife, and spermatozoa recovered from the other four patients were frozen for future use in intracytoplasmic sperm injection.
Case Report
Adult-onset idiopathic male hypogonadotropic hypogonadism (IMHH) is a rare endocrine disorder first reported in 1997 [Nachtigall et al. Citation1997]. Adult-onset IMHH patients undergo age-appropriate normal puberty but subsequently develop gonadotropin-releasing hormone (GnRH) deficiency without an identifiable cause. Adult-onset IMHH may present as decreased libido, sexual dysfunction, gynecomastia, osteoporosis, or infertility, while secondary sexual characteristics are typically normal.
An early report suggested that recombinant human follicle-stimulating hormone (rhFSH) in combination with human chorionic gonadotropin (hCG) was effective for inducing spermatogenesis in patients with congenital male hypogonadotropic hypogonadism [Matsumoto et al. Citation2009]. The present study was undertaken to examine the efficacy and safety of this combination therapy for inducing spermatogenesis in patients with adult-onset IMHH. Approval was obtained from the Institutional Review Board of Dokkyo Medical University, and all participating patients provided informed consent.
Seven male infertility patients (mean age, 38.1 ± 5.7 years; age range, 34–45 years) were referred to our hospital with chief complaints of azoospermia and/or sexual dysfunction including erectile dysfunction, loss of libido, and anejaculation within one year. All had normal secondary sexual characteristics and previously had normal sexual function. Hormone analysis revealed decreased serum testosterone levels in all patients. They all underwent endocrinologic examination and imaging studies including monitoring of pulsatile luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretions for 24 hours, GnRH and hCG tests, and brain magnetic resonance imaging. All received a diagnosis of adult-onset IMHH according to the following criteria: (1) complete pubertal development by age 18 years; (2) clinical symptoms of hypogonadism, including sexual dysfunction, and/or idiopathic infertility; (3) frankly hypogonadal serum testosterone levels (<130 ng/dl) in the presence of low or normal gonadotropin levels on multiple assessments; (4) otherwise normal anterior pituitary function; (5) normal radiographic imaging of the sella turcica; and (6) absence of predisposing anatomical or functional factors for hypogonadotropic hypogonadism.
Patient characteristics are shown in . All patients were referred for the evaluation and treatment of male infertility. Two were azoospermic, two had erectile dysfunction, two had ejaculatory dysfunction (anejaculation), and one had loss of libido. Three patients had conceived children before referral. Testicular volume ranged from 6 to 24 ml. Two patients had smaller than normal testis of < 12 ml (normal limit at our institution). All patients’ baseline levels of gonadotropin and androgen hormones before treatment were measured in our hospital laboratory using standard immunoassay kits. Serum LH and testosterone levels were low. Serum FSH levels were fairly low or normal. The GnRH and hCG tests showed a normal response indicating intact pituitary and testicular endocrine functions. Periodic 24 hour serum sampling demonstrated a loss of pulsatile secretion of LH (). MRI showed no pituitary or hypothalamic lesions. All patients had a normal male karyotype of 46,XY.
Figure 1. Periodic monitoring of serum levels of hormones. (A) luteinizing hormone, (B) follicle-stimulating hormone, and (C) testosterone. In case 7 no pulsatile secretion of luteinizing hormone was noted, and testosterone levels remained low.
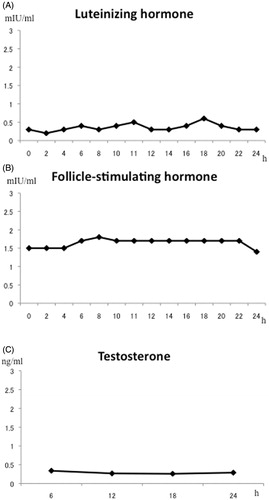
Table 1. Characteristics of the patients.
All patients were treated with 150 IU rhFSH (Follitropin Alpha, Gonale –F®, Merck Serono Co., Ltd., Tokyo, Japan) two times per week and 5,000 IU hCG (hCG, Gonatropin®, Aska Co, Tokyo, Japan) two times per week by self-injection. Physical examination, hormone and clinical laboratory measurements, and semen analysis were performed every three months during the treatment, and adverse events and local tolerance were recorded at each visit. The primary study endpoint was of the restoration of spermatogenesis. Pregnancy outcome during treatment was also investigated.
After treatment, all patients could ejaculate and spermatogenesis was restored in five of the men (71%). One patient achieved spontaneous pregnancy with his wife, and spermatozoa obtained from the ejaculates of the other four patients were frozen for future intracytoplasmic sperm injection. The longest administration period is 36 months in these patients. At present two patients achieved pregnancy with their wives, and the take home baby rate was 29%. No adverse effects were reported. Two patients of azoospermia who had hCG and rhFSH treatment for 12 months left the study.
In the few studies reporting adult-onset IMHH [Dwyer et al. Citation2010; Kobayashi et al. Citation2002; Mao et al. Citation2010; Whitten et al. Citation2006], the treatment aim was the induction of spermatogenesis to overcome male infertility and/or androgenization. Treatment strategy depends on the requirements for fatherhood. Men who wish to restore their reproductive capacity can choose between pulsatile GnRH or self-injected gonadotropin therapy. The latter is simpler than prolonged subcutaneous injections of GnRH, which require special equipment, and is also reportedly more effective than clomiphene citrate therapy [Whitten et al. Citation2006]. Combination treatment of hCG with human menopausal gonadotropin (hMG) has also been used. Recombinant products such as rhFSH have higher purity than hMG [Okada et al. Citation1992; Schopohl et al. Citation1991], and rhFSH has since been effectively combined with hCG to become a standard therapeutic regimen. A pharmacokinetic study in men with hypogonadotropic hypogonadism showed that rhFSH is well tolerated and shows dose-linear serum FSH levels [Mannaerts et al. Citation1996]. The chance of restoring spermatogenesis is notably higher in men with adult-onset IIHH than in those with congenital hypogonadotropic hypogonadism; in particular, the Sertoli and Leydig cells in the former condition, but not the latter one, are fully proliferated and developed [Nachtigall et al. Citation1997]. Testosterone replacement is another therapeutic option with good compliance. In the present study, the patient who achieved spontaneous pregnancy with his wife and the four patients who succeeded in producing sperm for cryopreservation had stopped hCG and rhFSH treatment after six months. The other two patients continued hCG and rhFSH treatment for 12 months but did not achieve spermatogenesis. Following hCG and rhFHS treatment, all patients were shifted to testosterone replacement therapy (125–250 mg intramuscular injection every 3–4 weeks).
In the present study, all patients were diagnosed at a male infertility clinic. It is noteworthy that five of the seven patients reported sexual dysfunction (e.g., erectile dysfunction, anejaculation, and loss of libido), indicating that adult-onset IMHH patients may present with some form of sexual dysfunction. Thus, clinicians should be aware that this rare but treatable disease may be encountered at standard urologic outpatient clinics.
Declaration of interest
This study was done using departmental funds. The authors have no declarations of interest.
Author contributions
Wrote the manuscript: YK, HO; Collected data from four patients (cases 1-4): KS, TI, RS, GA; Collected data from three patients (cases 5-7) TS, KN, HY, SS.
References
- Dwyer, A.A., Hayes, F.J., Plummer, L., Pitteloud, N. and Crowley W.F Jr. (2010) The long-term clinical follow-up and natural history of men with adult-onset idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 95:4235–4243
- Kobayashi, T., Okuno, H., Nishiyama, H., Nakamura, E., Ogawa, O., Kawahara, M., et al. (2002) Adult-onset idiopathic hypogonadotropic hypogonadism presented with erectile and ejaculatory disorder. Int J Urol 9:604–606
- Mannaerts, B., Fauser, B., Lahlou, N., Harlin, J., Shoham, Z., Bennink, H.C, et al. (1996) Serum hormone concentrations during treatment with multiple rising doses of recombinant follicle stimulating hormone (Puregon) in men with hypogonadotrophic hypogonadism. Fertil Steril 65:406–410
- Mao, J.F., Nie, M., Lu, S.Y. and Wu, X.Y. (2010) Adult-onset idiopathic hypogonadotropic hypogonadism: possible aetiology, clinical manifestations and management. Asian J Androl 12:611–614
- Matsumoto, A.M., Snyder, P.J., Bhasin, S., Martin, K., Weber, T., Winters, S., et al. (2009) Stimulation of spermatogenesis with recombinant human follicle-stimulating hormone (follitropin alfa; GONAL-f): long-term treatment in azoospermic men with hypogonadotropic hypogonadism. Fertil Steril 92:979–990
- Nachtigall, L.B., Boepple, P.A., Pralong, F.P. and Crowley W.F Jr. (1997) Adult-onset idiopathic hypogonadotropic hypogonadism—a treatable form of male infertility. N Engl J Med 336:410–415
- Okada, Y., Kondo, T., Okamoto, S. and Ogawa, M. (1992) Induction of ovulation and spermatogenesis by rMG/hCG in hypogonadotropic GH-deficient patients. Endocr J 39:31–43
- Schopohl, J., Mehltretter, G., Von Zumbusch, R., Eversmann, T. and Von Werder, K. (1991) Comparison of gonadotropin-releasing hormone and gonadotropin therapy in male patients with idiopathic hypogonadotropic hypogonadism. Fertil Steril 56:1143–1150
- Whitten, S.J., Nangia, A.K., Kolettis, P.N. (2006) Select patients with hypogonadotropic hypogonadism may respond to treatment with clomiphene citrate. Fertil Steril 86:1664–1668
