Abstract
The effect of oral administration of 50% ethanolic leaf extract of Citrus limon (500 and 1,000 mg/kg body weight/day) for 35 days on fertility and various male reproductive endpoints was evaluated in Parkes strain of mice. Testicular indices such as histology, 3β- and 17β-HSD enzymes activity, immunoblot expression of StAR and P450scc, and germ cell apoptosis by TUNEL and CASP- 3 expression were assessed. Motility, viability, and number of spermatozoa in the cauda epididymidis, level of serum testosterone, fertility indices, and toxicological parameters were also evaluated. Histologically, testes in extract-treated mice showed nonuniform degenerative changes in the seminiferous tubules. Treatment had adverse effects on steroidogenic markers in the testis and induced germ cell apoptosis. Significant reductions were noted in epididymal sperm parameters and serum level of testosterone in Citrus-treated mice compared to controls. Fertility of the extract-treated males was also suppressed, but libido remained unaffected. By 56 days of treatment withdrawal, alterations induced in the above parameters returned to control levels suggesting that Citrus treatment causes reversible suppression of spermatogenesis and fertility in Parkes mice. Suppression of spermatogenesis may result from germ cell apoptosis because of decreased production of testosterone. The present work indicated that Citrus leaves can affect male reproduction.
Introduction
In recent years, plants have attracted much interest towards their use in the regulation of male fertility because they can have a better compatibility with the human body, better cultural acceptability, and fewer side effects [Lohiya et al. Citation2001; Ogbuewu et al. Citation2011; Meerwal and Jain Citation2015]. Plants may have a viable future in the search of a contraceptive for use in men.
Citrus limon (C. limon, family, Rutaceae), commonly known as lemon, is an evergreen aromatic shrub mostly with thorny branches distributed throughout the tropical and temperate regions of the world. Infusions prepared with aerial (leaves) parts of lemon are used in folk medicines for the treatment of obesity, diabetes, blood lipid lowering, cardiovascular diseases, brain disorders, and certain types of cancer [Campelo et al. Citation2011]; the peel extracts are reported to possess antibacterial and antifungal activities [Johann et al. Citation2007]. Kulkarni et al. [Citation2012] have recently reported a reversible antifertility effect of alcoholic seed extract of lemon and its fraction in male albino rats. However, these authors did not describe the histological changes in the seminiferous tubules of rats treated with 200 mg/kg body weight of the extract and its fraction for 30 and 60 days, and only mentioned that the seminiferous tubules showed decreased diameter and atrophy. To the best of our knowledge, however, there is no detailed report of the effect of C. limon on the male reproductive system. The present study describes the effect of 50% ethanolic leaf extract of C. limon on the reproductive organs and fertility of the Parkes (P) strain male mice, which we have been using for an animal model [Verma and Singh Citation2014]. We assessed various male reproductive end points such as sperm parameters, testicular histology, testosterone assay and fertility indices, activities of steroidogenic enzymes viz. 3β- and 17β- hydroxysteroid dehydrogenases, immunoblot expression of steroidogenic acute regulatory protein, P450 side chain cleavage enzyme Caspase-3, and apoptosis of germ cells by TUNEL in the testis. Recovery and toxicological studies were also carried out.
Results
Mice were randomly allocated to six (I-VI) groups of five mice each, and were treated as follows. Group I were the untreated controls; II, controls treated with 1% Tween-80 then sacrificed 24 hours later; III, treated for 35 days with 500 mg/kg BW C. limon then sacrificed 24 hours later; IV, treated for 35 days with 1000 mg/kg BW C. limon then sacrificed 24 hours later; V, controls treated with 1% Tween-80 for 35 days then sacrificed 56 days later; and VI, treated with 1,000 mg/kg BW C. limon for 35 days then sacrificed 56 days later. No significant differences were found in the mean counts of RBC and WBC, level of Hb and in haematocrit value, serum levels of transaminases (ALT and AST), and creatinine in C. limon -treated mice compared to controls (data not presented). Additionally, no significant differences were noted between the initial and final body weights of C. limon-treated mice and controls (). Significant reductions were, however, found in the weights of testis, epididymis, and seminal vesicle in Citrus-treated mice compared to controls, but these had recovered to control levels by 56 days of treatment withdrawal. Weights of liver, spleen, kidney, adrenal gland, and brain were not affected by Citrus treatment ().
Table 1. Body weight (BW) and weights of the testis, epididymis, seminal vesicle, liver, spleen, kidney, adrenal gland, and brain in mice after Citrus limon (C. limon) treatment and following treatment withdrawal.
Fertility
When C. limon- treated males were caged with females at 24 hours, 2, 4, 6, and 8 weeks after cessation of treatment, libido was not affected at any interval (). Fertility of Citrus-treated males was, however, adversely affected at 24 hours post-withdrawal, as none of the females mated to these males showed live implants (). Two weeks after treatment withdrawal, live implants were seen in females, but the number was lower compared to controls and this remained so up to 6 weeks post-withdrawal. By 8 weeks of treatment withdrawal, all the five females impregnated by Citrus–treated males showed live implants, and their number was comparable to that of controls ().
Table 2. Fertility of male mice after treatment with C. limon (1000 mg/kg BW/days) or 1% Tween-80.
Significant reductions were found in motility, viability, and number of spermatozoa in cauda epididymidis of Citrus-treated mice compared to controls; the altered sperm parameters, however, had recovered to control levels by 56 days after cessation of treatment (). Histologic observations of testes in untreated controls () and 1% Tween-80-treated controls showed normal features in almost all the seminiferous tubules, except in a few (Group I: 4.68%; Group II: 5.40%; ) in which the tubules exhibited loosening of germinal epithelium or intraepithelial vacuolation. Detectable alterations were noticed in histoarchitecture of the testes in C. limon-treated mice (); the alterations in testis were, however, not uniform, and both affected and normal seminiferous tubules were observed in the same testis sections. In two of five mice treated with 1,000 mg dose of Citrus, degenerative changes were observed in all the seminiferous tubules (). The affected seminiferous tubules showed similar histologic changes in both dose groups. In general, the affected tubules showed loosening of germinal epithelium, intraepithelial vacuolation, exfoliation of germ cells, marginal condensation of chromatin in round spermatids, mixing of spermatids at different stages of spermatogenesis, and occurrence of giant cells ().
Figure 1. PAS–haematoxylin stained sections of mouse testis. (A) Control to show normal appearance of the seminiferous tubules. (B) After Citrus limon (C. limon) treatment, 500 mg/kg body weight/day for 35 days and sacrificed 24 hours after the last treatment to show loosening of germinal epithelium in the seminiferous tubules. (C) After the same treatment as shown in (B) to show exfoliation of germ cells in the lumen of a tubule. (D) After the same treatment as shown in (B). Note marginal condensation of chromatin in round spermatids (arrow) in a seminiferous tubule and appearance of intraepithelial vacuoles (broken arrows) in the tubules. (E) After C. limon treatment, 1000 mg/kg body weight/day for 35 days and sacrificed 24 hours after the last treatment. Note mixing of spermatids of different stages of spermatogenesis in a seminiferous tubule; steps 8, 9, and 10 spermatids are seen in the same tubule and an intraepithelial vacuole (broken arrow) is also seen in the tubule. (F) After the same treatment as shown in (E) to show occurrence of giant cells (arrow) containing 9 nuclei of round spermatids in the epithelium of a seminiferous tubule. (G) After the same treatment as shown in (E) to show severe degenerative changes in the seminiferous tubules. The tubule in the centre is lined by Sertoli cells and a few germ cells. (H) After C. limon treatment, 1,000 mg/kg body weight/day for 35 days and sacrificed 56 days after treatment withdrawal to show normal appearance of the seminiferous tubules (cf. part A). Scale bar = 50 μm.
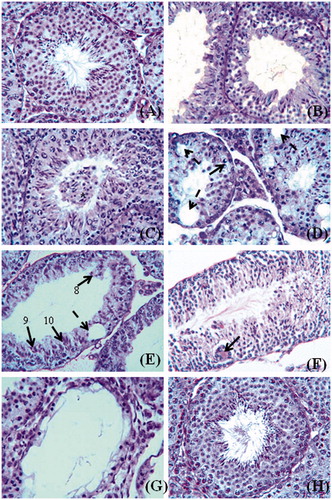
Table 3. Motility, viability, and number of spermatozoa in cauda epididymidis of mice after Citrus limon (C. limon) treatment and following treatment withdrawal.
Table 4. Diameter of seminiferous tubules, height of germinal epithelium, corrected count of germ cells in stage VII seminiferous tubules, and percentage of affected seminiferous tubules in mice testes after Citrus limon (C. limon) treatment and following treatment withdrawal.
Giant cells containing round spermatid nuclei were often seen in the seminiferous tubules, and such cells had two to nine nuclei of the spermatids (); however, giant cells containing two to five nuclei of spermatocytes were also not uncommon. In severe cases, the affected tubules were lined with only Sertoli cells and a few spermatogonia (). When quantitatively analyzed, testes in C. limon-treated mice showed a significant increase in the percentage of affected seminiferous tubules compared to that of controls (); further, the diameter of seminiferous tubules and height of germinal epithelium were also adversely affected in Citrus-treated mice (). Enumeration of germ cells in stage VII seminiferous tubules showed that there was a significant reduction in the number of pachytene spermatocytes and step seven spermatids in Citrus-treated mice compared to controls, though the number of type A spermatogonia and preleptotene spermatocytes was not affected by the treatment (). Fifty-six days after cessation of treatment, the alterations caused in the seminiferous tubules recovered to that of the control () and the testis presented a histology () similar to that of control ().
Activities of testicular steroidogenic enzymes
A significant reduction was noted in the activities of 3β– and 17β- HSD in the testis of Citrus-treated mice compared to controls. By 56 days after cessation of treatment, the enzyme activities had recovered to control levels (). Western blot analysis showed a single immunoreactive band of StAR protein and P450scc enzyme at ∼30 and ∼54 kDa, respectively (). A significant reduction was found in the expression of StAR protein in mice treated with a 1,000 mg dose of the extract, while the level of cytochrome P450scc decreased in testis of mice treated with both doses of Citrus compared to controls. Fifty-six days after discontinuing treatment, expression recovered to control levels (). A significant reduction was noted in serum level of testosterone in mice treated with 1,000 mg dose of C. limon compared to controls; the hormone level decreased in the 500 mg dose group of mice, but was not statistically significant in comparison to controls ().
Figure 2. Western blot analyses of (A) StAR protein and (B) P450scc enzyme in testes of control mice and after Citrus limon (C. limon) treatment (500 and 1,000 mg/kg body weight for 35 days and sacrificed 24 hours after the last treatment), and after treatment withdrawal. In recovery groups (CR and TR), mice were treated with 1% Tween-80 or with C. limon, 1,000 mg/kg body weight for 35 days, and thereafter the treatment was discontinued and mice sacrificed 56 days post-withdrawal (C, D). Each bar represents mean ± SEM for triplicate blots (n = 5). *Values of band intensity significantly different from controls (p < 0.05). IRDV = integrated relative density value.
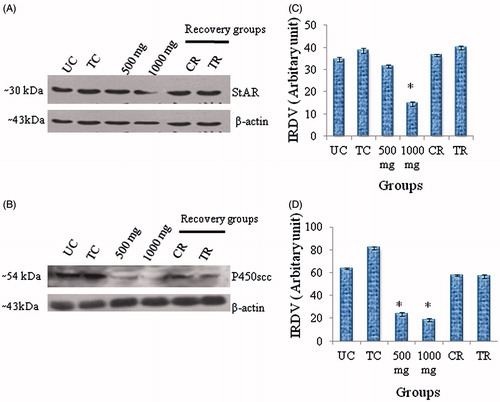
Table 5. Activities of steroidogenic enzymes (3β- and 17β-HSD) in the testis and serum testosterone level in mice after Citrus limon (C. limon) treatment and following treatment withdrawal.
Apoptosis
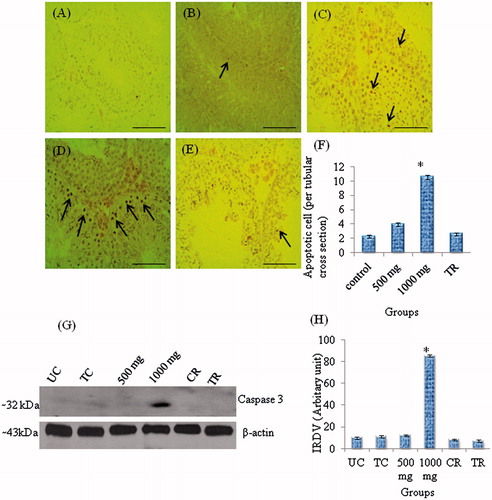
Western blot analysis showed a single immunoreactive band of CASP-3 at ∼32 kDa (). A significant increase in the level of CASP-3 was only found in testis of mice treated with the 1,000 mg dose of Citrus compared to the controls. This returned to control levels after treatment was discontinued for 56 days (). A very few apoptotic germ cells were observed in the testis of control mice (), while TUNEL positive cells were noticed in the seminiferous epithelium of testis in mice treated with 500 mg and 1,000 mg dose of Citrus (), though the number of these cells was significantly higher only in mice treated with 1,000 mg dose of the extract compared to controls. TUNEL-labelled germ cells in the seminiferous tubules of treated mice were mainly spermatocytes and occasionally round spermatids. By 56 days of treatment withdrawal, however, TUNEL-positive germ cells were seldom seen in the tubules ().
Figure 3. TUNEL staining of apoptotic germ cells in sections of mice testes and Western blot analysis of CASP-3 to detect germ cells apoptosis. (A) TUNEL reaction was conducted omitting TdT as a negative control. (B) Control (1% Tween-80); a TUNEL-positive germ cell (spermatocyte) is seen in a seminiferous tubule in the centre. (C) After Citrus limon (C. limon) treatment, 500 mg/kg body weight/day for 35 days and sacrificed 24 hours after the last treatment to show few TUNEL-positive germ cells (arrows) in the seminiferous tubules. (D) After C. limon treatment, 1,000 mg/kg body weight/day for 35 days and sacrificed 24 hours after the last treatment. Note many TUNEL-positive germ cells (arrows) in the seminiferous tubules. (E) After C. limon, treatment 1,000 mg/kg body weight for 35 days and sacrificed 56 days after treatment withdrawal (Treatment recovery, TR); note TUNEL staining in the seminiferous tubules as in controls (see part B). Scale bar = 50 μm. (F) Number of apoptotic germ cells per tubular cross section. Values are mean ± SEM; n = 5. * Significantly different from controls (p < 0.05). (G) Western blot analysis of CASP-3 in testis of control mice and after treatment with C. limon (500 and 1,000 mg/kg body weight for 35 days and sacrificed 24 hours after the last treatment), and following treatment withdrawal; in recovery groups, mice were treated with 1% Tweeen-80 (control recovery, CR) or C. limon, 1,000 mg/kg body weight for 35 days and thereafter the treatment was discontinued and mice sacrificed 56 days post-withdrawal (treatment recovery, TR). (H) Bar represents mean ± SEM of triplicate blots (n = 5). *Values of band intensity significantly different from controls (p < 0.05). IRDV: integrated relative density value.
Discussion
The results of the present study indicate that treatment with a 50% ethanolic leaf extract of C. limon caused a marked reduction in testis weight. This was accompanied by diverse degenerative changes in the seminiferous tubules, such as loosening of germinal epithelium, intraepithelial vacuolation, exfoliation of germ cells, marginal condensation of chromatin in round spermatids, mixing of spermatids at different stages of spermatogenesis, and the occurrence of giant cells. Similar histological changes have also been observed in testes of Parkes mice after treatment with Azadirachta indica [Mishra and Singh Citation2005], Allamanda cathartica [Singh and Singh Citation2008], Curcuma longa [Mishra and Singh Citation2009], Brahmi [Singh and Singh Citation2009], Dalbergia sissoo [Verma and Singh Citation2014], and several other antispermatogenic agents including gossypol tetra acetic acid [Singh and Rath Citation1990] and SC 12937 [Singh and Chakravarty Citation2003]. Multinucleated giant cells as noticed in the present study have also been observed in our earlier studies in mice testis after efferent duct ligation [Singh and Abe Citation1987] and treatment with aqueous leaf extracts of Allamanda cathartica [Singh and Singh Citation2008], Dalbergia sissoo [Verma and Singh Citation2014], and several antispermatogenic agents including gossypol tetra-acetic acid [Singh and Rath Citation1990] and SC 12937 [Singh and Chakravarty Citation2003]. These cells are suggested to be formed by fusion of germ cells because of widening/opening of the intercellular bridges [Singh and Abe Citation1987; Russell et al. Citation1987; Faridha et al. Citation2007], which connect groups of germ cells in the seminiferous tubules [Dym and Fawcett, Citation1971]. Though it is difficult to explain the mode of formation of giant cells in the present study, it is likely that these cells are formed by fusion of germ cells. Further, marked reductions were noted in the number of pachytene spermatocytes and step 7 spermatids in Stage VII seminiferous tubules in testes of C. limon-treated mice compared to controls. The serum level of testosterone also declined in treated mice, though not significantly in those treated with 500 mg dose of the extract. Since testosterone supports meiotic division of spermatocytes [O’Donell et al. Citation2006], reduction in the number of pachytene spermatocytes and step 7 spermatids as noted in the present study is likely to be caused due to the decreased level of the hormone. The observation that there were marked reductions in the diameter of the seminiferous tubules and in the height of the germinal epithelium in C. limon-treated mice in the present study is suggestive of the adverse effect of the treatment on spermatogenesis [D’Souza Citation2004]. This contention is also supported by histological changes in testis of treated mice.
The treatment also had adverse effects on motility, viability, and the number of spermatozoa in the cauda epididymidis. The diminution in the number of spermatozoa in Citrus-treated mice is probably caused by the adverse effect of the treatment on spermatogenesis as sperm number returned to the control level after recovery of the process following treatment discontinuation. Changes in motility and viability of spermatozoa in treated mice might have resulted from disturbances in epididymal functions [Rajalakshmi Citation1992]. Citrus treatment also caused significant reduction in the weight of seminal vesicles in treated mice compared to controls; this is likely due to the decreased serum level of testosterone in treated mice, because this gland is dependent on testosterone. Fertility of the Citrus-treated mice was also affected and this may be attributed to the poor quality of sperm; however, the libido was not affected by Citrus therapy. In this case serum testosterone levels and the sexual function do not appear directly correlated. This may be similar to men that have exhibited symptoms of hypogonadism even in the presence of normal testosterone concentrations [Travison et al. Citation2006]. Further, it is pertinent to mention that there is also a report of a marked decline in serum testosterone without an effect on libido in Parkes mice, e.g., after treatment with aqueous leaf extract of Dalbergia sissoo [Verma and Singh Citation2014]. The absence of any changes in the weights of the liver, spleen, kidney, adrenal gland, and brain, serum levels of ALT, AST, and creatinine, and in hematological parameters in C. limon-treated mice suggests that the treatment does not induce systemic toxic effect.
In the present study in Parkes mice, Citrus treatment caused a decrease in the serum level of testosterone in treated mice. This is also supported by results of reduced expressions of StAR and P450scc and in the activities of 3β- and 17β-HSD enzymes in the testes of C. limon-treated mice in the present study as these are indices of key roles in testicular androgenesis [Stocco and McPhaul Citation2006]. It is well known that testosterone is essential for the maintenance of spermatogenesis [O’Donell et al. Citation2006], and that this hormone protects germ cells against apoptosis [Hammami et al. Citation2009]. Thus, the suppression of spermatogenesis in Citrus-treated mice in the present study is likely to be caused by the diminished production of testosterone leading to apoptosis of germ cells. The observations of a marked increase in TUNEL-positive apoptotic germ cells and an increase in the expression of caspase-3 in testes of treated mice compared to controls as noted in the present study also lends support to the above view [Porter and Janicke Citation1999].
In conclusion, our results suggest that C. limon treatment causes suppression of spermatogenesis and fertility in male Parkes mice, without producing detectable signs of toxicity. Suppression of spermatogenesis may result from germ cell apoptosis due to the decreased production of testosterone. Further, the alterations induced in the male reproductive organs by Citrus treatment are reversible after withdrawal of therapy. Thus, C. limon may prove to have potential in the regulation of male fertility. However, further studies are required to be carried out to identify the active ingredient of C. limon which is responsible for the antifertility action.
Materials and Methods
Plant material and preparation of extract
Fresh leaves of C. limon were collected from the campus of the Banaras Hindu University and authenticated by experts from the Botany Department of the University. A voucher specimen (Rutaceae/11/13) was deposited in the herbarium of the Botany Department of the University. Dried and ground leaves were extracted with 50% ethanol (1,500 ml, w/v 1:15) in a soxhlet apparatus (Piviera Glass Pvt Ltd., Mumbai, India) for 24 h according to WHO [Citation1986] chemical guidelines. The filtrate was concentrated to dryness in a vacuum evaporator under reduced pressure and controlled temperature, and the yield of the extract was approximately 23 g, i.e., about 23% of the raw material. The lyophilized extract was then suspended in 1% Tween-80 and the doses were expressed as dry weight of the extract.
Animals and treatment
Adult (age 12-14 w) male mice belonging to the Parkes strain weighing 30-34 g were used in the study. Mice were from a closed and randomly bred colony maintained in the animal house of the Department of Zoology, Banaras Hindu University. Animals were housed in a well ventilated room at 23 ± 2°C with 12-h photoperiod and relative humidity of 50 ± 20%, and were maintained on pellet food (Mona Laboratory Animal Feeds, Varanasi, India) and drinking water ad libitum. Mice of a same group were housed together in a polypropylene cage (450 mm × 270 mm × 150 mm) and maintained according to the Guidelines of the Institutional Animal Ethics Committee.
The 50% ethanolic leaf extract of C. limon was suspended in 1% Tween-80 and administered through oral route, with the help of an oral gavage. Controls (Groups II and V) received an equivalent volume of 1% Tween-80 (0.5 mL/100 g BW per day) in a similar manner. Doses of C. limon were selected on the basis of a pilot study conducted in our laboratory in P mice. Mice in untreated (Group I) and 1% Tween-80-treated (Group II) control groups were sacrificed by decapitation under ether anaesthesia together with those treated with 500 mg/kg BW/day (Group III) and 1,000 mg/kg BW/day (Group IV) of C. limon for 35 d. Mice treated with 1,000 mg/kg BW/day of C. limon for 35 d and allowed to recover for 56 days (Group VI) and those that received 1% Tween-80 only in a similar way (Group V) were sacrificed together in a like manner. Testes were randomly excised from either the left or the right side of each mouse and fixed in Bouin's solution for histology and TUNEL assay, while testis on the other side was weighed and stored at −20 °C for enzyme assay and western blotting. Blood was collected, and serum was separated and stored at −20°C until further use. An aliquot of blood was immediately used for the assessment of haematological parameters.
Organ weight
Testis, epididymis, seminal vesicle, liver, spleen, kidney, adrenal gland, and brain were excised, blotted free of blood, and weighed.
Sperm analyses
At necropsy, spermatozoa were obtained from cauda epididymidis of each mouse in a group in physiological saline maintained at 37 °C [Singh and Chakravarty Citation2003]. Motility, viability, and number of spermatozoa were assessed according to WHO [Citation1999] laboratory manual. Sperm motility was assessed subjectively.
Histopathology of testis
For histological study, testes were randomly excised from either left or right side of each mouse, fixed in Bouin's fluid, dehydrated in graded ethanol series, cleared in benzene, and embedded in paraffin. Tissues were sectioned at 6 μm, and the sections were then stained with Periodic Acid-Schiff (PAS) and counterstained with haematoxylin.
Identification of stages of spermatogenesis in mouse testis was performed according to the criteria of Russell et al. [Citation1990]. For evaluation of quantitative changes in spermatogenesis caused by Citrus treatment, germ cell number at stage VII of the spermatogenic cycle was determined [Verma and Singh Citation2014]. The crude count of different germ cells (spermatogonia A, preleptotene spermatocytes, pachytene spermatocytes and step 7 spermatids) was corrected by Abercrombie's formula [Abercrombie Citation1946; Russell et al. Citation1990] and Sertoli cell nuclei were used as the reference structure. The diameter of the seminiferous tubules and height of the germinal epithelium were also measured in stage VII round or slightly oblique seminiferous tubules [Singh and Singh Citation2009]. Percentage of affected seminiferous tubules was also determined [Singh and Rath Citation1990].
Activities of steroidogenic enzymes in testis
Activities of 3β hydroxysteroid dehydrogenase (3β-HSD) and 17β hydroxysteroid dehydrogenase (17β-HSD) were measured according to methods of Talalay [Citation1962] and Jarabak et al. [Citation1962], respectively, with minor modifications [Mishra and Singh Citation2008].
Testosterone assay
Serum level of testosterone was measured by radioimmunoassay using a commercial kit, as per manufacturer’s instruction (Immunotech, Marseille, France). The sensitivity of the assay was 0.025 ng/mL. All samples were quantified in a single assay with intra- and interassay coefficient of variations being 14.8% and 15%, respectively.
Immunoblot expressions of steroidogenic acute regulatory (StAR) protein, P450 side chain cleavage (P450scc), and Caspase-3(CASP-3) enzymes
The testicular tissue was homogenized in 20% (w/v) ice cold buffer (1 M NaCl, 1 M tris Cl, pH 7.6, 0.1 M EDTA, pH 8.0, 0.1 M 100 µg/ml PMSF, and distilled water). Equal quantity of proteins of each sample of StAR (60 μg), P450scc (60 μg), and of CASP-3 (80 μg) as determined by Lowry’s [Citation1951] method was loaded on SDS–PAGE (12%) for electrophoresis [Laemmli Citation1970]. Thereafter, proteins were electrophoretically transferred to a nitrocellulose membrane (0.45 μm, Axiva Sichem Biotech, Mumbai, India) for overnight at 4 °C. The membrane was blocked in TBST (0.05% Tween-20 in Tris-50 mM, pH 7.6) for 4 h and incubated with specific primary antibodies (StAR, gift from Dr. D. M. Stocco, Texas Technical University, USA; P450scc, gift from late Dr. A. H. Payne, Southern Illinois University, USA; and for caspase-3, purchased from Selleck Chemicals, Texas, USA) at dilutions of 1:500 for StAR, 1:1000 for P450scc, and 1:1,500 for caspase-3 for overnight at 4°C. Membranes were then washed in TBS-T and incubated with horseradish peroxidase – conjugated anti-rabbit IgG (1:2,000 for StAR and P450scc, 1:5,000 for Caspase 3) for 2–3 h at room temperature. Signals were detected using an ECL kit (Thermo Scientific, Rockford, IL, USA) on X-ray films and resulting immunospecific bands were quantified by Image J Software (NIH, Bethesda, MD, USA) with β-actin as a loading control.
TUNEL analysis of apoptosis
Apoptotic germ cells were determined by TUNEL assay using apoptosis detection kit (FITC labelled POD, GenScript USA Inc., Piscataway, NJ, USA). The assay was performed on 6 μm thick paraffin –embedded sections according to the manufacturer’s protocol. A positive control for detection of DNA fragmentation was incubated by adding 100 μl DNase I (30,000 U/ml) solution for 10 min at room temperature. Negative controls had distilled water in place of TdT enzyme in TUNEL mixture. TUNEL-positive apoptotic germ cells in 20 seminiferous tubules at random from each mouse were counted, and mean apoptotic cell in each group was presented.
Blood and serum analysis
Blood was analyzed for red blood cell (RBCs) and white blood cell (WBCs) counts, hemoglobin (Hb) concentration and the hematocrit; serum was analyzed to measure the activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) according to the method of Reitmann and Frankel [Citation1957]. Serum level of creatinine was determined using a commercial kit (Span Diagnostics, India).
Fertility test
Ten adult (age 12-14 w) male mice of proven fertility were employed in the fertility test. Mice were administered 50% ethanolic leaf extract of C. limon or 1% Tween-80 (n = 5 per treatment). The 50% ethanolic leaf extract of C. limon was suspended in 1% Tween-80 and orally given at a dose of 1,000 mg/kg BW for 35 d, while the controls received 1% Tween-80 only in a similar way. Fifty adult female mice were employed in the fertility test. The fertility of C. limon or 1% Tween-80-treated males was assessed 24 h and 2, 4, 6, and 8 weeks after withdrawal of the treatment by allowing each male to cohabit with a coeval, virgin female showing regular cycle. Females in proestrus phase were kept with the males overnight. Females were checked the next morning for the presence of vaginal plug for an indication of mating. After 12/13 d of mating, the females were sacrificed by dislocation of cervical vertebrae under ether anaesthesia and then autopsied to record implantations in both the horns of the uterus. The males were considered fertile if females impregnated by them showed live implants. Index of libido and number of live implants were determined [Singh and Singh Citation2008].
Statistical analysis
All data, except for body weight and fertility test results, were analyzed by one-way analysis of variance (ANOVA) followed by Neuman-Keuls’ multiple range test for the comparison of group means. Body weight and fertility test data were analyzed by Student’s t test. Differences were considered significant at p < 0.05.
| Abbreviations | ||
| C. limon | = | Citrus limon |
| NaCl | = | sodium chloride |
| EDTA | = | ethylenediaminetetraacetic acid |
| PMSF | = | phenylmethanesulfonyl fluoride |
| SDS–PAGE | = | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TBS-T | = | tris-buffered saline and tween 20 |
| 3β- HSD | = | 3β- hydroxysteroid dehydrogenase |
| 17β-HSD | = | 17β hydroxysteroid dehydrogenase |
| StAR | = | steroidogenic acute regulatory protein |
| P450scc | = | P450 side chain cleavage enzyme |
| TUNEL | = | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| CASP-3 | = | Caspase-3 enzyme |
Acknowledgments
This work was supported by funds from the University Grants Commission through CAS in Zoology, Banaras Hindu University and partly by the Department of Science and Technology (grant No. SR/SO/AS-19/2010), New Delhi, to SKS. NS was a recipient of a Junior Research Fellowship in the Reproductive Biology Merged Scheme of the University Grants Commission to the Department of Zoology, Banaras Hindu University.
Declaration of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions
Designed the research study, performed the experiments, analyzed the data, and wrote the paper: NS; Contributed substantially to the research design and critically revised the manuscript: SKS.
References
- Abercrombie, M. (1946) Estimation of nuclear population from microtome sections. Anat Rec 94:239–247
- Campelo, L.M.L., de Almeida, A.A.C., de Freitas, R.L.M., Cerqueira, G.S., de Sousa, G.F., Saldanha, G.B., et al. (2011) Antioxidant and antinociceptive effects of Citrus limon essential oil in mice. J Biomed Biotechnol 2011:678673. doi:10.1155/2011/678673
- Dym, M. and Fawcett, D.W. (1971) Further observations on the number of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Bio Reprod 4:195–215
- D’Souza, U.J.A. (2004) Effect of tamoxifen on spermatogenesis and tubular morphology in rats. Asian J Androl 6:223–226
- Faridha, A., Faisal, K. and Akbarsha, M.A. (2007) Aflatoxin treatment brings about generation of multinucleate giant spermatids (symplasts) through opening of cytoplasmic bridges: Light and transmission electron microscopic study in Swiss mouse. Reproductive Toxicology 24:403–408
- Hammami, I., Amara, S., Benahmed, M., El May, M.V. and Mauduit, C. (2009) Chronic crude garlic-feeding modified adult male rat testicular markers: mechanisms of action. Reprod Biol Endocrinol 7:65. DOI: 10.1186/1477-7827-7-65
- Jarabak, J., Adams, J.A., Williams-Ashaman, H.G. and Talalay, P. (1962) Purification of a 17β hydroxysteroid dehydrogenase of human placenta and studies on its transhydrogenase function. J Biol Chem 237:345–357
- Johann, S., de Oliveira, V.L., Pizzolatti, M.G., Schripsema, J., Braz-Filho, R., Branco, A., et al. (2007) Antimicrobial activity of wax and hexane extracts from Citrus spp. peels. Mem Inst Oswaldo 102:681–685
- Kulkarni, T.R., Mateenuddin, M., Bodhankar S.L. and Saharabudhe, R.A. (2012) Reversible anti-fertility effect of lemon seeds (Citrus limonum) in male albino rats. Int J Res Pharm Biomed Sci 3:545–550
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
- Lohiya, N.K., Manivannan, B., Mishra, P.K. and Pathak, N. (2001) Prospects of developing a plant based male contraceptive pill. In Current Status in Fertility Regulation: Indigenous and Modern Approaches. Chowdhury S.R., Gupta, C.M. and Kamboj, V.P., eds. Lucknow: Central Drug Research Institute, pp. 99–119
- Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
- Meerwal, P. and Jain, G.C. (2015) Male fertility regulation with plant products: a review. IJPCBS 5:146–162
- Mishra, R.K. and Singh, S.K. (2005) Effect of aqueous leaf extract of Azadirachta indica on the reproductive organs in male mice. Indian J Exp Biol 43:1093–1103
- Mishra, R.K. and Singh, S.K. (2008) Safety assessment of Syzygium aromaticum flower bud (clove) extract with respect to testicular function in mice. Food Chem Toxicol 46:3333–3338
- Mishra, R.K. and Singh, S.K. (2009) Reversible antifertility effect of aqueous rhizome extract of Curcuma longa L. in male laboratory mice. Contraception 79:479–487
- Ogbuewu, I.P., Unamba-Oparah, I.C., Odoemenam, V.U., Etuk, I.F. and Okoli, I.C. (2011) The potentiality of medicinal plants as the source of new contraceptive principles in males. N Am J Med Sci 3:255–263
- O’Donell, L., Meachem, S.J., Stanton, P.G. and McLachlan, R.I. (2006) Endocrine regulation of spermatogenesis. In Knobil and Neill’s Physiology of Reproduction. Neill, J.D., ed. St Louis MO: Elsevier Academic Press, pp. 1017–1069
- Porter, A.G. and Jänicke, R.U. (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
- Rajalakshmi, M. (1992) Regulation of male fertility: Epididymis as a potential extragonadal site. In Frontiers in Reproductive Physiology. Ghosh, D. and Sengupta, J., eds New Delhi: Wiley Eastern Ltd., pp. 63–66
- Reitman, S. and Frankel, S. (1957) A colorimetric method for the determination of serum glutamate oxaloacetate and glutamic pyruvic transaminases. Amer J Clin Pathol 28:56–63
- Russell, L.D., Ettlin, R.A., Hikim, A.P.S. and Clegg, E.D. (1990) Histological and histopathological evaluation of the testis. Cache River Press; Clearwater, FL
- Russell, L.D., Vogl, A.W. and Weber, J.E. (1987) Actin localization in male germ cell intercellular bridges and the disruption of selected bridges by cytochalasin D. Am J Anat 180:25–40
- Singh, A. and Singh, S.K. (2008) Reversible antifertility effect of aqueous leaf extract of Allamanda cathartica L. in male laboratory mice. Andrologia 40:337–345
- Singh, A. and Singh, S.K. (2009) Evaluation of antifertility potential of Brahmi in male mouse. Contraception 79:71–79
- Singh, S.K. and Abe, K. (1987) Light and electron microscopic observations of giant cells in the mouse testis after efferent duct ligation. Arch Histol Jpn 50:579–585
- Singh, S.K. and Chakravarty, S. (2003) Antispermatogenic and antifertility effects of 20, 25-diazacholesterol dihydrochloride in mice. Reprod Toxicol 17:37–44
- Singh, S.K. and Rath, S.K. (1990) Histologic changes in the mouse testis after treatment with gossypol tetra-acetic acid. Arch Histol Cytol 53:393–396
- Stocco, D. and McPhaul, M. (2006) Physiology of testicular steroidogenesis. In Knobil and Neill’s Physiology of Reproduction. Neill, J.D., ed. St Louis, MO: Elsevier Academic Press, pp. 977–1016
- Talalay, P. (1962) Hydroxysteroid dehydrogenase. In: Methods in Enzymology. Colowick, S.P. and Kaplan, N.O, eds. New York: Academic Press, pp 512–516
- Travison, T.G., Morley, J.E., Araujo, A.B., O’Donnell, A.B. and McKinlay, J.B. (2006) The relationship between libido and testosterone levels in aging men. J Clin Endocrinol Metab 91:2509–2513
- Verma, H.P. and Singh, S.K. (2014) Effect of aqueous leaf extract of dalbergia sissoo Roxb. on spermatogenesis and fertility in male mice. Eur J Contracept Reprod Health Care 19:475–486
- WHO (1986) WHO Chemical Guidelines, CG-03, 1001A/ip. World Health Organization; Geneva .
- WHO (1999) World Health Organization laboratory manual for the examination of human semen and semen-cervical mucus interaction. University Press Cambridge; Cambridge .
