Abstract
Diabetes is known to be associated with erectile dysfunction, retrograde ejaculation, level of testicular hormone, and a decrease in semen quality, respectively. In this project, we aimed to investigate at the molecular level, the effects of NOS on testes pathology in diabetes and examine the effects of pentoxifylline on healing. A total of 50 Wistar albino male rats were divided into five groups: Group I control; Group II only diabetes; Group III and IV diabetes + pentoxifylline; Group V only pentoxifylline. Group III rats received 50 mg/kg/day pentoxifylline during two months. In comparison, Group IV rats received saline in the first month followed by 50 mg/kg/day of pentoxifylline for the following month. NOS expression in testicular tissue was assessed using qRT-PCR, western blot, and immunohistochemistry. The mean seminiferous tubule diameter, Johnsen's testicular biopsy score, and serum testosterone levels decreased compared to controls. In contrast, the number of apoptotic cells, the levels of nNOS, iNOS and eNOS mRNA, and protein increased when compared to the control. Upon pentoxifylline therapy NOS decreased suggesting that it contributes to this damage and treatment with pentoxifylline may be effective in reversing this damage.
Introduction
Diabetes mellitus (DM) is a serious disease that threatens human health in the modern world. According to the World Health Organization forecasts, the number of diabetic patients worldwide will reach up to 366 million in 2030. Diabetes is one of the most important chronic diseases and occurs either in the setting of insufficient production of insulin in the pancreas or when insulin is not used effectively in the body. Uncontrolled diabetes manifests as hyperglycemia, and after a long period of time begins to affect many organ systems, such as the nervous and vascular systems [Roessner et al. Citation2012].
Diabetic induced damage is caused by persistently high glucose levels. Hyperglycemia reflects cell damage by increased glycolysis, glucose oxidation, glycation end product activation, and polyol pathway formation [Rolo and Palmeira Citation2006; Yu et al. Citation2006]. About 90% of male diabetic patients experience a loss of libido, sexual impotency, and also sexual functions relating to infertility [Amaral et al. Citation2008]. DM interferes with male fertility on many levels. For example, endocrine control of spermatogenesis, penile erection, pubertal initiation, in addition to ejaculation distortion are all affected [La Vignera et al. Citation2012; Sexton and Jarow Citation1997]. Many studies of diabetic men have demonstrated pathologic changes in sperm count, motility, and morphology [Agbaje et al. Citation2007; Delfino et al. Citation2007]. This parrallels an increase in reactive oxygen species (ROS) production, indicating an increase in oxidative stress. There is a clear distortion in semen parameters and an increase in apoptosis [La Vignera et al. Citation2012].
Nitric oxide (NO) is a free radical gaseous molecule with a biological half-life of a few seconds. NO is generated from the guanidine nitrogen of L-arginine by three isoforms of nitric oxide synthase (NOS), i.e., neuronal NOS (nNOS, NOS-1), endothelial NOS (eNOS, NOS-3), and inducible NOS (iNOS, NOS-2). NO acts as an intracellular and intercellular messenger in different tissues [Sonmez et al. Citation2009a; Sonmez et al. Citation2012]. Hyperglycemia disrupts the electron transfer and activation in the mitochondria and causes an excessive production of ROS via the polyol pathway. ROS, in conjunction with NOS, shifts some intracellular compounds of the membrane components of protein, lipid, and carbohydrate structures [Abou-Seif and Youssef Citation2004].
The effect of diabetes on testicular damage is multifactorial and has yet to be fully elucidated. Recently, clinical and animal studies have shown the effect of DM on the male reproductive system and a disruption of molecular mechanisms. However, much of the interpretation of these data is still disputed. Therefore, we aimed to investigate the role and effect of NOS at the molecular level in testicular tissue damage induced by diabetes, in addition to the possible therapeutic role of pentoxifylline (PTX), which is a methyl xanthine derivative.
Results and Discussion
Diabetes-induced alterations
The effect DM on the male reproductive system has been known for years. In this study we investigated the effect of DM on testicular tissue histopathology, apoptosis, and NOS expression. At the beginning of the experiment, body weights and blood sugar levels of rats were close to each other. But at the end of the experiment in Group II (only streptozotocin-induced diabetic rats), Group III (rats administered PTX from the beginning of the experiment), and Group IV (rats received saline for the first month and underwent a PTX injection during the subsequent months) average body weights were significantly decreased when compared to Group I (control rats). In a similar way, the diabetes groups’ (Group II, Group III, and Group IV) blood sugar levels significantly increased compared to the control group. When looking at testicular weight, only Group II decreased (). All findings were similar in group V and group I.
Table 1. Body weight, testicular weight, blood glucose, and serum testosterone levels of control and streptozotocin (STZ)-induced diabetic rats.
To determine the extent of testicular tissue damage caused by diabetes, serum testosterone levels, testicular histomorphology, and apoptosis were evaluated. The microscopic examination of testicular tissue belonging to Group 1 subjects exhibited normal histomorphology. Group II, the STZ-induced diabetes, exhibited atrophic seminiferous tubules with shedding of epithelial seminiferous tubules to the lumen and irregularities of the epithelium of the seminiferous tubules. Although less often, similar findings were also observed in Groups III and IV testes tissue subjects. To score testes tissue damage, the average diameter of seminiferous tubule damage was measured as mean seminiferous tubule diameter (MSTD). To determine the degree of damage during spermatogenesis, Johnsen's tubular biopsy score (JTBS) was used (). In Group II, the JTBS and MSTD values decreased compared to the control subjects. No differences were observed between Group V and Group I. TUNEL staining was performed to determine the distribution of apoptotic cells within the testicular tissue as shown in (). Apoptotic cells were observed among the spermatogenic cells in seminiferous tubules. The number of apoptotic cells in testicular tissues increased in rats with induced diabetes as summarized in .
Figure 1. TUNEL staining of testicular tissue. (A) Group I (control), (B) Group II (STZ-induced diabetic rats), (C) Group III (STZ-induced diabetic rats +50 mg/kg/day PTX through the experiment), (D) Group IV (STZ-induced diabetic +50 mg/kg/day PTX for just second month), (E) Group V (only 50 mg/kg/day PTX administered to rats), and (F) Negative controls. TUNEL-positive cells (arrow) were mainly observed in germ cells of testis. nNOS: neuronal nitric oxide synthase, iNOS: inducible nitric oxide synthase, eNOS: endothelial nitric oxide synthase, STZ: streptozotosin, PTX: pentoxifylline.
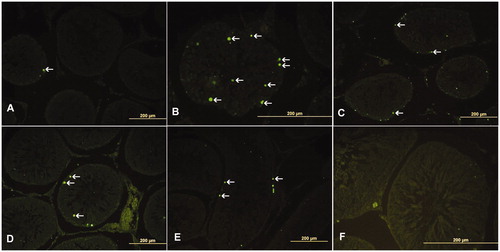
Table 2. Mean seminiferous tubule diameter (MSTD), Johnsen's score (JTBS), apoptotic index-1 (AI-1) and apoptotic index-2 (AI-2) of control and streptozotocin (STZ)-induced diabetic rats.
As reported by others [Alves et al. Citation2013], we showed that diabetes affects sperm production on many levels. The serum testosterone level in rats with induced diabetes was decreased compared to the control group. This showed that spermatogenesis is affected by endocrine metabolism. Together with histological seminiferous tubule distortion this showed that spermatogenesis is directly affected. In the present study, in the diabetic induced rat group apoptosis increased compared to the control group. Further, the Johnsen biopsy score and the average diameter of the seminiferous tubules decreased compared to the control group.
Analysis of NOS
Over the past 10 years, research on diabetic vascular diseases has primarily focused on NO [Toda et al. Citation2010], which is produced by endothelial cells and plays an important role in vascular homeostasis [Tousoulis et al. Citation2012]. NO is normally produced from L-arginine through the action of eNOS. The NO pathway leads to endothelial dependent vaso-dilatation. At the same time, NO has antiproliferative effects, and inhibits platelet and leukocyte adhesion to the vascular endothelium. Therefore, NO is considered a vascular protective molecule. However, it may easily react with superoxide, producing a product of peroxinitrite that is highly reactive. For this reason, NO’s chemical environment, e.g., the presence of oxygen, determines whether NO will be harmful or beneficial [Abou-Seif and Youssef Citation2004].
The pathophysiology of DM originates from hyperglycemia. A high concentration of glucose causes sorbitol production via the polyol pathway. In this pathway, aldose reductase enzyme activity is increased and intracellular NADPH is consumed. NADPH is required to translate oxidized glutation to the reduced form and synthesize NO. The cell’s antioxidant capacity is limiting as an active sorbitol pathway markedly reduces NADPHs. Reduced glutation and a reduction in NO synthesis have a clear contribution to diabetic vascular complications [Szabo Citation2009], while an increase in NO has been associated with diabetes [Abou-Seif and Youssef Citation2004].
There is a large volume of literature available on NO and its effect on the pathogenesis of diabetes [Khanna et al. Citation2014; Tousoulis et al. Citation2012]. Recently, it was shown that NOS, primarily eNOS, plays an important role in the pathogenesis of diabetic nephropathy [Dellamea et al. Citation2014]. Cardiovascular disease is one of the most important complications of diabetes. iNOS expression has been identified in studies of heart tissue from diabetic patients [Jo et al. Citation2011]. Similarly, an increase of eNOS expression has been observed in the aortic tissue of diabetic patients [Hink et al. Citation2001]. Diabetes is thought to be associated with erectile dysfunction, especially through the decrease of eNOS expression [Ohmasa et al. Citation2011].
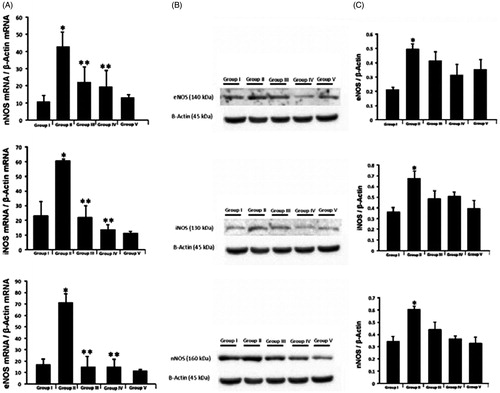
There are many studies showing the effects of DM on the expression of NOS in different tissues, and their different associated pathologies. In this study NOS activities were investigated using immunohistochemistry, western blot and qRT-PCR techniques in testicular tissue. Immunohistochemical staining was performed using the avidin-biotin method to determine the testicular tissue expression of NOS. The expression of nNOS (), iNOS () and eNOS () and was observed in interstitial testicular tissue. Interstitial NOS expression was evaluated in terms of the expression differences using the image J program [Sonmez et al. Citation2015]. The NOS expression in diabetic rats had increased statistically compared to the control group. The expression of all three testicular tissue NOS isoforms was statistically significantly higher in the diabetic rat Group II than the control group (). Diabetic rat NOS testes protein expression was analyzed with western blot, NOS immunohistochemicaly localized and mRNA levels assessed by RT-PCR. In the diabetic induced Group II (only STZ-induced rats), nNOS, iNOS, and eNOS mRNA levels had increased significantly compared to the control group (). The immunohistochemical stain clearly identifies eNOS, iNOS, and nNOS in the interstitial area of testicular tissue. Diabetes was associated with a significant increase of each of the three NOS’s compared to the control group as shown by western blot and PCR analyses.
Figure 2. nNOS expression. (A) Immunohistochemical localization of nNOS expression of the testicular tissue and semiquantitative results of nNOS content obtained by densitometric analysis of immunohistochemistry. (B) Immunohistochemical localization of iNOS expression of the testicular tissue and semiquantitative results of iNOS content obtained by densitometric analysis of immunohistochemistry. (C) Immunohistochemical localization of eNOS expression of the testicular tissue and semiquantitative results of eNOS content obtained by densitometric analysis of immunohistochemistry. (I) Group I (control), (II) Group II (STZ-induced diabetic rats), (III) Group III (STZ-induced diabetic rats +50 mg/kg/day PTX through the experiment), (IV) Group IV (STZ-induced diabetic +50 mg/kg/day PTX for just second month), (V) Group V (only 50 mg/kg/day PTX administered to rats), and (NC) Negative controls. nNOS (neuronal nitric oxide synthase), iNOS (inducible nitric oxide synthase), eNOS (endothelial nitric oxide synthase), STZ (streptozotosin) PTX (pentoxifylline). *p < 0.05 compared to Group I, **p < 0.05 compared to Group II.
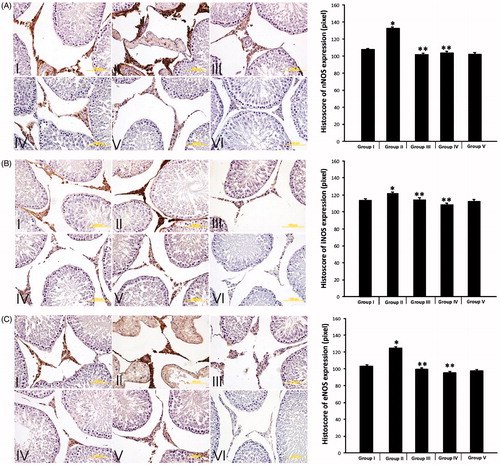
Figure 3. Neuronal, inducible, and endothelial NOS. (A) Neuronal, inducible and endothelial NOS mRNA/β-Actin mRNA levels of the testicular tissue in the different groups. (B) Endothelial, inducible and neuronal NOS expression of the testis in the different groups. Representative Western blots of NOS content determined in the testicular lysates. (C) Semiquantitative results of NOS content obtained by densitometric analysis of Western blots. Group I (control), Group II (STZ-induced diabetic rats), Group III (STZ-induced diabetic rats +50 mg/kg/day PTX through the experiment), Group IV (STZ-induced diabetic +50 mg/kg/day PTX for just second month), Group V (only 50 mg/kg/day PTX administered to rats). *p < 0.05 compared to Group I, **p < 0.05 compared to Group II.
Pentoxifylline therapy
In recent studies, various antioxidants were tested on testis damage of STZ-induced diabetes. Tang et al. [Citation2008] used fructose 1,6 diphosphate, an antioxidant, and showed that suppression of ROS yields fewer pathological changes in the testicular tissue. Guneli et al. [Citation2008] showed that melatonin also reduces pathological changes of the testes tissue and decreases the number of apoptotic cells. Using the diabetic rat model, Armagan et al. [Citation2006] demonstrated that melatonin regulates antioxidant enzyme activity by inhibiting lipid peroxidation in testicular tissue.
PTX is a derivative of methyl xanthine used for the regulation of blood flow [Horvath et al. Citation2002]. PTX inhibits transcription of some cytokines; such as tumor necrosis factor-alpha (TNF-α), and adhesion of platelets, and maintains endothelium derived vascular relaxation [Motawi et al. Citation2011; Wang et al. Citation2006]. It also has antioxidant properties [Garcia et al. Citation2014; Stosic-Grujicic et al. Citation2001]. Previously, it was shown that in STZ-induced diabetes in rats, the application of PTX application for eight weeks decreased lipid peroxidation in kidney tissue [Davila-Esqueda and Martinez-Morales Citation2004]. Chen et al. [Citation1999] showed that PTX decreases proteinuria by suppressing mesangial cell proliferation in the kidney. Guarrero-Romero et al. [Citation1995] showed that both in type 1 and type 2 diabetes, PTX showed reduced proteinuria. Liu and colleagues [Liu et al. Citation2006] showed that PTX significantly decreased the number of apoptotic cells and MDA levels in rats with testicular torsion. Savas et al. [Citation2002] demonstrated that PTX improves blood flow in the setting of a testicular torsion. As shown by others PTX can, to a certain extent, mitigate the effects of both non-diabetic testis tissue and other diabetic tissues. Accordingly, its effect in STZ-induced diabetic rat testis was examined.
The level of improvement upon PTX treatment was determined in MSTD values with PTX treatment; however, the JTBS and the number of apoptotic cells were not improved (; ). When we look at the NOS protein levels () and mRNA level () in the diabetic rat groups (Group III and Group IV) treated with PTX, NOS expression decreased significantly compared to the diabetic group. We also observed by western blotting that the PTX treatment decreased the level of NOS protein but that the difference was not statistically significant (). NOS expression was similar in Group V (non-diabetic only PTX given rats) compared to the control group. The improvement was determined in MSTD and NOS expression in PTX treatment given to Groups of III and IV. However, serum testosterone levels, the number of apoptotic cells, and JTBS values did not changed with PTX treatment. The effect of diabetes on testicular tissue is multifactorial. Left untreated due to high blood glucose levels diabetes damages testicular tissue. Increased NOS expression is thought to be responsible for the testicular tissue damage and PTX may reduce NOS.
Material and Methods
Sexually mature eight week old male Wistar rats obtained from the Hakan Çetinsaya Experimental and Clinic Research Center, Erciyes University, Kayseri, Turkey, were used for this study. They were housed in plastic cages placed in a well-ventilated room, allowed ad libitum access to rat chow and water, and were subjected to a natural photoperiod of 12-h light : dark cycle. This study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Erciyes (Permit Number: 12/32). The rats were randomly assigned to five groups of ten rats per group. Group I: served as control rats; Group II: STZ-induced diabetic rats; Group III: STZ-induced diabetic +50 mg/kg/day PTX through the experiment rats; Group IV: STZ-induced diabetic +50 mg/kg/day PTX for just second month rats; Group V: PTX administered rats.
Animals received 40 mg/kg intraperitoneal streptozotocin (sc-200719, Santa Cruz Biotechnology, CA, USA) to create diabetes [Rathinam et al. Citation2014]. Seventy-two h following application, blood glucose levels were measured from the tails of the rat. Blood glucose levels over 250 mg/dL were considered to be diabetic rats. PTX (Trental 100 mg/5 ml, Sanofi Aventis, Paris, France) 50 mg/kg was administrated by intraperitoneal injection to the rats [Queiroz-Junior et al. Citation2013]. Group III rats were administered PTX from the beginning of the experiment. Group IV rats received saline for the first month and underwent a PTX injection during the subsequent months. The control group subjects were administered the same amount of intraperitoneal saline.
At the end of the second month, before the rats were sacrificed, weights and blood glucose levels from their tails were measured again. Then the subjects were decapitated under ketamine (75 mg/kg) + xylazine (10 mg/kg) anaesthesia and their blood was collected and testicular tissue was removed and weighed. Serum testosterone levels were measured by ELISA. Some of the testicular tissues were left in Bouin fixative for histological examination, while some were stored in TRizol for RT-PCR and the rest placed into eppendorf tubes for further analyses including western blot and other biochemical investigations and stored at −80 °C.
Histopathological evaluation
The testicular tissue was examined and evaluated by a histologist in random order under blinded conditions by standard light microscopy. Mean seminiferous tubule diameter (MSTD) was measured in micrometers (Analysis LS Research Program). More than 20 seminiferous tubular sections per testis were each given a Johnsen's score (JTBS) from 1 to 10 as described previously [Johnsen Citation1970]. In this system of classification, all tubular sections in each section of the testicular biopsy are evaluated systematically and each is given a score from 1 to 10. Complete spermatogenesis with many spermatozoa present is evaluated as score 10.
Immunohistochemistry
Testes nNOS, eNOS, and iNOS were detected by immunohistochemistry using a rabbit polyclonal and the streptavidin–biotin peroxidase technique as previously described [Ozan et al. Citation2007]. Sections of 5–6 μm were taken and kept at 60 °C for one night, then first passed through xylene then through a serial series of increasing concentrations of alcohol, all tissues were then rehydrated. After rehydration, the sections were washed with phosphate buffer saline (PBS) three times within a 5 min time window. Then for the antigen retrieval, all sections were placed in 0.01 M sodium citrate buffer in a microwave oven at 350 W for 5 min. Then all sections were cooled in the same buffer at room temperature for 20 min. Sections were washed again with PBS to prevent endogenous peroxidase activity, then were treated with 3% hydrogen peroxide (H2O2) for 5 min. For the next stages, an ABC staining system (Santa Cruz Biotechnology, sc-2023) was used. All cross sections were washed with PBS and then block serum was applied for 20 min at room temperature to block nonspecific staining. Immediately following this, sections were incubated with eNOS (PA3-031A, 1/250 dilution, Pierce antibody product, Rockford, USA), iNOS (PA3-030A, 1/200 dilution, Pierce antibody product, Rockford, USA), and nNOS (PA3-032A, 1/200 dilution, Pierce antibody product, Rockford, USA) primary antibodies for a night at +4 °C. The following day they were incubated at room temperature for 20 min. As a negative control, PBS was used instead of primary antibody. After washing, sections were incubated with a biotinylated secondary antibody for 30 min and then the washing process was repeated. Then sections were treated with an enzyme bracket of Avidin-Biotin (AB) for 30 min. Afterwards, they were washed and the immunoreactive products visualized by incubating with diaminobenzydine (DAB) for 5 min, then washed with deionized water for 5 min. Contrast painted sections with Gill hematoxylin were washed several times with deionized water. For the final step, a serially increasing concentration of alcohol were used to remove water, passed from xylene, and at the end, sections were covered with an entellan. Images were obtained using an olympus BX51with a DP71digital camera. From each of the animals, five different areas were evaluated in terms of the expression differences using the image J program [Sonmez et al. Citation2009b].
Apoptosis (TUNEL)
The TUNEL method was utilized to assess apoptosis of testicular tissue. An in situ Cell Death Detection Kit Fluorescein’ Kit (Roche, Indianapolis, IN, USA) was used. For the process, first 5–6 µm thick testis tissues were obtained and after deparaffinized and rehydrating, they were washed with PBS. After washing for antigen retrieval, tissues were placed in a 0.01 M sodium citrate buffer in the microwave oven at 350 W for 5 min. Then, they were left for cooling for 20 min at room temperature. Having washed with PBS three times for 5 min, tissues were incubated with a TUNEL reaction mixture in a damp and dark place at 37 °C for 60 min. After washing with PBS three times for 5 min, tissues were contrast colored with 4,6-diamidine-2'-fenilindol. After covering the tissues with solution containing glycerol, they were all examined with the Olympus BX – 51 fluorescent microscope at 450–500 nm wavelength. At least 10 fields from each section were counted for tubules and positive stained cells. The apoptotic index (AI) was created in two ways. AI-1, each consist of 100 tubules stained positive for apoptotic cells; AI-2 was calculated from positive in every 100 tubules stained as apoptotic cells [Bayatli et al. Citation2013].
ELISA
Blood samples were taken into empty tubes to obtain sera and were centrifuged at 1,509 g for 10 min. The resulting sera were used for the determination of testosterone levels (ELISA CSB-E05100r, 96 Wells kit, CUSABIO Bıotech CO., LTD, Whuan, Hubei, P.R. China) using ELISA kits. Cusabio Biotech rat ELISA kit protocols were used. All products were kept at room temperature for 30 min just before their use. A washing solution was prepared by adding 15 mL concentrated washing solution to 285 ml distilled water to make a total of 300 mL. 50 µL of standard, or serum samples were added to each well. With the exception of the blind well, 50 µL HRP and 50 µL of antibody were added to each well and incubated for one hour at 37 °C. Afterwards, the plate was washed three times with wash solution in an automatic washing device. 50 µL substrates A and B were added to each well and then 50 µL of termination solution was added to each well and all microplates were read at 450 nm.
Analysis of gene expression with qRT-PCR
Tissues were taken and homogenized in 1 ml of TRIzol. Until RNA was isolated, samples were all kept at −80 °C. When the RNA isolation procedure was carried out, samples were removed from the freezer then incubated at 37 °C for 5 min. After the tissues were thawed, 0.2 mL of chloroform was added to the tissue TRIzol mixture for a total volume of 1 mL and vortexed thoroughly. Tissues were then centrifuged at 241.4 g for 15 min at 8 °C. At the end of the centrifugation, three phases appeared. The top layer containing RNA was carefully retrieved and placed in a new tube. 0.5 mL of isopropyl alcohol was added into this transparent phase and was thoroughly vortexed. Samples were incubated at −20 °C for 15–30 min. After the centrifugation (24,148 g for 15 min), white pellets appeared at the bottom of the tube and were reconstituted by adding 1 mL of 75% ethanol, vortexed, and were centrifuged at 9,433 g for 5 min at 8 °C. After the pellets were dried, 100 µL of RNAase-free water was added to them to dissolve the pellet thoroughly. To determine the quality of the resulting RNA, all measurements were made in the Nano drop spectrophotometer. Each sample had at least 200 ng of RNA in 1 µL, and then cDNA synthesis was performed from RNA using a cDNA kit (Complementary DNA). iNOS, eNOS, and nNOS mRNA expressions were obtained from cDNA and using housekeeping genes (β-actin and GAPDH) as primary probes, RT-qPCR analysis was performed for each individual sample (). The reaction was conducted in a LightCycler® 480 II (Roche) for 45 cycles; the reaction results were analyzed via an efficiency corrected advanced relative quantification algorithm on the LightCycler® 480 SW 1.5 software. Conditions of the reaction were as follows: denaturation 95 °C 5 min, annealing of primers 50 °C 15 s, elongation 72 °C 10 s. All samples were tested in duplicate.
Table 3. Sequences of primers and ID numbers of UPL probes used for qRT-PCR.
Western blot
The presence of testicular nNOS, eNOS, and iNOS proteins was determined by SDS-PAGE. Testicular tissue, was homogenized in lysis buffer (RIPA Lysis buffer, sc-24948, Santa Cruz Biotechnology), then centrifuged at 28,341 g for 30 min at 4 °C. The protein content of samples was determined by the Lowry method. Samples were heated in sample buffer for 5 min at 95 °C just before electrophoresis. Then each sample containing 100 µg protein was run on an 8% SDS-polyacrylamide gel at 100 mV for 3 h. The gel proteins were transferred to a PVDF (Westran® PVDF membranes Z671010 Aldrich Whatman®) membrane using a semidry blotter. The molecular weights of the immunoreactive protein bands were determined using protein standards (Precision Plus Protein Standards All Blue, Bio-Rad, Hercules, CA, USA, 250 – 10 kDa). The membrane was incubated in a TBS-T solution containing 5% non-fat milk on a shaker for 90 min. A mouse monoclonal antibody of anti-β-actin (1/1000; Cell signaling Technology, mAb # 3700) was used as an internal loading control. The membrane was stripped and re-probed. Then, the membrane was incubated with eNOS (1/1000; Pierce antibody product, PA3-031A), iNOS (1/1000; Pierce antibody product, PA3-030A), and nNOS (1/1000; Pierce antibody product, PA3-032A) primary antibodies overnight at 4 °C. The next day, the membrane was washed with TBS-T 5 x 15 min. Then the membranes were incubated in a blocking solution of 1/5,000 containing reconstituted goat anti rabbit IgG HRP (Santacruz sc-2030) in a container for one h at room temperature. After the conjugated antibody was poured, the membranes were washed five times for 15 min periods with a washing solution. Finally, membranes were treated with ECL detection reagents (Amersham, Buckinghamshire, UK) and a gel imaging system (Bio-Rad ChemicDoc MP Imaging System, CA, USA) was used to view the membranes. The differences between the groups were analyzed using the Image J program.
Statistical analysis
One-way analysis of variance (ANOVA) and post hoc Tukey test were used to determine differences between groups. Results are presented as mean ± S.E.M. Values were considered statistically significant if p < 0.05. The SPSS/PC program (Version 15.0; SPSS, Chicago, IL, USA) was used for the statistical analysis.
| Abbreviations | ||
| DM | = | diabetes mellitus |
| ROS | = | reactive oxygen species |
| NO | = | nitric oxide |
| NOS | = | nitric oxide synthase |
| nNOS | = | neuronal NOS |
| NOS | = | endothelial NOS |
| iNOS | = | inducible NOS |
| PTX | = | pentoxifylline |
| JTBS | = | Johnsen's tubular biopsy score |
| MSTD | = | mean seminiferous tubule diameter |
| DAB | = | diaminobenzydine |
| ANOVA | = | one-way analysis of variance |
Declaration of interest
This work was supported, by a research grant from The Scientific and Technological Research Council of Turkey (TUBITAK, 112S213). We declare that we have no conflict of interest.
Author contribution
Study conception and design, drafting of manuscript, and critical revision: MFS; Performed ELISA and Western blot: EK; Performed experimental diabetes, immunohistochemistry, and light microscopy: DK, KTC, ED; Performed RT-PCR: MD.
References
- Abou-Seif, M.A. and Youssef, A.A. (2004) Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta 346:161–170
- Agbaje, I.M., Rogers, D.A., McVicar, C.M., McClure, N., Atkinson, A.B., Mallidis, C., et al. (2007) Insulin dependant diabetes mellitus: Implications for male reproductive function. Hum Reprod 22:1871–1877
- Alves, M.G., Martins, A.D., Rato, L., Moreira, P.I., Socorro, S. and Oliveira, P.F. (2013) Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta 1832:626–635
- Amaral, S., Oliveira, P.J. and Ramalho-Santos, J. (2008) Diabetes and the impairment of reproductive function: Possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev 4:46–54
- Armagan, A., Uz, E., Yilmaz, H.R., Soyupek, S., Oksay, T. and Ozcelik, N. (2006) Effects of melatonin on lipid peroxidation and antioxidant enzymes in streptozotocin-induced diabetic rat testis. Asian J Androl 8:595–600
- Bayatli, F., Akkus, D., Kilic, E., Saraymen, R. and Sonmez, M.F. (2013) The protective effects of grape seed extract on MDA, AOPP, apoptosis and eNOS expression in testicular torsion: An experimental study. World J Urol 31:615–622
- Chen, Y.M., Chien, C.T., Hu-Tsai, M.I., Wu, K.D., Tsai, C.C., Wu, M.S., et al. (1999) Pentoxifylline attenuates experimental mesangial proliferative glomerulonephritis. Kidney Int 56:932–943
- Davila-Esqueda, M.E. and Martinez-Morales, F. (2004) Pentoxifylline diminishes the oxidative damage to renal tissue induced by streptozotocin in the rat. Exp Diabesity Res 5:245–251
- Delfino, M., Imbrogno, N., Elia, J., Capogreco, F. and Mazzilli, F. (2007) Prevalence of diabetes mellitus in male partners of infertile couples. Minerva Urol Nefrol 59:131–135
- Dellamea, B.S., Leitao, C.B., Friedman, R. and Canani, L.H. (2014) Nitric oxide system and diabetic nephropathy. Diabetol Metab Syndr 6:17
- Garcia, F.A., Pinto, S.F., Cavalcante, A.F., Lucetti, L.T., Menezes, S.M., Felipe, C.F., et al. (2014) Pentoxifylline decreases glycemia levels and TNF-alpha, iNOS and COX-2 expressions in diabetic rat pancreas. Springerplus 3:283
- Guerrero-Romero, F., Rodriguez-Moran, M., Paniagua-Sierra, J.R., Garcia-Bulnes, G., Salas-Ramirez, M. and Amato, D. (1995) Pentoxifylline reduces proteinuria in insulin-dependent and non insulin-dependent diabetic patients. Clin Nephrol 43:116–121
- Guneli, E., Tugyan, K., Ozturk, H., Gumustekin, M., Cilaker, S. and Uysal, N. (2008) Effect of melatonin on testicular damage in streptozotocin-induced diabetes rats. Eur Surg Res 40:354–360
- Hink, U., Li, H., Mollnau, H., Oelze, M., Matheis, E., Hartmann, M., et al. (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88:E14–22
- Horvath, B., Marton, Z., Halmosi, R., Alexy, T., Szapary, L., Vekasi, J., et al. (2002) In vitro antioxidant properties of pentoxifylline, piracetam, and vinpocetine. Clin Neuropharmacol 25:37–42
- Jo, H., Otani, H., Jo, F., Shimazu, T., Okazaki, T., Yoshioka, K., et al. (2011) Inhibition of nitric oxide synthase uncoupling by sepiapterin improves left ventricular function in streptozotocin-induced diabetic mice. Clin Exp Pharmacol Physiol 38:485–493
- Johnsen, S.G. (1970) Testicular biopsy score count–a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1:2–25
- Khanna, S., Singh, G.B. and Khullar, M. (2014) Nitric oxide synthases and diabetic cardiomyopathy. Nitric Oxide 43:29–34
- La Vignera, S., Condorelli, R., Vicari, E., D'Agata, R. and Calogero, A.E. (2012) Diabetes mellitus and sperm parameters. J Androl 33:145–153
- Liu, Z.M., Zheng, X.M., Yang, Z.W. and Li, S.W. (2006) Protective effect of pentoxifylline on spermatogenesis following testicular torsion/detorsion in rats. Zhonghua Nan Ke Xue 12:323–325, 329
- Motawi, T.K., Darwish, H.A. and Abd El Tawab, A.M. (2011) The relative efficacy of aminoguanidine and pentoxifylline in modulating endotoxin-induced cardiac stress. Cell Biochem Funct 29:694–702
- Ohmasa, F., Saito, M., Tsounapi, P., Dimitriadis, F., Inoue, S., Shomori, K., et al. (2011) Edaravone ameliorates diabetes-induced dysfunction of NO-induced relaxation in corpus cavernosum smooth muscle in the rat. J Sex Med 8:1638–1649
- Ozan, E., Sonmez, M.F., Ozan, S., Colakoglu, N., Yilmaz, S. and Kuloglu, T. (2007) Effects of melatonin and vitamin C on cigarette smoke-induced damage in the kidney. Toxicol Ind Health 23:479–485
- Queiroz-Junior, C.M., Bessoni, R.L., Costa, V.V., Souza, D.G., Teixeira, M.M. and Silva, T.A. (2013) Preventive and therapeutic anti-TNF-alpha therapy with pentoxifylline decreases arthritis and the associated periodontal co-morbidity in mice. Life Sci 93:423–428
- Rathinam, A., Pari, L., Chandramohan, R. and Sheikh, B.A. (2014) Histopathological findings of the pancreas, liver, and carbohydrate metabolizing enzymes in STZ-induced diabetic rats improved by administration of myrtenal. J Physiol Biochem 70:935–946
- Roessner, C., Paasch, U., Kratzsch, J., Glander, H.J. and Grunewald, S. (2012) Sperm apoptosis signalling in diabetic men. Reprod Biomed Online 25:292–299
- Rolo, A.P. and Palmeira, C.M. (2006) Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol 212:167–178
- Savas, C., Dindar, H., Aras, T. and Yucesan, S. (2002) Pentoxifylline improves blood flow to both testes in testicular torsion. Int Urol Nephrol 33:81–85
- Sexton, W.J. and Jarow, J.P. (1997) Effect of diabetes mellitus upon male reproductive function. Urology 49:508–513
- Sonmez, M.F., Karabulut, D., Kilic, E., Akalin, H., Sakalar, C., Gunduz, Y., et al. (2015) The effects of streptozotocin-induced diabetes on ghrelin expression in rat testis: Biochemical and immunohistochemical study. Folia Histochem Cytobiol 53:26–34
- Sonmez, M.F., Narin, F., Akkus, D. and Ozdamar, S. (2009a) Effect of melatonin and vitamin C on expression of endothelial NOS in heart of chronic alcoholic rats. Toxicol Ind Health 25:385–393
- Sonmez, M.F., Narin, F., Akkus, D. and Turkmen, A.B. (2012) Melatonin and vitamin C ameliorate alcohol-induced oxidative stress and eNOS expression in rat kidney. Ren Fail 34:480–486
- Sonmez, M.F., Narin, F. and Balcioglu, E. (2009b) Melatonin and vitamin C attenuates alcohol-induced oxidative stress in aorta. Basic Clin Pharmacol Toxicol 105:410–415
- Stosic-Grujicic, S., Maksimovic, D., Badovinac, V., Samardzic, T., Trajkovic, V., Lukic, M., et al. (2001) Antidiabetogenic effect of pentoxifylline is associated with systemic and target tissue modulation of cytokines and nitric oxide production. J Autoimmun 16:47–58
- Szabo, C. (2009) Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol 156:713–727
- Tang, X.Y., Zhang, Q., Dai, D.Z., Ying, H.J., Wang, Q.J. and Dai, Y. (2008) Effects of strontium fructose 1,6-diphosphate on expression of apoptosis-related genes and oxidative stress in testes of diabetic rats. Int J Urol 15:251–256
- Toda, N., Imamura, T. and Okamura, T. (2010) Alteration of nitric oxide-mediated blood flow regulation in diabetes mellitus. Pharmacol Ther 127:189–209
- Tousoulis, D., Kampoli, A.M., Tentolouris, C., Papageorgiou, N. and Stefanadis, C. (2012) The role of nitric oxide on endothelial function. Curr Vasc Pharmacol 10:4–18
- Wang, W., Zolty, E., Falk, S., Basava, V., Reznikov, L. and Schrier, R. (2006) Pentoxifylline protects against endotoxin-induced acute renal failure in mice. Am J Physiol Renal Physiol 291:F1090–1095
- Yu, T., Robotham, J.L. and Yoon, Y. (2006) Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 103:2653–2658
