Abstract
Purpose: This study evaluated the potential for prepared chitosan–plasmid DNA (pDNA) nanoparticles to transfer an exogenous gene into human bone marrow-derived mesenchymal stem cells (MSCs). Methods: Chitosan–pDNA nanoparticles were synthesized by the complex coacervation method. We used 18, 50 and 136 KD chitosan at concentrations of 0.05%, 0.1%, 0.5% and 1%, in addition to a pTracer-CMV2 plasmid that contained the green fluorescent protein (GFP) gene. To examine the complexation, samples were run through an agarose gel. The sizes and zeta potential of the nanoparticles were measured by a nanosizer. Scanning electron microscopy (SEM) imaging was used to observe the nanoparticle morphology. MSCs were prepared from human bone marrow and transfected with chitosan–pDNA nanoparticles. The cultures transfected by lipofectamine2000 were taken as the control. Cell viability was determined by MTT assay and transfection efficiency by flow cytometry. Results: The smallest size of complexes was obtained with 18 KD chitosan (211 nm) and the highest zeta potential was observed with 136 KD chitosan (29.61 mV). The best transfection rate (18.43%) was achieved with the 0.1% concentration of 18 KD chitosan nanoparticles versus 40.57% for commercial lipofectamine (p < 0.01). The MTT assay indicated an average of 95.5% cell viability for 0.1% concentration of 18 KD chitosan compared with approximately 60% of Lipofectamine2000. Conclusion: Nanoparticles produced by 18 KD chitosan at the 0.1% concentration and pDNA may be a promising gene delivery system for human marrow-derived MSCs. Although transfection efficiency of such nanoparticles is lower than that of Lipofectamine2000, however comparatively they possess less cytotoxic effects.
Introduction
With the advancement of genomic and proteomic technologies, the prospect for gene therapy has progressed rapidly. Gene therapy is the treatment of human disorders by introduction of genetic material into specific target cells of a patient, where an encoded protein will be produced (Corsi et al. Citation2003). Viral and non-viral vectors are the main systems that can be used to transfer foreign genetic material into a specific target cell (Quong and Neufeld Citation1998). Viruses such as retrovirus, herpes simplex virus, lentivirus, adenovirus and adeno-associated virus are the most common viral vectors in use (Oligino et al. Citation2000). In contrast to their high transfection efficiency compared with non-viral vectors (Quong and Neufeld Citation1998), clinical application of viral vectors is limited due to their immunogenicity, potential for infectivity, complicated production and inflammation (Smith Citation1995). Naked DNA is one of the simplest nonviral gene delivery systems, however only 1–3% of the cells in different target tissues take up this DNA, resulting in low production of the encoded protein (Mansouri et al. Citation2004). Cationic phospholipids and cationic polymers are two major types of non-viral gene delivery systems. Both types interact electrostatically with negatively charged DNA and produce complexes (lipo- or polyplexes). Low immunogenicity, ease of preparation, targetability and stability in storage are major advantages of cationic phospholipids and cationic polymers over viral vectors (Deshpande et al. Citation1998). Polyplexes possess low cytotoxicity and are more stable compared with lipoplexes (Leong et al. Citation1998).
Chitosan is a cationic polymer that would be a good gene carrier candidate due to its biocompatibility, biodegradability and cationic potential. In addition, chitosan can protect DNA against nuclease degradation. The chitosan molecule is a copolymer of N-acetyl-D-glucosamine and D-glucosamine. This polymer is a weak base that is insoluble at neutral and alkaline pH values. In acidic mediums, the amine groups will be positively charged (Hejazi and Amiji Citation2003). Advantages of chitosan are that it does not need sonication or organic solvents for its preparation, therefore minimizing possible damage to DNA during complexation. Complexes of chitosan–plasmid DNA (pDNA) have been found to be stable during storage (Leong et al. Citation1998).
Molecular weight of chitosan highly influences transfection efficiency, stability of the complex and post-endocytosis release of pDNA from the complex (Sato et al. Citation2000). Chitosan of lower molecular weight causes less cytotoxicity (Heller et al. Citation1996) and is more soluble in water (Lee et al. Citation2001). It also yields smaller size nanoparticles in contrast with a higher molecular mass (MacLaughlin et al. Citation1998). In terms of transfection efficiency, the optimum molecular mass of chitosan is cell-type dependent (Sato et al. Citation2000). One interesting cell type for potential application in gene therapy is mesenchymal stem cells (MSCs). These cells are defined as nonhematopoietic cells that reside in a multitude of adult tissues. MSCs have originally been isolated from bone marrow samples and described as fibroblastic cells capable of forming colonies. The most important characteristic of these cells is their potential to produce bone- and cartilage-like deposits in culture. MSCs possess two outstanding features, an extensive self-renewal proliferation capacity and multipotent differentiation potential. These cells could easily be isolated from tissue samples. Such features render MSCs as an attractive therapeutic tool for application in numerous fields, such as regenerative medicine and tissue engineering (Gao et al. Citation2009).
MSCs are also of much interest in the field of gene therapy in that transduced cells can be used to deliver genes to sites of injury. By date, a range of gene delivery vectors have been tested on MSCs. Viral vectors including adenovirus, adeno-associated virus, Herpes simplex virus and lentivirus seem to be most effective in the expression level but their clinical use is limited (McMahon et al. Citation2006). Nonviral vectors such as PLGA nanoparticles, PEI nanoparticles and their derivatives give moderate transfection levels but the negative effect of these nanoparticles on the MSCs’ viability is their major deficiency (Kim et al. Citation2011, Pimpha et al. Citation2010, Park et al. Citation2010). Chitosan as a natural polymer would be an appropriate gene-carrier candidate (Duceppe and Tabrizian Citation2010). Scant information exists regarding transfectability of MSCs using chitosan nanoparticles. In this regards, Corsi et al. have investigated 150, 400 and 600 KD chitosan at a concentration of 0.02% and found no significant difference between the applied chitosan and naked DNA. Given the importance of molecular weight of chitosan on transfection efficiency, in the current study, we compared MSC transfection using varying molecular weights (18, 50 and 136 KD) and concentrations (1%, 0.5%, 0.1%, 0.05%, 0.01%, 0.005% and 0.001%) of chitosan versus naked DNA and lipofectamine. We found chitosan nanoparticles as a promising vector for gene transfer into MSCs.
Methods
Materials
The following materials were used in the experiments: thiazolyl blue tetrazolium bromide (MTT, Sigma, M2128-1G), α-MEM (Gibco, 22571), FBS (Gibco, 10270), pen/strep (Gibco, 15070), trypsin/EDTA (Gibco, 25300), phosphate buffered saline (Gibco, 21600-051), Lipofectamine2000 (Invitrogen, 11668-019), pTracer-CMV2 plasmid (Invitrogen, V885-20), low-molecular weight chitosan (75–85% deacetylated, Sigma, 44886-9), tris (MP Biomedicals, 819623), ethidium bromide (EtBr, Invitrogen, 15585-011), dimethyl sulfoxide (DMSO, Merck, D2650), Qiagene HiSpeed Plasmid Maxi Kit (12663), Escherichia coli (DH5α) and DNA ladder (1 kb, Fermentas, #SM1343).
Preparation of chitosan at various molecular weights
A low-molecular weight chitosan solution was prepared at a concentration of 2% (w/v) in 6% acetic acid. In order to depolymerize chitosan, we gradually added 10 ml NaNO2 aliquots into 100 ml of the chitosan solution, separately, at concentrations of 1, 2.5 and 7 mg/ml. The mixtures were stirred for 1 h at room temperature. The chitosan depolymerization was stopped and the solution was allowed to precipitate by increasing the pH to 9 with 5N NaOH. The white-yellowish precipitate was filtered and washed thoroughly with acetone. The filterant was dissolved in a minimum volume of 0.6% acetic acid (v/v). The depolymerized polymer was dialyzed (MWCO: 12KD) and the purified product was freeze-dried. To determine the molecular weight of the original chitosan, we used the gel permeation chromatography technique according to Yomota et al. (Citation1993). The chitosan weighed 18, 50 and 136 KD.
Plasmid purification
The pTracer-CMV2 plasmid DNA that contained the green fluorescent protein (GFP) gene was used in this research. This plasmid was amplified in E. coli DH5α and purified by the Qiagene HiSpeed Plasmid Maxi Kit according to the manufacturer's instructions, after which the plasmid was resuspended in water. The quantity of plasmid DNA was assessed by UV absorption at 260 nm; its purity and quality were confirmed by 0.8% agarose gel electrophoresis.
Preparation of chitosan solution
Chitosan at molecular weights of 18, 50 and 136 KD was dissolved in 1% acetic acid. Each was then diluted to the following v/w concentrations: 1%, 0.5%, 0.1%, 0.05%, 0.01%, 0.005% and 0.001%. The pH was adjusted to 5.5–5.7 after which the solutions were filtered through 0.22 μ filters.
Preparation of chitosan–pDNA nanoparticles
Chitosan–pDNA nanoparticles were prepared according to the complex coacervation technique with a process similar to the protocol by Gao et al. (Citation2009). Equal volumes of each MW chitosan solution and plasmid DNA (0.2 μg/μl concentration) were warmed to 55°C and rapidly vortexed for 60 s. The resultant complexes were left for 30 min at room temperature to become stable.
Gel retardation assay
Naked DNA and chitosan–pDNA nanoparticles of different concentrations and molecular weights were loaded onto a 0.8% agarose gel that contained EtBr in a tris-borate EDTA buffer at pH 8. The samples were run through the gel at 80 V for 1 h, after which the gel was stained with EtBr and photographed.
Particle size and zeta potential measurements
A nanosizer instrument (Malvern Zetasizer laser, Malvern Instruments, UK) was used to measure the nanoparticle size and zeta potential. Nanoparticle size and surface charge are important factors that influence cell transfection. We measured the size and zeta potential for 21 chitosan samples of different molecular weights (18, 50 and 136 KD) and concentrations [0.005–1% (v/w)].
SEM
We used scanning electron microscopy (SEM) to study the morphology of the chitosan–pDNA nanoparticles. One drop of the complex was placed on aluminum foil and allowed to dry. The samples were then coated with a gold layer and visualized at an accelerating voltage using a Wega/Tescan SEM.
Cell culture
In this experiment, we used surplus MSCs derived from the bone marrow of a patient who received cell therapy treatment for knee osteoarthritis at Royan Institute Cell Therapy Center. To isolate the cells, the bone marrow sample was diluted with an equal volume of PBS, layered onto a Ficoll gradient and centrifuged at 400 g for 30 min at 4°C. Interphase mononuclear cells were then collected and seeded in a 25-cm culture flask at a density of 106/cm2 in an α-MEM supplemented with 15% fetal bovine serum, pen/strep(100 U/ml) and 2 mM L-glutamine. The cultures were incubated at 37°C in a humidified atmosphere of 5% CO2, with twice weekly changes of culture medium. The cultures were passaged at approximately 80% confluency. Before applying passaged-3 cells for the transfection experiment, we verified their ability to differentiate into skeletal cell lineages. For osteogenesis, confluent passaged-3 cultures were treated with α-MEM that contained the osteogenic supplements dexamethasone (10− 8 M), 10 mM beta-glycerophosphate (Sigma-Aldrich) and 50 μg/ml ascorbic acid-2-phosphate (Wako Chemicals). Similarly, for adipogenesis, passaged-3 cultures were treated by the same medium with adipogenic supplements that included 1 mM dexamethasone and 60 mM indomethacin (Sigma-Aldrich). For chondrogenesis, we used a micro mass culture system. Approximately 2 × 105 passaged-3 cells were centrifuged at 400 g for 5 min in a 15-ml polypropylene tube. The resultant pellet was placed in 500 μl of chondrogenic medium that contained 10 ng/ml transforming growth factor-β3, then it was incubated at 37°C and 5% CO2 for 3 weeks. At the end of this period, the pellets were histologically prepared and embedded in paraffin wax. Pellets were cut into 5-μm sections and stained with toluidine blue.
pH optimization
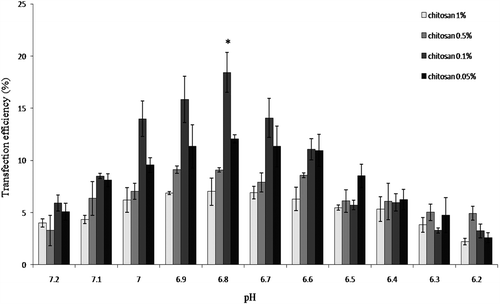
Passaged-3 MSCs were transfected using 18 KD chitosan at four concentrations of varying pH with the intent to determine the optimal pH for the transfection medium. Cells were seeded in a 24-well plate at a density of 105cells/well in 1 ml α-MEM supplemented with 15% FBS and 0.1% pen/strep. At 24 h after culture initiation, the medium was replaced with 1 ml fresh medium of varying pH (6.2, 6.3, 6.4, 6.5, 6.6, 6.7, 6.8, 6.9, 7, 7.1 and 7.2) and treated with 18 KD chitosan–pDNA nanoparticles that contained 10 μg of plasmid DNA. The cultures were incubated for 72 h at 37°C and 5% CO2.
In vitro transfection of MSCs
To determine the in vitro pDNA transfer capability of chitosan–pDNA nanoparticles, the MSC culture was established and transfected as previously mentioned using the nanoparticles. The pH of this medium was 6.8, which was determined to be the optimal pH according to the previous experiment. Separate cultures were established for each chitosan molecular weight and concentrations. The transfection efficacy of the nanoparticle groups was compared with those of commercial lipofectamine as well as naked DNA. Lipofectamine/pDNA was prepared according to the manufacturer's instructions. In brief pDNA and commercial lipofectamine were separately diluted in 50 μl of α-MEM, mixed gently and incubated for 5 min at room temperature. The diluted lipofectamine was subsequently added to the diluted pDNA and left for 20 min prior to application.
Evaluation of transfection efficiency and cell viability assay
The transfection rate was measured with flow cytometry. The wells of the culture plate were washed with PBS and then trypsinized. The solution was pelleted by centrifuging at 300 g for 5 min, then analyzed for GFP+ cells by a FACSCalibur cytometer equipped with a 488 nm argon laser. The cultures were also observed under an Olympus BX51 fluorescence microscope. To determine the effect of chitosan– pDNA nanoparticles on cell viability, MTT assay was used. In brief, the cultures were washed with PBS, then added to a solution composed of a 5:1 ratio of media and MTT solution (5 mg/mL in PBS) and incubated for 2 h at 37°C. Both the medium and MTT solution were removed and 0.5 ml of DMSO, an extraction solution, was added to solve the formazan precipitate. The absorbance of the supernatant was read with a microplate reader (BioTek ELX × 800) at 570 nm. Cell number was determined through a standard curve established by using a known number of cells counted by a Coulter counter.
Statistical analysis
All measurements were performed in three independent triplicates and presented as mean ± SD. The data were compared using one-way ANOVA. A p-value of < 0.05 was considered statistically significant.
Results
Chitosan–pDNA nanoparticles
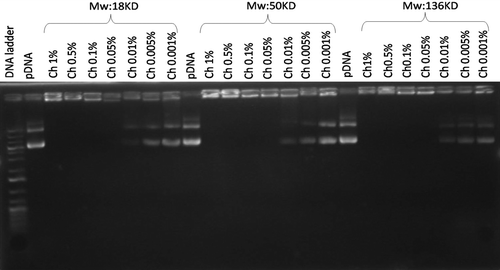
The chitosan–pDNA nanoparticles were obtained through the complex coacervation process. shows the migration of the complexes in agarose gel electrophoresis. The control, naked DNA, indicated no retardation. Chitosan–pDNA complexes in the concentration range of 0.05–1% were completely retarded in the agarose gel which indicated that chitosan could electrostatically neutralize the plasmid DNA's negative charge and deter electrophoretic mobility. This retardation was not observed at the 0.01–0.001% concentrations for the three molecular weights.
Size, zeta potential and SEM image of nanoparticles
Chitosan–pDNA nanoparticle characteristics are shown in . The size distribution of nanoparticles of 18 KD chitosan ranged from 215 to 322 nm. Nanosizer measurements of these nanoparticles showed zeta potential values of 13.7–18.4 mV. With the 50 KD chitosan, the nanoparticle sizes ranged from 274 to 435 nm with a zeta potential of 11.7–17.3 mV. Chitosan nanoparticles of this group were smaller than those of the two other groups. Nanoparticles of the 136 KD chitosan were 266–472 nm in size with a zeta potential of 14.6–29.6 mV. The highest zeta potential was achieved with this group. A highly positive zeta potential indicates excellent electrostatic stability and positive surface charge. shows the formation of complexes as observed by SEM. These complexes consisted of a spherical morphology and compacted structure.
Figure 2. SEM image of chitosan/pDNA complex. Representative image is the 18 KD chitosan at 0.1% concentration.
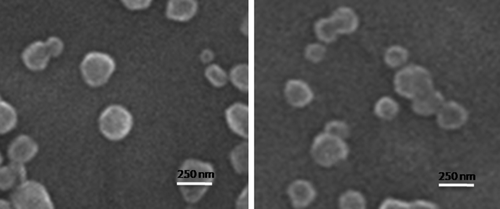
Table I. Molecular weight, concentration, particle size and zeta potential of chitosan nanoparticles.
MSC isolation and differentiation potential
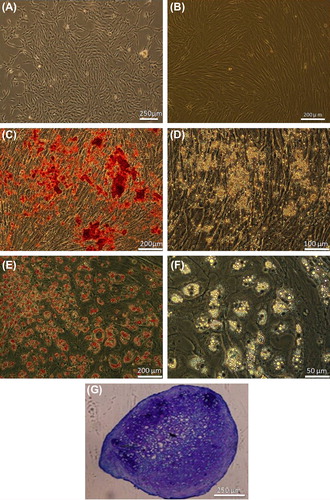
In primary cultures, there were some cells that adhered. These cells had an elongated morphology and began proliferating, leading to the formation of colonies which then grew larger and became confluent (). Fibroblast morphology was maintained during the subcultures. Passaged-3 cells tended to successfully differentiate along bone, adipose and cartilage cell lineages as evidenced in alizarin red, oil red and toluidine blue staining, respectively ().
Figure 3. Human MSC culture and differentiation. (A) Bone marrow cell at primary culture. (B) Confluent culture of passaged-3 cells. (C) Alizarin red staining of the osteogenic culture. (D) The same osteogenic culture without staining. (E) Oil red staining of the adipogenic culture. (F) Adipogenic culture without staining. (G) Toluidine blue staining of chondrogenic culture.
pH optimization
Transfection of passage-3 MSCs at different pH levels indicated that the maximum transfection rate could be achieved at pH 6.8 (). This finding was used in the following experiment.
Transfection efficacy and cell viability
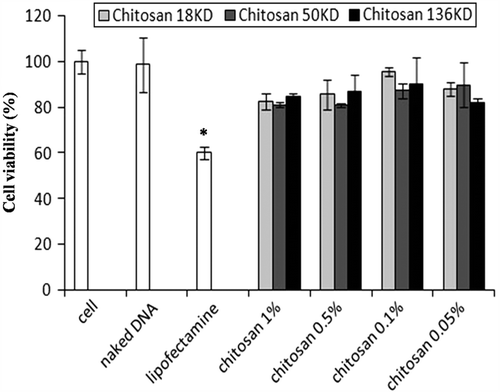
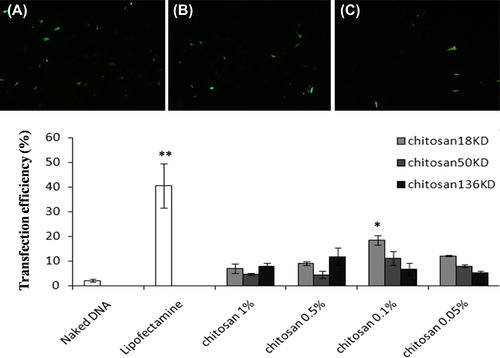
Transfected cells appeared as green cells under fluorescent microscopy. Lipofectamine tended to yield much better transfection than nanoparticles (p < 0.01). Nanoparticles produced significantly more transfection than naked DNA (p < 0.01). The mean chitosan–pDNA nanoparticle potential to transfect MSC is shown in . According to this data, nanoparticles formed by 18 KD of chitosan at the 0.1% concentration tended to have more transfection efficacy than the other studied nanoparticles. In this group, the efficacy was calculated as 18.43 ± 1.92%, which was significantly higher than for nanoparticles formed by 50 KD (11.06 ± 2.73%) and 136 KD (6.66 ± 2.50%) at the 0.1% concentration of chitosan.
Figure 5. Human MSC transfection with chitosan/pDNA nanoparticles. Transfected cells appear green as visualized by fluorescent microscope. (A) Commercial lipofectamine group. (B) Nanoparticle group (18 KD chitosan/pDNA at 0.1% concentration). (C) Naked DNA (original magnification: 10x). (D) Commercial lipofectamine tended to yield better transfection than nanoparticles. **p < 0.01. Nanoparticles produced significantly more transfection than naked DNA. Comparatively, nanoparticles formed by 18 KD chitosan at the 0.1% concentration tended to have more transfection efficacy than the other studied nanoparticles. *significant difference (p < 0.01).
As shown in , cell viability for the three molecular weights of chitosan nanoparticles was higher than 81% compared with 60% viability for commercial lipofectamine.
Discussion
In the present study, we evaluated 18, 50 and 136 KD molecular weight chitosan at several v/w concentrations for their ability to form complexes with plasmid DNA. The chitosan–pDNA nanoparticles were then examined for their transfecting potential using a human marrow-derived MSC culture. Our findings indicated that all three molecular weights (18, 50 and 136 KD) of chitosan at the 0.05%, 0.1%, 0.5% and 1% concentrations formed complete complexes with plasmid DNA. The best MSC transfection was achieved by nanoparticles formed by 18 KD chitosan at a concentration of 0.1%, which was comparable with the transfection efficacy of commercial lipofectamine. Importantly, the percentage of viable cells in the nanoparticle groups was significantly higher than the commercial lipofectamine system. The data have indicated that chitosan nanoparticles could be an appropriate vehicle for gene delivery into MSCs. Corsi et al. have attempted to transfect MSCs by chitosan–pDNA complexes using 150, 400 and 600 KD chitosan at a concentration of 0.02%. These authors reported no significant difference between the applied molecular weights of chitosan and naked DNA in terms of their transfection efficiency. This contrasted our findings which showed a comparatively increased transfection rate with nanoparticles composed of 18 KD chitosan at the 0.1% concentration. This discrepancy could possibly be attributed to the different molecular weights used in each study. We examined some smaller molecular weights in comparison with corsi et al.'s study. Additionally, a key regulator of transfection rate is the pH of the transfection medium which should be determined for each studied cell line (Sato et al. Citation2000). In contrast to Corsi et al., in the present study, we examined the effect of several pH values on MSCs transfection and found that a pH of 6.8 was associated with better results. Dependence of transfection efficiency on the pH of culture medium has been reported to be the result of the protonation of amines in chitosan. Since DNA/chitosan complexes are positively charged they can bind with negatively charged cells through electrostatic interaction at pH values below 7 (Sato et al. Citation2000).
In this study we tried to evaluate and optimize MSC transfection using chitosan nanoparticles. Finding an appropriate, optimized and safe gene delivery system would be of utmost importance for MSC application in gene therapy strategies (Kim et al. Citation2012). Furthermore such a system could be used to transfer certain genes into MSCs in order to enhance the cell proliferation (Lou et al. Citation1999) and differentiation capacity (Jeon et al. Citation2012) which are necessary for MSC-based tissue engineering and regeneration. Finally, gene transfer strategy would be used to increase MSC resistance to apoptosis, or manipulate the cell directional migration (Chen et al. Citation2011) which is desired in MSC-based treatment of tissue defects.
In the current investigation, we examined two low (18 and 50 KD) and one high (136 KD) molecular weight chitosan. We chose these molecular weights because of the question regarding the best molecular weight of these cationic polymers with regard to their efficacy in gene delivery. Low-molecular weight chitosan can easily be endocytosed by cells, therefore, it yields better transfection whereas high-molecular weight chitosans could more efficiently compact plasmid DNA. Based on former investigations, some authors have achieved a better in vitro transfection using the high-molecular weight of chitosan (MacLaughlin et al.1998, Kiang et al. Citation2004), however others had better success with low-molecular weight chitosan (Sato et al. Citation2000, Lee et al. Citation2001, Ishii et al. Citation2001, Hoggard et al. Citation2004). For this reason, we examined both low- and high-molecular weight chitosans, each in six concentrations. Based on our findings, a number of the six concentrations from each molecular weight failed to form complete complexes with pDNA because they were unable to electrostatically neutralize the plasmid DNA's negative charge. Thus, only the 0.05–1% concentrations successfully formed complexes. We determined that the low-molecular weight chitosan (18 KD) was more effective in transferring plasmid DNA into human marrow MSCs.
According to our findings, the chitosan–pDNA complexes composed from 136 KD chitosan tended to be larger in size compared with those derived from 18 KD chitosan. In general there has not been an agreement regarding the relationship between chitosan–pDNA complex size and the molecular weight of the applied polymer. Some groups have reported that high molecular weight polymers yield large complexes (MacLaughlin et al. Citation1998), while other groups obtained smaller complexes using high molecular weight polymers (Morimoto et al. Citation2003). Size distribution also seems to be dependent on the ratio of moles of the amine groups of chitosan to those of the phosphate groups of DNA (N/P ratio) of complexes. Mao et al. have reported that complexes with a N/P ratio of 2 or less do not lead to nanosize structure, in the N/P ratio above 2, submicron particles will be formed and increases in the N/P ratio to 4 or more yields nanosize particles (Mao et al. Citation2001). Few studies have researched the effect of nanoparticle size on transfection efficiency. Some have reported that complexes smaller than 100 nm could be enclosed in endocytic vesicles which lead to target cell entrance (Vinogradov et al. Citation2002). For chitosan complexes this range was reported to be 80–500 nm (MacLaughlin et al. Citation1998). Nikanishi et al. have indicated complexes of moderate size (0.4–1.4 μm) were the best transfectants.
In the current study, the best transfection rate was with the 18 KD chitosan nanoparticle which possessed a zeta potential of 12.3 Mv. The zeta potential of nanoparticles has a direct relation to the polymer molecular weight as well as the concentration of complexes. In this study, nanoparticles of 136 KD chitosan at the 1% concentration had the highest zeta potential, whereas the 18 KD chitosan at a concentration of 0.05% possessed the lowest zeta potential. These findings agreed with those of Huang et al. (Citation2004). Low zeta potential indicates the formation of more neutral particles that lead to aggregation of complexes which in turn result in a low transfection rate (Duceppe and Tabrizian Citation2009). High zeta potential increases the stability of the complexes (ASTM Standard Citation1985) which negatively affects release of plasmid DNA from the complexes. Our findings were consistent with these results. It seems that moderate zeta potential is more appropriate for achieving optimal transfection.
According to the results of this study, nanoparticles produced with 18 KD chitosan at the 0.1% concentration transfected MSCs at an efficacy rate of 18.43%. Although this rate could be promising, however it is insufficient. Possible ways to modify the nanoparticles and increase transfection rate include the following: (1) Hydrophobic modification could mediate a favorable gene transfer by modulating the interactions of nanoparticles with cells and facilitate intracellular pDNA association (Liu et al. Citation2010, Kurisawa et al. Citation2000). Reported modifications include: PEI (Wong et al. Citation2006), alkyl group (Liu et al. Citation2001), thiol group (Lee et al. Citation2007), deoxycholic acid (Chae et al. Citation2005) and stearic acid (Hu et al. Citation2006). (2) Hydrophilic modifications such as quaternization (Germershaus et al. Citation2008), trimethylation (Kean et al. Citation2005) and polyethylene glycol (PEGylation) (Park et al. Citation2001) would be an alternative way to enhance transfection efficiency due to improvements in pDNA release and increasing water solubility at physiological pH (Mao et al. Citation2010). (3) Specific ligand modification could be the third method to enhance the efficiency by improving both cell specificity and endocytosis efficiency via receptor-mediated endocytosis (Kim et al. Citation2007). Galactose (Cook et al. Citation2005), mannose (Hashimoto et al. Citation2006), folate (Dongwon et al. Citation2006) and transferrin (Zhang et al. Citation2006) are some ligands that have thus far been investigated. Each method can be used to further enhance chitosan nanoparticles for increased efficiency of MSC transfection.
Conclusion
Chitosan–pDNA nanoparticles of 18KD chitosan at 0.1% concentration would be a promising vector for gene transfer into MSC.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by a grant from Royan Institute. The authors wish to express their appreciation for this support.
References
- ASTM Standard. 1985. Zeta potential of colloids in water and waste water. Am Soc Testing Mater. 4187–4182.
- Chae SY, Son S, Lee M, Jang MK, Nah JW. 2005. Deoxycholic acid- conjugated chitosan oligosaccharide nanoparticles for efficient gene carrier. J Control Release. 109:330–344.
- Cook SE, Park IK, Kim EM, Jeong HJ, Park TG, Choi YJ, et al. 2005. Galactosylated polyethylenimine-graft-poly(vinyl pyrorolidone) as a hepatocyte-targeting gene carrier. J Control Release. 105: 151–163.
- Corsi K, Chellat F, Yahia LH, Fernandes JC. 2003. Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles. Biomaterials. 24:1255–1264.
- Chen XA., Zhang LJ., He ZJ., Wang WW., Xu B, Zhong Q, et al. 2011. Plasmid-encapsulated polyethylene glycol-grafted polyethylenimine nanoparticles for gene delivery into ratmesenchymal stem cells. Int J Nanomedicine. 6:843–853.
- Deshpande D, Blezinger P, Pillai R, Duguid J, Freimark B, Rolland A. 1998. Target specific optimization of cationic lipid-based systems for pulmonary gene therapy. Pharm Res. 15:1340–1347.
- Dongwon L, Richard L, Shyam M. 2006. Folate receptor-mediated cancer cell specific gene delivery using folic acid-conjugated oligochitosan. J Nanosci Nanotechnol. 6:2860–2866.
- Duceppe N, Tabrizian M. 2009. Factors influencing the transfection efficiency of ultra low molecular weight chitosan/hyaluronic acid nanoparticles. Biomaterials. 13:2625–2631.
- Duceppe N, Tabrizian M. 2010. Advances in using chitosan based nanoparticles for in vitro and in vivo drug and gene delivery. Expert Opin Drug Deliv. 7:1191–1207.
- Gao Y, Zhang Z, Chen L, Gu W, Li Y. 2009. Chitosan N-betainates/ DNA self-assembly nanoparticles for gene delivery: in vitro uptake and transfection efficiency. Int J Pharm. 371:156–162.
- Germershaus O, Mao S, Sitterberg J, Bakowsky U, Kissel T. 2008. Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: establishment of structureeactivity relationships in vitro. J Control Release. 125: 145–154.
- Hashimoto M, Morimoto M, Saimoto H, Shigemasa Y, Yanagie H, Eriguchi M, Sato T. 2006. Gene transfer by DNA/mannosylated chitosan complexes into mouse peritoneal macrophages. Biotechnol Lett. 28:815–821.
- Hejazi R, Amiji M. 2003. Chitosan-based gastrointestinal delivery systems. J Control Release. 89:151–165.
- Heller J, Liu LS, Duncan R, Richardson S. 1996. Alginate:chitosan microporous microspheres for the controlled release of proteins and antigens. Proc Int Symp Control Release Bioact Mater. 23:269–270.
- Hoggard MK, Varum KM, Issa M, Danielsen S, Christensen BE, Stokke BT, Artursson P. 2004. Improved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomers. Gene Ther. 11:1441–1145.
- Hu FQ, Zhao MD, Yuan H, You J, Du YZ, Zeng S. 2006. A novel chitosan oligosaccharide-stearic acid micelles for gene delivery: properties and in vitro transfection studies. Int J Pharm. 315:158–166.
- Huang M, Khor E, Lim LY. 2004. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm Res. 21:344–353.
- Ishii T, Okahata Y, Sato T 2001Mechanism of cell transfection with plasmid/chitosan complexes. Biochim Biophys Acta. 1514:51–64.
- Jeon SY, Park JS, Yang HN, Woo DG, Park KH. 2012. Co-delivery of SOX9 genes and anti-Cbfa-1 siRNA coated onto PLGA nanoparticles for chondrogenesis of human MSCs. Biomaterials. 33:4413–4423.
- Kean T, Roth S, Thanou M. 2005. Trimethylated chitosans as non- viral gene delivery vectors: cytotoxicity and transfection efficiency. J Control Release. 103:643–653.
- Kiang T, Wen J, Lim HW, Leong KW. 2004. The effect of the degree of chitosan deacetylation on the efficiency of gene transfection. Biomaterials. 25:5293–5301.
- Kim JH, Park JS, Yang HN, Woo DG, Jeon SY, Do HJ, et al. 2011. The use of biodegradable PLGA nanoparticles to mediate SOX9 gene delivery in human mesenchymal stem cells (hMSCs) and induce chondrogenesis. Biomaterials. 32:268–78.
- Kim TH, Jiang HL, Nah JW, Cho MH, Akaike T, Cho CS. 2007. Receptor-mediated gene delivery using chemically modified chitosan. Biomed Mater. 2:S95–S100.
- Kim SW, Lee DW, Yu LH, Zhang HZ, Kim CE, Kim JM, et al. 2012. Mesenchymal stem cells overexpressing GCP-2 improve heart function through enhanced angiogenic properties in a myocardial infarction model. Cardiovasc Res. 95:495–506.
- Kurisawa M, Yokoyama M, Okano T. 2000. Transfection efficiency increases by incorporating hydrophobic monomer units into polymeric gene carriers. J Control Release. 68:1–8.
- Lee M, Nah JW, Kwon Y, Koh JJ, Ko KS, Kim SW. 2001. Water-soluble and low molecular weight chitosan-based plasmid DNA delivery. Pharm Res. 18:427–431.
- Lee D, Zhang W, Shirley SA, Kong X, Hellermann GR, Lockey RF, Mohapatra SS. 2007. Thiolated chitosan/DNA nanocomplexes exhibit enhanced and sustained gene delivery. Pharm Res. 24: 157–167.
- Leong KW, Mao HQ, Truong-Le VL, Roy K, Walsh SM, August JT. 1998. DNA-polycation nanospheres as non-viral gene delivery vehicles. J Control Release. 53:183–193.
- Liu WG, Yao KD, Liu QG. 2001. Formation of a DNA/N-dodecylated chitosan complex and salt-induced gene delivery. J Appl Polym Sci. 82:3391–3395.
- Liu Z, Zhang Z, Zhou C, Jiao Y. 2010. Hydrophobic modifications of cationic polymers for gene delivery. Prog Polym Sci. 35:1144–1162.
- Lou J, Xu F, Merkel K, Manske P. 1999. Gene therapy: adenovirus- mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 17:43–50.
- MacLaughlin F, Mumper R, Wang J, Tagliaferri J, Gill I, Hinchcliffe M, Rolland A. 1998. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J Control Release. 56:259–272.
- Mansouri S, Lavigne P, Corsi K, Benderdour M, Beaumont E, Fernandes JC. 2004. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur J Pharm Biopharm. 57:1–8.
- Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, et al. 2001.Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 70:399–421.
- Mao S, Sun W, Kissel T. 2010. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev62:12–27.
- McMahon JM, Conroy S, Lyons M, Greiser U, O'shea C, Strappe P, et al. 2006. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Dev. 15:87–96.
- Morimoto K, Nishikawa M, Kawakami S, Nakano T, Hattori Y, Fumoto S, et al. 2003Molecular weight-dependent gene transfection activity of unmodified and galactosylated polyethyleneimine on hepatoma cells and mouse liver. Mol Ther. 7:254–261.
- Oligino TJ, Yao Q, Ghivizzani SC, Robbins P. 2000. Vector systems for gene transfer to joints. Clin Orthop Relat Res. 379: S17–S30.
- Park IK, Kim TH, Park YH, Shin BA, Choi ES, Chowdhury EH, et al. 2001. Galactosylated chitosan-graft poly(ethylene glycol) as hepatocyte-targeting DNA carrier. J Control Release. 76: 349–362.
- Park JS, Na K, Woo DG, Yang HN, Kim JM, Kim JH, et al. 2010. Non-viral gene delivery of DNA polyplexed with nanoparticles transfected into human mesenchymal stem cells. Biomaterials. 31: 124–132.
- Pimpha N, Sunintaboon P, Inphonlek S, Tabata Y. 2010. Gene delivery efficacy of polyethyleneimine-introduced chitosan shell/poly(methyl Methacrylate) core nanoparticles for rat mesenchymal stem cells. J Biomaterials Sci. 21:205–223.
- Quong D, Neufeld RJ. 1998. DNA protection from extracapsular nucleases, within chitosan- or poly-L-lysine-coated alginate beads. Biotechnol Bioeng. 60:124–134.
- Sato T, Ishii T, Okahata Y 2000. In vitro gene delivery mediated by chitosan. Effect of pH, serum,and molecular mass of chitosan on the transfection efficiency. Biomaterials. 22:2075–2080.
- Smith AE. 1995. Viral vectors in gene therapy. Annu Rev Microbiol49:807–838.
- Vinogradov SV, Bronich TK, Kabanov AV. 2002.Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 54:135–147.
- Wong K, Sun G, Zhang X, Dai H, Liu Y, He C, Leong KW. 2006. PEIg-chitosan, a novel gene delivery system with transfection efficiency comparable to polyethylenimine in vitro and after liver administration in vivo. Bioconjugate Chem. 17:152–158.
- Yomota C, Miyazaki T, Okada S. 1993. Determination of the viscometric constants for chitosan and the application of universal calibration procedure in its gel permeation chromatography. Colloid Polym Sci. 271:76–82.
- Zhang H, Mardyani S, Chan WCW, Kumacheva E. 2006. Design of biocompatible chitosan microgels for targeted pH-mediated intracellular release of cancer therapeutics. Biomacromolecules. 7:1568–1572.