Abstract
Pancreatic trypsin was immobilized by cross-linked enzyme aggregates (CLEA) which is a carrier free immobilization method. Ammonium sulfate was chosen for enzyme precipitation which was followed by cross linking of formed aggregates via glutaraldehyde. Concentrations of precipitant and cross linker were respectively optimized as 60% ammonium sulfate and 1% glutaraldehyde. Optimum pH and temperature for CLEA was increased compared to free enzyme. Furthermore, pH, thermal and storage stability were improved. Presence of additives had no effects on enzyme activity. Prepared cross-linked trypsin aggregates are convenient for in situ protein fragmentation and can be used for protein identification.
Introduction
Trypsin (E.C. 3.4.21.4) is an important proteolytic enzyme which cleaves selectively at the lysine and arginine residues. Production of peptides in a range ideal for mass mapping through mass spectroscopy makes the enzyme suitable for protein fragmentation in order for identification and characterization of proteins (CitationMassolini and Calleri 2005). Furthermore trypsin is used in many processes such as preparation of bioactive peptides, treatment of milk (CitationBryjak and Kolarz 1998), cutaneous dressing (CitationMonteiro et al. 2007), and as an additive in detergent industry (CitationKtari et al. 2012).
Immobilization of enzymes facilitates their recovery and provides ease of reuse, increases operational and thermal stability (CitationRoessl et al. 2010), and improves enzyme performance under optimum reaction conditions (CitationBrady and Jordaan 2009). Trypsin has been immobilized by many different methods so far: covalent binding of enzymes to water-insoluble carrier (CitationBryjak and Kolarz 1998, CitationAbbas et al. 2009), adsorption (CitationWu et al. 2006), and entrapment (CitationMonteiro et al. 2007). Moreover, enzymes can be immobilized carrier free. Cross-linked enzyme aggregates (CLEA) method consists of precipitation of enzyme molecules and following cross linking of formed aggregates. Cross linking of these aggregates via free amino groups on the protein surface with a bifunctional reagent renders the aggregates permanently insoluble while maintains its catalytic activity (CitationSheldon 2011). Lack of a carrier eliminates the dilution of enzyme activity which is encountered frequently in carrier bound enzyme immobilization (CitationSchoevaart et al. 2004).
In this study, commercial pancreatic trypsin was used to prepare trypsin CLEA. Within this scope, conditions of trypsin immobilization were optimized and formed trypsin CLEA was characterized from the point of optimum temperature and pH, enzyme stability, and morphology.
Methods
Chemicals
Ammonium sulfate, glutaraldehyde (25%), N-α- benzoyl-DL-arginine-p-nitroanilide (BAPNA) were obtained from Sigma-Aldrich, Trypsin (3.4.21.4) was purchased from Novo Nordisk. All other reagents were of analytical grade. New Brunswick Innova® 40 shaker was applied for cross-linking procedure. Thermo Multiscan FC was used for optical absorbance measurements. All experiments were performed in triplicate.
Enzyme activity and protein concentration assays
Trypsin activity was measured using a hydrolytic assay with the chromogenic substrate BAPNA prepared in dimethylsulfoxide (CitationDaglioglu and Zihnioglu 2012). The reaction mixture was incubated at room temperature for 10 min. The release of yellow p-nitroaniline (p-NA) was monitored at 405 nm in a microplate reader. One unit (U) is the amount of enzyme hydrolyzing 1 μmol of N-α-benzoyl-DL-arginine-p-nitroanilide per minute. Bradford method was used to estimate the amount of trypsin (CitationBradford 1976).
Preparation of trypsin CLEA
CLEA preparation was carried out in two steps: Enzyme precipitation and cross linking (CitationSchoevaart et al. 2004). Trypsin solution (10 mg/mL) was prepared in 0.1 M sodium phosphate buffer, pH 7.5 and centrifugated for 10 min at 10,000 rpm. The supernatant was collected and used for enzyme precipitation. Trypsin aggregates were obtained by slowly adding ammonium sulfate under stirring condition. Different ammonium sulfate concentrations (50%, 60%, 70% and 80%) were tested. The saturated solution was kept for 24 h. The aggregates separated by centrifugation (10,000 rpm, 10 min) and dissolved in the same buffer. Cross linking performed with 1 mL of trypsin aggregate and glutaraldehyde ranging in 1–3% concentrations at various cross linking time (15, 30, 45 and 60 min). Effects of additives on CLEA activity was studied by addition of sodium dodecyl sulfate (SDS), Triton X-100, CHAPS (0.1% and 0.5% w/v), and Bovine serum albumin (BSA) (10–100 mg/mL) to enzyme solution. CLEAs were prepared as described above and activities measured. All steps were carried out at 4°C.
Effect of temperature and pH on enzyme activity
Optimum temperature of the free enzyme and CLEAs were estimated by measuring enzyme activities in a temperature range of 20°C–70°C. The effect of pH on enzyme activity was studied in a pH range of 4.0–11.0 (sodium acetate–acetic acid: 4.0–6.0; sodium phosphate: 6.0–8.0; tris/HCl: 8.0–9.0 and sodium bicarbonate–sodium hydroxide: 9.0–11.0).
Stability tests
For all stability tests, activities were measured as described above. Initial activity was taken to be 100% and the relative activity (%) was calculated by the ratio of the residual activity to the initial activity of each sample.
Thermal stability
Thermal stability was monitored by incubating the CLEAs and free trypsin in the absence of substrate in aqueous buffer (0.1 M sodium phosphate, pH 7.5) at different temperatures (40°C, 50°C and 60°C) and time dependently (60, 90 and 120 min) in a shaking condition (175 rpm). After each time point, samples were taken and enzyme activities measured.
pH stability
For monitoring stability at different pH points, CLEAs and free enzymes were incubated for 30 min in 0.1 M buffer (sodium acetate–acetic acid: 6.0; sodium phosphate: 7.0–8.0; sodium bicarbonate–sodium hydroxide: 9.0–11.0) After incubation, activities were measured under standard assay conditions.
Storage stability
Both free and immobilized trypsin were kept in sodium phosphate buffer (100 mM, pH 7.5) at 4°C for 4 weeks. Storage stabilities were determined by measuring the residual activity of both enzymes after each week.
Operational stability
Operational stability of the trypsin CLEA was examined in a reaction medium containing 4 mL 0.1 M sodium phosphate buffer (pH 7.5), 0.5 mL 0.9% NaCl, 2.5 mL 0.1% BAPNA, and 0.5 mL trypsin CLEA. The reaction was maintained at 37°C and shaken continuously for 6 h. The activities of samples taken at regular time intervals were measured. The relationship between operation time and conversion was determined and also the half-life of the biocatalyst was calculated.
Reusability
To evaluate the reusability of immobilized trypsin, CLEAs were washed with buffer after each use and resuspended in a fresh reaction mixture in order to measure enzyme activity.
Scanning electron microscopy images
Trypsin CLEA suspension was lyophilized and forming CLEA powder was mounted on the metal stub and sputtered with gold. Scanning electron microscopy (SEM) images were recorded on JEOL JSM 6060.
Results and discussion
Preparation of trypsin CLEA
Ammonium sulfate which has been used by other workers was chosen for precipitation of trypsin (CitationLópez-Serrano et al. 2002, CitationYu et al. 2006). After 24 h of precipitation, 66% of initial activity was obtained with 60% ammonium sulfate saturation. Precipitated protein molecules held together as physical aggregates by noncovalent bonds, and cross linking of these aggregates brings them into an insoluble state (CitationSheldon 2007). Glutaraldehyde is a commonly preferred cross-linker due to its low cost and availability. Moreover, use of glutaraldehyde to stabilize proteins was reported (CitationChang 1971). In order to obtain the highest activity recovery, 1 mL of trypsin aggregate was cross linked via different glutaraldehyde concentrations in 15 min cross linking time. At 1% (v/v) glutaraldehyde concentration the highest activity recovery was achieved. Above this concentration activity was decreased. The optimum time for cross linking was observed as 30 min. Higher cross linker ratio and cross linking time may have led to excessive cross linking which causes a complete loss of enzyme activity and restricts mass transfer due to enlarged particle size (CitationSheldon 2011). BSA was reported as a proteic feeder to increase free amino group concentration for optimal cross linking (CitationShah et al. 2006). CLEAs which were prepared containing 10–100 mg/mL of BSA showed a dramatic decrease in activity. It is possible that presence of BSA may disturb the internal structure of CLEAs and limit the mass transfer due to narrowed channels (CitationCabana et al. 2007). The activation of lipases by additives such as surfactants, crown ethers, or amines is well documented (CitationGupta et al. 2009, CitationLópez- Serrano et al. 2002) Effects of SDS, Triton X-100, and CHAPS on CLEA activity was investigated. In our case presence of additives led to lower CLEA activity (Data not shown).
Characterization of trypsin CLEA
Effect of temperature on trypsin activity
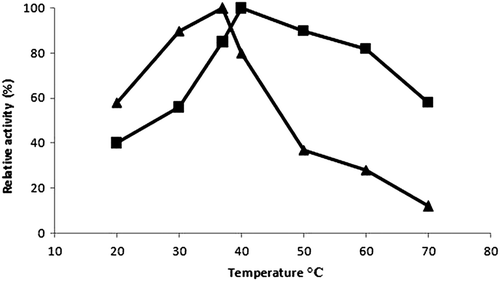
Temperature has an important effect on activity of soluble enzyme. The effect of temperature on the activity of free and immobilized enzyme was investigated in a temperature range of 20°C–70°C and the results presented in . The activity of free enzyme increased gradually with temperature and the maximum activity was obtained at 37°C. Above this temperature an expeditious decrease was observed due to denaturation. CLEAs demonstrated maximum activity in a range of 40°C–50°C and possessed a resistance to temperature more than free enzyme. Cross linking of amino groups via covalent bonds might reduce the conformational flexibility and results in higher temperature requirement for the molecule to reorganize the proper conformation for binding to the substrate and also rigid structure of CLEAs protects the molecule from denaturation (CitationPark et al. 2010).
Effect of pH on trypsin activity
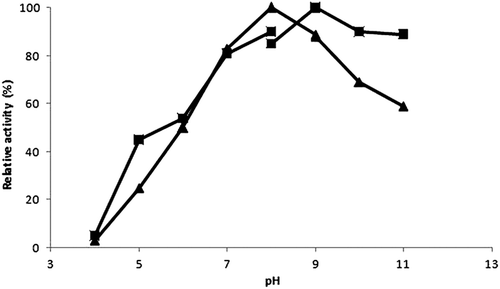
The effect of pH variations in a range of 4.0–11.0 on the free and immobilized trypsin activities was examined as shown in . Optimum pH values for free and immobilized enzyme were observed as 8.0 and 9.0, respectively. This shift could be as a result of the change of the total electrostatic charge of the enzyme after cross linking via free basic groups. This shift in optimum pH has also been described for other CLEAs, such as subtilisin (CitationSangeetha and Abraham 2008) and lipase (CitationKartal et al. 2011).
Thermal and pH stability
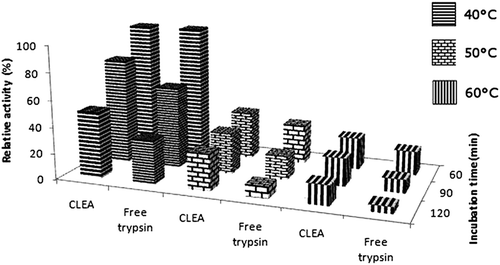
The activity profiles of free trypsin and CLEAs at different temperatures and time intervals are represented in . Increasing temperature resulted in gradual decreases of residual activities among free and immobilized enzymes, yet the levels of decreases were different. Results showed that thermal stability of CLEAs was higher than the free enzyme. After 2 h of incubation at 60°C, the free enzyme retained only 5% residual activity while CLEAs retained 15% of its initial activity. The increased thermal stability may be due to inter- and intramolecular covalent cross links which by increasing the rigidity of the structure prevents conformational changes (CitationMigneault et al. 2004).
On the other hand, from pH 6 to pH 9, CLEA and free trypsin showed similar stability to temperature. Between pH 9 and pH 11 CLEA exhibited better stability compared to free enzyme.
Operational stability
Enzymes are intrinsically labile therefore their operational stability has importance for any bioprocess. Operational stability corresponds to the change in the activity of the enzyme during the reaction period. The time during which the enzyme loses its 50% of its initial activity is termed as half-life. Half-life can be calculated by the following equations:
To analyse the operational stability of immobilized trypsin, a batch-stirred system was used. The operating temperature was set to 37°C and samples taken from the reactor at different time intervals were measured for enzyme activity. The half-life (t1/2) of trypsin CLEA was estimated as 44 min.
Reusability and storage stability
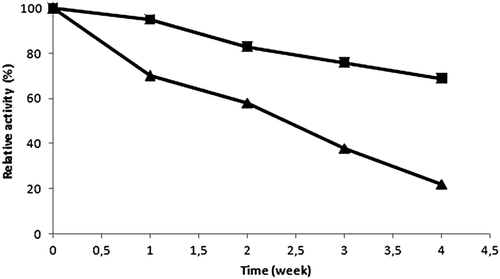
Enzymes have many benefits for chemical industries including high reaction rates and chemo-, regio-, and enantioselectivities. Nevertheless commercial use of enzymes can be limited due to their low storage stability and lack of reusability. Repeated enzyme use on the activity of the trypsin CLEA was examined, and immobilized enzyme maintained its activity up to 65%. Activity lost can result from conformational and morphological changes due to repeating centrifugations (CitationDalal et al. 2007). shows storage stability of free and immobilized trypsin. After 4 weeks of storage, immobilized trypsin and free trypsin maintained 69% and 22% of their initial activity, respectively. This indicates that the stability of trypsin was improved noticeably after the immobilization.
SEM analysis
The ratio of cross linker also plays a role in determining the particle size of CLEAs. Particle size affects mass transfer of substrates to contact with the inner enzymes of CLEA (CitationYu et al. 2006). shows the SEM micrographs of trypsin CLEA with a magnification of 7500x. The surface morphology showed a uniform structure of the CLEA particles of 0.2–0.7 microns in size. SEM of trypsin CLEAs also showed Type 1 structure of aggregates similar to the reports of CitationSchoevaart et al. (2004). This typical surface morphology of CLEAs offers higher surface area which represents more catalytic sites and reduces diffusion limitations.
Conclusions
CLEAs were prepared from commercial pancreatic trypsin and characterized through a series of stability tests. Overall results of immobilization of trypsin caused a decrease in the activity.
Although CLEA was reported with higher activity recoveries compared to other methods, immobilization can influence the enzyme negatively. In this case, cross linking of enzyme aggregates can cause a restricted conformational freedom for binding substrate or cross linking of residues close by the active site which results in activity loss. Nevertheless, immobilization of trypsin led to an obvious increase in stability in every respect which is an important factor for industrial use of enzymes.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abbas A, Vercaigne-Marko D, Supiot P, Bocquet B, Vivien C, Guillochon D. 2009. Covalent attachment of trypsin on plasma polymerized allylamine. Colloid Surface B. 73:315–324.
- Bradford M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254.
- Brady D, Jordaan J. 2009. Advances in enzyme immobilisation. Biotechnol Lett. 31:1639–1650.
- Bryjak J, Kolarz BN. 1998. Immobilisation of trypsin on acrylic copolymers. Process Biochem. 33:409–417.
- Cabana H, Jones JP, Agathos SN. 2007. Preparation and characterization of cross-linked laccase aggregates and their application to the elimination of endocrine disrupting chemicals. J Biotechnol. 132:23–31.
- Chang TMS. 1971. Stabilization of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross linking with glutaraldehyde. Biochem Biophys Res Commun. 44:1531–1536.
- Daglioglu C, Zihnioglu F. 2012. Covalent immobilization of trypsin on glutaraldehyde-activated silica for protein fragmentation. Artif Cell Blood Sub. 40:378–384.
- Dalal S, Kapoor M, Gupta MN. 2007. Preparation and characterization of combi-CLEAs catalyzing multiple non-cascade reactions. J Mol Catal B Enzym. 44:128–132.
- Gupta P, Dutt K, Misra S, Raghuwanshi S, Saxena RK. 2009. Characterization of cross-linked immobilized lipase from thermophilic mould Thermomyces lanuginosa using glutaraldehyde. Bioresour Technol. 100:4074–4076.
- Kartal F, Janssen MHA, Hollmann F, Sheldon RA, Kılınc A. 2011. Improved esterification activity of Candida rugosa lipase in organic solvent by immobilization as Cross-linked enzyme aggregates (CLEAs). J Mol Catal B Enzym. 71:85–89.
- Ktari N, Ben Khaled H, Nasri R, Jellouli K, Ghorbel S, Nasri M. 2012. Trypsin from zebra blenny (Salaria basilisca) viscera: purification, characterisation and potential application as a detergent additive. Food Chem. 130:467–474.
- López-Serrano P, Cao L, van Rantwijk F, Sheldon RA. 2002. Cross-linked enzyme aggregates with enhanced activity: application to lipases. Biotechnol Lett. 24:1379–1383.
- Massolini G, Calleri E. 2005. Immobilized trypsin systems coupled on-line to separation methods: recent developments and analytical applications. J Sep Sci. 28:7–21.
- Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC. 2004. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 37:790–802.
- Monteiro FMF, Silva GMM, Silva JBR, Porto CS, Carvalho LB Jr , Lima Filho JL, et al. 2007. Immobilization of trypsin on polysaccharide film from Anacardium occidentale L. and its application as cutaneous dressing. Process Biochem. 42: 884–888.
- Park J, Kim M, Lee D, Lee K, Min J, Kim Y. 2010. Immobilization of the cross-linked para-nitrobenzyl esterase of Bacillus subtilis aggregates onto magnetic beads. Process Biochem. 45:259–263.
- Roessl U, Nahalka J, Nidetzky B. 2010. Carrier-free immobilized enzymes for biocatalysis. Biotechnol Lett. 32:341–350.
- Sangeetha K, Abraham TE. 2008. Preparation and characterization of cross-linked enzyme aggregates (CLEA) of subtilisin for controlled release applications. Int J Biol Macromol. 43:314–319.
- Schoevaart R, Wolbers MW, Golubovic M, Ottens M, Kieboom APG, Van Rantwijk F, et al. 2004. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol Bioeng. 87:754–762.
- Shah S, Sharma A, Gupta MN. 2006. Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal Biochem. 351:207–213.
- Sheldon RA. 2007. Cross-linked enzyme aggregates (CLEAs): stable and recyclable biocatalysts. Biochem Soc Trans. 35:1583–1587.
- Sheldon RA. 2011. Cross-linked enzyme aggregates as industrial biocatalysts. Org Process Res Dev. 15:213–223.
- Wu J, Luan M, Zhao J. 2006. Trypsin immobilization by direct adsorption on metal ion chelated macroporous chitosan-silica gel beads. Int J Biol Macromol. 39:185–191.
- Yu HW, Chen H, Wang X, Yang YY, Ching CB. 2006. Cross- linked enzyme aggregates (CLEAs) with controlled particles: Application to Candida rugosa lipase. J Mol Catal B Enzym. 43: 124–127.