Abstract
This study evaluates the suitability of a macroporous three-dimensional chitosan/hydroxyapatite (CS/HA) composite as a bone tissue engineering scaffold using MC3T3-E1 cells. The CS/HA scaffold was produced by freeze-drying, and characterized by means of SEM and FTIR. In vitro findings demonstrated that CS/HA supported attachment and proliferation of cells, and stimulated extracellular matrix (ECM) production. Tissue biocompatibility and osteogenic capacity of the cell-laden constructs were evaluated in an ectopic Wistar rat model. In vivo results showed that the MC3T3-E1 cell-laden CS/HA was essentially histocompatible, promoted neovascularization and calcified matrix formation, and secreted osteoblast-specific protein. We conclude that the composite scaffold evaluated has potential for applications in bone regeneration.
Introduction
The treatment of bone damage caused by aging, demineralization, trauma, biochemical disorders, and developmental deformities represents a common clinical challenge, since current orthopedic surgical techniques still have inherent limitations such as auto-graft insufficiency, donor site morbidity, risk of disease transmission, adverse host immune reactions in allografts/xenografts, cost, and so on (CitationMiclau et al. 2007). Scientists have been focusing on alternative strategies to develop bone graft substitutes capable of repairing bone tissue with specific cells, to meet the challenges and ensure adequate and successful treatment (CitationSalgado et al. 2004, MallicCitationk 2014). The development of bone graft with the desired surface chemistry, topography, and porosity to allow cell adhesion, migration, and proliferation is a critical parameter for bone tissue engineering (BTE). Several natural and synthetic biopolymers and certain inorganic compounds have been explored as scaffolding materials for BTE applications (CitationZhou and Xu 2011, CitationZhang et al. 2012, CitationBonilla et al. 2014, CitationPark et al. 2014). The ideal bone scaffolding materials are described by their biocompatibility, osteoconductivity, bioactivity, biodegradability, and mechanical properties. A three-dimensional (3D) and highly interconnected porous structure to promote attachment and migration of cells, and flow of nutrients and metabolic wastes, must also be taken into consideration (CitationElcin 2004, CitationChung and King 2011, CitationAllori et al. 2013).
Due to their adequate biocompatibility and relatively non-toxic degradation products, natural biomaterials have the potential to create a suitable template for tissue engineering applications. Among several choices of natural polymers, chitosan (CS), the partially de-acetylated derivative of chitin, has been used as implantable material in a broad range of biomedical applications (CitationDurkut et al. 2006, CitationPillai et al. 2009, CitationYhee et al. 2014, CitationAubert-Viard et al. 2015). CS provides a hydrated microenvironment which may assist cell adhesion, proliferation, and metabolite transport. It also facilitates the reconstruction and vascularization of damaged tissue by interacting with negatively charged proteins and anionic polysaccharides, thanks to its cationic nature (CitationSeol et al. 2004, CitationKiroshka et al. 2014).
CS has been commonly combined with inorganic compounds in an attempt to fabricate a binary system, with some functional properties to stimulate the 3D osteogenic growth by improving its mechanical and biological properties (CitationZhang et al. 2007, CitationBudiraharjo et al. 2012). With its osteoinductive property, hydroxyapatite (HA) is considered as a convenient bioceramic for this purpose (CitationLee et al. 2008). When the advantages of CS and HA are combined, the result is a composite construct with improved mechanical properties, tissue integration, and biocompatibility (CitationZhang et al. 2007, CitationTeng et al. 2009).
The generation of 3D polymeric scaffolds with desired properties could be achieved by using various methods, such as phase separation, freeze-drying, particulate-leaching, gas foaming, or fiber bonding (CitationLiu and Ma 2004). A method combining phase separation and freeze-drying is commonly used to produce highly porous composite scaffolds, with pore sizes that can be controlled by changing the polymer concentration and the freezing speed (CitationKong et al. 2005).
In this study, the suitability of a macroporous 3D chitosan/hydroxyapatite (CS/HA) composite as a BTE scaffold using MC3T3-E1 cells was evaluated. The in vitro studies were performed to investigate cell attachment and proliferation, as well as ECM production. The in vivo evaluation of the capacity for ectopic bone formation was conducted, and the biocompatibility of the construct was assessed. Our findings demonstrated the potential of the composite scaffold for application in bone regeneration.
Materials and methods
Chemicals
CS of medium molecular weight (Mr ∼ 400,000; > 85% deacetylation) was purchased from Fluka Chemical Company (Milwaukee, WI, USA). Hydroxyapatite (Type I, in 0.001 M phosphate buffer; pH 6.8), α-modified Eagle's minimal essential medium (α-MEM), fetal bovine serum (FBS), ascorbic acid, ß-glycerophosphate, dexamethasone, and antibiotics were obtained from Sigma Chemical Company (St. Louis, MO, USA), except otherwise stated.
Fabrication of the CS/HA scaffold
The CS/HA scaffold was fabricated by using the freeze-drying method. Briefly, a 2.0% solution of CS in 1.0% acetic acid was freshly prepared. HA (ratio 1:9) was introduced under thorough mixing and overnight stirring at room temperature, to obtain a homogeneous suspension. Subsequently, the mixture was placed into 24-well cell culture plates and stored in a freezer at −20°C until frozen. The solidified samples were lyophilized in a freeze dryer (Alpha 1–4 LD, Christ, Germany) at −80°C for 24 h. Later, the scaffold was neutralized in 1.0 M NaOH solution and rinsed several times with double-distilled water, and then freeze-dried again to obtain the final porous composite construct. The constructs were sterilized in 90% ethanol (previously sterile-filtered, 0.2 μm) overnight and washed successively with sterile phosphate-buffered saline (PBS; pH 7.4).
Characterization of the CS/HA scaffold
The surface/cross-sectional pore morphology of the CS/HA scaffold was examined using scanning electron microscopy (SEM). The samples were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 h; then they were washed with phosphate buffer, placed through a series of graded ethanol dehydrations, and allowed to air-dry (CitationEmin et al. 2008). Finally, the samples were sputter-coated with gold and analyzed under a Jeol-JSM model scanning electron microscope (Tokyo, Japan).
The structural characterization of pure CS and HA, and the CS/HA scaffolds, was carried out using Fourier transform infrared spectroscopy (FTIR) (Hitachi; Tokyo, Japan). The samples were pressed into powder, mixed with KBr (1:10), and compressed into a pellet prior to examinations. The spectra were measured over the range of 4000–400 cm− 1.
In vitro studies
MC3T3-E1 cells were kindly provided by Dr. G. Finkenzeller (University of Albert-Ludwig, Freiburg, Germany). The cells were cultured utilizing the α-MEM supplemented with 10% FBS, and 1% penicillin streptomycin in a humidified 5% CO2 balanced-air incubator at 37°C. The 10th–12th passage cells were used in the experiments. After ∼80–90% confluence, the cells were digested by 0.05% trypsin/0.53 mM EDTA, and the cell density was adjusted to 1.0 × 107/mL. Fifty μL of cell suspension was incorporated dropwise onto each construct placed in the wells of a 24-well culture plate, and agitated at 37°C for 30 min using an orbital shaker to assure initial cell adhesion. Following the placement of cell-laden scaffolds in a new culture plate, wells were filled up with the osteogenic culture medium consisting of α-MEM supplemented with 10% FBS, 1% penicillin streptomycin, 10 nM dexamethasone, 50 mg/mL L-ascorbic acid, and 10 mM β-glycerophosphate (CitationInanc et al. 2007a). The culture was set at 37°C, in a humidified atmosphere with 5% CO2 for up to 21 days. The culture medium was refreshed every 3 days. Cell-laden scaffolds were collected at 7, 14, and 21 days. While some samples were evaluated by histology (hematoxylin & eosin, von Kossa), others were analyzed by immunohistochemistry (IHC; osteopontin) and by SEM.
In vivo studies
All animals were treated in accordance with the principles of the Guide for The Care and Use of Laboratory Animals. Adult Wistar rats (weighing 250–300 g) were used in the animal experiments (n = 16). All subjects were immunosuppressed by daily subcutaneous injections (15 mg/kg body wt) of cyclosporin A (Sandimmun, Novartis), starting 2 days prior to transplantation and during the study (CitationKoc et al. 2014).
The surgical procedures were performed in aseptic conditions under general anesthesia (300 mg/kg Avertin, 1.25%). The groin areas (both sides) of the rats were shaved and disinfected with iodine and isopropanol. Prior to implantation, cell-laden scaffolds were incubated overnight in a CO2 incubator under standard culture conditions. Then, they were implanted into the epigastric fasciovascular flaps of the rats (CitationElcin et al. 2003a). Each animal received two constructs, one with cells and the other without cells, serving as control. Wounds were closed with 4-0 silk sutures. All surgical incisions healed without evidence of infection or other complications. The rats were sacrificed and the implants were removed from the groins together with surrounding tissue at 7, 14, and 21 days after surgery.
Histology and immunohistochemistry
MC3T3-E1 cell-laden scaffolds retrieved from the in vitro and in vivo studies were prepared for histological and IHC analyses using standard methods. The samples were retrieved at predetermined time intervals and soaked in 0.1 M potassium phosphate buffer (pH 7.4) containing 15% sucrose and tissue-freezing medium (Tissue-Tek, Sakura Finetek, The Netherlands), respectively. The specimens were embedded in tissue-freezing medium for 30 min and frozen at − 80°C. The tissue blocks were sectioned at 5 μm using a cryostat (Leica CM 1900, Wetzlar, Germany) and stained with hematoxylin and eosin (H&E) to visualize the formation of the new tissue (CitationKoc et al. 2008). The matrix mineralization was demonstrated by von Kossa staining. IHC was performed to detect synthesis of osteopontin (OSP) (Santa Cruz Biotechnology) in the cell-laden scaffolds. Finally, specimens were visualized under a Leica DM 4000B model digital light microscope (Wetzlar, Germany).
Results
Material characterization
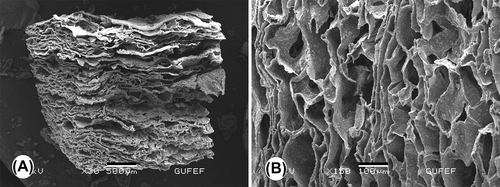
The surface morphology of the composite scaffold was examined using SEM. and show the surface and cross-sectional morphology of the CS/HA scaffold. SEM observations revealed that the construct had a highly macroporous structure. The pore walls had rough structures as a result of the distribution of HA microparticles inside the polymer matrix (). The surface roughness supports cell attachment, proliferation, and differentiation by increasing the scaffold's contact surface area, and shortens the time for bone tissue formation. The pore size of the constructs were approximately 100–300 μm, which is known to be sufficient for good interconnection and exchange of nutrients and to allow the penetration of cells.
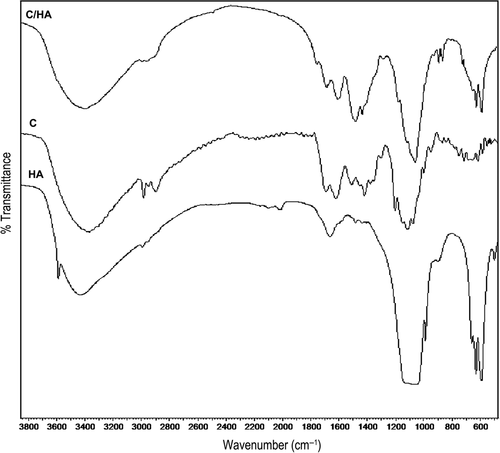
The FTIR spectra of CS, HA, and the CS/HA composite scaffold are shown in . The spectra of pure CS exhibit characteristic peaks at 2962 cm− 1 and 1380 cm− 1 due to the protonated amide stretch (). The C–O stretching vibration bands are exhibited at 1160 cm− 1, 1067 cm− 1, and 1031 cm− 1. Characteristic bands derived from N–H stretching at 3400 cm− 1 are evident in the spectrum (CitationSudo et al. 1983). The FTIR spectrum of CS/HA gathers all the characteristic absorption peaks of HA and CS. The sharp peaks at around 1660, 1583, and 1456 cm− 1 are attributed to amide groups present in the composite scaffold. The absorption bands in the composite at 963 cm− 1 and 602 cm− 1 are attributed to a phosphate group from HA. The FTIR observations indicate the presence of HA particles in the CS matrix.
In vitro findings
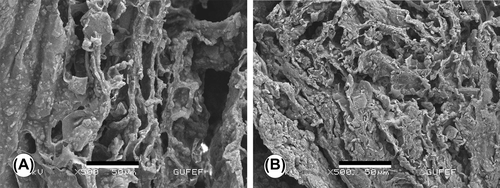
SEM was employed to assess the distribution and morphology of cells on the macroporous scaffold. The adhesion of the cultured cells within the pores of the sponges at days 7 and 21 is depicted in and . These SEM micrographs demonstrate that the MC3T3-E1 cells were attached tightly to the surface and walls of the construct. As shown in , the cells proliferated well and almost covered the entire surface of the scaffold, and filled the voids over time. This indicates that the composite scaffold is essentially non-toxic and suitable for the attachment and growth of pre-osteoblasts.
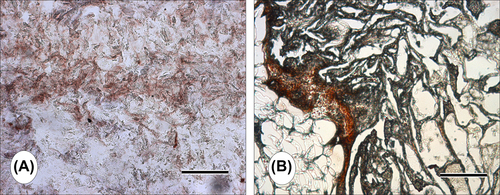
Histochemical analysis provided information about the attachment and proliferation of the cells on the construct (). Consistent with SEM analysis, H&E histochemistry revealed that MC3T3-E1 cells were widespread and had continued to proliferate extensively within the pores of the CS/HA composite scaffold over a short period of time (). The distribution of cells inside the pores was uniform, which is an important parameter in scaffold-based tissue engineering. The cells proliferated prevalently and covered the entire surface of the materials after 21 days of culture (). The cellularization and extracellular matrix deposition were increased as the culture proceeded from 7 days to 21 days. Von Kossa-stained histochemical sections demonstrated that the pores of the CS/HA construct were predominantly covered with tissue containing mineralized matrix after 21 days of culture ( and ). Cell-laden constructs demonstrated distinctively higher level of staining than the empty ones (not shown). These findings indicated that the CS/HA scaffold provided a suitable template for the proliferation and mineralization of MC3T3-E1 cells.
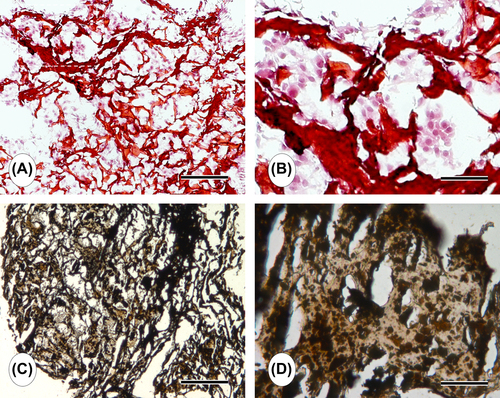
The in vitro differentiation of MC3T3-E1 cells into the osteoblastic phenotype was qualitatively evaluated using anti-OSP staining at the 21 st day of the culture. Results demonstrated that MC3T3-E1 cells seeded on the macroporous CS/HA scaffold under osteogenic culture conditions differentiated into the osteoblastic cells and produced OSP ( and ).
In vivo findings
Tissue biocompatibility and the capacity to form ectopic bone-like tissue, seen in the composite CS/HA scaffold, were investigated by a short-term implantation into the epigastric fasciovascular flap of Wistar rats. The duration of the experiment was planned to be short, in order to avoid the risk of animal resistance against immunosuppressive treatment. All animals survived during the experimental period. There was no infection, extrusion, or significant inflammatory reaction, indicating that the scaffold was essentially non-toxic, at least for the duration of the experiments. Both empty and cell-laden scaffolds maintained their characteristic morphology and preserved initial shape for the course of the experiment.
Histological observations confirmed that the scaffold allowed cell growth, while exhibiting a typical acute inflammatory response in the early stages post-implantation. The natural antibacterial property of CS may be responsible from this mild inflammatory response. The cell-laden CS/HA scaffold was infiltrated with fibrous connective tissue (). We observed the formation of small capillaries (∼10–20 μm) in the newly forming tissue, developing inside the construct pores (). Von Kossa staining demonstrated the presence of calcium released; thus bone-like tissue formation could be observed within the pores of the scaffold ( and ). This indicates that the scaffold induced an osteogenic environment and provided a suitable template for ectopic (subcutaneous) bone-like tissue formation in rats.
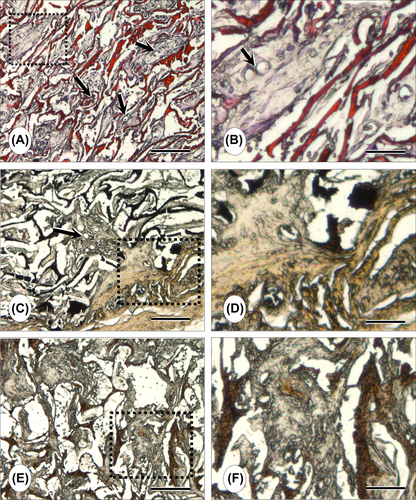
Immunohistochemical staining with anti-OPN was performed on the 21-day specimens. Strong positive staining to OSP was observed, indicating that the MC3T3-E1 cell-laden CS/HA scaffold secreted osteoblast-specific proteins ( and ).
Discussion
Restoration of damaged bone is one of the major subjects in the field of human healthcare. Despite early success with the use of autografts and allografts, development of alternative materials to replace defective bone is considered an important issue, and many studies have been proposed involving tissue engineering scaffolds based on composites of bioceramics and synthetic or natural polymers (CitationOliveira et al. 2009, CitationBaykan et al. 2014, CitationDemirdögen et al. 2014). From the clinical point of view, calcium phosphate ceramics have usually been preferred for BTE, thanks to their physiochemical and unique biological properties. HA is the most prevalent calcium phosphate and has been used extensively in clinical applications, as it is a natural bone mineral which supports osteoinduction (CitationKilpadi et al. 2001, CitationWahl and Czernuszka 2006). It has been used in different forms, such as porous granules, blocks, and as a coating material for metal implants. HA can be easily combined with polymeric matrices in order to obtain an operative tissue engineering biomaterial (CitationTeixeira et al. 2010). Owing to their properties of biocompatibility, biodegradability and hydrophilicity, CS, collagen, and gelatin have been frequently used as polymeric matrices in creating particle composite materials (CitationKim et al. 2005, CitationWahl and Czernuszka 2006, CitationKong et al. 2006, CitationInanc et al. 2007b).
It is known that surface wettability, roughness, and chemistry of a scaffold should be optimized to facilitate cell attachment and differentiation (CitationDowling et al. 2011). Studies have shown that composites of CS enhance osteoblast differentiation and matrix mineralization. As a bone grafting material, CS/HA templates allow cells to proliferate and express their specific functions, induce bone formation and enhance bonding between the existing bone tissue (CitationTeng et al. 2009). As observed in other studies, the presence of HA on the CS scaffold increases the level of serum protein absorption and facilitates cell attachment by increasing the binding sites of the material surfaces (CitationKilpadi et al. 2001). Dissolution of HA results in the formation of calcium and phosphate ions. These ions support the process of bone formation by affecting the surrounding cell population (CitationBagambisa et al. 1993). It has been shown that HA-composited polymer biomaterials have good osteoconductivity and biodegradation rates, with favorable mechanical strength for orthopedic use (CitationYamaguchi et al. 2001, CitationThein-Han and Misra 2009). Likewise, we have opted for CS and HA to produce a composite scaffold that could mimic native bone tissue via freeze-drying, for BTE.
Porosity and the pore size are important parameters for facilitating tissue in-growth and new bone formation (CitationKarageorgiou and Kaplan 2005, CitationElcin et al. 2003b). These properties enhance bone formation by affecting cellular behavior. A scaffold pore size ranging from 150–500 μm is generally accepted as suitable by many groups as favoring cellular and vascular penetration, while also ensuring tissue in-growth into the pores (CitationLinn et al. 2003, CitationKarageorgiou and Kaplan 2005) and facilitating connection between implants and the surrounding tissue (CitationTeixeira et al. 2010, CitationBaykan et al. 2014). In the freeze-drying technique, ice crystals are formed in the polymer solution while polymer molecules aggregate in interstitial spaces; then the ice crystals are removed by sublimation, leaving behind the porous material (CitationKong et al. 2005). As indicated by SEM analysis, the CS/HA scaffold produced by this technique was found to be highly porous and had interconnections between the pores, which ranged in size from 100–300 μm. As a whole, SEM analysis, histological studies, and IHC observations showed that cells had attached, dispersed, and proliferated extensively within the pores of the construct, secreted ECM, and expressed osteogenic proteins.
Osteoprogenitor cells originated from calvaria, periosteum, bone marrow and osteoblastic cell lines such as MC3T3-E1 and Saos-2, have usually been utilized in BTE experiments. In this study, we used the clonogenic MC3T3-E1 cell line which was derived from the newborn mouse calvaria (CitationSudo et al. 1983). Bone tissue development can be followed by histochemical staining, such as von-Kossa or Alizarin Red S, or more specifically by osteoblastic markers such as Cbfa1/Runx2, OSP, bone-sialoprotein, high levels of alkaline phosphatase, and osteocalcin (CitationChristenson 1997). We have evaluated osteogenic differentiation by mineralized ECM production, in terms of von Kossa staining, both in vitro and in vivo. While HA also stains with von Kossa, we have observed a gradual increase in calcium deposition with time after the cells reached confluence on the constructs. OSP is a phosphorylated glycoprotein secreted to the mineralizing ECM by osteoblasts during bone development. Besides histochemical analyses, IHC revealed that MC3T3-E1 cells on the composite scaffold expressed OSP both in vitro and in vivo, indicating bone-like tissue formation. H&E histochemistry also demonstrated that the cell-laden composite scaffold supported neovascularization in the growing osteogenic tissue inside the construct pores. The size range of the newly forming capillaries was around 10–20 μm, basically composed of primitive endothelial tubes.
The combined results of in vitro and in vivo experiments revealed that preosteoblasts had attached to the material surfaces, expressed their functions, and maintained a trend of optimal proliferation in the CS/HA composite scaffold, thus providing a cytocompatible environment for bone engineering.
Acknowledgments
The support from DAAD and Baden-Württemberg Fellowships, Germany (to A.K.), and The Turkish Academy of Sciences (TÜBA), Ankara, Turkey (to Y.M.E.) is acknowledged. We also thank Ms. Beate vom Hoevel for excellent technical assistance.
Declaration of interest
The authors declare no competing interests. The authors alone are responsible for the content and writing of the paper.
References
- Allori AC, Davidson EH, Reformat DD, Sailon AM, Freeman J, Vaughan A, et al. 2013. Design and validation of a dynamic cell-culture system for bone biology research and exogenous tissue-engineering applications. J Tissue Eng Regen Med. Published online: 11 SEP 2013, DOI: https://doi.org/10.1002/term.1810.
- Aubert-Viard F, Martin A, Chai F, Neut C, Tabary N, Martel B, Blanchemain N. 2015. Chitosan finishing nonwoven textiles loaded with silver and iodide for antibacterial wound dressing applications. Biomed Mater. 10:015023.
- Bagambisa FB, Joos U, Schilli W. 1993. Mechanisms and structure of the bond between bone and hydroxyapatite ceramics. J Biomed Mater Res. 27:1047–1055.
- Baykan E, Koc A, Elcin AE, Elcin YM. 2014. Evaluation of a biomimetic poly(ε-caprolactone)/β-tricalcium phosphate multispiral scaffold for bone tissue engineering: In vitro and in vivo studies. Biointerphases. 9:029011.
- Bonilla, CEP, Trujillo S, Demirdogen B, Jairo E, Perilla JE, Elcin YM, Ribelles JL. 2014. New porous polycaprolactone-silica composites for bone regeneration. Mat Sci Eng C Mat Biol Appl. 40:418–426.
- Budiraharjo R, Neoh KGN, Kang ET. 2012. Hydroxyapatite-coated carboxymethyl chitosan scaffolds for promoting osteoblast and stem cell differentiation. J Colloid Interface Sci. 366:224–232.
- Christenson RH. 1997. Biochemical markers of bone metabolism: an overview. Clin Biochem. 30:573–593.
- Chung S, King MW. 2011. Design concepts and strategies for tissue engineering scaffolds. Biotechnol Appl Biochem. 58:423–438.
- Demirdögen B, Bonilla CE, Trujillo S, Perilla JE, Elçin AE, Elcin YM, Ribelles JL. 2014.Silica coating of the pore walls of a microporous polycaprolactone membrane to be used in bone tissue engineering. J Biomed Mater Res A. 102:3229–3236.
- Dowling DP, Miller IS, Ardhaoui M, Gallagher WM. 2011. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma modified polystyrene. J Biomater Appl. 26:327–347.
- Durkut S, Elcin AE, Elcin YM. 2006. Biodegradation of chitosan-tripolyphosphate beads: In vitro and in vivo studies. Artif Cell Blood Substit Immoobil Biotechnol. 34:263–276.
- Elcin YM. 2004. Stem cells and tissue engineering. Adv Exp Med Biol. 553:301–316.
- Elcin YM, Elcin AE, Bretzel RG, Linn T. 2003a. Pancreatic islet culture and transplantation using chitosan and PLGA scaffolds. Adv Exp Med Biol. 534:255–264.
- Elcin YM, Elcin AE, Pappas GD. 2003b. Functional and morphological characteristics of bovine adrenal chromaffin cells on macroporous poly (D,L-lactide-co-glycolide) scaffolds. Tissue Eng. 9:1047–1056.
- Emin N, Koc A, Durkut S, Elcin AE, Elcin YM. 2008. Engineering of rat articular cartilage on porous sponges: Effects of TGF-beta1 and microgravity bioreactor culture. Artif Cell Blood Substit Immoobil Biotechnol. 36:123–137.
- Inanc B, Elcin AE, Elcin YM. 2007a. Effect of osteogenic induction on the in vitro differentiation of human embryonic stem cells cocultured with periodontal ligament fibroblasts. Artif Organs. 31:792–800.
- Inanc B, Elcin AE, Koc A, Balos K, Parlar A, Elcin YM. 2007b. Encapsulation and osteoinduction of human periodontal ligament fibroblasts in chitosan-HA microspheres. J Biomed Mater Res A. 82:17–926.
- Karageorgiou V, Kaplan D. 2005. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 26:5474–5491.
- Kilpadi KL, Chang PL, Bellis SL. 2001. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J Biomed Mater Res A. 57:258–267.
- Kim HW, Kim HE, Salih V. 2005. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin–hydroxyapatite for tissue engineering scaffolds. Biomaterials. 26:5221–5230.
- Kiroshka VV, Yurchuk TA, Repin NV, Petrova VA, Gofman IV, Skorik YA, et al. 2014. Adhesion, growth, and proliferation of endothelial cells on biopolymer extracellular film matrices. Bull Exp Biol Med. 158:153–158.
- Koc A, Emin N, Elcin AE, Elcin YM. 2008. In vitro osteogenic differentiation of rat mesenchymal stem cells in a microgravity bioreactor. J Bioact Compat Pol. 23: 244–261.
- Koc A, Finkenzeller G, Elcin AE, Stark GB, Elcin YM. 2014. Evaluation of adenoviral vascular endothelial growth factor-activated chitosan/hydroxyapatite scaffold for engineering vascularized bone tissue using human osteoblasts: In vitro and in vivo studies. J Biomater Appl. 29:748–760.
- Kong L, Gao Y, Cao W, Gong Y, Zhao N, Zhang X. 2005. Preparation and characterization of nanohydroxyapatite/chitosan composite scaffolds. J Biomed Mater Res A. 75:275–282.
- Kong L, Gao Y, Lu G, Gong Y, Zhao N, Zhang X. 2006. A study on the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur Polym J. 42:3171–3179.
- Lee KW, Wang S, Yaszemski MJ, Lu L. 2008. Physical properties and cellular responses to crosslinkable poly(propylene fumarate)/hydroxyapatite nanocomposites. Biomaterials. 29:2839–2848.
- Linn T, Erb D, Schneider D, Kidszun A, Elcin AE, Bretzel GB, Elcin YM. 2003. Polymers for induction of revascularisation in the rat fascial flap: application of vascular endothelial growth factor and pancreatic cell lines. Cell Transplantation. 12:769–778.
- Liu X, Ma PX. 2004. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 32: 477–486.
- Mallick K (Ed.). 2014. Bone Substitute Biomaterials. Woodhead Publishing. ISBN 978-0-85709-497-1.
- Miclau T, Bozic K, Tay BK, Kim HG, Colnot C, Puttlitz C, et al. 2007. Bone injury, regeneration and repair. In: Einhorn T, O’Keefe RJ, Buckwalter JA, Eds. Orthopaedic Basic Science, 3rd ed. American Academy of Orthopaedic Surgeons.
- Oliveira JM, Costa SA, Leonor IB, Malafaya PB, Mano JF, Reis RL. 2009. Novel hydroxyapatite/carboxymethyl chitosan composite scaffolds prepared through an innovative “autocatalytic” electroless coprecipitation route. J Biomed Mater Res A. 88:470–480.
- Park H-J, Yu SJ, Yang K, Jin Y, Cho A-N, Kim J, et al. 2014. Paper-based bioactive scaffolds for stem cell-mediated bone tissue engineering. Biomaterials. 35:9811–9823.
- Pillai CKS, Paul W, Sharma CP. 2009. Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Poly Sci. 34:641–678.
- Salgado AJ, Coutinho OP, Reis RL. 2004. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 4:743–765.
- Seol YJ, Lee JY, Park YJ, Lee YM, Young K, Rhyu IC, et al. 2004. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol Lett. 26:1037–1041.
- Sudo H, Kodama H, Amagai Y. Yamamoto S, Kasai S. 1983. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 96:191–198.
- Teixeira S, Fernandes H, Leusink A, van Blitterswijk C, Ferraz MP, Monteiro FJ, de Boer J. 2010. In vivo evaluation of highly macroporous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res A. 93:567–575.
- Teng SH, Lee EJ, Yoon BH, Shin DS, Kim HE, Oh JS. 2009. Chitosan/nanohydroxyapatite composite membranes via dynamic filtration for guided bone regeneration. J Biomed Mater Res A. 88: 569–580.
- Thein-Han WW, Misra RD. 2009. Biomimetic chitosan-nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 5:1182–1197.
- Wahl DA, Czernuszka D. 2006. Collagen hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 11:43–56.
- Yamaguchi I, Tokuchi K, Fukuzaki H, Koyama Y, Takakuda K, Monma H, Tanaka J. 2001. Preparation and microstructure analysis of chitosan/hydroxyapatite nanocomposites. J Biomed Mater Res. 55:20–27.
- Yhee JY, Son S, Kim SH, Park K, Choi K, Kwon IC. 2014. Self-assembled glycol chitosan nanoparticles for disease-specific theranostics. J Control Release. 193:202–213.
- Zhang YF, Cheng XR, Chen Y, Shi B, Chen XH, Xu DX, Ke J. 2007. Three-dimensional nanohydroxyapatite/chitosan scaffolds as potential tissue engineered periodontal tissue. J Biomater Appl. 21:333–349.
- Zhang Q, Mochalin VN, Neitzel I, Hazeli K, Niu J, Kontsos A, et al. 2012. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials. 33: 5067–5075.
- Zhou H, Xu HH. 2011. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials. 32:7503–7513.