ABSTRACT
The production of waste printed circuit boards (WPCBs) has drawn increasing global concern, especially because the high bromine (Br) content (5–15%) places obstacles in the way of simple disposal techniques. Microwave-induced pyrolysis of WPCBs provides a promising way to dispose of these hazardous and resource-filled wastes. The transference rules for Br during microwave-induced pyrolysis have been investigated experimentally. It was found that the microwave energy could be used more efficiently to accelerate the heating rate and improve the final pyrolysis temperature by adding some activated carbon (AC) as microwave absorbents. The high temperature and rapid pyrolysis process promoted the yields of gaseous products and the decomposition of brominated compounds into hydrogen bromide and then benefited the capture of Br and the debromination of byproducts. The application of a calcium carbonate (CaCO3) layer overhead led to over 95% debromination of the liquid products and over 50% capture of the total Br. It can be concluded that the presented method is suitable for the control of Br transference in the recycling of WPCBs. This method can also be extended to the disposal of the electronic scraps.
Development of environmentally sound treatments for the comprehensive recycling of WPCBs and even waste electrical and electronic equipment (WEEE) is a hot issue all over the world. New ideas and technologies will be continuously developed. The idea of recycling WPCBs by microwave-induced pyrolysis coupled with the debrominating of pyrolysis products by a CaCO3 layer is just a novel one. It has great potential for industrial practice and its implementation will enrich the choices for WEEE recycling.
INTRODUCTION
The problems associated with waste electrical and electronic equipment (WEEE) have caused increasing concern all over the world. Printed circuit boards (PCBs) are dumped more and more often because of rapid updating or redundancy of electronic devices. They cause great damage to the environment and potential risks to human health. Although PCBs are of many types, they generally contain approximately 40% valuable metals, 30% resins, and 30% glass fiber.Citation1 Taking the rare metals as examples, their contents in PCBs are usually much higher than those in the corresponding ores. However, hazardous materials such as brominated flame retardants (BFRs) and heavy metals make them difficult to recycle. Thus, waste printed circuit boards (WPCBs) are valuable as well as polluting. At present, pyrometallurgical,Citation2,Citation3 hydrometallurgical,Citation3–5 and mechanical-physical processesCitation1,Citation6,Citation7 are widely applied to recycle metals in WEEE, but their pollution of air, water, and soil are serious. Thus, the development of an environmentally friendly technology for the disposal of WPCBs is eagerly awaited.
Pyrolysis is regarded as a potential means to dispose of or recycle wastes, especially organic polymers. It has the distinct advantages of volume reduction, pollutant removal, and reclamation of resources. Using pyrolysis, the resins in WEEE can be recovered in the form of fuel gases, oils, or other chemical materials, and the metals can be enriched for easier separation.
Pyrolysis of WPCBs has been investigated using a thermogravimetric analyzer, a rotary kiln, a fluidized bed, or a fixed-bed reactor under an atmosphere of nitrogen or in a vacuum, etc.Citation8–12 However, most previous studies were carried out in externally heated devices. In contrast, microwave-induced pyrolysis may be regarded as an internal heating method because the materials are heated mainly because of the dielectric loss responding to the microwave radiation rather than by conductive heat flow from outside. Thus, microwave heating is a volumetric process and easy to control. The radiation energy is dissipated within the samples more or less uniformly, giving rise to much higher heating rates. Furthermore, when WPCBs are treated by microwave heating, a corona discharge may also be produced because of the existence of metals with edges and corners. By coupling the microwave absorption ability of the WPCBs themselves and some additional microwave absorbents with corona discharge from certain metals, the heating process can be greatly accelerated and the energy input can be utilized more efficiently. The authors' working group has already carried out some researches into the microwave-induced pyrolysis of WEEE, showing that it promises to achieve an efficient energy utilization, recycling of metals, product re-use, and effective avoidance of pollution by coupling the corona discharge with microwave heating.Citation13,Citation14
However, the bromine (Br) content is usually very high in PCBs, which limits the utilization of the pyrolysis byproducts. The approximately 5–15 wt % Br in WPCBs can cause operational difficulties in handling hydrogen bromide (HBr), toxic brominated polycyclic aromatic hydrocarbons, and dioxins,Citation8,Citation15 any of which can be generated. Thus, the destruction of the organobrominated compounds to recover useful Br-free chemicals or fuels via thermochemical processes has become an important research area.Citation10,Citation16–20 So far, the fate of Br has been studied only for routine pyrolysis conditions as mentioned above.Citation15 However, the transference rules for Br during the microwave-induced pyrolysis of WPCBs have rarely been studied. Without understanding these rules, it will be difficult to promote the practical application of microwave-induced pyrolysis of WPCBs.
In this paper, WPCBs that contain BFRs are selected as experimental materials and activated carbon (AC) is used as microwave absorbent. The Br distribution among the three-phase products is traced, and the influence of calcium carbonate (CaCO3) on the capture of Br is also investigated.
MATERIALS AND METHODS
Materials
WPCBs obtained from a local PCB factory were used as experimental materials. The WPCBs have a multilayered structure, containing epoxy resin, woven glass fiber, brominated epoxy resin flame retardants, and copper. Approximately 20-kg WPCBs were prepared for the experiments. They were first shredded into fragments of approximately 5 × 5 cm and then ground to reduce their particle size down to below 1 mm. The main composition of WPCBs is given in . The light elements were analyzed by an elemental analyzer (VarioEL III CHNS) and the other elements were analyzed using wavelength-dispersive X-ray fluorescence spectrometry (Bruker S8 TIGER). Note that the Br content was determined by ion chromatography (IC) after some transformation, as described in Determination of Br Content. Analytical pure CaCO3 and calcium oxide (CaO) with a specific surface area of 5 and 3.4 m2/g, respectively, were used as the reagents to capture Br. Sodium hydroxide (NaOH) was used as reagent to transform Br present. AC was adopted as the microwave absorbent. The particle size and specific surface area of the selected powder and granular AC were 380 and 61 μm and 1237.112 and 915.668 m2/g, respectively.
Table 1. Composition of WPCBs
Experimental Setup and Safeguard
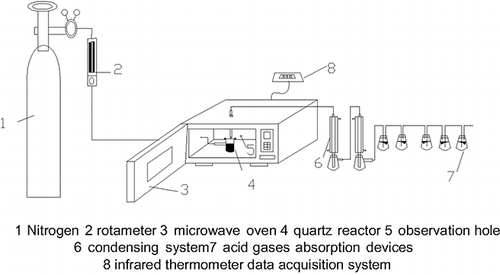
The microwave-induced pyrolysis experiments were carried out using a domestic microwave oven. A self-made quartz device was used to contain the sample, and a set of polytetrafluoroethylene (PTFE) flanges with silicone gaskets clamped by a set of four bolts were used to seal the quartz device. The inlet and outlet of the device were connected, respectively, with a nitrogen tank and condensing devices by silicone tubes. An infrared (IR) thermometer was used to detect the temperature of the material surface through an observation hole on the oven wall. To represent the average temperature of the bulk sample quite accurately, the IR thermometer was previously calibrated for different temperatures in separate experiments by switching off the microwaves and immediately introducing a thermocouple in the center of the bulk sample. Then, the emissivity parameter of the IR thermometer was set in such a way that the temperature measured by the optical pyrometer and thermocouple was the same.Citation21 shows a schematic diagram of the microwave-induced pyrolysis apparatus, which includes the microwave oven, quartz reactor, temperature monitor system, condensing system, and acid gas absorption system.
Because safety should always be the first consideration, preventive and protective measures should be taken before operation. All users should follow the safety rules of remote control operation, shielding the working area, wearing special antimicrowave radiation suits and gas masks, standing at least 1 m away during the operation, and so forth.
EXPERIMENTAL METHODS
In each experimental run, 20-g WPCBs were placed in the homemade quartz device. The device was then centered in the microwave oven and sealed and purged with pure nitrogen (99.999%) at a flow rate of 100 ml/min for 30 min to expel oxygen before the pyrolysis process. The samples were then irradiated with a microwave, and the nitrogen flow was cut off. The temperature was monitored online during the process. After escaping from the quartz reactor, the volatile fractions passed through a series of seven glass bottles in an ice-bathed cooler. The first two bottles were used to collect the liquid products, and the other five, containing an aqueous solution of NaOH, were used to absorb the acid gas. Here, the weight of the homemade quartz device, condensing system, and connecting pipes were recorded before and after pyrolysis to calculate the yields of solid and liquid products. The yield of gaseous products was obtained by subtracting the yields of solid and liquid products from the 20 g according to the conservation of mass. When the pyrolysis was finished, the solid products could be separated easily from the container, but the liquid products were difficult to collect because they tended to adhere to the walls. Absolute alcohol was used to help wash the devices. To precisely determine the Br content, it was measured using a Dionex DX100 ion chromatograph fitted with a Dionex AS11-HC column. The detailed disposal processes of the products are described in Determination of Br Content.
Test Conditions
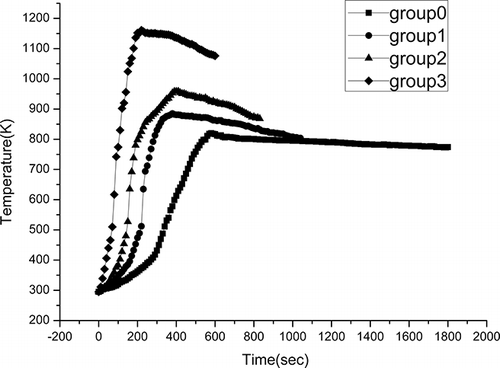
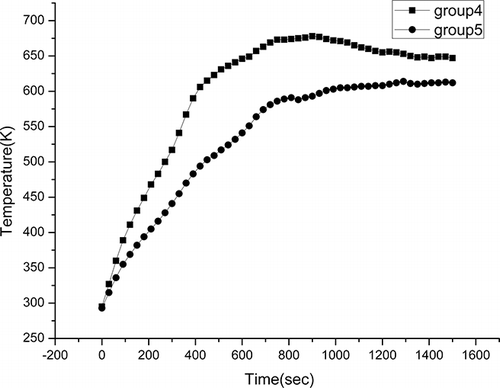
First, considerable work was done to determine the time needed to pyrolyze all of the organic components using WPCBs mixed with 0, 5, 12.5, and 25 wt % powdered AC at an output power of 560 W. Powdered AC is preferred because AC powder has a relatively large surface area and can penetrate the samples more uniformly compared with the same weight of granular AC. The test mixtures described above were labeled groups 0, 1, 2, and 3, respectively. In this work, temperature was monitored online to compare the heating processes. The objective of this work was to investigate the effect of AC on the heating rate and pyrolysis temperature and then select suitable conditions. Second, WPCB samples were mixed with 50 wt % CaCO3 and 28 wt % CaO to co-pyrolyze at a microwave output power of 560 W, labeled as groups 4 and 5, respectively, and their temperatures were also monitored online. This work was done to determine the feasibility of microwave co-pyrolysis of WPCBs with CaCO3 or CaO. Lastly, groups 1, 2, and 3 were selected to guarantee a rapid heating rate, and a layer of 25-wt % (5 g) CaCO3 was placed on top of the mixed samples to change the pathway of Br and make it more convenient to be reclaimed. These test conditions were labeled groups 1′, 2′, and 3′, correspondingly.
Yields of Pyrolysis Products
The yields of solid, liquid, and gaseous products were obtained on the assumption that there was no loss of AC using the following equations:
Determination of Br Content
The precise measurement of Br content was the most difficult aspect of this study. Much work has been done to determine the best methods for the disposal of WPCBs and their pyrolysis products. A combination of bomb combustion and IC has been used for the determination of Br in solid recovered fuels and has been standardized by the European Committee for Standardization (CEN/TS 15408: 2006). However, this does not suit the disposal of WPCBs because of their inferior flammability. Generally, because of incomplete combustion, the results detected by this method are 15–25% lower than the actual contents.Citation22 Actually, no systematic methods have been established so far to determine the contents of Br in WPCBs and their pyrolysis products. In this study, the determination of Br content consisted of the conversion of the Br present and the detection of bromide ion by IC. The mechanism of the conversion is to transform brominated compounds into bromide ion; thereupon, a set of conversion techniques were set up by referring to the literature.Citation11,Citation20,Citation22–25
Determination of Br Content in WPCBs
The main forms of Br in WPCBs are organobrominated compounds, which are almost insoluble in water, so these cannot be directly detected by IC. Meanwhile, the compositions of WPCBs are so complicated that Br can react with other substances during the thermochemical conversion. Substances like copper, which has a considerable amount in WPCBs, can react with Br to generate insoluble cuprous bromide and confuse the quantification of Br. Thus, to determine the Br content more precisely, copper needs to be removed first. Here, iron(III) chloride (FeCl3) was used to remove copper using the reaction
Meanwhile, some hydrochloric acid was added to remove any copper(II) oxide (CuO) present. After 24 hr of reaction and filtration, the residues were carefully rinsed with deionized water and dried, and the filtrate was diluted and measured by IC to determine the content of possible inorganic Br, c 1. Then, approximately 0.5-g residues were put in a nickel crucible and covered by excess of powdered NaOH (∼10 g) and then maintained at 650 °C for 30 min in a high-temperature furnace to produce a carbonization reaction and melting. Here, the quantity of NaOH re-agent, the temperature, and the retention time were optimized by referring to the literature published by ZhaoCitation25 and several tests. This method is commonly known as the high-temperature alkali fusion method. The substance obtained was dissolved in deionized water after cooling. The mechanism of this conversion is
Determination of Br Distribution in the Solid Residues
The Br distribution in the solid products was also difficult to determine because these contained inorganic brominated compounds and more complicated organic brominated compounds. The determination of the inorganic Br content in the solid residues followed the method published by Peng et al.,Citation11 whereas the organic Br was measured using the method of high-temperature alkali fusion and IC measurement described above.
Similarly, the content of Br captured by the CaCO3 layer consisted of organic and inorganic Br. The CaCO3 layer was carefully rinsed with deionized water to obtain the content of inorganic Br. However, the quantitative determination of organic Br was very difficult. Because of their low flammability, the bomb combustion method was not suitable. Furthermore, few methods related to the transformation of the organobrominated compounds captured by CaCO3 into bromide ion have been reported or standardized. Thus, the quantity of organic Br captured by the CaCO3 layer was classified as a transformation loss.
Determination of Br Distribution in the Liquid Products
The determination of Br distribution in the liquid products was much easier. The liquid products can be mixed with NaOH after the high-temperature alkali fusion method to obtain the total Br content or be carefully rinsed with deionized water to obtain the inorganic Br content. Then the organic Br content can be obtained by subtracting the inorganic Br content from the total.
Determination of Br Distribution in the Gaseous Products
The Br distribution in the gaseous products can be obtained by subtracting the Br transferred into the solid residues and liquid products from the total Br content of the WPCBs. Because of the inevitable loss during the transformation and the neglected organic Br captured by CaCO3, the Br distribution in the gases was inevitably higher than might be expected. However, the results were useful for comparison.
RESULTS AND DISCUSSIONS
The Effects of AC on Pyrolysis of WPCBs
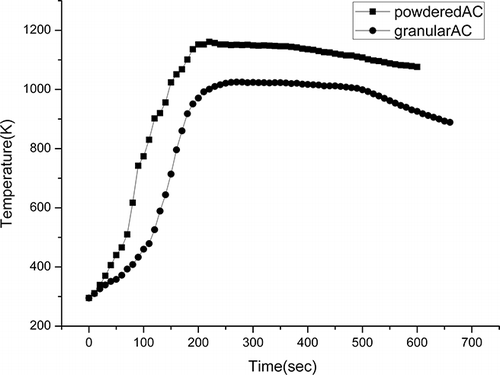
shows the weight loss results and pyrolysis time results as a function of different weight percentages of powdered AC using a microwave power of 560 W. It was found that the pyrolysis time can be significantly shortened and the weight loss rate increased somewhat with the increase of AC. When the percentage of AC reached 25 wt %, the process was so fast that effluents were generated in a large amount and instantly escaped from the reactor. Consequently, part of the AC was carried away by the effluents and adhered to the pipes or was transferred into byproducts. When 25 wt % powdered AC was replaced by 25 wt % granular AC, the escape of AC could be kept under control. However, the process was slower, mainly because of the smaller specific surface area of granular AC and less effectively mixed with WPCBs, compared with powdered AC. This is in accordance with the temperature changes illustrated in .
Table 2. The determination of pyrolysis time of each group
Along with the reduction of pyrolysis time, increasing the proportion of powdered AC accelerated the heating process and greatly enhanced the final temperature, as shown in . This result was consistent with our previous research.Citation14 In summary, by microwave irradiation and the addition of AC, the slow heating process by using a conventional external heat supply can be replaced by a dramatically effective internal heating process. For the sake of efficient microwave energy utilization, a moderate flow rate of effluents, and economic AC consumption, it was suggested that WPCBs be mixed with between 5 and 12.5 wt % powdered AC to achieve rapid heating and high pyrolysis temperature.
The Effect of Co-Pyrolysis with CaO and CaCO3 on Heating Rates
The co-pyrolysis of WPCBs with 50 wt % CaCO3 and 28 wt % CaO were investigated, and shows the temperature profile obtained. It was found that the heating process was severely disrupted by the addition of CaCO3 and CaO, and the highest temperature was below 673 K. This is mainly because CaO and CaCO3 are not good microwave absorbents and their addition can increase resistance to heat transfer. Thus, the pyrolysis of WPCBs will be affected by mixing with CaO or CaCO3, so some of the advantages of microwave heating may be lost. The main function of CaCO3 or CaO is to absorb HBr in the effluents to partially change the Br pathway and debrominate the byproducts. The higher temperature of the effluents is more beneficial to the reaction of HBr with CaCO3 or CaO. Because CaCO3 is cheap, easy to obtain, and has a larger specific surface area, it was therefore suggested that a layer of CaCO3 be placed on the top of the mixture of WPCBs and AC to effectively capture Br from the effluents. This provided a more convenient method for Br reclamation, in which the negative effects on the pyrolysis process were small and the advantages of microwave heating remained. Moreover, the overhead CaCO3 layer would exert little negative effects on the metals and glass fiber because they have little contact.
Br Transference Rules under Microwave Irradiation
Effect of AC and CaCO3 Layer on the Yields of Products
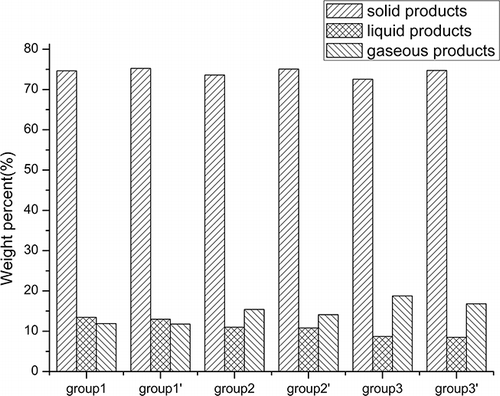
To study the distribution of Br among the products, it is necessary to obtain data on the yields of solid, liquid, and gaseous products. gives the results. With increasing amounts of AC, the yield of solid products decreased slightly, from 74.6 to 72.6 wt %. In stark contrast, the liquid and gaseous yields clearly changed. The yield of liquid products decreased from 13.5 to 8.5 wt %; conversely, the yield of gaseous products increased from 11.8 to 18.7 wt %. The striking changes of liquid and gaseous yields were because the high-molecular-weight effluents, which tend to condense, were degraded further into low-molecular-weight components before they escaped from the reactor with the acceleration of the heating process and the enhancement of pyrolysis temperature. This is in accordance with the results of other research on the pyrolysis yield of solid waste as a function of temperature.Citation9
By placing a layer of CaCO3 on the top of the mixture of WPCBs and AC, the yield of solid products increased slightly whereas the yields of liquid and gaseous products decreased slightly. From one perspective, these results could be explained in terms of the reaction
The molecular weight of calcium bromide (CaBr2) is twice that of CaCO3 and the molecular weights of water and carbon dioxide (CO2) are less than that of HBr. Furthermore, because of the CaCO3 layer, the escaping rate of the effluents was slowed down. As a result, a proportion of the effluents would be deposited in the solid products or adhere to the surface of the CaCO3.
Br Distribution among the Pyrolysis Products
From the measurements described above, the Br content of WPCBs is 10.88%. To trace the transference rules for the Br, it is necessary to obtain the Br distribution in the three product phases. Because of the complicated chemical structures, the quantity of Br transferred to the three product phases could not be obtained directly. Because the quality of liquid products was a main focus for WPCB thermal recycling techniques, it was the Br distribution in the liquid products that was investigated first. Subsequently, the Br distribution in the solid residues was obtained. Simultaneously, the quantity of Br transferred to the CaCO3 layer was measured to study the removal efficiency. The Br distribution in gaseous products was obtained by subtracting the total Br transferred into the solid residues, CaCO3, and liquid products from the total Br content of the WPCBs. Finally, the Br distribution in the pyrolysis products and the transference rules for Br could be determined.
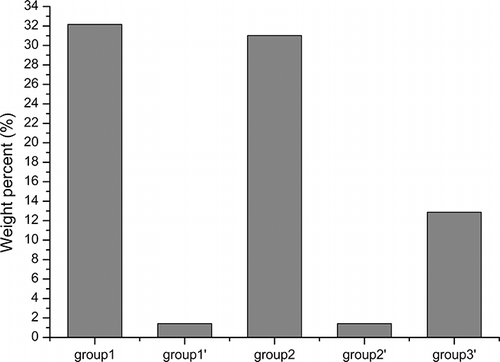
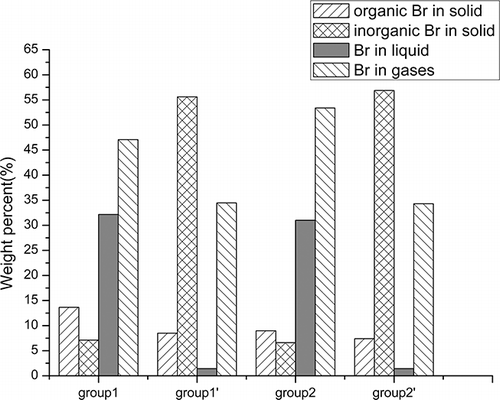
illustrates the Br distribution in liquid products from groups 1, 1′, 2, 2′, and 3′. Group 3 was excluded because the rapid process resulted in little liquid yield and it was difficult for this to be collected because it adhered to the walls of pipes and condensing devices. In addition, some solid products such as AC were also transferred into the liquid. The Br distributions in the liquid products of groups 1 and 2 were as high as more than 31 wt % and were decreased to 1.42 wt % for groups 1′ and 2′ after adding a layer of CaCO3—a debromination of over 95%. However, the Br distribution in the liquid products of group 3′ was still significant at 12.87 wt %. This was mainly because the process was so fast that part of the effluents failed to react with CaCO3 before it escaped from the reactor. Another factor may be that part of the CaCO3 layer may be above 1000 K, at which temperature CaBr2 begins to melt and decompose.Citation26 Thus, from the perspective of the production and the debromination of liquid products, the proportion of AC must be chosen carefully. Compared with group 3′, groups 1′ and 2′ prevent more Br from transferring into the liquid products. By adding a layer of CaCO3, the quality of the pyrolysis oils generated could be improved greatly.
When the liquid products of group 2 were rinsed with deionized water to obtain the inorganic Br content, this was found to be 27.94 wt %, which was approximately 3.08 wt % lower than the total Br distribution in the liquid products. The fact that this proportion of organic Br was still higher than the Br distribution in the liquid products of group 2′ indicated that the CaCO3 layer could also absorb organobrominated compounds.
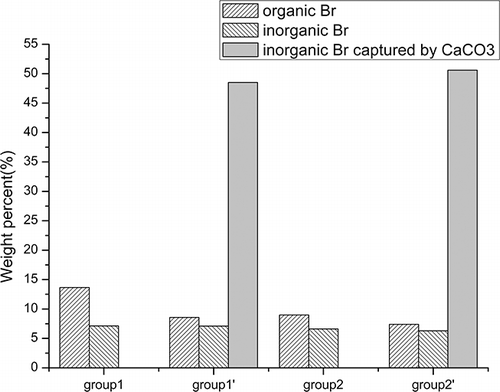
shows the Br distribution in the solid products from groups 1, 1′, 2, and 2′. With the increase of the percentage of AC, organic Br was decreased remarkably whereas inorganic Br was decreased slightly, by less than 1 wt %. Conversely, the proportion of inorganic Br absorbed by the CaCO3 layer increased from 48.5 to 50.6 wt%. These results show that with the accelerated pyrolysis process and the enhanced pyrolysis temperature, the escape of brominated compounds from the solid residues was promoted. Furthermore, the brominated compounds were benefited to decompose further into bromine hydride and then escape from the residues and as such can be recycled easily from the effluents. The decrease of the proportion of organic Br in the solid residues after adding a layer of CaCO3 indicated that organobrominated compounds can also be absorbed by the CaCO3 layer. It is more beneficial for CaCO3 to capture organobrominated compounds when the process is slowed down to have a long residence time. In this work, the content of organic Br in the CaCO3 layer was not measured and was classified as a transformation loss. On the basis of the obtained data, it was assessed to be approximately 1.5–3.5 wt % for group 2′ and a little more for group 1′. In conclusion, the CaCO3 layer could capture more than 50% Br and more desirable byproducts can be obtained.
More importantly, because an increase of the proportion of AC can accelerate the pyrolysis process and improve the pyrolysis temperature, and thereupon can result in more gaseous products and greater transformation of brominated compounds into HBr, aqua ammonia is an economic alternative for the disposal of the gas thus obtained.Citation27 According to the authors' previous research, the gas obtained by microwave pyrolysis of WPCBs contains approximately 80% combustible gases such as hydrogen, methane, carbon monoxide, etc.,Citation13,Citation14 which is meaningful to make it more suitable as fuel gas. However, whether the advantages of efficiency improvement and production of fuel gases overwhelm the increased investment of AC as well as the inevitable sacrifice of liquid products needs further investigation.
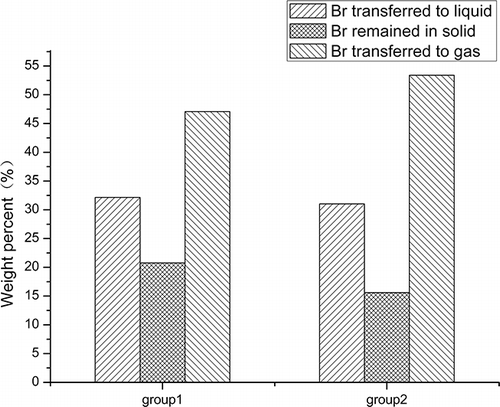
The Br transference rules can be drawn from . Most Br was transferred into the liquid and gaseous products, whereas only approximately one-fifth was left in the solid residues. Furthermore, more Br in group 2 entered the gaseous products and less Br remained in the solid residues than in group 1, which indicated that more brominated compounds were released from the solid residues and entered the volatiles with the increase of heating rate and pyrolysis temperature. The final Br distribution in the pyrolysis products is shown in . After adding a layer of CaCO3 overhead, the Br distribution in the gaseous and liquid products decreased significantly. Although the Br distribution obtained for gaseous products was higher than the actual value because of the causes previously mentioned, an appropriate comparison could be made. Thus, it is more convenient to reuse the byproducts. In addition, with the improvement of pyrolysis temperature, more brominated compounds were cracked into HBr and released from the residues; therefore, it is much easier to dispose of and recover metals from the solid residues and capture Br from the effluents. In summary, the microwave-induced pyrolysis of WPCBs can be modified to utilize the microwave energy more efficiently and obtain more desirable byproducts as well as reclaim Br resources more easily.
After the pyrolysis stage, the metals contained in WPCBs are enriched and the pyrolysis residues are more fragileCitation28 and less hazardous compared with WPCBs. Moreover, the metallic and nonmetallic components are different in properties such as conductivity, magnetism, and density. Consequently, metals can be easily recovered by crushing and mechanical separations. The nonmetallic components can be used as filler in cement because of its high content of silicon and aluminum. Thus, it opens a more convenient way to recycle metals and make use of the less hazardous residues.
CONCLUSIONS
Microwave-induced pyrolysis of WPCBs was proved to be a promising way to dispose of this kind of hazardous and resource-rich waste. Microwave energy can be utilized more efficiently to accelerate the heating rate and improve the final pyrolysis temperature by adding some AC.
This is beneficial for the further decomposition of organic polymers and BFR to generate more HBr and combustible gases such as hydrogen, methane, carbon monoxide, etc., and promoting them to escape from the solid residues. Consequently, it is advantageous for the debromination of products, reclaimation of Br resources, and disposal of solid residues.
The transference rules of Br under the influence of microwave-induced pyrolysis were studied by these experiments. Under the high temperature and rapid pyrolysis induced by microwave, more than 50 wt % BFR in WPCBs can be transformed into HBr and captured by the overhead CaCO3 layer, which is effective and efficient to react with the high-temperature HBr. As a result, the pyrolysis oils can achieve a debromination of over 95%. Thus, it opens a simple way to recycle Br-free pyrolysis byproducts and reclaim Br resources without adding more difficulty in the disposal of residues or exerting negative effects on the valuable metals.
In summary, from the perspective of the greatest recovery of resources and the simplicity of the process, the gas fraction should be suitable for fuel recovery, the liquid products for chemical recuperation or fuel production, the residues for noble and common metal recovery, and the additives should not increase the postprocessing difficulty. As far as WPCBs are concerned, nearly all of these goals are matched by the combination of microwave-induced pyrolysis and CaCO3 removal treatments.
ACKNOWLEDGMENTS
The authors thank the support of the Program for New Century Excellent Talents in University (NCET-10-0529).
REFERENCES
- Xu , M. , Li , G. , He , W. and Li , H. 2008 . Recovering Metals from Printed Circuit Board Scrap by a Mechanical Separation Process . J. Chongqing Univ. , 2 : 100 – 106 .
- Szczygiel , Z. , Lara , C. , Escobedo , S. and Mendoza , O. 1998 . The Direct Reduction of Sulfide Minerals for the Recovery of Precious Metals . J. Met. , 50 : 55 – 59 .
- Cui , J. and Zhang , L. 2008 . Metallurgical Recovery of Metals from Electronic Waste: a Review . J. Hazard. Mater. , 158 : 228 – 256 .
- Mecucci , A. and Scott , K. 2007 . Leaching and Electrochemical Recovery of Copper, Lead and Tin from Scrap Printed Circuit Boards . J. Chem. Technol. Biotechnol. , 77 : 449 – 457 .
- Oishi , T. , Koyama , K. , Alam , S. , Tanaka , M. and Lee , J.C. 2007 . Recovery of High Purity Copper Cathode from Printed Circuit Boards Using Ammoniacal Sulfate or Chloride Solutions . Hydrometallurgy , 89 : 82 – 88 .
- Veit , H.M. , Diehl , T.R. , Salami , A.P. , Rodrigues , J.S. , Bernardes , A.M. and Tenório , J.A.S. 2005 . Utilization of Magnetic and Electrostatic Separation in the Recycling of Printed Circuit Boards Scrap . Waste Manage. , 25 : 67 – 74 .
- Li , J. , Lu , H. , Guo , J. , Xu , Z. and Zhou , Y. 2007 . Recycle Technology for Recovering Resources and Products from Waste Printed Circuit Boards . Environ. Sci. Technol. , 41 : 1995 – 2000 .
- Mattila , H. , Virtanen , T. and Ruuskanen , J. 1992 . Emissions from Combustion of Waste Plastic Material in Fixed Bed Boiler . Chemosphere , 25 : 1599 – 1609 .
- Sun , L.S. 2004 . Experimental Study on the Pyrolysis Characteristics and Resource Recycling of the Products of Printed Circuit Board Scrap , Wuhan , , China : Huazhong University of Science and Technology .
- Hall , W.J. and Williams , P.T. 2006 . Pyrolysis of Brominated Feedstock Plastic in a Fluidized Bed Reactor . J. Anal. Appl. Pyrol. , 77 : 75 – 82 .
- Peng , K. , Li , S. , Chen , B. and Yao , Q. 2009 . Fate of Bromine during the Pyrolysis of Waste Printed Circuit Board . J. Combust. Sci. Technol. , 2 : 114 – 118 .
- Long , L. , Sun , S. , Zhong , S. , Dai , W. , Liu , J. and Song , W. 2010 . Using Vacuum Pyrolysis and Mechanical Processing for Recycling Waste Printed Circuit Boards . J. Hazard. Mater. , 177 : 626 – 632 .
- Sun , J. , Wang , W. , Ma , C. and Dong , Y. 2010 . “ An Exploratory Study of Electronic Waste Treatment: Microwave-Induced Pyrolysis ” . In Power and Energy Engineering Conference (APPEEC), 2010 Asia-Pacific 1 – 4 . Chengdu , , China
- Sun , J. , Wang , W. , Ma , C. and Dong , Y. Study on Pyrolysis Characteristics of Electronic Waste . Proceedings of the 2009 International Conference on Chemical, Biological and Environmental Engineering . pp. 13 – 16 . Hackensack , NJ : World Scientific Publishing .
- Chien , Y.C. , Wang , H.P. , Lin , K.S. , Huang , Y.J. and Yang , Y.W. 2000 . Fate of Bromine in Pyrolysis of Printed Circuit Board Wastes . Chemosphere , 40 : 383 – 387 .
- Luijk , R. , Govers , H.A.J. , Eijkel , G.B. and Boon , J.J. 1991 . Thermal Degradation Characteristics of High Impact Polystyrene/Decabromodiphenylether/Antimony Oxide Studied by Derivative Thermogravimetry and Temperature Resolved Pyrolysis-Mass Spectrometry: Formation of Polybrominated Dibenzofurans, Antimony (Oxy)Bromides and Brominated Styrene Oligomers . J. Anal. Appl. Pyrol. , 20 : 303 – 319 .
- Bhaskar , T. , Kaneko , J. , Muto , A. , Sakata , Y. , Jakab , E. , Matsui , T. and Uddin , M.A. 2004 . Pyrolysis Studies of PP/PE/PS/PVC/HIPS-Br Mixed with PET Plastics and Dehalogenation (Br, Cl) of Liquid Products . J. Anal. Appl. Pyrol. , 72 : 27 – 33 .
- Brebu , M. , Bhaskar , T. , Murai , K. , Muto , A. , Sakata , Y. and Uddin , M.A. 2005 . Removal of Nitrogen, Bromine, and Chlorine from PP/PE/PS/PVC/ ABS-Br Pyrolysis Liquid Products Using Fe- and Ca-Based Catalysts . Polym. Degrad. Stabil. , 87 : 225 – 230 .
- Hall , W.J. and Williams , P.T. 2007 . Analysis of Products from the Pyrolysis of Plastics Recovered from the Commercial Scale Recycling of Waste Electrical and Electronic Equipment . J. Anal. Appl. Pyrolysis , 79 : 375 – 386 .
- Onwudili , J.A. and Williams , P.T. 2009 . Alkaline Reforming of Brominated Fire-Retardant Plastics: Fate of Bromine and Antimony . Chemosphere , 74 : 787 – 796 .
- Domínguez , A. , Fernández , Y. , Fidalgo , B. , Pis , J.J. and Menéndez , J.A. 2008 . Bio-Syngas Production with Low Concentrations of CO2 and CH4 from Microwave-Induced Pyrolysis of Wet and Dried Sewage Sludge . Chemosphere , 70 : 397 – 403 .
- Belevi , H. and Moench , H. 2000 . Factors Determining the Element Behavior in Municipal Solid Waste Incinerators. 1. Field Studies . Environ. Sci. Technol. , 12 : 2501 – 2506 .
- Gao , C. , Cai , R. and Zhang , Y. 2009 . Study on the Bromine Analytical Methods of Brominated Flame Retardants . J. Salt Chem. Ind. , 38 : 21 – 24 .
- Zhou , W. , Chen , L. , Guan , G. and Huang , H. 2009 . Study on Copyrolysis and Dehalogenation of Waste Printed Circuit Boards and Calcium Carbonate . Chinese J. Environ. Engineer. , 1 : 169 – 176 .
- Zhao , Z. 1999 . Determination of Br Content in Decabromodiphenyl Ether by Alkali Fusion-Potentiometric Titration . Jiangsu Prov. Salt Sci. Technol. , 4 : 11 – 13 .
- Peng , S. , Chen , L. and Cai , M. 2006 . Forming and Scavenging of Hydrogen Bromide during Pyrolysis of Waste Printed Circuit Boards . J. South China Univ. Technol. , 10 : 15 – 20 .
- Brebu , M. , Jakab , E. and Sakata , Y. 2007 . Effect of Flame Retardants and Sb2O3 Synergist on the Thermal Decomposition of High-Impact Polystyrene and on Its Debromination by Ammonia Treatment . J. Anal. Appl. Pyrol. , 79 : 346 – 352 .
- Li , J. , Duan , H. , Yu , K. and Wang , S. 2010 . Interfacial and Mechanical Property Analysis of Waste Printed Circuit Boards Subject to Thermal Shock . Journal of the Air & Waste Management Association , 60 : 229 – 236 . doi: 10.3155/1047–3289.60.2.229