Abstract
All current drugs approved to treat schizophrenia appear to exert their antipsychotic effects through blocking the dopamine D2 receptor. Recent meta-analyses and comparative efficacy studies indicate marginal differences in efficacy of newer atypical antipsychotics and the older drugs, and little effects on negative and cognitive symptoms. This review integrates findings from postmortem, imaging, and drug-challenge studies to elucidate a corticolimbic “pathologic circuit” in schizophrenia that may be particularly relevant to the negative symptoms and cognitive impairments of schizophrenia. Potential sites for pharmacologic intervention targeting glutatatergic, GABAergic, and cholinergic neurotransmission to treat these symptoms of schizophrenia are discussed.
Todos los fármacos actuales aprobados para tratar la esquizofrenia parecen ejercer sus efectos anti psicóticos a través del bloqueo del receptor de dopamina D2. Recientes estudios de meta-análisis y de eficacia comparativa muestran diferencias menores entre la eficacia de los antipsicóticos atípicos más nuevos y los más antiguos, y escasos efectos sobre los síntomas negativos y cognitivos. Esta revisión integra los hallazgos de estudios postmortem, de imágenes y de pruebas con fármacos para aclarar un “circuito patológico” córtico-límbico en la esquizofrenia que pueda ser de particular importancia para los síntomas negativos y los deterioros cognitivos de la esquizofrenia. Se discuten los potenciales sitios para la intervención farmacológica de blancos en la neurotransmisión glutamatérgica, gabaérgica y colinérgica para tratar estos síntomas de la esquizofrenia.
Tous les médicaments actuels autorisés dans le traitement de la schizophrénie exercent leur action antipsychotique en bloquant le récepteur D2 à la dopamine. Selon des métaanalyses récentes et des études d'efficacité comparatives, les différences d'efficacité entre les antipsychotiques atypiques les plus récents et les médicaments les plus anciens sont marginales et les effets sur les symptômes négatifs et cognitifs sont faibles. Cet article présente les résultats d'études d'imagerie, d'études postmortem, et de tests de compétition pharmacologique afin de trouver un « circuit pathologique » corticolimbique dans la schizophrénie, qui serait particulièrement pertinent pour les symptômes négatifs et les déficits cognitifs dans cette maladie. Les sites potentiels de l'action pharmacologique visant la neurotransmission glutamatergique, GABAergique et cholinergique pour traiter ces symptômes sont analysés.
Limitations of antipsychotic medications in schizophrenia
To this day, the pharmacological management of schizophrenia is based upon the serendipitous discovery, over 50 years ago, of the antipsychotic effects of chlorpromazine.Citation1 Subsequent drug discovery for schizophrenia treatments was directed at identifying agents with comparable properties inferred by quite indirect criteria such as protection against apomorphine-induced canine vomiting or improvement in the conditioned avoidance response, while at the same time seeking increased potency and attenuated neurologic side effects.Citation2 CarlsonCitation3 proposed that antipsychotic drugs produced their therapeutic effects by blocking dopamine receptors. Advances in ligand-binding techniques led Snyder and Seemen to demonstrate that there was a specific and highly robust correlation between the clinical potencies of antipsychotics and their ability to block the dopamine D2 receptor.Citation5-Citation5 With the target of therapeutic action clearly identified, pharmacologists could then “build” into new agents other neurotransmitter receptor interactions to minimize side effects. However, these modifications, while virtually eliminating extrapyramidal side effects, introduced other serious problems including weight gain, hyperlipidosis, and glucose intolerance.Citation6
The introduction of antipsychotic medications was associated with the progressive decline in the number of patients held in state mental hospitals. The vast majority of these suffered from psychotic disorders, and the inference was that the antipsychotic medications had a profound impact on their care, permitting this deinstitutionalization. A less sanguine view would note that currently half of the homeless suffer from serious mental illness,Citation7 and that the number of prison beds on a percapita basis has largely replaced the closed mental hospital beds, consistent with a shift in the locus of confinement.Citation8 So, in spite of the semblance of substantial improvements in treatment of schizophrenia and related psychotic disorders, schizophrenia, which affects approximately 1% of the population, remains the seventh most costly medical illness to society, and is still associated with a life-long disability for the vast majority of patients suffering from the disease.Citation9
Results of recent clinical studies further raise concern over the modest advances that have been achieved over the last five decades in developing more effective drugs for treating schizophrenia. While meta-analyses comparing the first-generation antipsychotics to the secondgeneration antipsychotics do suggest some modest superiority of the second-generation antipsychotics, these effects are limited to positive symptoms known to be sensitive to D2 receptor antagonism.Citation10 In the large-scale CATIE (Clinical Antipsychotic Trials of Intervention Effectiveness) trial, Lieberman et alCitation11 compared several second-generation antipsychotics with a first-generation antipsychotic, perphenazine. The majority of patients in each group discontinued their antipsychotics owing to inefficacy or intolerable side effects. When clozapine was compared with other second-generation antipsychotics, it did exhibit modest but significant superiority over these other drugs. A separate study carried out in England, Cost Utility of the Latest Antipsychotic Drugs and Schizophrenia Study (CUtLASS 1), also found few differences in effectiveness between first-generation antipsychotics and second-generation antipsychotics in non-refractory patients.Citation12 As pointed out by Lieberman,Citation13 both the CATIE and the CUtLASS studies are “effectiveness” studies, which examine the therapeutic response in real-world clinical situations. This design is markedly different from the randomized clinical trial of “efficacy,” in which a new drug is compared with placebo in a very select group of patients subject to a myriad of exclusionary criteria. Thus, basing a drug discovery effort for schizophrenia on the assumption that it is primarily a disorder of dopaminergic dysfunction has led to the introduction of antipsychotics that are marginally more efficacious than their “progenitors,” chlorpromazine and haloperidol.
Starting about 20 years ago, psychopharmacologists began to focus on other components of schizophrenia rather than just the antipsychotic responsive positive symptoms (ie, hallucinations, delusion, and thought disorder). Negative symptoms including apathy, poverty of thought, anhedonia, lack of drive, disorganization, and social isolation were observed to covary independently of positive symptoms, be much more enduring, and correlate inversely with outcome.Citation14,Citation15 With advances in neuropsychology, much more rigorous testing delineated the specific impairments in memory, problem-solving, and executive functions, which were noted a century ago with the designation of “dementia praecox.”Citation16,Citation17 At the same time, progress in both structural and functional brain imaging revealed substantial cortical involvement in schizophrenia. On average, cortical volume is reduced and lateral ventricular volume is increased in individuals with a first episode of schizophrenia, and these differences increase over the next 5 to 10 years.Citation18,Citation19 Functional imaging studies demonstrate impairments in the ability to perform tasks that engage the prefrontal cortex or the hippocampus, which corresponds with their inability to activate these areas.Citation20 Diffusion tensor imaging (DTI) has shown abnormalities in white-matter tracts of frontotemporal, frontoparietal, and temporooccipital connections,Citation21,Citation22 providing further evidence for the presence of structural disconnectivity in schizophrenia. Finally, event-related potentials reveal disruption in cortical processing of sensory stimuli regardless of modalityCitation22 Thus, the preponderance of evidence supports the notion that schizophrenia is a progressive disorder that diffusely affects the corticolimbic system.
The N-methyl-D-aspartate receptor and schizophrenia
Dissociative anesthetics such as ketamine and phencyclidine (PCP) have been known since their introduction a half-century ago to produce in adults a syndrome difficult to distinguish from schizophrenia.Citation23-Citation24 While these drugs have complex interactions in the nervous system, Javitt and ZukinCitation25 noted that the psychotomimetic effects of PCP occurred at plasma concentrations that cause a noncompetitive, use-dependent antagonism of N-methyl-D-aspartate (NMDA) receptors.Citation26 Ketamine infused in normal volunteers at doses that do not cause delirium/dementia produced the full range of signs and symptoms of schizophrenia, with positive symptoms, negative symptoms, and the selective cognitive deficits.Citation27,Citation28 Subsequent studies showed that low-dose ketamine caused in normal volunteers the physiologic abnormalities associated with schizophrenia, including abnormal event-related potentials,Citation29 eye-tracking abnormalitiesCitation30 and enhanced subcortical dopamine release.Citation31 Individuals with stabilized schizophrenia exhibited marked sensitivity to ketamine with recurrence of individual specific symptoms.Citation32
With a greater availability of brain tissue for histologic and neurochemical analyses, a number of findings have crystallized over the last 15 years as they have been confirmed in different laboratories using a variety of techniques including quantitative neurochemistry, immunocytochemistry, in situ hybridization, and DNA chip arrays. One of the first neurochemical abnormalities described in postmortem studies in schizophrenia was a reduction in the cortical activity of glutamate decarboxylase (GAD), the enzyme that synthesizes γ-amino butyric acid (GAB A), in the cortex.Citation33 More recent studies have revealed a much more selective effect primarily on the parvalbumin (PV+) -expressing, fast-firing GABAergic interneurons in the intermediate layers of the cortex and in subsectors of the hippocampus that provide recurrent inhibition to the pyramidal cells.Citation34 Citation35 Thus, the reduction in the expression of GAD67, PV, and the GABA transporter has been demonstrated in this neuronal population.Citation36 That the downregulation of these presynaptic markers reflects reduced activity of these GABAergic neurons is inferred by the compensatory upregulation of postsynaptic GABAA receptors and its a2-containing subunit.Citation37 Another recurrent finding from Golgi-stain studies and more recent immunocytochemistry of spinophilin, a protein enriched in dendritic spines, is the reduction in dendritic complexity and spine density on pyramidal neurons in several cortical regions, consistent with the overall cortical atrophy in schizophrenia.Citation38,Citation39
These core pathologic features of schizophrenia have been linked to NMDA receptor hypofunction. Several studies have demonstrated that subacute treatment of rats with dissociative anesthetics results in a downregulation of GAD67 and PV expression in the GABAergic neurons in the intermediate layers of the cortex and a consequent disinhibition of pyramidal neuronal firing.Citation40 Citation41 This disinhibition of the pyramidal neurons is consistent with the results of functional imaging studies in the hippocampus, as well as the elevated evoked subcortical dopamine release in normal individuals challenged with ketamine.Citation31 The paradoxically reduced firing of the PVGABAergic interneurons may be secondary to the decreased flux of calcium through their NMDA receptors, which causes a misperception of reduced excitatory drive.Citation42 NMDA receptors also play an important role in dendritic elaboration and spine development.Citation43 Mice that are homozygotes for a null mutation of serine racemase, the enzyme that synthesizes D-serine, exhibit marked reduction in NMDA receptor function.Citation44 Cortical pyramidal neurons of these serine racemase knockout mice have significantly reduced dendritic complexity and spine density, as compared with their wild-type littermates, with the pathology quite similar to that observed in schizophrenia.Citation45
Schizophrenia is a disorder with a high degree of heritability, and recent genetic studies have provided support for a role for NMDA receptors in this disorder. Most of the evidence is derived from association studies, although that strategy has come under criticism by advocates of “unbiased” genome -wide association study (GWAS) strategy. Meta-analysis has strongly implicated the gene encoding D -amino acid oxidase (DAAO), which regulates the availability of D-serine, as well as G72, a gene encoding a protein that binds to and inhibits DAAO (for review, see ref 42). Meta-analysis has also pointed to NR2B, a component of the NMDA receptor, as a risk gene for schizophrenia.Citation46 Other risk genes include neuregulin 1, which among other actions directly modulates NMDA receptor activity,Citation47 and dysbindin, which is concentrated in glutamatergic terminals.Citation48 Integrating the postmortem, genetic, and animal modeling results has suggested a plausible pathologic circuit in schizophrenia (Figure 1) . Hypofunction of corticolimbic NMDA receptors on the fast-firing PV+-GABAergic interneurons in the intermediate layers of the cortex results in downregulation of GAD67 and PV expression, reduced inhibitory postsynaptic potentials (IPSPs), and disinhibition of the postsynaptic pyramidal cells.Citation42 NMDA receptor hypofunction can be due to elevated endogenous inhibitors such as kynurenic acid or N-acetyl aspartyl glutamate (NAAG), reduced availability of the endogenous co-agonist D-serine, or heritable abnormalities in NR2B expression or function. Electrophysiological correlates include loss of gamma-band responses to sensory stimuli and elevated neuronal activity in the default mode.Citation49 Disinhibition of glutamatergic output from the ventral hippocampus would drive the firing of dopaminergic neurons in the ventral tegmental area and enhanced subcortical dopamine release, which in PET studies correlates with psychosis.Citation50 Thus, in this model, psychosis is a downstream event.
Figure 1. Schematic representation of the synaptic circuitry relevant to the pathophysiology of schizophrenia. NMDA receptor hypofunction can be produced by exogenous antagonists such as ketamine, endogenous antagonists such as N-acetyl aspartyl glutamate (NAAG) or kyneurenic acid, reduced availability of D-serine due to increased activity of D-amino acid oxidase (DAAO) or mutant NR2B. This results in dendritic dysplasia on pyramidal neurons and reduced activity of the parvalbumin positive GABAergic interneurons. Reduced recurrent inhibition disrupts cortical processing, causing cognitive impairment and negative symptoms and increased excitatory drive to the ventral tegmental area (VTA), leading to psychosis. An allelic variant of the gene encoding the α7 nicotinic receptor causes reduced expression and disrupts sensory gating. NMDA, N-methylD-aspartate; GABA, γ-aminobutyric acid; DA, dopamine; NBM, nucleus basalis of Meynert; nAchR, nicotinic acetylcholine receptor; Glu, glutamate
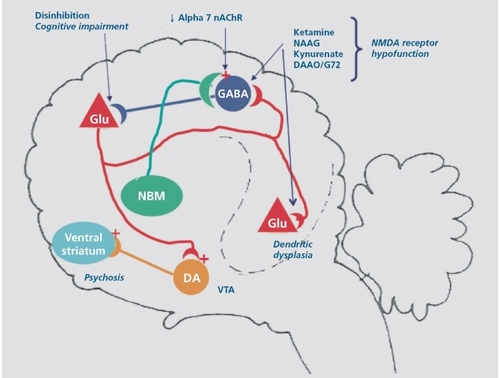
Hypofunction of NMDA receptors could account for other aspects of the disorder. First, given the role of NMDA receptors in neuronal migration,Citation51 it could account for the finding of abnormal distribution of cortical GABAergic interneurons in some cases.Citation52 Secondly, persistent hypofunction of NMDA receptors is consistent with the reduced pyramidal neuron dendritic complexity, reduced spine density, and net compaction of the neuropil in schizophrenia.Citation37
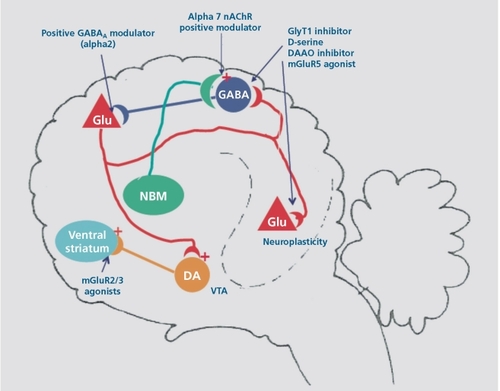
Obviously, the pathophysiology of schizophrenia is much more complex and nuanced than suggested by this simplified model. Indeed, a number of putative risk genes encode transcriptional factors that affect brain development.Citation53 Other risk genes encode products involved in myelination.Citation54 Furthermore, in recognition of the variation in symptoms among patients who satisfy the diagnostic criteria for schizophrenia and its complex genetics, where literally hundreds of genes of modest effect might be involved, the proposed “pathologic circuit” represents at best a crude first approximation of the pathophysiology of schizophrenia. Nevertheless, it does yield a host of potential targets for therapeutic intervention, and many of these are under investigation by the pharmaceutical industry. It is these potential therapeutic targets related to this circuit that are the subject of this review (Figure 2). Of particular interest is the fact that these targets would intervene in the primary cortical pathology of schizophrenia and thus potentially treat the negative symptoms and cognitive deficits.
Figure 2. Potential pharmacologic interventions to treat schizophrenia: (i) Enhance NMDA receptor function by increasing synaptic glycine concentrations with an inhibitor of GlyT1 , administering exogenous D-serine, inhibiting D-amino acid oxidase or by treating with an mGluR5 agonist that augments NMDA receptor function; (ii) Increase the excitability of the parvalbumin-positive GABAergic interneurons with a c 7nicotine receptorpositive modulator; (iii) Reduce pyramidal neuron excitability with GABAA receptor-positive modulator. (iv) Decrease disinhibited pyramidal neuron glutamate release with an mGluR2/3 agonist. NMDA, N-methyl-D-aspartate; GABA, γ-aminobutyric acid; DA, dopamine; NBM, nucleus basalis of Meynert; mAchR, metabotrophic acetylcholine receptor; Glu, glutamate; DAAO, D-amino acid oxidase
Targeting the glutamatergic synapse
Structure and function of the NMDA receptor
The NMDA receptor, with its triple gate for activation, is a critical postsynaptic mediator of activity-dependent synaptic plasticity. Throughout most of the brain, the heterotetrameric receptor is composed of two NR1 subunits and two NR2 subunits, all of which contribute transmembrane domains to the pore of the ion channel. The NR1 subunit has eight different splice variants, which may affect channel function differently by associating with different intracellular signaling pathways.Citation55 NR2 subunits may be expressed in four different forms (NR2AD), and in some regions of the nervous system may be substituted by two different forms of NR3 subunits, each of which confer different biophysical and pharmacologic properties to the channel.Citation56 Mg2 occludes the ion channel at resting membrane potential. Hence, opening of the “voltage gate” by expelling Mg2+ with depolarization of the postsynaptic cell is one requirement for conductance through the channel. A second requirement is opening of the “ligand gate” by agonist binding at glutamate binding sites on the NR2 subunits. A third requirement is agonist binding at glycine modulatory sites (GMS, also the Glycine B receptor) on the NR1 channel-encoding subunit.Citation57 Endogenous polyamines also modulate NMDA receptors by potentiating the action of glutamate.Citation58 Dissociative anesthetics gain access to and bind within the NMDA receptor channel pore when the channel is open, and as such are both noncompetitive and usedependent antagonists.Citation59,Citation60
The key roles that the NMDA receptor is known to play in neurodevelopment and in activity-dependent plasticity make it all the more plausible as a contributor to the pathophysiology of schizophrenia, particularly deficits in cognitive function. Because it opens only when the postsynaptic neuron receives several simultaneous excitatory inputs to sufficiently depolarize it so as to relieve the Mg2+ blockade, the NMDA receptor functions as a molecular coincidence detector. The NMDA receptor ion channel is characterized by high Ca2+ permeability, and the influx of Ca2+ triggers a cascade of intracellular events that mediate local, acute synaptic plasticity as well as changes in gene expression that influence long-term neural plasticity and have trophic effects.Citation61,Citation62 Whether or not symptoms of schizophrenia are caused in part by hypofunctional signaling through NMDA receptormediated pathways, enhancing NMDA receptor-mediated activity may improve cognition and neural plasticity, thereby reducing the debilitating negative and cognitive symptoms. On the other hand, a significant risk in pursuing NMDA receptor activation as a therapeutic pathway is that of excitotoxic damage to the brain, which can result from excessive activation of NMDA receptors.Citation63 With this caveat in mind, efforts to treat symptoms of schizophrenia through the NMDA receptor have focused on positive modulation of the receptor rather than increasing agonist binding at the glutamate site.
The glycine modulatory site
The GMS of the NMDA receptor is a potentially rich target for therapeutics. Despite the presence of endogenous high potency agonists glycine and D-serine,Citation64,Citation65 the GMS is not saturated in vivo,Citation66,Citation67 supporting the idea that administration of GMS agonists could benefit patients by enhancing activation of NMDA receptors. Furthermore, evidence of reduced cerebrospinal fluid (CSF) and serum D-serine levels in schizophrenic patientsCitation68,Citation69 as well as evidence of elevated levels of the endogenous GMS antagonist kynurenate in postmortem brain and CSFCitation70,Citation71 suggest that the GMS occupancy is downshifted or shifted toward antagonism in the disease state.
There have been more than 80 clinical trials of agents that increase agonist occupancy of the GMS in schizophrenia, including D-serine, glycine, D-cycloserine, Dalanine, and sarcosine. Several of these studies have reported significant improvements over multiple symptom domains while others have not. Aside from intrinsic differences in efficacy between candidate GMS regulators, methodological factors likely contribute to the variability in results among these trials, most notably small sample sizes, variability in concomitant typical and atypical antipsychotic use, and subject compliance. Also, important to consider from the point of view of evaluating the promise of the GMS strategy, the majority of these trials have been conducted using glycine and/or the partial GMS agonist D-cycloserine, which are not the most potent agonist of the site. Studies employing cloned NMDA receptors expressed in a Xenopus oocyte system suggest the potency of D-serine is about three times that of glycine,Citation72 and D-cycloserine is a partial agonist with only about half the efficacy of glycine at the GMS.Citation73 Still, glycine and D-cycloserine have been more widely tested than D-serine due to historical approval of these agents for human use, glycine as a nonessential amino acid, and D-cycloserine as a second-line antibiotic effective against Mycobacterium tuberculosis.
A recent meta-analysis of strategies to enhance NMDA receptor-mediated neurotransmission in schizophrenia reported the striking finding that NMDA-enhancing molecules as a whole exerted statistically significant effects on total psychopathology, depressive symptoms, negative symptoms, cognitive symptoms, positive symptoms, and general psychopathology in descending order of effect size.Citation74 The meta-analysis included results from 26 double-blind, placebo-controlled clinical trials in which the treatment lasted at least 4 weeks. Agents tested were glycine, D-cycloserine, D-serine, sarcosine, and D-alanine. Pooling of data from different studies was made possible by including only those for which enough data were available to calculate a standardized metric of the degree of improvement seen in a particular symptom domain relative to placebo, or the effect size (ES). There was some heterogeneity in the trials that were included, in that patients enrolled were administered concomitant typical or atypical antipsychotics and in others were not. Also, trials of chronic stable and acutely exacerbated schizophrenia were included. When the effects of different molecules were assessed separately, glycine was found to have significant effects on total psychopathology, positive symptoms, and depressive symptoms. D-serine was found effective on total psychopathology, negative symptoms, and cognitive symptoms. Sarcosine, an endogenous inhibitor of the glycine transporter, was effective on total psychopathology, negative symptoms, and general psychopathology. D-cylcoserine was not effective on any domain of schizophrenia symptoms. However, if the trials that use clozapine as the antipsychotic are excluded, the duration of exposure restricted and compliance controlled the data suggest that D-cycloserine significantly reduces negative symptoms.Citation63
The findings of the meta-analysis by Tsai and LinCitation74 provide some interesting new illumination for the results of the largest individual study to date of glycine and Dcycloserine, a multicenter trial called the Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST). The CONSIST study found no statistically significant effects of either glycine or D-cyloserine on negative symptoms or cognitive performance in patients with chronic schizophrenia. Previous smaller studies of high doses of glycine administered concurrently with typical and atypical antipsychotics had reported improvements negative and cognitive symptoms.Citation75-Citation77 High doses were purported to be required to achieve sufficiently high serum glycine levels for clinical efficacy, and difficulty with compliance was noted. The CONSIST study did report a significant effect of site (P<0.01), as well as lower serum levels of glycine than was achieved in previous studies. Thus, one of the concerns raised about the interpretation of this study with respect to its results with glycine was that variability in patient compliance between inpatient and outpatient clinics could account for the negative result. Indeed, restricting the results to those obtained with inpatients, for whom compliance was not in question, both glycine and D-cycloserine significantly (P<0.03) reduced negative symptoms. However, the results of the meta-analysis, which includes the CONSIST study, showed that when double-blind, placebo-controlled trials with glycine were considered together, glycine still had no significant effect on negative symptoms, but rather was effective on positive and depressive symptoms, which were not assessed by the CONSIST. Nonetheless, as serum levels of glycine were not part of the meta-analysis, the lack of any significant dose-response with glycine on negative symptoms, positive symptoms, or total psychopathology is still open to the question of whether compliance is a major issue in evaluating outcomes. Despite the negative findings of glycine efficacy with respect to negative and cognitive symptoms, the issues of compliance and serum levels may still be highly relevant to schizophrenia therapy, given the significant effects of glycine on positive and depressive symptoms, particularly for patients who do not respond to antipsychotics or are experiencing adverse side effects of clozapine.
Consistent with its action as a partial agonist, an initial dose-finding study with D-cycloserine added on to conventional neuroleptics reported a U-shaped doseresponse curve with an intermediate dose that improved negative as well as cognitive symptoms.Citation78 Given that the GMS is not saturated in vivo, one might speculate that a partial agonist would augment the activity of the NMDA receptor up to a point and then actually begin to compete with the endogenous agonist. Furthermore, this point of inflection in the nature of the D-cylcoserine effect may vary depending on the individual patient's level of GMS saturation. D-cylcoserine may in any case be an impractical approach for prolonged treatment, as NMDA receptor desensitization has been observed with chronic administration.Citation79 The apparent lack of consistent success of D-cycloserine use in schizophrenia stands in contrast to the positive results observed with it in extinction therapy for specific phobias. The extinction of a conditioned fear memory is an NMDA-dependent process,Citation80 which can be enhanced by positive modulation of the GMS.Citation81,Citation82 D-cycloserine has been effective in treating acrophobia in combination of a virtual reality-based cognitive behavioral therapyCitation83,Citation84 Thus, a key difference between the successful application of D-cycloserine in anxiety disorders and the unsuccessful application in schizophrenia may be that the in the former it is used acutely or subchronically as an adjunct to concomitant activation of specific brain circuitry related to specific fear or phobia.
D-serine itself is a potential therapy, as it has been shown in rodents to be relatively efficient at crossing the blood-brain barrier upon peripheral administration compared to glycine,Citation85 and can persist in cortex thereafter.Citation86 In contrast to other GMS agonists, there is direct indication that D-serine is affected in schizophrenia, as it is decreased in CSFCitation69 and serum from patients.Citation68 The significant effects of D-serine on total psychopathology, negative symptoms, and cognitive symptoms found in the Tsai and LinCitation74 meta-analysis are based only on small trials that tested it as an add-on therapy to typical or atypical antipsychotics. In the case of D-serine as well as other agents, testing in conjunction with typical or atypical antipsychotics may occlude potential effects on positive symptoms, which are relatively well controlled with available antipsychotics. Large Phase II trials of D-serine in schizophrenia and schizophrenia prodrome are currently underway, both as an add-on to antipsychotics and as a monotherapy.
An intriguing pattern in the literature on GMS agonists, corroborated by the meta-analysis, is that they are ineffective when combined with clozapine as opposed to other antipsychotics. These results together could be explained by an effect of clozapine on GMS occupancyCitation75 The mechanism of putative clozapine interaction with NMDA receptors is not yet known, but increased NMDA-mediated currents have been observed in the presence of clozapine in rat frontal cortex,Citation87 and effects on glycine transport have been proposed.Citation88 If clozapine, which is superior to other atypical antipsychotics in treating negative and cognitive symptoms,Citation10 works through the GMS, it may be possible to achieve comparable benefits without the troubling side effects of clozapine such as agranulocytosis, weight gain, and metabolic syndrome by using other agents that enhance GMS occupancy.
D-amino acid oxidase
The peroxisomal enzyme D-amino acid oxidase (DAAO) converts D-serine to hydroxy-pyruvate in the brain, yielding hydrogen peroxide as a by-product.Citation89 DAAO expression was originally believed to be restricted to astrocytes in the mammalian cerebellum,Citation90 but has since been observed in neurons.Citation91 Inhibitors of DAAO would be expected to increase D-serine in the brain, and could thereby increase GMS occupancy. Direct evidence of involvement of DAAO in schizophrenia is somewhat controversial. DAAO has been implicated as a putative schizophrenia gene by linkage and association methods, but meta-analyses have revealed that the disease-associated variants of the gene are different across studies,Citation92,Citation93precluding a simple functional hypothesis based on the findings. Postmortem studies of brain DAAO expression in schizophrenia have reported elevated transcript levels and enzyme activity.Citation69,Citation94-Citation96 G72, a mysterious putative interacting protein of DAAO, is coded for in a linkage region identified for schizophrenia by multiple studies, and considered one of the strongest genetic risk factors for schizophrenia identified using linkage analysis. The link between G72 and DAAO originates from a yeast 2-hybrid study from which DAAO emerged as a G72 inter-actor.Citation97 An in vitro functional assay suggested that G72 protein is an activator of DAAO; but more recent studies demonstrate that it inhibits DAAO. According to this conceptualization, mutations in G72 would result in disinhibition of DAAO, thereby reducing the availability of D-serine. However, despite significant attention paid to it pursuant to its repeated appearance in the schizophrenia genetic literature, to date the protein has been observed only in heterologous expression systems. It should be noted that DAAO activity is not specific to D-serine, so manipulating the activity of this enzyme can affect the levels of other D-amino acids.
Several pharmaceutical companies have established DAAO inhibitor programs. While there are no published clinical data, preclinical studies have revealed promising behavioral effects. Adage et alCitation98 reported that DAAO inhibitor, AS057278, significantly increased cortical Dserine, corrected PCP induced prepulse inhibition (PPI) deficits and normalized PCP-induced hyperactivity, a behavioral surrogate for psychosis. Hashimoto et alCitation99 found that combining D-serine with the DAAO inhibitor, 5-chloro-benzo[d]isoxazol-3-ol (CBIO), markedly increased cortical D-serine levels and corrected dizocilpine-induced (MK801) PPI deficits. However, another DAAO inhibitor, in spite of elevating CSF D-serine levels, failed to normalize amphetamineinduced hyperactivity and MK801-induced disruption of cognition. As D-serine treatment was effective, it appears that DAAO inhibition must be greater than 80%, the upper limit achieved by their drug.Citation100
D-Serine synthesis and transport
D-serine, the highest-affinity endogenous GMS agonist, is synthesized from L-serine by the pyridoxal 5'-dependent enzyme serine racemase. Polymorphisms in the 5' untranslated region of the serine racemase gene, which may be functionally related to levels of its promoter activity, have been associated with schizophrenia.Citation101-Citation103 Like DAAO, serine racemase was originally believed to be restricted to astrocytes in its localizationCitation104 but has since been observed in neurons.Citation105,Citation106 Genetic knockout of serine racemase leads to a reduction of 80% to 90% in brain D-serine in mice.Citation107 The origin of the remaining 10% to 20% is unknown but may be diet and/or bacterial flora. D-serine levels within the synapse are regulated by the arginine-serine-cysteine transporter, ASC-1,Citation108 which is localized to neuronal somata and dendrites.Citation109,Citation110 Inhibitors of ASC-1 have been proposed as therapeutics in schizophrenia,Citation111 as they would presumably elevate levels of extracellular brain D-serine. On a cautionary note, constitutive ACS-1 gene deletion in mice has been shown to cause tremors, seizures, and early postnatal death.Citation112
GlyT1 inhibitors
The concentration of glycine in mammalian CSF is high relative to its dissociation constant (Kd) for the GMS, but local glycine levels are functionally regulated at the synapse by the sodium-dependent glycine transporter-1 (GlyT1) expressed in astrocytes.Citation113,Citation114 The activity of GlyT1 is itself endogenously regulated by sarcosine (Nmethylglycine), an intermediate and byproduct in glycine synthesis and degradation. Electrophysiological studies in rodents suggest that inhibition of GlyT1 is more effective than exogenous application of glycine at potentiating NMDA receptor-mediated neurotransmission. For example, in an acute hippocampal slice preparation, NMDA receptor-mediated excitatory postsynaptic potentitals (EPSPs) in CA1 hippocampal pyramidal neurons were potentiated robustly by the sarcosine analog N[3-(4'-fluorophenyl)-3-(4'-phenylphenoxy)-propyl] sarcosine (NFPS), whereas perfusion with high concentrations of glycine (1 or 10 µM) had relatively little effect.Citation67 Similar findings have been reported in acute frontal cortical slices.Citation115 Systemic treatment with NFPS increased NMDA receptor currents and LTP in the dentate gyrus and enhanced prepulse inhibition of the acoustic startle response.Citation116
Sarcosine administered to patients in conjunction with antipsychotics has shown some promise for treatment of schizophrenia. A meta-analysis of clinical trials testing the efficacy of GMS-enhancing agents found it effective on total psychopathology, negative symptoms, and general psychopathology.Citation74 However, undesirable side effects of sarcosine-derived GlyT1 inhibitors have also been noted, including ataxia, hypoactivity, and decreased respiration, prompting the development of novel classes of non-sarcosine-based inhibitors of GlyT1.Citation117 Several GlyT1 inhibitors are in the early stages of clinical trials; and Hoffman-LaRoche has reported that their GlyT1 inhibitor caused significant reductions in overall symptoms and especially negative symptoms in a Phase-II clinical trial in schizophrenia.
Metabotropic glutamate receptors (mGluRs) as therapeutic targets
Characteristics of mGluRs
While ionotropic glutamate (iGlu) receptors (AMPA, kainate and NMDA subtypes) serve as the mediators of excitatory (glutamatergic) signaling, G-protein coupled metabotropic glutamate (mGlu) receptors act as modulators of excitatory signaling. Given the increased interest in the pathophysiological impact of dysfunctional glutamate signaling and their role as modulatory receptors, mGluRs have become a major target for the development of therapeutics for schizophrenia and other psychiatric disorders.Citation118-Citation121 The mGluRs are members of Class C of the G-protein coupled receptor superfamily. Eight subtypes of mGluRs have been identified and divided into three groups, based upon pharmacology, sequence homology, and G protein coupling: Group I (mGlu1 and mGlu5), Group II (mGluR2 and mGluR3), and Group III (mGluR4, mGluR6, mGluR7, and mGluR8) (for review, see ref 122). Each of these receptors possesses a distinct expression pattern that relates to physiological control over glutamatergic neurotransmission at various levels including neurotransmitter release, function of postsynaptic iGluRs, glial function, and neuroplastic changes in postsynaptic neurons. These discrete functions make these receptors very attractive targets for pharmacological intervention. Group I and II receptors have notably risen in interest as potential treatments for schizophrenia because of their ability to normalize dysfunctional glutamatergic neurotransmission thought to be a core feature of the disorder.
Group II mGluRs
Group II mGluRs are promising therapeutic targets because of their role as autoreceptors in the regulation of glutamate release from nerve terminals. Activation of Gai/o-coupled mGlu2/3 receptors attenuate electrically evoked excitatory neurotransmission.Citation123 Pharmacologically evoked and spontaneous excitatory currents are attenuated by mGluR2/3 activation, with effects predominantly on the frequency of currents, supporting a presynpatic mode of activityCitation124,Citation125 Preclinical observations have been made that psychotomimetic drugs that act as noncompetitive blockers of NMDA receptors (eg, PCP, ketamine, MK801) cause an increase in synaptic glutamate levels in the prefrontal cortex (PFC).Citation126,Citation127 As reviewed above, the deficient PV+-GABAergic neuron function found in postmortem studies in schizophrenia has led to the hypothesis that the pathophysiology of schizophrenia involves a disinhibition of cortical glutamatergic neurons and that group II mGluR-mediated reduction in glutamate release account for their antipsychotic action.Citation128-Citation130 The development of ligands selective for mGlu2/3 receptors has allowed for the examination of this hypothesis in preclinical models of schizophrenia.
(1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane4,6-dicarboxylic acid (LY379268) and (1S,2S,5R,6S)-2-Aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid) LY354740 are highly selective agonists of mGlu2/3 receptors possessing >100-fold selectivity over other subtypes of mGluRs.Citation131 These ligands have been shown to reverse the behavioral disruptive effects of the psy-chotomimetic PCP in numerous paradigms including stereotypy and hyperactivity,Citation126,Citation132-Citation136 social interactions and cognition.Citation137,Citation138 These ligands also display apparent antipsychotic efficacy by inhibiting the behavioral effects of psychedelic hallucinogens that influence glutamater-gic signaling via serotonin 2A receptors,Citation139 an effect linked to the inhibition of glutamate release from nerve terminals.Citation124 A structurally related compound, (-)-(1R, 4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic (LY404039), administered via a prodrug form, exhibited promising efficacy in a Phase II clinical trial, reversing positive and negative symptoms in schizophrenic patients as a standalone therapy.Citation140 This therapeutic efficacy was similar to that of olanzapine and was achieved without any of the side effects of commonly prescribed antipsychotics such as elevated prolactin, weight gain, and extrapyramidal symptoms. The achievement of this clinical trial is twofold; it: (i) provides proof of concept for the development and application of glu-tamatergic based therapeutics and (ii) demonstrates the predictive validity of the PCP/ketamine model of schizophrenia. This second point was initially supported by research demonstrating that the cognitive-disruptive effects of ketamine in humans were indeed attenuated by an mGlu2/3 receptor agonist.Citation141
The work with mGluR2/3 receptor agonists also highlights another key mechanistic point about potential schizophrenic therapies: they need not reverse hyper-dopaminergic neurotransmission. All current therapies block D2 receptors to some degree, which has been assumed to be necessary for therapeutic efficacy. The work of Moghaddam and AdamsCitation127,Citation138 illustrates that the major element of psychotomimetic drug (PCP or ketamine) action is to stimulate glutamatergic neurotransmission (paradoxical to the action of these drugs as NMDA receptor blockers), with dopamine release coincidental. Notably, mGluR2/3 agonists achieve behavioral effects that are paralleled by inhibition of drug-induced glutamate efflux without affecting drug-induced increases in extracellular dopamine levels measured by in vivo microdialysis.Citation126 Single-unit recordings in awake rats are further illustrative; mGluR2/3 receptor agonists reversed NDMA receptor blocker-induced disinhibition and dysregulation of prefrontal pyramidal neuron firing.Citation142 The results of these studies suggest that antipsychotic efficacy can be achieved in the absence of a direct effect on forebrain dopamine, an effect alluded to in earlier research showing a temporal disconnect between the behavioral effects of PCP and modulation of DA, but not glutamate, brain levels.Citation126
Positive allosteric modulation of mGlu2 receptors
Efforts to refine the mGlu2/3 agonists have focused upon finding a ligand that selectively activates mGlu2 receptors. Discriminating between mGluR2 and mGluR3 subtypes has been difficult, as they share >90% sequence homology. Expression studies suggest mGluR2 are predominately localized to presynaptic sites,Citation143 while mGluR3 are localized more postsynaptically and in glial cells.Citation144 Using mGluR2-deficient mice, the apparent antipsychotic effects of mGluR2/3 agonists have been attributed to mGluR2 activation.Citation145,Citation146 These studies demonstrate the potential for selective activiation of mGluR2; however, efforts to develop agonists of the glutamate-binding (orthosteric) site have not surprisingly fallen short. Recently, greater efforts have been undertaken to pursue ligands that activate the receptor through sites other than agonist binding site, termed allosteric sites. The success of these efforts illustrate that while the orthosteric site is highly conserved between the two receptors, allosteric sites are located in less conserved regions of the receptor and can be selectively targeted to modulate agonist-induced signaling.Citation147
Allosteric modulators can be either positive or negative in direction of activity, causing an increase or decrease, respectively, in the activity of orthosteric ligand induced signaling by altering agonist affinity and/or efficacy of G-protein coupling.Citation148 In the case of mGlu2 receptors, efforts have been directed towards identifying positive allosteric modulators (PAMs). To date, numerous PAMs haven been identified and shown to possess selective efficacy to enhance agonist activity at mGlu2 receptors with dramatic selectivity over other targets.Citation135,Citation149,Citation150 These ligands increase the ability of endogenous glutamate and exogenous agonists to reduce evoked excitatory postsynaptic potentials in brain slice preparations.Citation135,Citation139,Citation149,Citation151 Behavioral studies show that mGlu2 receptor PAMs possess efficacy similar to that of mGluR2/3 agonists, reducing PCP induced locomotion,Citation134,Citation135 decreasing fearpotentiated startleCitation150,Citation151 and diminishing hallucinogen-induced stereotypies.Citation139 Interestingly, one study showed that one PAM, biphenyl-idanone A (BINA), was capable of uniquely reducing PCP-induced deficits in PPI. These studies demonstrate the validity and therapeutic potential of selectively targeting mGluR2. While issues of in vivo potency remain for currently available ligands, PAMs possess potential benefits. The selective potentiation of endogenous signaling would work to enhance the activity-dependent neurotransmission, while avoiding the potentially deleterious effects of persistent receptor activation, notably desensitization and tolerance.Citation135
Cystine-glutamate exchanger
Discussion of mGluR2 activation has focused on the development of ligands that directly target these receptors with either direct agonists or PAMs that require endogenous glutamate for activity. An additional way to enhance activity at these receptors is through the modulation of extrasynaptic glutamate levels. The cystineglutamate exchanger maintains 60% of the extra-synaptic glutamate concentration.Citation152 The exchanger is located on the glial cell membrane and releases glutamate in a 1:1 ratio with the import of cystine.Citation153 Activation of this exchanger produces a reduction in excitatory neurotransmission by mGluR2/3-dependent mechanism.Citation154 The compound N-acetylcysteine (NAC) is a substrate for this exchanger that substitutes for cystine. The work of Kalivas and colleagues has done much to demonstrate the behavioral effects of NAC in a preclinical model of drug-seeking behavior used to understand addiction. In these studies, NAC reduces drug-seeking behaviors and reinstatement of drug consumption after extinctionCitation152 Citation155 in a manner that is similar to the effects of an mGluR2/3 agonist.Citation156 More germane to the current review, recent work has demonstrated the ability of NAC to reverse PCP-induced deficits in cognition and social interactions, as well as PCP-induced activation of glutamate release in the prefrontal cortex of rodents.Citation157
These findings provide an additional context for the interpretation of results of a recent clinical trial with NAC (or placebo control) given as an adjunct therapy to schizophrenic patients.Citation158 The investigators saw a significant improvement over placebo in Positive and Negative Symptom Scale (PANSS) negative scale and overall Clinical Global Impression (CGI-S) after 24 weeks of treatment. General functioning (Scale of Global Assessment of Functioning) improved within a comparison of NAC-treated patients, but not as a comparison to placebo treatment (NB, within-group comparison of placebo treatment was not significant). The investigators undertook this clinical trial to test the effect of restoring glutathione deficiency in schizophrenia as a treatment. Cystine and correspondingly NAC are precursors to the production of glutathione, a molecule necessary for the protection against the effects of reactive oxygen species.Citation153 Is the physiological effect of NAC treatment prevention of oxidative damage or a restoration of glutamatergic tone on presynaptic mGluR2/3? In spite of preclinical studies demonstrating that mGluR2/3 antagonists block the effects of NAC, further studies need to be done to clarify this point. Perhaps the beneficial effects of NAC are twofold at the molecular level. Given the sensitivity of NMDA receptor function to redox state,Citation159,Citation160 this might be an ideal way to use a single therapy to target NMDA receptor hypofunction and oxidative stress.
Activation of mGlu5 receptors
While development of ligands targeting group II mGluRs is focused on reversing excessive, dysfunctional glutamate release downstream of cortical disinhibition, mGluR5 selective activators are sought to directly reverse NMDA receptor hypofunction though enhancement of the ionotropic receptor activity. A functional link is formed between Gaq -coupled postsynaptic mGlu5 receptors and NMDA receptors by the scaffolding protein Homer and Shank interacting with the postsynaptic densityCitation161 NMDA receptor signaling in hippocampal slices is selectively potentiated by the mGlu5 agonist (RS)-2-Chloro5-hydroxyphenylglycine (CHPG).162163The specificity for mGluR5 versus mGluR1, of this effect on NMDA receptor currents is further demonstrated by the absence of potentiated signaling in the presence of mGluR5 (but not mGluR1) antagonists.Citation163,Citation164
Available mGluR5 agonists suffer from poor brain penetration. As a result, much of the in vivo preclinical work demonstrating the role of mGlu5 receptors was done using the centrally active mGluR5-selective antagonist 2-Methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP). MPEP potentiates the locomotor hyperactivityCitation165-Citation167 and PPI disruptionCitation165-Citation167 caused by either PCP or MK801. These effects were seen without any effect on activity or PPI in the absence of PCP/MK801. MPEP also enhances the detrimental effects of PCP/
MK801 in cognitive tasks of working memory and instrumental learning.Citation167,Citation168 In vivo single-unit recordings show that MPEP enhances the MK801-induced increase in neuronal activity, thereby linking the behavioral findings back to the electrophysiology.Citation169
Like the Group II mGluRs, recent research demonstrates that the most effective strategy to selectively activate mGlu5 versus mGlu1 may be through the use of PAMs. Two unique PAMs, 3-Cyano-N-(1,3-diphenyl1H-pyrazol-5-yl)benzamide (CDPPB) and (S)-(4fluorophenyl)-(3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol5-yl]piperidin-1-yl)methanone (ADX47273), have been developed and shown to display dramatic mGluR5-selectivity and the ability to increase the efficacy of glutamate to activate mGlu5-mediated potentiation of NMDA receptor signaling.Citation166,Citation170 Furthermore, the PAMs are systemically active and display antipsychotic-like properties, blocking amphetamine-induced hyperactivity,Citation166,Citation170,Citation171 PCPinduced hyperactivity,Citation170 and amphetamine/apomorphineinduced disruption of PPI.Citation166,Citation171 In the 5-choice serial reaction time task, ADX47273 reduced impulsive errors.Citation170 Taken together these results demonstrate the potential antipsychotic-like ability of mGlu5 receptor PAMs to reduce the behavioral effects of multiple classes of psychotomimetics as well as produce procognitive effects. The efficacy in models of disrupted PPI is a divergence from that of mGluR2/3 agonists and suggests that these two approaches might have distinct therapeutic profiles. The limited preclinical testing and absence of any clinical demonstration of mGluR5 activation as a therapeutic target in schizophrenia temper enthusiasm. However, the demonstrated ability to enhance NMDA receptor signaling at the neuronal level will encourage the future development and testing of mGluR5 ligands.
GABAA receptors as therapeutic targets for schizophrenia
GABAergic pathology in schizophrenia
There is now substantial evidence that GABA signaling is deficient in corticolimbic regions, particularly in the dorsal lateral prefrontal cortex (DLPFC) and hippocampus, of patients with schizophrenia. One of the most consistent postmortem findings in schizophrenia is a reduction in the mRNA expression level of GAD67 in PV+-GABAergic interneurons, as well as reductions in PV expression itself.Citation172 PV+ interneurons exhibit fast-spiking firing properties and target the spike -initiating region of pyramidal neuron axons, and are therefore thought to play a key role in controlling the overall firing properties of brain networks. Recent pharmacological, immunological, and genetic evidence from animal models suggests that inflammatory cytokine exposure (increased oxidative stress) and NMDA receptor hypofunction occurring during cortical development leads to permanent disturbances in neuronal circuits, specifically in the population of PV-containing interneurons.Citation173
The reduced GABA signaling by PV+-interneurons onto pyramidal neurons could contribute to the working memory deficits observed in schizophrenia. PVinterneurons control the rate of pyramidal cell firing, thereby synchronizing oscillatory activity of cortical pyramidal neurons in the gamma band range (30 to 80 Hz).Citation174 Gamma oscillations regulate working memory and the transmission of information between cortical regions. Therefore, it is hypothesized that the asynchronous pyramidal neuronal activity resulting from aberrant PV+ GABAergic signaling contributes to the cognitive dysfunction observed in schizophrenia. It is because of this hypothesis that GABAA receptors are now being considered a viable pharmacologic target for treating the cognitive disturbances associated with schizophrenia.Citation172
GABAA receptors are membrane proteins that form a heteropentameric GABA-gated chloride channel, which mediate largely tonic and phasic inhibition. They are composed of several classes of subunits (α1-6, β1-3, γ1-3, δ, ε, θ, π, ρ1-3), but generally consist of three types of subunits (α, β, γ). The majority of GABAA receptors are characterized by their sensitivity to benzodiazepines. These receptors contain subunits (α1, α2, α3, or α5), a β subunit (mainly β2 or β3), and in almost all cases the γ2 subunit in a 2:2:1 stoichiometry. Benzodiazepineinsensitive receptors contain α4, α6, or δinstead of γ2. In addition to their structural diversity, GABAA receptor subtypes have different expression patterns, pointing to unique roles for these receptor subtypes in regulating neuronal activity.Citation175
Postmortem and genetic evidence suggest that α2/α3containing GABAA receptors are the most relevant targets for the treatment of cognitive dysfunction in schizophrenia. It is the α2-containing GABAA receptors that are up regulated on the postsynaptic axon initial segments of pyramidal neurons in schizophrenia.Citation176 In mice, deletion of the α3 subunit results in mild hyperactivity and a pronounced deficit in PPI of the acoustic startle response, suggesting a hyperdopaminergic phenotype.Citation177 Targeting these specific receptor subtypes would circumvent the adverse cognitive and sedative effects associated with nonspecific agonists, like benzodiazepines, which are attributable to their affinity for α1 and/or α5containing GABAA receptors.Citation178
α2-GABAA receptors
A recent proof-of-concept trial was conducted with MK0777, a benzodiazepine-like compound selective for GABAA receptors containing α2 or α3 subunits, to determine whether selective enhancement of GABAergic transmission would improve cognitive functions and gamma oscillations in patients with schizophrenia.Citation179 MK0777 improved the performance of patients in several working memory tasks, and was associated with increased gamma band power in the frontal cortex during task performance. However, MK-0777 did not significantly alter scores on the Brief Psychiatric Rating Scale (BPRS) or Repeatable Battery for the Assessment of Neuropsychological status, except for improvement in the delayed memory index in the latter test.
α3-GABAA receptors
Mouse genetics supports the hypothesis that a3GABAA receptors are involved in sensorimotor gating,Citation177 a process that is disrupted in schizophrenia. Compounds that would selectively augment signaling through these receptors would be potentially beneficial in treating this schizophrenia endophenotype. As the a3containing GABAA receptor is the major subtype expressed on dopaminergic and other monoaminergic neurons,Citation180 agonists at this receptor might augment the inhibitory tone of these neurons and reverse their hyperfunctioning state in psychosis.
Cholinergic therapeutic targets
Muscarinic receptors
Muscarinic acetylcholine (mACh) receptors are widely distributed throughout the neocortex and are promising targets for numerous neurological and psychiatric disorders.Citation181 Five isoforms (M1-M5) of these G-protein coupled metabotropic receptors have been identified and characterized.Citation182 The therapeutic potential for muscarinic receptor activation in schizophrenia is fueled in large part by the efficacy of acetylcholine esterase inhibitors, which elevate synaptic acetylcholine levels, in reducing behavioral disturbances in Alzheimer's disease patients that are reminiscent of symptoms of schizophrenia.Citation183,Citation184 These effects are in addition to the primary cognitive enhancement due to the therapy. Efficacy of these treatments could likely be due to downstream activation of mACh receptors. Consistent with the hypothesized therapeutic impact of mACh receptor activation is a small clinical trial in schizophrenia showing antipsychotic efficacy of the putative M1/M4 selective mACh receptor agonist xanomaline.Citation185
Current cholinergic therapeutics are limited in their applicability because of aversive side-effect profiles that are attributed to peripheral activation of M2 and M3 mACh receptors.Citation186,Citation187 For this reason, the development of subtype selective ligands has been a major interest. M1 and M4 subtypes are of greatest interest in schizophrenia, given the efficacy of xanomaline (an M1/M4-pref erring agonist) and postmortem findings of reduced M1 and M4 receptor densities in schizophrenia.Citation188,Citation189 Studies with mutant mice support the targeting of M1 and M4 receptors. Null deletion mutants of M1 receptors display deficits in working memory and social memory,Citation190 as well as elevated baseline dopamine turnover and increased sensitivity to the behavioral and neurochemical effects of amphetamine.Citation191 Likewise M4 null mutant mice display hypersensitivity to amphetamine and PCP-induced increases in nucleus acccumbens dopamine, consistent with an involvement of NMDA receptors.Citation192
In the absence of selective pharmacological tools, mutant animal studies have been used to improve our understanding of the neurophysiological role of mACh receptors.Citation187 M4 null mutant mice display enhanced baseline ACh efflux with in vivo dialysis in various brain regions, consistent with a prominent role as an autoreceptorCitation193 The finding that M1 null mutation abolishes ACh-mediated LTP of pyramidal neurons in the hippocampusCitation194 complements earlier work suggesting a similar role for M1 receptors in the potentiation of NMDA receptor currents.Citation195 Taken together, these studies suggest that M1 mACh receptors possess activity similar to that of mGlu5 receptors, modulating NMDA receptor signaling postsynaptically mACh receptors, like mGluRs, have proven to be difficult to selectively target at the orthosteric site. The agonist xanomaline, though often touted as M1/M4-selective, possesses prominent affinity for other subtypes. Recent progress has been made in the development of M1 and M4 PAMs and allosteric agonists for mACh receptors.Citation196 As with mGlu receptors, allosteric modulation appears to be a promising route for achieving pharmacological selectivity. Recent studies describe the activity of a M1-selective allosteric agonist, 1-(1'-2-methylbenzyl)-1,4'-bipiperidin4-yl)-1H-benzo[ d]imidazol-2(3H)-one (TBPB) and a PAM, benzylquinolone carboxylic acid (BQCA). In experiments that further elucidate the physiological roles of M1 receptors, TBPB enhances NMDA receptor currents; BQCA enhances the frequency and amplitude of spontaneous excitatory neurotransmission in the cortex.Citation197 In evidence of in vivo activity, TBPB reduced amphetamineinduced hyperactivityCitation198 and BQCA enhanced reversal learning in a murine transgenic model of Alzheimers' disease.Citation199 Likewise, the selective targeting of M4 receptors has proven successful via allosteric modulation.Citation197,Citation200,Citation201 These PAMs display in vivo efficacy, reducing amphetamine-induced hyperactivity (VU0152099201; and apomorphine-induced disruption of PPI (LY2033298).Citation200 These limited pharmacological studies serve as merely a proof of concept. As these compounds (and others with optimized pharmacokinetics) are more widely tested, we are likely to gain a better understanding of the function of and therapeutic potential for targeting M1 and M4 ACh receptors.
Nicotine and schizophrenia
The involvement of nicotinic acetylcholine receptors (nAChRs) in the pathophysiology of schizophrenia was initially suggested by behavioral and biochemical data. People with schizophrenia, in both inpatient and outpatient settings, smoke cigarettes at a rate (80%) more than threefold higher than the general population smoking rate in the United States.Citation202 They are also heavier smokersCitation203 and extract more nicotine per cigarette smoked than the general population.Citation204 Their motivation to quit smoking is lowCitation205 and the smoking cessation rates are lower than the rates of the general population.Citation203 Furthermore, in schizophrenic patients, cigarette smoking normalized their deficits in sensory gating.Citation206 Patients with schizophrenia also have reductions in the numbers of [3H]-cytisine and [125I]-abungarotoxin binding sites in the hippocampus as well as elevated serum levels of nAChR antibodies compared with controls.Citation207
The high rate and heavy level of smoking in schizophrenic subjects suggest that they might be medicating themselves with nicotine to reduce cognitive impairments associated with the disorder and/or antipsychotic treatment. Patients report that they smoke as a sedative, to reduce negative symptoms, and to counteract medication side effects.Citation208 Studies have demonstrated that nicotine administration produces positive effects on sensory gating, eye movements, negative symptoms, some cognitive tasks, and movement disorders. Although nicotine is therapeutic for certain aspects of schizophrenia, it has several limitations that hinder its clinical utility. Nicotine induces tachyphylaxis and carries abuse liability. The long-term risks of chronic treatment are unknown but might include carcinogenic features and cerebro- or cardiovascular risks. Therefore, novel nicotinic agonists have been developed that are more selective than nicotine for particular nAChR subtypes, and may provide cognitive benefits similar to nicotine, with fewer adverse side effects.
nAChRs
Neuronal nAChRs are widely expressed in the central nervous system and mediate fast synaptic signaling and the release of other neurotransmitters. They are involved in numerous physiological functions including cognition (attention and working/associative memory performance), neuronal development, particularly in the sensory cortex, and reward mechanisms via the mesocorticolimbic system.Citation209 Cholinergic modulation also plays a critical role in the functioning of neural circuits, including those involving glutamatergic, GABAergic, and dopaminergic innervations.
nAChRs are excitatory neurotransmitter-gated ion channels that belong to a superfamily that includes other ionotropic receptors for 5-HT, glycine, and GABA. This family of receptors is comprised of 16 different subunits in humans (α1 -7, α9-10, β1-4, δ, ε, γ). This wide variety of subtypes of nAChRs arising from combinations of subunits displays a range of different functional and pharmacological properties. Neuronal nAChRs are assembled from five transmembrane subunits that are arranged around a central water-filled pore. Neuronal subunits that form nAChRs in αβ combinations include α2-α6 and β2-β4. Although most nAChRs subunits assemble only into heteropentameric receptor ion channel combinations, the α7 subunits are able to generate functional homomeric nAChRs.Citation209 nAChRs composed of α4β2 and α7 subunits make up the majority of the nAChRs in the brain. There are two ACh binding sites per receptor. Mammalian nAChRs are cation-selective, being permeable to small monovalent and divalent cations like Ca2+. Nicotinic receptor activity causes depolarization, and the divalent cation permeability plays an important physiological role by supplying ionic signals, including Ca2+.
α7nAChRs
α7 nAChRs are abundantly expressed in the hippocampus and cortex. They have distinct characteristics due to their homopentameric composition that distinguishes them from the other nAChR subtypes. α7 nAChRs are rapidly desensitizing, are an order of magnitude less sensitive to nicotine as an agonist, and have a higher calcium permeability than other nAChRs.Citation209
Because cholinergic innervation arises from projections that send diffuse afferents to a broad range of brain areas, nicotinic activity is a modulatory signal that subtly influences many neurotransmitter systems and contributes to the overall efficiency of various neural circuits. Cholinergic fibers innervate the entire hippocampus with synaptic contacts made onto granule cells, pyramidal cells, interneurons, and neurons of the hilus.Citation210 The hippocampus expresses a wide variety of nAChR subunits, but the α7, α4, and β2 subunits predominate. The GABAergic interneurons more densely express nAChRs than do the glutamatergic cells. Activation of a7nAChRs on presynaptic terminals of glutamatergic pyramidal neurons increases intraterminal Ca2+ levels to facilitate glutamate release.Citation211 α7nAChRs are also present in high density at postsynaptic sites on PV+-GABAergic interneuronsCitation212 that are vulnerable in schizophrenia,Citation130 where they mediate fast cholinergic excitatory transmission.Citation213 In the cortex, cholinergic innervation sparsely reaches all layers, but layer V is the most heavily innervated, especially in the motor and sensory areas. The manner in which nicotinic signaling affects cortical activity is dependent on which part of the pyramidal cell the nAChRs are activated. Activation of nAChRs on distal apical dendrites depolarizes the cell and promotes action potential firing, while activation on proximal apical dendrites reduces membrane impedance and shunts signals from the apical tuft.Citation209 Midbrain dopamine neurons in the substantia nigra and ventral tegmental area (VTA) express a variety of nAChR subunits (α4-α7 and β2), with β2 subunit containing nAChRs dominating (~40% of rat dopaminergic VTA neurons express the a7nAChR subunit.Citation214 Cholinergic afferents into the midbrain enhance glutamate transmission via mainly presynaptic oc7 nAChRs on glutamatergic terminals,Citation215 thereby influencing the firing frequency and firing modes of DA neurons.Citation216
Association of α7nAChRs with schizophrenia
α7 nAChRs have been associated with schizophrenia across several domains. A linkage was found between the α7 nAChR and schizophrenia on chromosome 15q13-14,Citation206 a region containing the gene that encodes for the oc7 nAChR (CHRNA-7). Although no amino acid-coding region polymorphisms have been found, multiple single-nucleotide polymorphisms (SNPs) in the promoter region of CHRNA-7 as well as a partial duplication of CHRNA-7, have been characterized, with certain alleles more frequently present in people with schizophrenia.Citation217 Reduced α7 receptor binding was found in the reticular nucleus of the thalamus,Citation218 hippocampus,Citation219 and cingulate cortex.Citation220 Moreover, there were reduced a7 subunit levels in the DLPFC,Citation221 as well as reduced mRNA expression of α7 in peripheral blood lymphocytesCitation222 of patients with schizophrenia. In addition to the clinical data, preclinical evidence implicates α7nAChR function in regulating cognition. Mice deficient in α7nAChRs have impaired sustained attention,Citation223 while administration of α7nAChR antagonistsCitation224 and agonistsCitation225 impair and enhance, respectively, working memory in rodents.
α7nAChR full agonists
The α7nAChR agonist, (-)-spiro[1-azabucyclo[2,2,2]octane3,5'-oxazolidin-2'-one] (ARR 17779), significantly improved learning and memory in rats,Citation225 while an α7nAChR agonist with 5-HT3 receptor antagonist properties, improved the inhibition of the P50 response in schizophrenia.Citation226 A novel selective oc7nAChR agonist, 5-morpholin-4-yl-pentaoic acid (4-pyridin-3-yl-phenyl)-amide (SEN12333), with only weak antagonist activity at α3-containing receptors, was shown to have procognitive properties in rats across several domains, including episodic memory, attention, and perceptual processing.Citation227
α7nAChR partial agonists
3-(2,4 Dimethoxy)benzylidene-anabaseine (DMXBA) is one of a series of compounds derived from anabaseine, an alkaloid found in marine worms. DMXBA is a partial agonist at the α7nAChR and is a weak competitive antagonist at the α4/32 nAChR and at the 5-HT3 receptor. The metabolites of DMXBA are also active at these receptors, but their biological effect may be limited due to their greater polarity, and therefore greater difficulty in crossing the blood-brain barrier. In preclinical animal models, DMXBA was shown to improve learning and memory-related behaviors in multiple paradigms, including nonspatial avoidance task,Citation228 delayed matching to sample,Citation229 Morris water maze,Citation228 and classic eye-blink conditioning.Citation230 DMXBA also normalizes auditory gating in the DBA/2 mouse, a strain with no sensory inhibition under routine experimental conditions.Citation231
Because of the success of DMXBA in preclinical trials, its effects on cognition were initially evaluated in normal subjects.Citation232 DMXBA significantly improved simple reaction time, correct detection during digit vigilance, both immediate and delayed word recall, word and picture recognition memory, and performance speed on a numeric and spatial working memory task.Citation233 A second Phase I trial was conducted in persons with schizophrenia.Citation234 This double-blind study found that DMXBA normalized auditory evoked responses in both the P50 ratio and the test wave amplitude in patients. DMXBA also improved performance on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and the Attention subscale, with effect sizes more favorable when compared with second-generation antipsychotics. However, DMXBA did not produce changes in the BPRS and therefore did not affect positive, negative, or anxiety related symptoms.
An initial Phase II trial recently assessed the clinical effects of DMXBA on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, as well as the Scale for the Assessment of Negative Symptoms (SANS) and Brief Psychiatric Rating Scale (BPRS).Citation235 Although DMXBA did not significantly improve MATRICS cognitive measures, patients reported significant improvements on the SANS total score, most notably on the anhedonia and alogia subscales. fMRI was also conducted in this trial to ascertain if DMXBA would have an effect on hippocampal activityCitation236 In schizophrenia, increased hippocampal hemodynamic activity is often observed during many tasks, including smooth pursuit eye movements, and is thought to be the result of hippocampal interneuron dysfunction. DMXBA (150 mg) reduced hippocampal activity in patients during pursuit eye movements consistent with the established function of α7nAChRs on hippocampal inhibitory interneurons.
R3487/MEM3454 is a partial α7nAChR agonist and a 5HT3 receptor antagonist. R3487/MEM3454 has been shown to be efficacious in multiple animal behavioral paradigms that evaluate episodic, spatial, and working memory function as well as sustained attention.Citation237 4-bromophenyl-1 ,4-diazabicyclo[3 ,2,2]nonane-4-carboxylatehydrochloride (SSR1 80711) is a partial a7 nAChR agonist, with no significant binding and/or functional activity at other human nAChRs. This compound produced electrophysiological, biochemical, and behavioral effects predictive of cognitive benefit in schizophrenia.Citation238 Citation239 SSR180711 also normalized abnormally persistent latent inhibition produced by an acute pharmacologic model (MK801) and a neurodevelopmental model (inhibition of nitric oxide production during the very early postnatal period), which are used as models of impaired cognitive flexibility in schizophrenia.Citation240 Moreover, SSR180711 reversed amphetamine -induced disruption of latent inhibition, an effect considered to be predictive of activity against the positive symptoms of schizophrenia.Citation240
Positive allosteric modulators of α7nAChRs
Positive allosteric modulators of α7nAChRs have attracted interest as potential compounds for the treatment of cognitive deficits associated with schizophrenia. α7nAChRs PAMs have been classified as either type I or type II compounds. Type I compounds mainly affect the peak current response, while type II compounds affect both the peak current response, as well as the kinetics of agonist-evoked responses.Citation241 1-(5-chloro-2, 4-dimethoxyphenyl)-3-(5-methyl-isoxazol-3-yl)-urea (PNU-120956) is a prototypical type II PAM with little or no activity on most other nAChR subtypes.Citation242 LY-2087101 is a recently discovered allosteric potentiator of nAChRs that is less selective for α7 nAChRs than PNU-120956, with properties similar to type I PAMs.Citation243 There are five amino acids in three a-helical transmembrane regions of the α7nAChR that are critical in facilitating the potentiaton of agonist evoked responses by PNU-120956 and LY2087101.Citation244 In addition to amplifying or unmasking α7nAChR responses to exogenous agonist, PAMs can potentially augment the effects of endogenous agonist, especially PNU-120956, since it reduces α7nAChR desensitization.Citation242
Genetic, biochemical, and behavioral findings have linked α7nAChRs to schizophrenia, particularly the cognitive and sensory processing components of the disease.Citation245 The ability of α7nAChR agonists (partial and full) and PAMs to improve a wide range of cognitive processes preclinically, and to a lesser extent clinically, makes them attractive targets for mitigating the cognitive deficits associated with schizophrenia that are not responsive to current first- and second-generation antipsychotics.
Conclusion
While this review is hardly exhaustive, it does identify a number of potential drug discovery targets that could address the symptoms most resistant to current treatments available for schizophrenia. As psychosis is a downstream consequence of a primary cortical dysfunction, it is possible that some of these interventions might not only affect the cognitive deficits and negative symptoms, but also positive symptoms. In this regard, the mGluR2/3 agonist, LY21 40023, which has no direct effects on dopaminergic neuronal function, exhibited antipsychotic effects comparable to the positive control, olanzapine.Citation140 Alternatively, other interventions might have only selective effects on negative symptoms and/or cognition, and thus would require the coadministration of an antipsychotic to reduce positive symptoms, much in the way that the combination of a mood stabilizer and an antipsychotic are used to treat bipolar disorder.
As the complex genetics of schizophrenia are resolved, it may be possible in the future to link risk genes to drugs that directly address their mechanisms. For example, an a7nAChR positive modulator might be particularly effective in those patients found to have an allelic variant of the CHRNA7 promoter that is associated with reduced expression.Citation246 Genetic studies indicate that individual risk genes such as common alleles of GABAA receptors are associated with elevated risk for schizophrenia, bipolar disorder, and autism-spectrum disorders.Citation247 Such shared risk genes or shared copy number variants provide face validity for the conviction that drug discovery around these targets may yield a much broader therapeutic impact than just in schizophrenia. However, in keeping with the complex genetics of neuropsychiatric disorders, drugs targeting these pathways will likely be useful only in particular subgroups of patients with schizophrenia, bipolar disorder, and autism-spectrum disorders.
Selected abbreviations and acronyms
| DAAO | = | D-amino acid oxidase |
| DMXBA | = | 3-(2,4 dimethoxy) benzylidene-anabaseine |
| GABA | = | γ-aminobutyric acid |
| GMS | = | glycine modulatory site |
| NAC | = | N-acetylcysteine |
| nAChR | = | nicotinic acetylcholine receptor |
| NMDA | = | N-methyl-D-aspartate |
| PAM | = | positive allosteric modulator |
Some of the research findings discussed in this article were supported by USPHS grants to Joseph T. Coyle, MD, including R01 MH51290 and P50MH06045. JTC holds a patent on the use of D-serine for the treatment of schizophrenia that is owned by Partners Healthcare and has consulted with Abbott, Bristol Meyer Squibb, Cephalon, and Lilly on drug discovery. The authors gratefully acknowledge the contributions of Debbie Johnson.
REFERENCE
- DelayJDenickerPNeuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955161041 1214392209
- JanssenPAAwoutersFHIs it possible to predict the clinical effects of neuroleptics from animal data? Part V: From haloperidol and pipamperone to risperidone. Arzneimittelforschung. 1994442692777514873
- CarlssonADoes dopamine play a role in schizophrenia? Psychol Med. 1977758359722890
- CreeseIBurtDRSnyderSHDopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 19761924814833854
- SeemanPLeeTChau-WongMWongKAntipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976 261717719945467
- MeltzerHYIlluminating the molecular basis for some antipsychotic drug-induced metabolic burden. Proc Natl Acad Sci U S A. 20071043019302017360600
- LehmanAFCordrayDSPrevalence of alcohol, drug and mental disorders among the homeless: one more time. Contemporary Drug Problems. 199320355384
- HartvigPKjelsbergEPenrose's law revisited: the relationship between mental institution beds, prison population and crime rate. Nord J Psychiatry. 200963515618985517
- WuEQBirnbaumHGShiLEt al.The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005661122112916187769
- DavisJMChenNGlickIDA meta-analysis of the efficacy of secondgeneration antipsychotics. Arch Gen Psychiatry. 20036055356412796218
- LiebermanJAStroupTSMcEvoyJPet al.Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 20053531209122316172203
- JonesPBBarnesTRDaviesLet al.Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006631079108717015810
- LiebermanJAComparative effectiveness of antipsychotic drugs: a commentary on: Cost Utility Of The Latest Antipsychotic Drugs In c Schizophrenia Study (CUtLASS 1) and Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE). Arch Gen Psychiatry. 2006631069107217015808
- dAmatoTRochetTDaleryJet al.Relationship between symptoms rated with the Positive and Negative Syndrome Scale and brain measures in schizophrenia. Psychiatry Res. 19924455621461947
- ArangoCBuchananRWKirkpatrickBCarpenterWTThe deficit syndrome in schizophrenia: implications for the treatment of negative symptoms Eur Psychiatry. 200419212614969777
- Mesholam-GatelyRIGiulianoAJGoffKPFaraoneSVSeidmanLJNeurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 20092331 5336
- HeinrichsRWZakzanisKKNeurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998124264459673998
- VelakoulisDWoodSJWongMTet al.Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultrahigh-risk individuals. Arch Gen Psychiatry. 20066313914916461856
- SalisburyDFKurokiNKasaiKShentonMEMcCarleyRWProgressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 20076452152917485604
- PearlsonGDCalhounVDConvergent approaches for defining functional imaging endophenotypes in schizophrenia. Front Hum Neurosci. 200933719956400
- KyriakopoulosMFrangouSRecent diffusion tensor imaging findings in early stages of schizophrenia. Curr Opin Psychiatry. 20092216817619553871
- CamchongJMacDonaldAW 3rdBellCMuellerBALimKOAltered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2009Nov17 [Epub ahead of print]
- JavittDCSensory processing in schizophrenia: neither simple nor intact. Schizophr Bull. 2009351059106419833806
- ItilTKeskinerAKiremitciNHoldenJMEffect of phencyclidine in chronic schizophrenics. Can Psychiatr Assoc J . 1967122092126040448
- LubyEDCohenBDRosenbaumGGottliebJSKelleyRStudy of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry 195981363369
- JavittDCZukinSRRecent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991148130113081654746
- AnisNABerrySCBurtonNRLodgeDThe dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983795655756317114
- KrystalJHKarperLPSeibylJPet al.Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994511992148122957
- NewcomerJWFarberNBJevtovic-TodorovicVet al.Ketamineinduced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999201061189885791
- UmbrichtDSchmidLKollerRVollenweiderFXHellDJavittDCKetamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000571139114711115327
- RadantADBowdleTACowleyDSKharaschEDRoy-ByrnePPDoes ketamine-mediated N-methyl-D-aspartate receptor antagonism cause schizophrenia-like oculomotor abnormalities? Neuropsychopharmacology 1998194344449778665
- KegelesLSAbi-DarghamAZea-PonceYet al.Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 20004862764011032974
- LahtiACWeilerMATamaraMichaelidisBAParwaniATammingaCAEffects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 20012545546711557159
- SpokesEGAn analysis of factors influencing measurements of dopamine, noradrenaline, glutamate decarboxylase and choline acetylase in human post-mortem brain tissue. Brain. 1979102333346455043
- LewisDAHashimotoTVolkDWCortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005631232415803162
- BenesFMEvidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 19994658959910472413
- HashimotoTBazmiHHMirnicsKWuQSampsonARLewisDAConserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 200816547948918281411
- VolkDWPierriJNFritschyJMAuhSSampsonARLewisDAReciprocal alterations in pre- and post-synaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002121063107012217970
- GlantzLALewisDADecreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 200057657310632234
- SweetRAHenteleffRAZhangWSampsonARLewisDAReduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 20093437438918463626
- BehrensMMAliSSDaoDNLuceroJShekhtmanGQuickKLDuganLLKetamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 20073181645164718063801
- ZhangYBehrensMMLismanJEProlonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J Neurophysiol. 200810095996518525022
- LismanJECoyleJTGreenRWet al.Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 20083123424218395805
- UltanirSKKimJEHallBJDeerinckTEllismanMGhoshARegulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci U S A. 2007104195531955818048342
- BasuACTsaiGEMaCLet al.Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 20091471972719065142
- BaluDBasuACCoyleJTAltered cortical dendritic morphology in serine racemase knockout mice, a genetic model of NMDA receptor hypofunction. Soc Neurosci. 2009Abs. No. 443.16
- AllenNCBagadeSMcQueenMBet al.Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 20084082783418583979
- HahnCGWangHYChoDSet al.Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 20061282482816767099
- JentschJDTrantham-DavidsonHJairlCTinsleyMCannonTDLavinADysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology. 2009342601260819641486
- Whitfield-GabrieliSThermenosHWMilanovicSet al.Hyperactivity and hyperconnectivity of the default network in schizophrenia and in firstdegree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 20091061279128419164577
- GraceAADopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010 (Epub ahead of print)
- KomuroHRakicPModulation of neuronal migration by NMDA receptors. Science. 199326095978096653
- AkbarianSBunneyWE JrPotkinSGWigalSBHagmanJOSandmanCAJonesEGAltered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry. 1993501691777679891
- MargolisRLRossCANeuronal signaling pathways: genetic insights into the pathophysiology of major mental illness. Neuropsychopharmacology. 20103535035120010716
- RietkerkTBoksMPSommerIEde JongSKahnRSOphoffRANetwork analysis of positional candidate genes of schizophrenia highlights myelin-related pathways. Mol Psychiatry. 20091435335519308022
- BradleyJCarterSRRaoVRWangJFinkbeinerSSplice variants of the NR1 subunit differentially induce NMDA receptor-dependent gene expression. J Neurosci. 2006261065107616436592
- LynchDRGuttmannRPNMDA receptor pharmacology: Perspectives from molecular biology. Curr. Drug Targets. 20012215231
- BergerAJDieudonneSAscherPGlycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J Neurophysiol. 199880333633409862928
- RansomRWStecNLCooperative modulation of [3H]MK-801 binding to the N-methyl-D-aspartate receptor-ion channel complex by L-glutamate, glycine, and polyamines. J Neurochem 1988518308362457653
- FosterACWongEHThe novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br J Pharmacol. 1987914034092886170
- WongEHKempJAPriestleyTKnightARWoodruffGNIversenLLThe anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 198683710471083529096
- HongSJLiHBeckerKGDawsonVLDawsonTMIdentification and analysis of plasticity-induced late-response genes. Proc Natl Acad Sci U S A. 20041002145215014766980
- LiuLWongTPPozzaMFet al.Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 20043041021102415143284
- CoyleJTGlutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 20062636538416773445
- JohnsonJWAscherPGlycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 19873255295312433595
- KlecknerNWDingledineRRequirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 19882418358372841759
- ThomsonAMWalkerVEFlynnDMGlycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature. 19893384224242538754
- BergeronRMeyerTMCoyleJTGreeneRWModulation of N-methylD-aspartate receptor function by glycine transport. Proc Natl Acad Sci U S A. 1 998951 57301 5734
- HashimotoKFukushimaTShimizuEet al.Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the Nmethyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 20036057257612796220
- BendikovINadriCAmarSPanizzuttiRDe MirandaJWoloskerHAgamGA CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res. 200790415117156977
- SchwarczRRassoulpourAWuHQMedoffDTammingaCARobertsRCIncreased cortical kynurenate content in schizophrenia. Biol Psychiatry. 20015052153011600105
- NilssonLKLinderholmKREngbergGet al.Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 200515;8031 5322
- MatsuiTSekiguchiMHashimotoATomitaUNishikawaTWadaKFunctional comparison of D-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J Neurochem. 1995654544587790891
- WatsonGBBolanowskiMABaganoffMPDeppelerCLLanthornTHD-cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Res. 199026;510158160
- TsaiGELinPYStrategies to enhance N-methyl-D-aspartate receptormediated neurotransmission in schizophrenia, a critical review and metaanalysis. Curr Ph arm Des. 201016522537
- BuchananRWJavittDCMarderSRet al.The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 20071641593160217898352
- Heresco-LevyUJavittD CErmilovMordelCSilipoGLichtensteinMEfficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 19995629369892253
- JavittDCSilipoGCienfuegosAShelleyAMBarkNParkMet al.Adjunctive high-dose glycine in the treatment of schizophrenia. Int J Neuropsychopharmacol. 20014385391 11806864
- GoffDCTsaiGManoachDSCoyleJTDose-finding trial of D-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry. 1995152121312157625475
- QuartermainDMowerJRaffertyMFHertingRLLanthornTHAcute but not chronic activation of the NMDA-coupled glycine receptor with Dcycloserine facilitates learning and retention. Eur J Pharmacol. 1994257712
- BakerJDAzorlosaJLThe NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1 9961106186208889007
- WalkerDLResslerKJLuKTDavisMFacilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of Dcycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002222343235111896173
- MyersKMCarlezonWA JrD-cycloserine facilitates extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. Biol Psychiatry. 201067858719782965
- ResslerKJRothbaumBOTannenbaumLet al.Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004611 1361 144
- NorbergMMKrystalJHTolinDFA meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapyBiol Psychiatry. 2008631118112618313643
- OldendorfWHUptake of radiolabeled essential amino acids by brain following arterial injection. Proc Soc Exp Biol Med. 19711363853865544467
- HashimotoAChibaSEffect of systemic administration of D-serine on the levels of D- and L-serine in several brain areas and periphery of rat. Eur J Pharmacol. 200449515315815249164
- NinanIJardemarkKEWangRYDifferential effects of atypical and typical antipsychotic drugs on N-methyl-D-aspartate- and electrically evoked responses in the pryramidal cells of the rat medial prefrontal cortexSynapse. 200348667912619040
- JavittDCDuncanLBallaASershenHInhibition of system A-mediated glycine transport in cortical synaptosomes by therapeutic concentrations of clozapine: implications for mechanisms of action. Mol Psychiatry. 20051027528715278098
- NagataYInvolvement of D-amino acid oxidase in elimination of Dserine in mouse brainExperientia. 1992487537551355447
- HoriikeKTojoHAraiRYamanoTNozakiMMaedaTLocalization of D-amino acid oxidase in Bergmann glial cells and astrocytes of rat cerebellum. Brain Res Bull. 1987195875962891417
- VerrallLBurnetPWBettsJFHarrisonPJThe neurobiology of Damino acid oxidase and its involvement in schizophrenia. Mol Psychiatry. 20101512213719786963
- Detera-WadleighSDMcMahonFJG72/G30 in schizophrenia and bipolar disorder: review and meta-analysis. Biol Psychiatry. 20066010611416581030
- ShiJBadnerJAGershonESLiuCAllelic association of G72/ G30 with schizophrenia and bipolar disorder: a comprehensive meta-analysis. Schizophr Res. 200898899718023149
- KapoorRLimKSChengAGarrickTKapoorVPreliminary evidence for a link between schizophrenia and NMDA-glycine site receptor ligand metabolic enzymes, d-amino acid oxidase (DAAO) and kynurenine aminotransferase-1 (KAT-1). Brain Res. 2006110620521016828464
- MadeiraCFreitasMEVargas-LopesCWoloskerHPanizzuttiRIncreased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res. 2008101768318378121
- BurnetPWEastwoodSLBristowGCGodlewskaBRSikkaPWalkerMHarrisonPJD-amino acid oxidase activity and expression are increased in schizophrenia. Mol Psychiatry. 2008365866018560437
- ChumakovIBlumenfeldMGuerassimenkoOCavarecLPalicioMAbderrahimHet al.Genetic and physiological data implicating the new human gene G72 and the gene for Damino acid oxidase in schizophrenia. Proc Natl Acad Sci U S A. 200299136751368012364586
- AdageTTrillatACQuattropaniAet al.In vitro and in vivo pharmacological profile of AS057278, a selective d-amino acid oxidase inhibitor with potential anti-psychotic properties. Eur Neuropsychopharmacol. 20081820021417681761
- HashimotoKFujitaYHorioMet al.Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol Psychiatry. 2009651103110619217074
- SmithSMUslanerJMYaoLet al.The behavioral and neurochemical effects of a novel D-amino acid oxidase inhibitor compound 8 [4H-thieno [3,2-b]pyrrole-5-carboxylic acid] and D-serine. J Pharmacol Exp Ther. 200932892193019088300
- GoltsovAYLosevaJGAndreevaTVealPolymorphism in the 5'-promoter region of serine racemase gene in schizophrenia. Mol Psychiatry. 20061132532616446740
- MoritaYUjikeHTanakaYOtaniKet al.A genetic variant of the serine racemase gene is associated with schizophrenia. Biol Psychiatry. 2007611200120317067558
- LabrieVFukumuraRRastogiAet al.Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet. 2009183227324319483194
- WoloskerHBlackshawSSnyderSHSerine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A. 199996134091341410557334
- KartvelishvilyEShleperMBalanLDuminEWoloskerHNeuronderived D-serine release provides a novel means to activate N-methyl-Daspartate receptors. J Biol Chem. 2006281141511416216551623
- MiyaKInoueRTakataYet al.Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol. 200820;510641654
- LabrieVFukumuraRRastogiAFickLJWangWBoutrosPCKennedyJLSemeralulMOLeeFHBakerGBBelshamDDBargerSWGondoYWongAHRoderJCSerine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet. 2009183227324319483194
- RutterARFradleyRLGarrettEMet al.Evidence from gene knockout studies implicates Asc-1 as the primary transporter mediating d-serine reuptake in the mouse CNS. Eur J Neurosci. 2007251757176617432963
- HelboeLEgebjergJMøllerMThomsenCDistribution and pharmacology of alanine-serine-cysteine transporter 1 (asc-1) in rodent brain. Eur J Neurosci. 2003182227223814622183
- MatsuoHKanaiYTokunagaMet al.High affinity D- and L-serine transporter Asc-1: cloning and dendritic localization in the rat cerebral and cerebellar cortices. Neurosci Lett. 200435812312615026164
- YangCRSvenssonKAAllosteric modulation of NMDA receptor via elevation of brain glycine and D-serine: the therapeutic potentials for schizophrenia. Pharmacol Ther. 200812031733218805436
- XieXDumasTTangLet al.Lack of the alanine-serine-cysteine transporter 1 causes tremors, seizures, and early postnatal death in mice. Brain Res. 2005105221222116026768
- GuastellaJBrechaNWeigmannCLesterHADavidsonNCloning, expression, and localization of a rat brain high-affinity glycine transporter. Proc Natl Acad Sci U S A. 199289718971931353889
- ZafraFGomezaJOlivaresLAragónCGiménezCRegional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur J Neurosci. 19957134213527582108
- ChenLMuhlhauserMYangCRGlycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol 20038969170312574447
- KinneyGGSurCBurnoMet al.The glycine transporter type 1 inhibitor N-[3-(4_-fluorophenyl)-3-(4_- phenylphenoxy)propyl]sarcosine potentiates NMDA receptor-mediated responses in vivo and produces an antipsychotic profile in rodent behavior. J Neurosci. 20032375867591 12930797
- LindsleyCWWolkenbergSEKinneyGGProgress in the preparation and testing of glycine transporter type-1 (GlyT1) inhibitors. Curr Top Med Chem. 200661883189617017963
- SwansonCBuresMJohnsonMLindenAMonnJSchoeppDMetabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005413114415665858
- PaluchaAPilcAMetabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharm Ther. 2007115116147
- KalivasPWThe glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 20091056157219571793
- ConnPJLindsleyCWJonesCKActivation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009302531 19058862
- ConnPJPinJPharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997372052379131252
- ScanzianiMSalinPAVogtKEMalenkaRCNicollRAUse-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 19973856306349024660
- MarekGJWrightRASchoeppDDMonnJAAghajanianGKPhysiological antagonism between 5-hydroxytryptamine 2A and group II metabotropic glutamate receptors in prefrontal corotexJ Pharmacol Exp Ther. 2000292768710604933
- DietrichDKralTClusmannHFriedlMSchrammJPresynaptic group II metabotropic glutamate receptors reduce stimulated and spontaneous transmitter release in human dentate gyrus. Neuropharmacology. 20024229730511897108
- MoghaddamBAdamsBReversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998281134913529721099
- MoghaddamBAdamsBVermaADalyDActivation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 199717292129279092613
- LewisDAHashimotoTVolkDWCortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005631232415803162
- LewisDAMoghaddamBCognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006631372137617030651
- LismanJECoyleJTGreenRWet al.Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 20083123424218395805
- SchoeppDDJaneDEMonnJAPharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999381431147610530808
- CartmellJMonnJASchoeppDDThe metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 199929116117010490900
- CartmellJSchoeppDDRegulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 20007588990710936169
- GaliciREchemendiaNGRodriguezALConnPJA selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther. 200531 51 1811 187
- GaliciRJonesCKHemstapatKNongYEchemendiaNGWilliamsLCde PaulisTConnPJBiphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J Pharmacol Exp Ther. 200631817318516608916
- Rorick-KehnLMHartJCMcKinzieDLPharmacological characterization of stress-induced hyperthermia in DBA/2 mice using metabotropic and ionotropic glutamate receptor ligands. Psychopharmacology (Berl). 200518322624016175401
- GrecoBInvernizziRWCarliMPhencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU(2/3) receptor agonist LY379268. Psychopharmacology (Berl). 2005179687615678361
- AdamsBMoghaddamBCorticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 199815;1855455554
- BenneyworthMAXiangZSmithRLGarciaEEConnPJSanders-BushEA selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 20077247748417526600
- PatilSTZhangLMartenyiFet al.Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007131102110717767166
- KrystalJHAbi-SaabWPerryEet al.Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl). 200517930330915309376
- HomayounHJacksonMEMoghaddamBActivation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol. 2005931989200115590730
- higemotoRKinoshitaAWadaEet al.Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 199717750375229295396
- TamaruYNomuraSMizunoNShigemotoRDistribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sitesNeuroscience. 200110648150311591452
- CortiCBattagliaGMolinaroGet al.The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci. 20071;2782978308
- poorenWPGaspariniFvan derPutten HKollerMNakanishiSKuhnRLack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice. Eur J Pharmacol. 2000397R1R210844118
- 147 ConnPJLindsleyCWJonesCKActivation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009302531 19058862
- ConnPJChristopoulosALindsleyCWAllosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 20098415419116626
- SchaffhauserHRoweBAMoralesSet al.Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2. Mol Pharmacol. 20036479881014500736
- JohnsonMPBaezMJagdmannGEet al.Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2- trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J Med Chem. 2003463189319212852748
- JohnsonMPBardaDBrittonTCet al.Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology. 20051 7927128315717213
- BakerDAMcFarlandKLakeRWShenHTodaSKalivasPWN-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003100334935114684458
- McBeanGJCerebral cystine uptake: a tale of two transportersTrends Pharmacol Sci. 20022329930212119142
- MoranMMMcFarlandKMelendezRlKalivasPWSeamansJKCystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005256389639316000629
- ZhouWKalivasPWN-acetylcysteine reduces extinction respondi d induces enduring reductions in cue- and heroin-induced drug-seekin Biol Psychiatry. 20086333834017719565
- PetersJKalivasPWThe group II metabotropic glutamate recept onist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl). 200618614314916703399
- BakerDAMadayagAKristiansenLVMeador-WoodruffJHHaroutunianVRajuIContribution of cystine-glutamate antiporters e psychotomimetic effects of phencyclidine. Neuropsychopharmacology. 08331760167217728701
- BerkMCopolovDDeanOet al.N-acetyl cysteine as a glutathione precursor for schizophrenia-a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 20086436136818436195
- KôhrGEckardtSLûddensHMonyerHSeeburgPHNMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron. 199412103110407514425
- BehrensMMSejnowksiTJDoes schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 20095719320019523965
- TuJCXiaoBNaisbittSet al.Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 19992358359210433269
- DohertyAJPalmerMJHenleyJMCollingridgeGLJaneDE(RS)-2chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlul, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997362652679144665
- PisaniAGubelliniPBonsiPet al.Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 200110657958711591458
- AwadHHubertGWSmithYLeveyAlConnPJActivation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000207871787911050106
- HenrySALehmann-MastenVGaspariniFGeyerMAMarkouAThe mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002431 199120912213254
- KinneyGGBurnoMCampbellUCet al.Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther. 200330611612312660307
- HomayounHStefaniMRAdamsBWTamaganGDMoghaddamBFunctional interaction Between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004291259126915010696
- CampbellUCLalwaniKHernandezLKinneyGGConnPJBristowLJThe mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology (Berl). 200417531031815024550
- HomayounHMoghaddamBBursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex. 2006169310515843630
- LiuFGrauerSKelleyCNavarraRet al.ADX47273 [S-(4-fluorophenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther. 200832782783918753411
- SchlumbergerCPietraszekMGraviusAet al.Comparison of the mGlu receptor positive allosteric modulator ADX47273 and the mGlu(2/3) receptor agonist LY354740 in tests for antipsychotic-like activity. Eur J Pharmacol. 2009623738319765575
- HashimotoTBazmiHHMimicsKWuQSampsonARLewisDAConserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 200816547948918281411
- BehrensMMAliSSDuganLLlnterleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 200828139571396619091984
- WhittingtonMATraubRDInterneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 20032667668214624852
- MohlerHRole of GABAA receptors in cognitionBiochem Soc Trans. 2009371328133319909270
- CruzDAWeaverCLLovalloEMMelchitzkyDSLewisDASelective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in subjects with schizophrenia. Neuropsychopharmacology. 2009342112212419322171
- YeeBKKeistRvon BoehmerLet al.A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci U S A. 2005102171541715916284244
- RudolphUMohlerHGABA-based therapeutic approaches: GABAA receptor subtype functionsCurr Opin Pharmacol. 20066182316376150
- LewisDAChoRYCarterCSet al.Subu nit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 20081651585159318923067
- 0kadaHMatsushitaNKobayashiKKobayashiK10. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J Neurochem. 20048971415030384
- LangmeadCJWatsonJReavillCMuscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 20081 1723224318082893
- CaulfieldMPBirdsallNJInternational Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998502792909647869
- GrossbergGTEffect of rivastigmine in the treatment of behavioral disturbances associated with dementia: review of neuropsychiatrie impairment in Alzheimer's disease. Curr Med Res Opin. 2005211631163916238903
- FigielGSadowskyCA systematic review of the effectiveness of rivastigmine for the treatment of behavioral disturbances in dementia and other neurological disorders. Curr Med Res Opin. 20082415716618036286
- ShekharAPotterWZLightfootJet al.Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 20081651033103918593778
- BymasterFPCarterPAYamadaMet al.Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003171403141012713643
- WessJEglenRMGautamDMuscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Wat Rev Drug Discov. 20076721733
- CrookJMTomaskovic-CrookECopolovDLDeanBLow muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann's areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am J Psychiatry. 200115891892511384900
- DeanBMcLeodMKeriakousDMcKenzieJScarrEDecreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 200271083109112476323
- AnagnostarasSGMurphyGGHamiltonSEet al.Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 20036515812483218
- GerberDJSotnikovaTDGainetdinovRRHuangSYCaronMGTonegawaSHyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc Natl Acad Sci U S A. 200198153121531711752469
- TzavaraETBymasterFPDavisRJet al.M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004181410141215231726
- TzavaraETBymasterFPFelderCCet al.Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol Psychiatry. 2003867367912874603
- ShinoeTMatsuiMTaketoMMManabeTModulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 200525111941120016319319
- MarinoMJRouseSTLeveyAIPotterLTConnPJActivation of the genetically defined m1 muscarinic receptor potentiates N-methyl-Daspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 19989511465114709736760
- ConnPJJonesCKLindsleyCWSubtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 20093014815519201489
- ShireyJKXiangZOrtonDet al.An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat Chem Biol. 20084425018059262
- JonesCKBradyAEDavisAAet al.Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 200828104221043318842902
- ShireyJKBradyAEJonesPJet al.A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 200929142711427819906975
- ChanWYMcKinzieDLBoseSet al.Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci U S A. 2008105109781098318678919
- BradyAEJonesCKBridgesTMet al.Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamineinduced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 200832794195318772318
- HughesJRHatsukamiDKMitchellJEDahlgrenLAPrevalence of smoking among psychiatric outpatients. Am J Psychiatry. 19861439939973487983
- KellyCMcCreadieRGSmoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry. 19991561751175710553739
- StrandJENybackHTobacco use in schizophrenia: a study of cotinine concentrations in the saliva of patients and controls. Eur Psychiatry. 200520505415642444
- AddingtonJel-GuebalyNAddingtonDHodginsDReadiness to stop smoking in schizophrenia. Can J Psychiatry. 19974249529040923
- AdlerLEOlincyAWaldoMet al.Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998241892029613620
- MukherjeeSMahadikSPKorenovskyALaevHSchnurDBReddyRSerum antibodies to nicotinic acetylcholine receptors in schizophrenic patients. Schizophr Res. 1994121311368043523
- ForchukCNormanRMallaAet al.Schizophrenia and the motivation for smoking. Perspect Psychiatr Care. 200238414912132630
- DaniJABertrandDNicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 20074769972917009926
- FrotscherMLeranthCCholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 19852392372464044938
- GrayRRajanASRadcliffeKAYakehiroMDaniJAHippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 19963837137168878480
- KrenzIKalkanDWeversAet al.Parvalbumin-containing interneurons of the human cerebral cortex express nicotinic acetylcholine receptor proteins. J Chem Neuroanat. 20012123924611382535
- FrazierCJBuhlerAVWeinerJLDunwiddieTVSynaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 199818822882359763468
- KlinkRde Kerchoved'Exaerde AZoliMChangeuxJPMolecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001211452146311222635
- MansvelderHDKeathJRMcGeheeDSSynaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 20023390591911906697
- SchilstromBFagerquistMVZhangXet al.Putative role of presynaptic alpha7: nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 20003837538311044884
- GaultJRobinsonMBergerRet al.Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7). Genomics. 1998521731859782083
- CourtJSpurdenDLloydSet al.Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999731590159710501205
- FreedmanRHallMAdlerLELeonardSEvidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 19953822337548469
- arutleAZhangXCourtJet al.Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 20012211512611470559
- Martin-RuizCMHaroutunianVHLongPet al.Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2003541222123314643090
- PerlOIlaniTStrousRDLapidusRFuchsSThe alpha7 nicotinic acetylcholine receptor in schizophrenia: decreased mRNA levels in peripheral blood lymphocytes. FASEB J. 2003171948195012897067
- YoungJWCrawfordNKellyJSet al.Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007171451 5516650968
- LevinEDBradleyAAddyNSiguraniNHippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 200210975776511927157
- LevinEDBettegowdaCBlosserJGordonJAR-R17779, and alpha7 nicotinic agonist, improves learning and memory in rats. Behav Pharmacol. 19991067568010780509
- KoikeKHashimotoKTakaiNet al.Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr Res. 200576677215927799
- RoncaratiRScaliCComeryTAet al.Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. J Pharmacol Exp Ther. 200932945946819223665
- MeyerEMTayETPapkeRLMeyersCHuangGLde FiebreCM3[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat alpha7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 199776849569369300
- BriggsCAAndersonDJBrioniJDet al.Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav. 1997572312419164577
- Woodruff-PakDSMecamylamine reversal by nicotine and by a partial alpha7 nicotinic acetylcholine receptor agonist (GTS-21) in rabbits test th delay eyeblink classical conditioning. Behav Brain Res. 200314315916712900042
- StevensKEKemWRMahnirVMFreedmanRSelective alpha7-nic nic agonists normalize inhibition of auditory response in DBA mice Psychopharmacology (Berl). 19981363203279600576
- KitagawaHTakenouchiTAzumaRet al.Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 20032854255112629535
- MartinLFFreedmanRSchizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 20077822524617349863
- OlincyAHarrisJGJohnsonLLet al.Proof-of-concept trial of an alpha7 nicotinic agonist in schizophreniaArch Gen Psychiatry. 20066363063816754836
- FreedmanROlincyABuchananRWet al.Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 20081651040104718381905
- TregellasJROlincyAJohnsonLet al.Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology. 20103593894219956085
- RezvaniAHKholdebarinEBrucatoFHCallahanPMLoweDALevinEDEffect of R3487/MEM3454, a novel nicotinic alpha7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Prog Neuropsychopharmacol Biol Psychiatry. 20093326927519110025
- BitonBBergisOEGalliFet al.SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: binding and functional profile. Neuropsychopharmacology. 20073211617019409
- PichatPBergisOETerranovaJPet al.SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 200732173416936709
- BarakSAradMDe LevieABlackMDGriebelGWeinerIPro-cognitive and antipsychotic efficacy of the alpha7 nicotinic partial agonist SSR180711 in pharmacological and neurodevelopmental latent inhibition models of schizophrenia. Neuropsychopharmacology. 2009341753176319158670
- BertrandDGopalakrishnanMAllosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007741155116317707779
- HurstRSHajosMRaggenbassMet al.A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005254396440515858066
- BroadLMZwartRPearsonKHet al.Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J Pharmacol Exp Ther. 200631 81 1081 117
- YoungGTZwartRWalkerASSherEMillarNSPotentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A. 2008105146861469118791069
- FreedmanRGoldowitzDStudies on the hippocampal formation: From basic development to clinical applications: Studies on schizophrenia. Prog Neurobiol. 20109026327519853005
- LiuJPearlsonGWindemuthARuanoGPerrone-BizzozeroNICalhounVCombining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum Brain Mapp.20093024125518072279