Abstract
Psychiatric disorders are complex multifactorial disorders involving chronic alterations in neural circuit structure and function. While genetic factors play a role in the etiology of disorders such as depression, addiction, and schizophrenia, relatively high rates of discordance among identical twins clearly point to the importance of additional factors. Environmental factors, such as stress, play a major role in the psychiatric disorders by inducing stable changes in gene expression, neural circuit function, and ultimately behavior. Insults at the developmental stage and in adulthood appear to induce distinct maladaptations. Increasing evidence indicates that these sustained abnormalities are maintained by epigenetic modifications in specific brain regions. Indeed, transcriptional dysregulation and associated aberrant epigenetic regulation is a unifying theme in psychiatric disorders. Aspects of depression can be modeled in animals by inducing disease-like states through environmental manipulations, and these studies can provide a more general understanding of epigenetic mechanisms in psychiatric disorders. Understanding how environmental factors recruit the epigenetic machinery in animal models is providing new insights into disease mechanisms in humans.
Los trastornos psíquíátricos son complejas enfermedades multífactoriales que íncluyen alteracíones crónicas en la estructura y funcíón de los círcuitos neurales. Aunque los factores genéticos juegan un papel en la etíología de trastornos como la depresíón, las adicciones y la esquizofrenia, las frecuencías relativamente altas de díscordancia entre gemelos ídéntícos apuntan claramente a la importancia de otros factores. Los factores ambientales, como el estrés, juegan un ímportante papel en los trastornos psíquíátricos al inducír cambíos estables en la expresíón géníca, en la funcíón de los circuítos neurales y fínalmente en la conducta. Lesíones en la etapa del desarrollo y en la adultez parece que inducen dístíntas malas adaptacíones. Hay creciente evídencía que índica que estas persistentes anormalídades se mantienen por modíficacíones epigenétícas en regiones cerebrales específicas. Ciertamente, la falta de regulacíón en la transcrípcíón y la regulacíón epígenética aberrante asociada son temas comunes en los trastornos psíquíátricos. Algunos aspectos de la depresíón se pueden modelar en anímales al inducir estados que simulan la enfermedad mediante manipulaciones ambientales; y estos estudios pueden aportar una comprensión más general de los mecanismos epígeneticos en los trastornos psíquíátricos. La comprensíón de cómo los factores ambientales reclutan la maquínaría epigenética en los modelos animales está aportando nuevas perspectivas en los mecanísmos del enfermar en humanos.
Les troubles psychiatriques sont complexes et multifactoriels et ils sont associés à des modifications chroniques dans la structure et la fonction des circuits neuronaux. Les facteurs génétiques jouent un rôle dans l'étiologie des troubles comme la dépression, l'addiction et la schizophrénie, mais des taux relativement élevés de discordance parmi les vrais jumeaux indiquent clairement l'importance de facteurs supplémentaires. Des facteurs environnementaux, comme le stress, jouent un rôle majeur dans les troubles psychiatriques en provoquant des modifications stables de l'expression des gènes, de la fonction des circuits neuronaux et enfin du comportement. Des lésions au cours du développement ou à l'âge adulte peuvent entraîner des inadaptations particulières. Selon des données de plus en plus nombreuses, ces anomalies prolongées sont maintenues par des modifications épigénétiques dans des régions cérébrales spécifiques. En effet, la dysrégulation transcriptionnelle et la régulation épigénétique aberrante associée sont un thème commun des troubles psychiatriques. Certains aspects de la dépression peuvent être modélisés chez les animaux en induisant des états mimant la maladie grâce à des manipulations environnementales. Ces études permettent une compréhension plus générale des mécanismes épigénétiques dans les troubles psychiatriques. Connaître la façon dont les facteurs environnementaux recrutent la machinerie épigénétique dans les modèles animaux apporte une nouvelle perspective des mécanismes pathologiques chez l'homme.
Introduction
Psychiatric disorders are complex and heterogeneous disorders arising from the interaction of several factors based on neurobiology, genetics, culture, and life experience. Advances in the last decade have identified epigenetic mechanisms as important actors in psychiatric conditions. Indeed, being at the center of gene regulation, epigenetic mechanisms are ideal candidates for the study of these conditions. Epigenetic mechanisms refer to chemical modifications of DNA (without a change in nucleotide sequence) and to a host of protein and RNA molecules that bind to DNA, and which regulate transcription. Epigenetic mechanisms, including DNA methylation, histone modifications, and microRNAs, are a particularly attractive explanation for how environmental factors, such as stress, exert life-long effects on neuropsychiatric phenomena. Addiction-relevant transcriptional regulation can be studied in rodents by exposing animals to drugs of abuse. To date, most studies have focused on experimenter-administered drug exposure and it should be noted that, although many effects are recapitulated in self-administration models, clearly some mechanisms are distinct. DNA methylation, histone modifications, and noncoding RNAs have all been implicated in regulating addiction processes, and this literature has been extensively reviewed in several recent publications.Citation1-Citation4 In contrast, the complex symptomatology of schizophrenia has proven more difficult to model in rodents, hindering efforts to advance the understanding of epigenetic mechanisms. However, limited data from human postmortem brains, complemented by findings from available animal models, suggest a potential role for DNA methylation of genomic targets implicated in this disorder, including glutamic acid decarboxylase67 (GAD67) and Reelin (RELN), as well as alterations in histone acetylation. For a detailed discussion, we refer the reader to several recent reviews.Citation5-Citation7 The current review focuses on the growing literature detailing modification of epigenetic regulation in the context of depression. The majority of these findings come from stress-related animal models of depression, although interesting insights in humans are starting to accumulate.
The complexity and heterogeneity of depression, as well as the ubiquity of many of the symptoms of depression, render clear identification of its etiology very difficult. Stressful life events represent a major risk factor in determining an individual's vulnerability to depression. Animal models offer a useful approach to study these questions. The development of chronic stress paradigms, combined with the ability to objectively measure anhedonia and certain stress susceptibility symptoms in rodents, have helped clarify the neural circuitry and neuroadaptations underlying depression. Chronic stress exposure induces functional and transcriptional alterations in several limbic regions implicated in regulating stress and reward responses . Citation4,Citation8-Citation12 Research, to date, has focused on relatively distinct neural circuits in exploring consequences of developmental vs adult stress. The present review brings together findings relating to epigenetic mechanisms in depression from both adult and developmental studies—in animal models and in humans—and elaborates the potential offered by epigenetic analyses to understand this complex disorder better.
![Figure 1. Epigenetic dysregulation in the HPA axis and reward circuitry is implicated in psychiatric disorders. A majority of research on altered epigenetic regulation in depression and other stress-related disorders has focused on changes within the HPA axis (A) and the brain's reward circuitry (B), depicted here in the rodent brain. Studies examining the effects of early-life manipulations on epigenetic regulation of behavior have focused on changes within the HPA axis, in contrast to adult studies, which have concentrated on epigenetic alterations in the reward circuitry. A) Main components of the HPA axis: CRF and AVP from the paraventricular nucleus of the PVN stimulates ACTH release from the anterior pituitary, which induces glucocorticoid (cortisol [human] or corticosterone [rodent]) release from the adrenal cortex. GRs in the HPC and other brain regions mediate negative feedback to reduce the stress response. B) Depicted are the major components of the limbic-reward circuitry: dopaminergic neurons (green) project from the VTA to the NAc, PFC, AMY, and HPC, among other regions. The NAc receives excitatory glutamatergic innervation (red) from the HPC, PFC, and AMY. ACTH, adrenocorticotropic hormone; AMY, amygdala; AVP, vasopressin; CRF, corticotropin releasing factor; GRs, glucocorticoid receptors; HPA, hypothalamic-pituitary-adrenal axis; HPC, hippocampus; PFC, prefrontal cortex; PVN, hypothalamus; VTA, ventral tegmental area.](/cms/asset/10d095a5-5d18-4620-b541-8b9c0c36d8b9/tdcn_a_12130967_f0001_oc.jpg)
Overview of epigenetic regulatory mechanisms
Epigenetic modes of gene regulation can be grouped into three general domains: (i) histone post-translational modifications (PTMs) and histone variant exchange; (ii) chromatin remodeling; and (iii) DNA methylation. While individually important, these mechanisms work together to orchestrate precise phenotypic outputs in mammalian cells. Also important for epigenetic control is the regulation of noncoding RNAs, which is not discussed here due to space limitations.
Histone modifications
The best-characterized mode of epigenetic regulation in brain is the post-translational, covalent modifications of histones.Citation13 Histones are proteins that stably interact with DNA to form nucleosomes, which package DNA into chromatin . The nucleosome consists of DNA wrapped around an octamer of core histone proteins, two copies each of H3, H4, H2A, and H2B. For each of the core histones in mammals, with the exception of H4, variants exist that can exhibit significantly distinct structures, temporal regulation, and cell-type specificity from their canonical counterparts. Histone variants may also provide an alternative mechanism of encoding and transmitting epigenetic information.
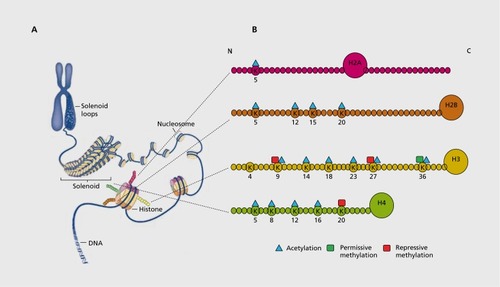
Interactions between DNA and core histone proteins can be altered by covalent modifications to histone N-terminal and C- terminal tails.Citation13 A large variety of histone PTMs have been identified, including phosphorylation, acetylation, methylation, adenosine diphosphate (ADP) ribosylation, ubiquitination, crotonylation, and small ubiquitin-like modifier (SUMO)ylation. Histone acetylation and phosphorylation decrease the affinity of histone octamers for DNA to loosen chromatin structure. This relaxed chromatin state, referred to as euchromatin, allows the transcriptional machinery, DNA binding proteins, and chromatin remodeling complexes access to genes and is often associated with active gene transcription. Methylation of lysine or arginine residues in histone tails is generally thought to be more stable than other histone PTMs, and plays roles in both transcriptional activation and repression depending on the residue being methylated. Histone methylation can also exist in multiple valence states (eg, mono-, di-, or trimethylation), with each state associated with distinct molecular consequences. The roles of histone ADP ribosylation, ubiquitylation, crotonylation, and SUMOylation are less well understood.
The enzymes that mediate histone modifications and their reversal can be understood as “writers” and “erasers,” respectively. For example, histone acetyltransferases (HATs) catalyze acetylation and histone deacetylases (HDACs) catalyze removal (deacetylation) of this mark. Similarly, histone methyltransferases (HMTs) catalyze methylation and histone demethylases (HDMs) catalyze removal of methylation marks. Proteins that bind to specific modified residues, termed “readers,” mediate the functional consequences of histone PTMs through effecting transcriptional change. Distinct roles for histone PTMs, along with their writers, readers, and erasers, led scientists to develop what is commonly referred to as the “histone code hypothesis,” which proposes that specific histone modifications work sequentially or in combination to form a code that can be read by other proteins to effect downstream changes in gene expression.Citation14 While it is true that certain histone PTMs are read in this way, it is becoming increasingly clear that a histone code per se does not work in isolation to direct the complex mechanisms of epigenetic regulation. Rather, this code cooperates with many other mechanisms, such as DNA methylation and chromatin remodeling, to produce a given phenotype.
Chromatin remodeling
With or without histone PTMs, nucleosomes themselves function as physical barriers to transcription. The transcription start sites (TSS) of actively transcribed genes are commonly depleted of nucleosomes; conversely, occlusion of TSSs by nucleosomal occupancy is often associated with gene repression. The precise positions of nucleosomes along DNA are controlled by chromatin remodeling complexes, which act to insert, slide, and eject histone octamers from the chromatin template. These multi-subunit complexes regulate the expression of many transcription factors. Chromatin remodelers also regulate alternative splicing, events that occur cotranscriptionally. It is likely that interactions between remodelers and associated transcription factors, other DNA binding proteins, histone PTMs, and DNA methylation, work together to direct remodeling activity in a manner that alters nucleosome positioning to affect gene transcription. Several major families of chromatin remodeling proteins have been described in cultured cell systems and are just now being studied in nervous tissue.
DNA methylation
Historically, the most studied epigenetic modification is the direct methylation of DNA, involving the addition of a methyl group to cytosine.Citation15 DNA methylation is classically regarded as a highly stable epigenetic mark and can be maintained throughout the lifetime of an organism. DNA methyltransferase (DNMT) catalyzes DNA methylation and occurs most commonly at CpG dinucleotides. DNA methylation generally exerts a repressive effect on gene transcription, as exemplified by the X chromosome inactivation in females and genomic imprinting, where hypermethylation of one parental allele for a given gene results in monoallelic expression.Citation15 There are instances, however, in which DNA methylation may promote gene expression. One mechanism through which DNA methylation inhibits gene expression is through the masking of DNA sequences to prevent their recognition by activating transcription factors. Additionally, methylated DNA is recognized by methyl-CpG-binding domain (MBD) proteins, such as MECP2 (protein-coding) and MBD1, whose binding can further recruit histone modifying enzymes and chromatin-remodeling complexes to compact nucleosomes and inhibit gene expression. DNA methylation has also been associated with alternative splicing although the mechanisms are still unclear.Citation16
Recently, additional DNA modifications have been discovered, including 5-hydroxymethylcytosine (5hmC), 5-formylcytosine, and 5-carboxylcytosine.Citation17,Citation18 These chemical modifications are thought to be derived from 5-methylcytosine through oxidation steps catalyzed by members of the ten-eleven translocation (TET) enzyme family, potentially representing a process of active DNA demethylation. DNA methylation in the brain may be more dynamic than in other tissues. Support for this idea comes from the discovery that: (i) the de novo DNA methyltransferase, DNMT3a, is the main DNMT expressed in neuronsCitation19; (ii) the highest levels of oxidized forms of methylcytosine are found in the brainCitation17; and (iii) active DNA repair results in demethylated DNA in nondividing neurons.Citation20 There is also evidence that the primary effect of 5-hmC is to promote gene expression through mechanisms that remain poorly understood.
Epigenetics and depression: studies in adulthood
Our understanding of the role of epigenetics in adult depression comes primarily from animal models. While acute stress paradigms are designed to evaluate an animal's initial coping response, chronic stress paradigms involve prolonged exposure to either physicalCitation21 or psychological stressors, such as social subordination.Citation22 Such chronic stress paradigms recapitulate certain behavioral features of human depression. Chronic stress produces anhedonia-like symptoms, characterized by a decrease in reward-related behaviors such as reduced preference for sucrose and social interactionCitation22-Citation23 that are rarely seen following acute stress. Additionally, certain behavioral alterations induced by chronic stress are long-lasting and can be effectively reversed by chronic, but not acute, treatment with existing antidepressant medications,Citation22,Citation23 a treatment course comparable with that required in humans. Together, these findings suggest that chronic stress paradigms are more effective at modeling at least certain features or subtypes of the human depression syndrome, while acute studies may provide insight into neuronal adaptations that regulate short-lived responses to stressful events.
Histone modifications
As mentioned above, modifications of histone tails widely affect gene expression and stress is believed to interfere profoundly with this process. While these effects are not perfectly understood, ongoing research is providing important insights into the role that these marks may have in the pathophysiology of depression (Table I)
Table I. Effect of stress on histone post-translational modifications and gene expression. Stress at different ages alters histone modifications within different brain regions, as well as proteins and enzymes responsible for these marks. General: global changes in total cellular levels of these marks. Green indicates increases associated with stress and blue indicates decreases with stress.
Histone acetylation
The potential importance of histone acetylation in depression was initially suggested by observations that HDAC inhibition alone, or in combination with, antidepressant treatment ameliorated depression-like behaviors in rodents.Citation24-Citation31 In mice, chronic social defeat stress (CSDS) induces genome -wide reprogramming of transcriptional profiles.Citation25 These changes associate with a transient decrease in H3K14 acetylation in the nucleus accumbens (NAc) and with a persistent reduction in HDAC2Citation25; both findings are also seen in the NAc of depressed humans. Interestingly, fluoxetine treatment, intra-NAc infusion of a class I HDAC inhibitor, or overexpression of an Hdac2 dominant-negative mutant yields antidepressant-like effects, suggesting that the persistent increase in histone acetylation in the NAc may facilitate adaptation to chronic stress.Citation25,Citation31 However, expression of the class II HDAC, Hdac5, is decreased in the NAc of mice susceptible to CSDS and increased by chronic imipramine treatment, and Hdac5 knockout mice are more susceptible to CSDS.Citation32 In view of these opposing effects, it is likely that HDAC2 and HDAC5 regulate distinct populations of genes and, based on HDAC5's shuttling between the cytoplasm and nucleus, HDAC5 could also regulate nonhistone targets.
Chronic stress paradigms also robustly regulate histone acetylation in the hippocampus (HPC), a brain region implicated in the regulation of stress responses. In contrast to effects in the NAc, CSDS transiently increases, then persistently decreases, global H3K14 acetylationCitation33; effects that are reversed by chronic imipramine. As well, imipramine induction of brain-derived neurotrophic factor (BDNF) in the HPC is associated with increased H3 acetylation at Bdnf promoters.Citation24 However, the fact that intra-HPC HDAC inhibition does not restore social interaction in defeated mice suggests that other mechanisms may be involved, most likely histone methylation. In addition, whereas Hdac5 expression in the NAc is antidepressant, in the HPC, Hdac5 is downregulated by imipramine in defeated mice and HPC overexpression of Hdac5 blocks the antidepressant actions of imipramine. These findings underscore the region-specific influence of epigenetic proteins on complex behavior.
In a genetic rat model of stress susceptibility, high responders (HR), which exhibit basal reductions in anxiety and increased sucrose preference relative to low responders (LR), have increased cAMP response element-binding protein (a type of HAT), lower HDAC3, and higher global H3 and H2B acetylation levels in the HPC.Citation34 However, HR rats are behaviorally more susceptible to CSDS than LR rats and, after CSDS, HR rats have decreased the global H3 and H2B acetylation in the HPC. In contrast, LR rats have increased H3 acetylation, suggesting that dynamic regulation of H3 acetylation in the HPC regulates depression-like behavior. Repeated electroconvulsive seizures (ECS) are robustly antidepressant, and regulate H3 and H4 acetylation at the Bdnf, c-Fos, and Creb promoters in a time-dependent manner that correlates with gene expression changes. Downregulation of c-Fos was linked to reduced H4 acetylation, whereas sustained induction of Bdnf was linked to increased H3 acetylation.Citation35 Potentially, H3 and H4 acetylation differentially modulates depression-like states in the HPC, further highlighting the complexity of histone mechanisms in depression.
H3K14 acetylation is transiently increased in the amygdala after CSDS.Citation36 Intra-amygdala HDAC inhibition reverses social avoidance, but not sucrose-preference deficits, suggesting that histone acetylation in the amygdala and the HPC may regulate different aspects of depression-like behavior. Chronic unpredictable stress in rats reduced Hdac5 expression in the amygdalaCitation37 and acute, but not chronic, defeat transiently decreased amygdala H3 acetylation.Citation38 Data on the role of histone acetylation in depression in the prefrontal cortex (PFC) is limited. Although some studies report no global change in acetylation levels,Citation36,Citation38 conflicting reports suggest that CSDS increases global H3 acetylation in the PFC.Citation39 A recent study found that rats less resilient to CSDS had increased levels of H3K18 acetylation in the PFC.Citation40 At this point, the lack of data documenting effects of manipulating histone acetylation in the amygdala and PFC prohibits a clearer understanding of the importance of potential stress-induced acetylation changes in these brain regions.
Histone methylation
CSDS robustly decreases global levels of the repressive H3K9me2 in the NAc, with the coincident downregulation of the histone methyltransferases G9a and G9a-like protein.Citation33 Overexpression of G9a in the NAc is antidepressantCitation33 and increased H3K9me2 at specific gene promoters is implicated in the antidepressant effect of fluoxetine.Citation41 Indeed, chronic exposure to fluoxetine reduces Camkii expression by reducing H3 acetylation and increasing H3K9me2 levels at the Camkiia promoter in the NAc. Interestingly, similar effects are found in the NAc of depressed humans exposed to antidepressants, suggesting that the stress-induced loss of repressive methylation is maladaptive and that the therapeutic effects of antidepressant drugs may act via the reinstatement of these marks at specific gene loci. The decreases in H3K9me2 in the NAc would be expected to mediate a more permissive transcriptional state similar to the effect of the global increases in H3 acetylation. However, manipulations that decrease repressive methylation induce susceptibility, whereas manipulations that increase acetylation induce resilience. Genome-wide promoter microarrays revealed dynamic changes in H3K9me2 and H3K27me2 levels in the NAc after CSDS or protracted social isolation, with more genes evidencing increased H3 methylation. Interestingly, approximately 20% of genes were similarly regulated in both stress models.Citation42 This opposes the finding of reduced global levels of H3K9me2 in the NAc of susceptible mice.Citation33 Another repressive histone mark, H3K27me3, is increased upstream of the Rac1 gene promoter in the NAc of susceptible mice and this is associated with a sustained reduction in transcript expression that influences characteristic dendritic spine changes in defeated mice.Citation43 These findings are corroborated in humans as H3K27me3 levels are inversely correlated with RAC1 expression, which is also decreased in the NAc of depression patients.Citation43
Stress regulates histone methylation in the HPC in a complex, time-dependent manner, potentially reflecting different processes of initial stress adaptation subsiding into eventual maladaptations with sustained stress.Citation44 For instance, acute stress increases global H3K27me3 and H3K9me3 levels, but decreases H3K9me1, effects that return to basal levels with chronic stress. In addition, subchronic and chronic stress has opposing effects on global H3K4me3 levels, which can be reversed by antidepressant treatment. Acute stress also increases H3K9me3 levels at transposable elements, which may be important in limiting potential genomic instability.Citation45 Whole forebrain overexpression of Setdb1, a histone methyltransferase that catalyzes H3K9me3, reduced depression-like behavior,Citation46 suggesting that the increase in H3K9me3 seen after acute stress may represent an adaptive response. However, experimental manipulations of such modifications are needed to interpret the functional consequences of these adaptations.
Aside from the few examples cited above, human postmortem studies examining histone modifications in depression are sparse. Elevated H3K4me3 levels were reported in the synapsin gene family, which was associated with higher expression of SYNAPSIN 2 in the PFCCitation47 from depression, but not bipolar disorder cases, suggesting that these changes may be specific to major depression. Similarly, higher levels of H3K4me3 in the polyamine gene OAZ were associated with higher expression in the PFC of suicide completers.Citation48 Decreased expression of the tyrosine receptor kinase B (TRKB), the receptor for BDNF in the PFC of depressed cases is also associated with an enrichment of H3K27me3 levels in the promoter of both TRKB and its astrocytic variant, TRKB.T1. Citation49,Citation50 The elevated H3K27me3 levels are associated with changes in DNA methylation in the gene promoter, suggesting the presence of dual epigenetic control over TRKB.T1 expression. In addition, mice overexpressing TRKB.T1 are more susceptible to CSDS,Citation51 which raises the possibility that epigenetic changes at the TRKB.T1 promoter could define vulnerability to chronic stress and the development of depression. Human postmortem studies also suggest that antidepressants promote open chromatin by decreasing H3K27me3 levels at BDNF promoters in the PFC of a depression sample,Citation52 an effect supported by follow-up studies in peripheral blood revealing an inverse correlation between H3K27me3 levels and both BDNF IV expression levels and symptom severity.Citation53
DNA methylation
A growing body of evidence supports a role for DNA methylation in mediating the impact of stress. Several of these alterations have been described in different animal models and more recently in human brains (Table II).
Table II. Effect of stress on DNA methylation and gene expression. Stress at different ages alters DNA methylation at specific genes within different brain regions, as well as proteins and enzymes related to DNa methylation. Green indicates increases associated with stress and blue indicates decreases with stress.
CSDS increases transcript levels of Dnmt3a in the NAc. Overexpressing Dnmt3a in NAc increases depression-like behavior after submaximal social defeat and intra-NAc infusion of a DNMT inhibitor, RG108, reverses defeat-induced social avoidance.Citation54 DNMT3a activity is generally associated with transcriptional repression, suggesting that susceptibility may be associated with downregulation of transcriptional expression in the NAc. Genome-wide analysis of DNA methylation will be important in establishing the precise mechanisms of this epigenetic modification in defeat-induced susceptibility. DNA methylation in NAc may play a role in regulating Gdnf (glial cell-derived neurotrophic factor).Citation31 Chronic mild stress induces depression-like behavior and increases Gdnf expression in the NAc of a stress-sensitive mouse line (BALB/C), but increases Gdnf expression in a more resilient line (C57BL/6). Although DNA methylation was increased at the Gdnf promoter in the NAc of both mouse lines, MECP2 was reported to complex with different proteins in the two lines and to thereby repress vs facilitate transcription. The authors suggest that these differences are mediated by differing patterns of methylation at the Gdnf promoter, although more work is required to confirm this speculation and elucidate the underlying mechanisms involved.
DNA methylation is implicated in the regulation of corticotropin-releasing factor (CRF) in the paraventricular nucleus of the hypothalamus (PVN)Citation37,Citation55 (see Figure 1 A). CRF is a critical regulator of the HPA-axis activation and other stress actions in the brain. CRF is increased in the PVN of mice that are susceptible to social defeat and this is accompanied by decreased DNA methylation at the Crf promoter. Both effects are reversed by chronic imipramine treatment.Citation55 DNA methylation is also increased at the Crf promoter in the PVN of female rats subjected to chronic mild stress, suggesting that DNA methylation may play a role in determining sex-specific regulation of the HPA-axis function.Citation37 Knockout of Mecp2 in PVN results in an exaggerated physiological stress response, however, the precise mechanism of MECP2 action remains to be fully elucidated.Citation56
The studies elaborated above highlight epigenetic modifications targeted toward specific genes frequently associated with behavioral alterations. However, it is clear that stress effects are not restricted to a subset of candidate genes. Genome-wide studies mapping DNA methylation alterations induced by stress are lacking in animals. A series of recent genome-wide studies address this issue in humans in the context of early-life adversity, as discussed below. Furthermore, analysis of the PFC of psychotic and bipolar cases reported numerous sites of differential methylation that were enriched in various functions such as glutamatergic and GABAergic neurotransmission, brain development, and response to stress.Citation57 Importantly, these studies compared different tissues (blood vs brain) and brain regions (HPC vs PFC) and globally suggest that stress-induced epigenetic adaptations are region specific and cell-type specific, which is consistent with the emerging principle of epigenetic heterogeneity across tissuesCitation58 and cell types.Citation59,Citation60
Epigenetics and depression: development vulnerability
It is well established that adults who experienced childhood stress or maltreatment are at a significantly greater lifetime risk for a range of mood or other disorders.Citation61-Citation64 Early-life adversity is modeled in animals using maternal separation (MS) or maternal deprivation. Natural variations in maternal care—maternal licking and grooming (LG)—likewise associate with differential stress responses among adult offspring.Citation65 Research in the last decade suggests that epigenetic mechanisms partly mediate these effects by altering the expression of key genes in response to subsequent environmental perturbations, thereby enhancing vulnerability to psychiatric disorders. This section describes epigenetic alterations resulting from developmental (in utero, early postnatal, and periadolescent) exposures to stress, with distinct consequences for depression and related disorders.
Histone modifications
Very little is known about the prenatal effects of stress on histone modifications. Treatment with the nonspecific HDAC inhibitor valproic acid, which has many additional pharmacological actions, after prenatal stress was shown to ameliorate several behavioral measures,Citation66 although more work is needed to elucidate the mechanisms responsible for these effects. More is known concerning the consequences of postnatal adversity in the form of maternal separation. In stress-susceptible BALB/C mice, MS reduces levels of Hdac1, -3, -7, -8, and -10 in the forebrain in adulthoods, and increases acetylation of histone H4.Citation67 Adult male rats that underwent maternal separation had reduced levels of Hdac1 mRNA,Citation68 consistent with the elevated H3 and H4 acetylation levels reported in the HPC of juvenile mice after maternal separation.Citation69 Adolescent fluoxetine treatment potentiated effects of maternal separation, but coadministration of fluoxetine with an HDAC inhibitor ameliorated the effects of maternal separation.Citation67 These findings suggest that adolescence may be a relevant period for pharmacological intervention and that it may be possible to erase at least some of the damaging epigenetic signature of early-life stress. Similarly, low maternal LG is associated with decreased HPC H3K9 acetylation at the glucocorticoid receptor (Gr) exon 17 promoter.Citation28,Citation70,Citation71,Citation72 These modifications are associated with the expression of depressive-like symptoms, reduced gene expression, and changes in DNA methylation spanning large regions of the genome.Citation71 Treatment with the nonselective HDAC inhibitor trichostatin A, infused either intracerebroventricularly (ICV) or intra HPC, reversed both the molecular and behavioral effects of low maternal care.Citation28,Citation73
DNA methylation
Prenatal stress
Under normal conditions, the developing fetus is largely protected from maternal glucocorticoids by the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which converts active glucocorticoids to their inactive form. However, 10% to 20% of maternal Cortisol is estimated to pass through the placenta to the fetus.Citation74 Evidence suggests that maternal stress during pregnancy induces a hypermethylation of Hsd11b2 in the placenta and hypomethylation in the fetal hypothalamusCitation75 that may consequently interfere with 11β-HSD2 enzymatic activityCitation76,Citation77 and induce heightened stress responses among offspring.Citation78,Citation79
Exposure to early prenatal stress is also associated with persistent changes in DNA methylation in the brains of adult miceCitation80 Within the hypothalamus, early prenatal stress increases DNA methylation in Gr noncoding exon 17 and decreases DNA methylation in the Crf promoter.Citation80 In humans, hypermethylation in the GR promoter 1F was likewise found in infant cord blood from mothers who experienced depression during the third trimester of pregnancyCitation81,Citation82 and from mothers reporting intimate partner violence during their pregnancy.Citation83 These effects were not reversed by antidepressant treatment. Altered DNA methylation at the serotonin transporter (SERT, SLC6A4) in offspring cord blood is likewise associated with prenatal maternal depression.Citation84 However, a recent study found no association between maternal depression and altered DNA methylationCitation85 with modest methylation differences in two genes (TNFRSF21 and CHRNA2) associated with maternal antidepressant use.
In addition, elevated levels of Dnmt3a mRNA were found in the placenta of rats exposed to in utero stress, while elevated Dnmt1 mRNA was found within the cortex of offspring at gestational day 20.Citation75 Mice exposed to prenatal stress had elevated levels of Dnmt3a and Dnmt1 mRNA in the PFC and HPC at birth, changes that persisted at postnatal day 7, 14, and 60.Citation66 Furthermore, prenatal stress increased binding of DNMTl and MECP2, along with increased 5-methylcytosine and 5-hydroxymethylcytosine, within the Reelin and Gad67 promoters.Citation66
Thus, existing evidence points to a role of prenatal stress in altering adult vulnerability to depression, in part via changes in DNA methylation. These alterations occur in specific genes and in specific brain regions, highlighting the difficulty in using peripheral tissues to predict functionally relevant changes within the brain. Genome-wide analyses should facilitate comparisons of potential epigenetic biomarkers in peripheral tissues with epigenetic and gene expression changes in specific brain regions. Even if changes that occur in the periphery are different from those in brain, it is conceivable that specific peripheral changes might predict central regulation; this is ultimately an empirical question. In addition, the inconsistencies reported among studies reflect the need for more precise tools to manipulate epigenetic gene regulation in functional preclinical studies.
Postnatal stress
Postnatal experience, particularly variations in the level and quality of maternal care, alters DNA methylation levels in genes thought to be critically involved in behavioral stress responses. For instance, the offspring of low-LG mothers, compared with those reared by high-LG mothers, exhibit robust DNA methylation changes that colocalized with chromatin modifications.Citation86,Citation87 This coincides with lower HPC expression of several variants of Gr, including the HPC specific variant 17.Citation28,Citation71 These alterations preferentially affect promoters, as evidenced in the cluster of protocadherin genes, and follow a nonrandom, discontinuous pattern across large genomic regions.Citation86 However, it is still unclear how both DNA methylation and chromatin conformation are coregulated in the context of stress. Similar alterations have been reported in the HPC of suicide completers with a history of child abuse. Individuals with a history of abuse who committed suicide exhibit lower expression levels of the 1B, 1C, and 1F variants of Gr compared with nonabused suicides and controls.Citation88,Citation89 These changes coincide with altered DNA methylation within respective promoters that may interfere with transcription factor binding. Furthermore, similar alterations within Gr positively correlate with different features of child abuse in individuals with major depressive disorders.Citation90,Citation91 Importantly, these alterations appear to be specific to early-life adversity, as negative findings have been reported in the brains of depressed patients with no history of child abuse.Citation92
Low maternal care in rats also reduces Gad1 and Grm1 expression in the HPC.Citation70,Citation72 These expression changes are accompanied by promoter hypermethylation and lower levels of H3K9Ac compared with pups raised by high-LG dams. As these findings have been associated with elevated levels of Dnmt1, it is believed that this hypermethylated state originates from the overactivity of different DNMTs. Indeed, human studies in schizophrenia and bipolar disorder reported correlations between promoter hypermethylation and lower expression of REELIN and GAD1 genesCitation93-Citation97 that are associated with altered levels of all three DNMTs (DNMT1, 3A, and 3B). Citation98
Exposure to early life stress likewise alters epigenetic gene regulation within the HPA axis and reward circuitry (Figure 1). Early maternal separation is associated with Avp overexpression and hypomethylation of an Avp enhancer region (rather than promoter) in the PVN of mice 6 weeks, 3 months, and 1 year after stress.Citation99 Interestingly, gene expression, but not enhancer methylation changes were found within 10 days of stress, suggesting a dual regulatory mode depending on the timing of stress and highlighting the importance of investigating mechanisms beyond the traditional promoter-centric focus.
Stress also alters epigenetic marks beyond the early neonatal period. Three weeks of adolescent isolation induced depressive-like behaviors accompanied by a sustained (12 weeks) hypermethylation of the tyrosine hydroxylase (Th) gene promoter in the VTA of a Disc1 mutant mouse.Citation100 Promoter hypermethylation was associated with both Disc1 mutations and adolescent isolation, and these effects were additive, although only in specific cell populations.Citation100 Importantly, these changes were rescued by treatment with a GR antagonist, suggesting that GR-mediation of the stress response in this chronic adolescent stress paradigm underlies stress-induced alterations in the mesocortical reward pathway.Citation100
DNA methylation is also altered by extreme childhood adversity in the form of abuse. Recently, hundreds of differentially methylated sites were identified in the HPC of suicide completers with a history of abuse (childhood sexual or physical) compared with healthy controls.Citation101 Interestingly, DNA methylation levels in gene promoters were inversely correlated with gene expression at a genome-wide level, supporting the globally repressive role of DNA methylation at promoters, as reported by other groups.Citation57,Citation102 The impact of abuse becomes obvious when assessing the gene functions enriched with differential methylation: differential methylation in the abused suicide group is enriched in genes related to cellular plasticity, while learning and memory genes were particularly affected in suicide. This suggests that among depressed suicide completers, intense early-life adversity might induce distinct longlasting epigenetic alterations. Importantly, the changes in DNA methylation levels reported in these studies occurred in specific cell types as most of the methylation changes were found exclusively in neuronal DNA. Rodent studies have likewise found DNA methylation alterations subsequent to maternal abuse (tramping, dragging, rough treatment). Rat maternal maltreatment decreases expression of Bdnf transcripts III and IV in the HPC and PFC and is associated with chronic hypomethylation at the Bdnf exon IX gene promoter in the PFC.Citation103,Citation104 These effects were at least partially rescued by ICV treatment for 7 days with zebularine, a DNA methylation inhibitor.
These studies suggest that the experience of stress, whether during early-life or adulthood, has profound, genome-wide epigenetic consequences in the brain and peripheral tissues. Modifications of DNA methylation signatures in different regions of the brain are a plausible mechanism to explain how stress can induce enduring behavioral alterations. Peripheral tissues may provide biomarkers of stress exposure and vulnerability, although this remains to be determined. Future studies should investigate correlated regulation of genome-wide DNA methylation between blood and brain and across numerous brain regions in animal models to facilitate interpretation of peripheral-based assays in humans.
Limitations, future directions, and concluding remarks
A wealth of data from animal models and evolving evidence from postmortem human samples has established the important role of diverse epigenetic regulatory mechanisms in mediating the transcriptional abnormalities that contribute importantly to depression. However, as our understanding of epigenetic mechanisms improves, several new questions emerge. Understanding how diverse epigenetic mechanisms, including histone modifications, DNA methylation, and noncoding RNAs, interact to ultimately orchestrate the characteristic abnormalities in gene expression is critical. There is an urgent demand for increased specificity in studies attempting to understand the interplay of developmental stress, epigenetics, and adult neuropsychiatry disorders. It is also imperative that the field move beyond the study of individual candidate genes and make far greater use of genome-wide determinations of stress- and depression-associated epigenetic abnormalities. Indeed, recent efforts have begun to generate significant data sets profiling genome-wide alterations in DNA methylation and other epigenetic patterns after stress. The current challenge is to refine these analyses to understand how epigenetic regulation of multiple genes and gene networks in specific brain regions relate to depression-related outcomes, first in animal models and ultimately in the human brain.
Although existing studies document how early developmental stress leads to life-long changes in gene expression and behavior, to understand these altered trajectories fully, it will be important to profile the epigenetic landscape across development. For instance, certain epigenetic modifications could conceivably be altered proximal to the time of stress, whereas other epigenetic modifications may incubate over time, but may be more stably maintained. A more precise understanding of such temporal dynamics obtained from genome-wide studies will facilitate attempts to identify critical windows for therapeutic intervention.
Selected abbreviations and acronyms
| CSDS | = | chronic social defeat stress |
| DNMT | = | DNA methyltransferase |
| HDAC | = | histone deacelytase |
| HPC | = | hippocampus |
| NAc | = | nucleus accumbens |
| PFC | = | prefrontal cortex |
| PTM | = | post-translational modification |
REFERENCES
- FengJ.NestlerEJ.Epigenetic mechanisms of drug addiction.Curr Opin Neurobiol.20132352152823374537
- PenaCJ.BagotRC.LabonteB.NestlerEJ.Epigenetic signaling in psychiatric disorders.J Mol Biol. 2014 Apr 5. Epub ahead of print.
- BaliP.KennyPJ.MicroRNAs and drug addiction.Front Genet.201344323717324
- RobisonAJ.NestlerEJ.Transcriptional and epigenetic mechanisms of addiction.Nat Rev Neurosci.20111262363721989194
- MahgoubM.MonteggiaLM.Epigenetics and psychiatry.Neurotherapeutics.20131073474124092614
- HasanA.MitchellA.SchneiderA.HaleneT.AkbarianS.Epigenetic dysregulation in schizophrenia: molecular and clinical aspects of histone deacetylase inhibitors.Eur Arch Psychiatry Clin Neurosci.201326327328423381549
- DempsterE.VianaJ.PidsleyR.MillJ.Epigenetic studies of schizophrenia: progress, predicaments, and promises for the future.Schizophr Bull.201339111623211398
- CislerJM.JamesGA.TripathiS.et alDifferential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress.Psychol Med.20134350751822781311
- SequeiraA.KlempanT.CanettiL.et alPatterns of gene expression in the limbic system of suicides with and without major depression.Mol Psychiatry.20071264065517353912
- DumanRS.AghajanianGK.Synaptic dysfunction in depression: potential therapeutic targets.Science.2012338687223042884
- McEwenBS.Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders.Ann NY Acad Sci.200410321715677391
- ResslerKJ.MaybergHS.Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic.Nat Neurosci.2007101116112417726478
- JenuweinT.AllisCD.Translating the histone code.Science.20012931074108011498575
- StrahlBD.AllisCD.The language of covalent histone modifications.Nature.2000403414510638745
- KloseRJ.BirdAP.Genomic DNA methylation: the mark and its mediators.Trends Biochem Sci.200631899716403636
- MaunakeaAK.NagarajanRP.BilenkyM.et alConserved role of intragenic DNA methylation in regulating alternative promoters.Nature.201046625325720613842
- KriaucionisS.HeintzN.The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain.Science.200932492993019372393
- TahilianiM.KohKP.ShenY.et alConversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MIL partner TET1.Science.200932493093519372391
- FengJ.ChangH.LiE.FanG.Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system.J Neurosci Res.20057973474615672446
- MaDK.GuoJU.MingGL.SongH.DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation.Cell Cycle.200981526153119377292
- AapolaU.KawasakiK.ScottHS.et alIsolation and initial characterization of a novel zinc finger gene, DNMT3L, on 21q22.3, related to the cytosine-5-methyltransferase 3 gene family.Genomics.20006529329810857753
- BertonO.McClungCA.DileoneRJ.et alEssential role of BDNF in the mesolimbic dopamine pathway in social defeat stress.Science.200631186486816469931
- WallaceDL.HanMH.GrahamDL.et alCREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits.Nat Neurosci.20091220020919151710
- TsankovaNM.BertonO.RenthalW.KumarA.NeveRL.NestlerEJ.Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action.Nat Neurosci.2006951952516501568
- CovingtonHE.MazeI.LaPlantQC.et alAntidepressant actions of histone deacetylase inhibitors.J Neurosci.200929114511146019759294
- SchroederFA.LinCL.CrusioWE.AkbarianS.Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse.Biol Psychiatry.200762556416945350
- SembaJ.KurodaY.TakahashiR.Potential antidepressant properties of subchronic GABA transaminase inhibitors in the forced swimming test in mice.Neuropsychobiology.1989211521562559361
- WeaverIC.CervoniN.ChampagneFA.et alEpigenetic programming by maternal behavior.Nat Neurosci.2004784785415220929
- YamawakiY.FuchikamiM.MorinobuS.SegawaM.MatsutnotoT.YamawakiS.Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus.World J Biol Psychiatry.20121345846721812623
- ZhuH.HuangQ.XuH.NiuL.ZhouJN.Antidepressant-like effects of sodium butyrate in combination with estrogen in rat forced swimming test: involvement of 5-HT(1A) receptors.Behav Brain Res.200919620020618817816
- UchidaS.HaraK.KobayashiA.et alEpigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events.Neuron.20116935937221262472
- RenthalW.MazeI.KrishnanV.et alHistone Deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli.Neuron.20075651752917988634
- CovingtonHE.MazeI.SunH.et alA role for repressive histone methylation in cocaine-induced vulnerability to stress.Neuron.20117165667021867882
- MollisF.DuclotF.GunjanA.KabbajM.Individual differences in the effect of social defeat on anhedonia and histone acetylation in the rat hippocampus.Norm Behav.201159331337
- TsankovaNM.KumarA.NestlerEJ.Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures.J Neurosci.2004245603561015201333
- CovingtonHE 3rdVialouVF.LaplantQ.OhnishiYN.NestlerEJ.Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition.Neurosci Lett.201149312212621335060
- SterrenburgL.GasznerB.BoerrigterJ.et alChronic stress induces sex-specific alterations in methylation and expression of corticotropinreleasing factor gene in the rat.PLoS One.20116e28128
- HollisF.WangH.DietzD.GunjanA.KabbajM.The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats.Psychopharmacology.2010211697720454892
- HinwoodM.TynanRJ.DayTA.WalkerFR.Repeated social defeat selectively increases SFosB expression and histone H3 acetylation in the infralimbic medial prefrontal cortex.Cereb Cortex.20112126227120513656
- KenworthyCA.SenguptaA.LuzSM.et alSocial defeat induces changes in histone acetylation and expression of histone modifying enzymes in the ventral hippocampus, prefrontal cortex, and dorsal raphe nucleus.Neuroscience.2014264889823370319
- RobisonAJ.VialouV.SunHS.et alFluoxetine epigenetically alters the CaMKIIa promoter in nucleus accumbens to regulate AFosB binding and antidepressant effects.Neuropsychopharmacology.2014391178118624240473
- WilkinsonMB.XiaoG.KumarA.et alImipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models.J Neurosci.2009297820783219535594
- GoldenSA.ChristoffelDJ.HeshmatiM.et alEpigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression.Nat Med.20131933734423416703
- HunterRG.McCarthyKJ.MilneTA.PfaffDW.McewenBS.Regulation of hippocampal H3 histone methylation by acute and chronic stress.Proc Natl Acad Sci U S A.2009106209122091719934035
- HunterRG.MurakamiG.DewellS.et alAcute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response.Proc Natl Acad Sci U S A.2012109176571766223043114
- JiangY.MatevossianA.HuangHS.StraubhaarJ.AkbarianS.Isolation of neuronal chromatin from brain tissue.BMC Neurosci.200894218442397
- CruceanuC.AldaM.NagyC.FreemantleE.RouleauGA.TureckiG.H3K4 tri-methylation in synapsin genes leads to different expression patterns in bipolar disorder and major depression.Int J Neuropsychopharmacol.20131628929922571925
- FioriLM.GrossJA.TureckiG.Effects of histone modifications on increased expression of polyamine biosynthetic genes in suicide.Int J Neuropsychopharmacol.2012151161116622008221
- ErnstC.ChenES.TureckiG.Histone methylation and decreased expression of TrkB.TI in orbital frontal cortex of suicide completers.Mol Psychiatry.20091483083219696771
- ErnstC.DelevaV.DengX.et alAlternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers.Arch Gen Psychiatry.200966223219124685
- RazzoliM.DomeniciE.CarboniL.et alA role for BDNF/TrkB signaling in behavioral and physiological consequences of social defeat stress. Genes Brain Behav.20111042443321272243
- ChenES.ErnstC.TureckiG.The epigenetic effects of antidepressant treatment on human prefrontal cortex BDNF expression.Int J Neuropsychopharmacol.20111442742921134314
- LopezJP.MamdaniF.LabonteB.et alEpigenetic regulation of BDNF expression according to antidepressant response.Mol Psychiatry.20131839839922547115
- LaPlantQ.VialouV.CovingtonHE.et alDnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens.Nat Neurosci.2010131137114320729844
- ElliottEE.Ezra-NevoGG.RegevLL.Neufeld-CohenAA.ChenAA.Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice.Nat Neurosci.2010131351135320890295
- FyffeSL.NeulJL.SamacoRC.et alDeletion of Mecp2 inSitnl-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress.Neuron.20085994795818817733
- MillJ.TangT.KaminskyZ.et alEpigenomic profiling reveals DNAmethylation changes associated with major psychosis.Am J Hum Genet.20088269671118319075
- Ladd-AcostaC.PevsnerJ.SabunciyanS.et alDNA methylation signatures within the human brain.Am J Hum Genet.2007811304131517999367
- DeatonAM.WebbS.KerrAR.et alCell type-specific DNA methylation at intragenic CpG islands in the immune system.Genome Res.2011211074108621628449
- IwamotoK.BundoM.UedaJ.et alNeurons show distinctive DNA methylation profile and higher interindividual variations compared with non-neurons. Genome.Res.201121688696
- HeimC.NemeroffCB.The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies.Biol Psychiatry.2001491023103911430844
- HolmesSJ.RobinsLN.The influence of childhood disciplinary experience on the development of alcoholism and depression.J Child Psychol Psychiatry.1987283994153597564
- EnochMA.The role of early life stress as a predictor for alcohol and drug dependence.Psychopharmacology.2011214173120596857
- RiggsS.AlarioAJ.Adolescent substance use and the role of the primary care provider.R I Med J.1990732532572193360
- MeaneyMJ.Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations.Annu Rev Neurosci.2001241161119211520931
- MatriscianoF.TuetingP.DalaiI.et alEpigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice.Neuropharmacology.20136818419422564440
- LevineA.WorrellTR.ZimniskyR.SchmaussC.Early life stress triggers sustained changes in histone deacetylase expression and histone H4 modifications that alter responsiveness to adolescent antidepressant treatment.Neurobiol Dis.20124548849821964251
- BlazeJ.RothTL.Exposure to caregiver maltreatment alters expression levels of epigenetic regulators in the medial prefrontal cortex.Int J Dev Neurosci.20133180481024120634
- XieL.KorkmazKS.BraunK.BockJ.Early life stress-induced histone acetylations correlate with activation of the synaptic plasticity genes Arc and Egr1 in the mouse hippocampus.J Neurochem.201312545746423413876
- BagotRC.ZhangTY.WenX.et alVariations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat.Proc Natl Acad Sci U S A.2012109172001720723045678
- McGowanPO.SudermanM.SasakiA.et alBroad epigenetic signature of maternal care in the brain of adult rats.PLoS One.20116e1473921386994
- ZhangTY.HellstromIC.BagotRC.WenX.DiorioJ.MeaneyMJ.Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus.J Neurosci.201030131301313720881131
- WeaverIC.MeaneyMJ.SzyfM.Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood.Proc Natl Acad Sci U S A.20061033480348516484373
- GitauR.CameronA.FiskNM.GloverV.Fetal exposure to maternal Cortisol.Lancet.19983527077089728994
- JensenPeña C.MonkC.ChampagneFA.Epigenetic effects of prenatal stress on 11[J-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain.PLoS One.20127e39791e3979122761903
- MairesseJ.LesageJ.BretonC.et alMaternal stress alters endocrine function of the feto-placental unit in rats.Am J Physiol Endo Metab.2007292E1526E1533
- O'DonnellKJ.BuggeJensen A.FreemanL.KhalifeN.O'ConnorTG.GloverV.Maternal prenatal anxiety and downregulation of placental 11P-HSD2.Psychoneuroendocrinology.20123781882622001010
- HolmesMC.AbrahamsenCT.FrenchKL.PatersonJM.MullinsJJ.SecklJR.The mother or the fetus? 11beta-hydroxysteroid dehydrogenase type 2 null mice provide evidence for direct fetal programming of behavior by endogenous glucocorticoids.J Neurosci.2006263840384416597738
- WelbergLA.SecklJR.HolmesMC.Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxietylike behaviour in the offspring.Eur J Neurosci.2000121047105410762336
- MuellerBR.BaleTL.Sex-specific programming of offspring emotionality after stress early in pregnancy.J Neurosci.2008289055906518768700
- OberlanderTF.WeinbergJ.PapsdorfM.GrunauR.MisriS.DevlinAM.Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant Cortisol stress responses.Epigenetics.200839710618536531
- HompesT.IzziB.GellensE.et alInvestigating the influence of maternal Cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood.J Psychiatr Res.20134788089123566423
- RadtkeKM.RufM.GunterHM.et alTransgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor.Transl Psychiatry.20111e2122832523
- DevlinAM.BrainU.AustinJ.OberlanderTF.Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth.PLoS One.20105e1220120808944
- SchroederJ.SmithA.BrennanP.et alDNA methylation in neonates born to women receiving psychiatric care.Epigenetics.2012740941422419064
- SudermanM.McGowanPO.SasakiA.et alConserved epigenetic sensitivity to early life experience in the rat and human hippocampus.Proc Natl Acad Sci U S A.2012109172661727223045659
- PeñaCJ.NeugutYD.ChampagneFA.Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats.Endocrinology.20131544340435124002038
- LabonteB.YerkoV.GrossJ.et alDifferential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse.Biol Psychiatry.201272414822444201
- McGowanPO.SasakiA.D'AlessioAC.et alEpigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse.Nat Neurosci.20091234234819234457
- PerroudN.Paoloni-GiacobinoA.PradaP.et alIncreased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma.Transl Psychiatry.20111e5922832351
- TyrkaAR.PriceLH.MarsitC.WaltersOC.CarpenterLL.Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults.PLoS One.20127e3014822295073
- AltSR.TurnerJD.KlokMD.et alDifferential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed.Psychoneuroendocrinology.20103554455619782477
- GuidottiA.AutaJ.DavisJM.et alDecrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study.Arch Gen Psychiatry.2000571061106911074872
- VeldicM.CarunchoHJ.LiuWS.et alDNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains.Proc Natl Acad Sci U S A.200410134835314684836
- KundakovicM.ChenY.CostaE.GraysonDR.DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes.Mol Pharmacol.20077164465317065238
- GraysonDR.JiaX.ChenY.et alReelin promoter hypermethylation in schizophrenia.Proc Natl Acad Sci U S A.20051029341934615961543
- TamuraY.KunugiH.OhashiJ.HohjohH.Epigenetic aberration of the human REELIN gene in psychiatric disorders.Mol Psychiatry.200712593600
- PoulterMO.DuL.WeaverIC.et alGABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes.Biol Psychiatry.20086464565218639864
- MurgatroydC.PatchevAV.WuY.et alDynamic DNA methylation programs persistent adverse effects of early-life stress.Nat Neurosci.2009121559156619898468
- NiwaM.Jaaro-PeledH.TankouS.et alAdolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids.Science.201333933533923329051
- LabonteB.SudermanM.MaussionG.et alGenome-wide epigenetic regulation by early-life trauma.Arch Gen Psychiatry.20126972273122752237
- UddinM.AielloAE.WildmanDE.et alEpigenetic and immune function profiles associated with posttraumatic stress disorder.Proc Natl Acad Sci U S A.20101079470947520439746
- RothTL.LubinFD.FunkAJ.SweattJD.Lasting epigenetic influence of early-life adversity on the BDNF gene.Biol Psychiatry.20096576076919150054
- RothTL.ZoladzPR.SweattJD.DiamondDM.Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of posttraumatic stress disorder.J Psychiatr Res.20114591992621306736
- SunH.KennedyPJ.NestlerEJ.Epigenetics of the depressed brain: role of histone acetylation and methylation.Neuropsychopharmacology.20133812413722692567
