Abstract
Autism spectrum disorder (ASD) is a heterogeneous condition that affects 1 in 68 children. Diagnosis is based on the presence of characteristic behavioral impairments that emerge in the second year of life and thus is not typically made until 3 to 4 years of age. Recent studies of early brain and behavior development have provided important new insights into the nature of this condition. Autism-specific brain imaging features have been identified as early as 6 months of age, and age-specific brain and behavior changes have been demonstrated across the first 2 years of life, highlighting the developmental nature of ASD. New findings demonstrate that early brain imaging in the first year of life holds great promise for presymptomatic prediction of ASD. There is a general understanding in medicine that earlier treatment has better outcomes than later treatment, and in autism, there is an emerging consensus that earlier intervention results in more successful outcomes for the child. Examining early brain and behavior trajectories also has the potential to parse the etiologic heterogeneity in ASD, a well-recognized impediment to developing targeted, mechanistic treatments. This review highlights the current state of the science in the pursuit of early brain and behavioral markers of autism during infancy and examines the potential implications of these findings for treatment of this condition.
El trastorno del espectro autista (TEA) es una condición heterogénea que afecta a uno entre 68 niños. El diagnóstico está basado en la presencia de alteraciones conductuales características que aparecen durante el segundo año de vida y que no son totalmente típicas hasta los tres o cuatro años. Estudios recientes sobre el desarrollo precoz, tanto cerebral como conductual, han aportado novedosos conocimientos respecto a la naturaleza de este cuadro. En los estudios de imágenes, ya a los seis meses de edad se han identificado características que son específicas para el autismo; también se han observado cambios conductuales y cerebrales específicos para la edad durante los dos primeros años de vida, lo que resalta la naturaleza evolutiva del TEA. Hay nuevos hallazgos que demuestran que imágenes cerebrales precoces durante el primer año de vida constituyen una gran promesa para la predicción del TEA previo a la aparición de los síntomas. En medicina existe el concepto que un tratamiento más precoz tiene mejor resultado que uno más tardío, y en el autismo ha surgido el consenso que una intervención más precoz obtiene resultados más exitosos para el niño. La evaluación de las manifestaciones conductuales y cerebrales precoces también tiene el potencial de analizar la heterogeneidad etiológica del TEA, la cual constituye un impedimento bien reconocido para el desarrollo de tratamientos específicos. Esta revisión destaca el estado actual de la ciencia en cuanto a la búsqueda de marcadores conductuales y cerebrales precoces de autismo durante la infancia y analiza las potenciales implicancias de estos hallazgos en el tratamiento de esta patología.
Le trouble du spectre de l'autisme (TSA) est une maladie hétérogène qui touche 1 enfant sur 68. Le diagnostic, rarement posé avant 3 ou 4 ans, est basé sur la présence de déficits comportementaux caractéristiques, apparaissant lors de la deuxième année de vie. De récentes études sur le développement précoce du cerveau et du comportement ont éclairé différemment la nature de cette maladie. Une imagerie cérébrale spécifique de l'autisme est identifiée dès l'âge de 6 mois et les modifications du cerveau et du comportement liées à l'âge mises en évidence lors des 2 premières années de vie, soulignent la nature développementale du TSA. D'après des données récentes, l'imagerie cérébrale précoce dans la 1re année de vie est très prometteuse en termes de prédiction présymptomatique du TSA. En médecine, il est généralement admis qu'un traitement précoce donne de meilleurs résultats qu'un traitement tardif et dans l'autisme, le consensus actuel est qu'il est bénéfique pour l'enfant d'intervenir tôt. L'examen des trajectoires précoces du cerveau et du comportement permet d'analyser l'hétérogénéité étiologique du TSA, frein bien connu au développement de traitements mécanistiques ciblés. Cet article met en lumière l'état actuel de la science dans la recherche de marqueurs précoces cérébraux et comportementaux de l'autisme pendant l'enfance et il analyse les implications potentielles de ces résultats pour le traitement de cette maladie.
Introduction
A critical challenge in child psychiatry is the need for the early detection of autism. Although autism spectrum disorder (ASD) affects 1 in 68 children, the average age of diagnosis in the United States is not until 4 years or older.Citation1 The current diagnostic criteria are hampered by the reliance on behaviorally defined impairments in social interaction and communication, along with the occurrence of restricted interests and repetitive behaviors (Diagnostic and Statistical Manual of Mental Disorders, fifth edition [DSM-5]).Citation2 which are not readily present in the first 2 years of life. Behavioral symptoms at the first year of life are not specific to ASD,Citation3-Citation5 and the symptoms that are diagnostic of ASD gradually unfold in the second year of life.Citation4-Citation6 The lack of early behavioral markers is a critical unmet challenge because early detection allows for early intervention, which is effective in decreasing impairmentsCitation7 and results in more positive long-term outcomes for the child.Citation8,Citation9
Given that the diagnostic behavioral features are not present until 2 years of age or later, how do we identify earlier markers of ASD? The solution is the advent of the “infant sibling” study design. This study design leverages the recurrence risk in siblingsCitation10 of about 20%: if an older sibling is already diagnosed with ASD, a subsequent younger sibling has a 15-to-20-fold greater risk of developing the condition than the general population. In addition, 20% to 30% of the remaining younger siblings who do not develop ASD will develop other developmental delays or psychiatric outcomes.Citation11-Citation13 Infant sibling studies longitudinally follow the younger “high-risk” (HR) siblings starting in infancy until the age when ASD can be reliably diagnosed. Here, we refer to such HR siblings who develop ASD as HR-ASD, and those who do not as HR-negative. In infant sibling studies, HR-ASD infants are compared with HR-negative infants and “low-risk” control infants with no family history of ASD or psychiatric disorders (LR-negative). Behavior assessments are conducted at regular intervals throughout infancy (eg, 6-12-24 months). At least one study, the Infant Brain Imaging Study (IBIS) Network, collects longitudinal magnetic resonance imaging (MRI) throughout the infant interval, whereas other studies collect electroencephalogram (EEG) and other electrophysiological measures. Thus, the infant sibling study paradigm offers an efficient strategy for prospectively studying the earliest behavioral and neural features of autism, before diagnosis. The ultimate goal is to detect early behavioral and biological markers of ASD before behavioral diagnosis is currently possible.
Early behavioral signs
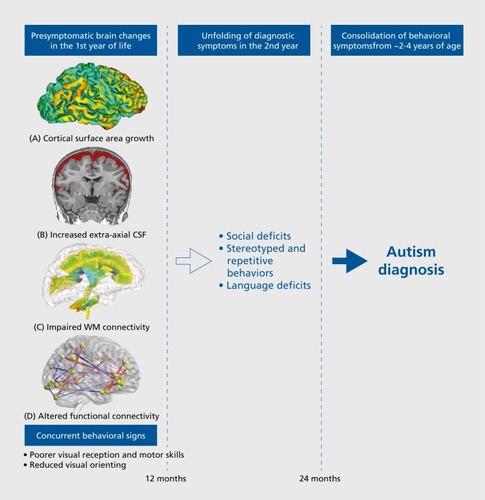
The extant literature of infant sibling studies of autism indicates that diagnostic symptoms, such as social be havior, are normal at 6 months of age and unfold and emerge over the first 2 to 3 years of life.Citation4 To date, studies of HR infants have not identified differences in the diagnostic symptoms of autism at 6 months in infants who ultimately receive an ASD diagnosis.Citation3-Citation5 Even symptoms that are exhibited in the first year of life are not specific to autism and thus do not differentiate infants who will develop ASD from those with other developmental delays.Citation14 By 12 months of age, repetitive behaviors associated with ASD are present in infants who later meet criteria for ASD.Citation15-Citation17 Thus, symptoms more fully diagnostic of autism appear to develop gradually between 12 and 24 months.Citation4-Citation6
However, differences in other developmental areas have been reported in the first year of life, such as motor skills,Citation18,Citation19 visual reception,Citation18 language,Citation20 and eye gaze patterns of social scenes and faces.Citation21-Citation23 Deficits in fine and gross motor skills at 6 months of age in HR-ASD infantsCitation18 suggest that motor development in the first year of life may have a role in the development of autism.Citation18,Citation19 Coupled with the presence of increased motor stereotypies in HR-ASD infants at 12 months of age,Citation15 this points to abnormal development of motor systems between 6 and 12 months of age. In addition, diminished language skills have been reported as early as 12 months of age, followed by atypical receptive-expressive language profiles at 24 months.Citation20
Behavioral signs in the first 2 years of life have been insufficient to accurately predict a later autism diagnosis. At 12 months, parental concerns predicted ASD diagnosis with a positive predictive value (PPV) of 58%.Citation24 At 18 months, behavioral profiles predicted ASD diagnosis with a PPV of 50%. Citation25 Even in a large community sample of toddlers 16 to 23 months old, the Modified Checklist for Autism in ToddlersCitation26—a parent-report questionnaire used as a screening tool for ASD in toddlers—had a PPV of 28%.Citation27,Citation28
Taken together, behavioral research indicates that the defining diagnostic features of autism are not present at 6 months of age, but begin to unfold in the second year of life and consolidate between 18 and 36 months. Although early behavioral markers alone are not sufficiently sensitive or specific to predict later autism diagnosis, the presence of early behavioral differences suggest that the emergence of behavioral features may be preceded by aberrant development of neural features.
Early biological signs
Structural neuroimaging studies of ASD in infancy
One of the most consistent findings from early studies of brain development in ASD has been that head size is normal at birth, but by 2 to 3 years of age, brain size is significantly enlarged. For example, a retrospective head circumference and prospective brain imaging study found indirect evidence that brain enlargement was not present at birth but emerged at the end of the first and second year of life.Citation29 This finding of brain enlargement at 2 to 3 years of age has been confirmed by other studiesCitation30-Citation33; however, until recently, there was a dearth of studies directly measuring brain development in the infant period, between birth and toddlerhood. The first direct MRI evidence of brain enlargement in infancy was reported for a sample of 55 infants (10 of whom developed ASD) who were longitudinally imaged between 6 and 24 months of age.Citation34 The HR-ASD group had significantly faster growth trajectories of total brain volume, such that by 12 to 24 months of age, the group had larger brain volumes on average. This was the first study to prospectively measure brain volume during infancy in ASD; however, the sample size was relatively small and thus did not attempt to tease apart individual growth trajectories.Citation34
A significant advance on elucidating the early brain development of ASD was achieved by a recently published study by the IBIS Network.Citation35 In this study, the individual trajectories of 15 HR-ASD infants were followed with three serial MRI scans at 6, 12, and 24 months of age. In addition to measuring total brain volume, this study also decomposed brain volume into precise anatomical measures of cortical surface area and cortical thickness, which both contribute to overall brain volume but are controlled by distinct genetic mechanisms.Citation36 The rationale for decomposing brain volume into surface area and cortical thickness is further underscored by previous evidence in toddlers with ASD that showed that increased brain volume was associated with an increase in cortical surface area but not cortical thickness,Citation29 a finding that was confirmed in a separate sample.Citation37
The IBIS studyCitation35 reported that HR-ASD infants had an increased rate of surface area expansion from 6 to 12 months, followed by an increased growth rate of total brain volume from 12 to 24 months, in comparison with both the HR-negative and LR groups. No group differences in cortical thickness were observed across the 6- to 24-month interval. In addition, there were specific brain-behavior relationships with temporal specificity: higher growth rates of brain volume between 12 and 24 months (but not between 6 and 12 months) were associated with greater autism severity scores in the social domain at 24 months (but not in the repetitive behavior domain). By virtue of following each infant longitudinally, and generating precise measures of cortical surface area, this study was able to generate a machine-learning algorithm that relied primarily on measures of cortical surface area growth between 6 and 12 months of age to predict on an individual basis an ASD diagnosis at 24 months. This algorithm correctly predicted subsequent ASD diagnosis with a sensitivity of 88%, specificity of 95%, and PPV of 81%. The findings of this study emphasized the importance of moving beyond group-level differences toward individual-level prediction and indicated that brain differences are present at 6 to 12 months of age, before the onset of the defining behavioral features of autism described above.
Two recent studies have detected another brain anomaly in the first year of life in infants who are subsequently diagnosed with ASD. In the first study,Citation34 infants who later developed ASD (HR-ASD; n=10) had an excessive amount of cerebrospinal fluid (CSF) in the subarachnoid space surrounding the cortical surface of the brain (ie, “extra-axial CSF”). The amount of extra-axial CSF (EA-CSF) at 6 months of age preceded the onset of autistic symptoms and was predictive of both their later autism diagnosis and severity of autism symptoms at 3 years of age. These findings were recently replicated in a larger, independent cohort of infants in the IBIS Network (N=343, of which n=47 were HR-ASD).Citation38 In this second study, infants who later developed ASD had 18% more EA-CSF at 6 months than the control groups (HR-negative and LR groups). EA-CSF volume remained persistently elevated through 24 months of age, relative to controls. Infants in who were later diagnosed with the most severe autism symptoms had a more pronounced increase of EA-CSF—nearly 25% greater EA-CSF at 6 months than the controls.Citation38 In addition, excessive EA-CSF at 6 months was associated with early motor deficits in the first year of life, suggesting that increased EA-CSF may affect motor development during the prodromal period in autism, before behavioral diagnostic signs of ASD typically arise. Finally, a fully cross- validated machine-learning algorithm relying on the amount of EA-CSF at 6 months predicted later ASD diagnosis at 24 months with 66% sensitivity and 68% specificity.Citation38 This prediction algorithm was then externally validated in the 2013 sampleCitation34 (in order to test the algorithm on an independent data set), which yielded 80% sensitivity and 67% specificity in predicting an ASD diagnosis at 24 months on the basis of EA-CSF volume at 6 months.Citation38 Thus, although these prediction metrics are not yet strong enough to be clinically useful as a single stand-alone marker to predict individual outcomes, the replication and reliability of the findings between the two independent data setsCitation34,Citation38 indicates that increased EA-CSF at 6 months could be a potential early stratification marker of a biologically based, etiologically homogenous subtype of ASD.
Taken together, these studies show that HR-ASD infants have elevated levels of EA-CSF at 6 months,Citation34,Citation38 increased growth rate of the cortical surface between 6 and 12 months,Citation35 and total brain volume overgrowth between 12 and 24 months of age.Citation35 Thus, brain changes are present during the prodromal period in ASD before diagnosis and precede (or coincide with) behavioral differences. At 6 months of age, brain size is normal, but there is an excessive amount of EA-CSFCitation34,Citation38 This is a time when the first behavioral alterations start becoming detectable, including motor delays.Citation5,Citation18,Citation28 Indeed, excessive EA-CSF at 6 months was found to be associated with early motor deficits at 6 months.Citation38 Between 6 and 12 months of age, there is rapid expansion of cortical surface area,Citation35 which is concurrent with the onset of sensory and attention problems, such as deficits in visual receptionCitation18 and orienting to salient social cues in the environment.Citation39 Interestingly, hyperexpansion of surface area between 6 and 12 months was observed in cortical areas associated with sensorimotor processing.Citation35 Between 12 and 24 months, there is an increased growth rate of total brain volume,Citation34,Citation35 which is concurrent with the emergence of social deficits.Citation4 Indeed, the rate of brain volume growth between 12 and 24 months was associated with autism-specific social deficits.Citation35 Thus, early changes in brain development coincide with the age when early sensorimotor and visual orienting differences emerge, which may have downstream effects on later social development and the consolidation of behaviors that are diagnostic of ASD.
How might increased EA-CSF in infancy and early brain overgrowth be related? Is it possible that accumulation of stagnant CSF over the surface of the brain influences cortical brain development? CSF has many important functions, including the removal of potentially harmful byproducts of brain metabolism, such as β-amyloid and tau.Citation40,Citation41 Stagnation of CSF leads to the accumulation of waste byproducts and neuromodulators in brain tissue that may alter the extracellular environment of neurons and impact their function.Citation42,Citation43 CSF also serves as a means of transporting important cytokines, growth factors, and other signaling molecules, such as insulin-like growth factor (IGF1 and IGF2), which are required for the normal development of the neocortex.Citation40,Citation44-Citation46 An imbalance of CSF production and absorption alters the concentration of these factors and could lead to serious consequences on cortical development.Citation47 For example, stagnation of CSF flow in animal models leads to an alteration of neurogenesis and premature migration of progenitor cells.Citation47 Indeed, there is evidence that the composition of CSF drawn from the subarachnoid space in infants with increased EACSF has a higher protein concentration than CSF drawn from the ventricles or spinal column,Citation48 and CSF in normal infants.Citation49 Of course, in the context of a research study, it would be too invasive to draw CSF from the brain in children with ASD. Future studies are needed to test the hypothesis that stagnant or elevated EA-CSF has a different composition of trophic growth factors (IGF1, IGF2) and neuroinflammatory proteins (β-amyloid, tau).Citation40,Citation44-Citation46
White matter brain connectivity
On the basis of diffusion tensor imaging (DTI) tractography, HR-ASD infants have been reported to have abnormalities in white matter (WM) organizational structure as early as 6 months of age in multiple fiber tracts across the brain.Citation50,Citation51 Aberrant WM integrity has been observed by 6 months in the genu of the corpus callosum,Citation51 and WM integrity in the genu and cerebellar peduncles is significantly associated with abnormal sensory responsiveness at 24 months, a clinical domain particularly affected in individuals with ASD.Citation52 These reports of reduced WM organization in HR-AD infants are consistent with studies of toddlers with ASD.Citation53,Citation54
Functional brain differences in infancy
Functional connectivity MRI (fcMRI) is another type of MRI scan that can be acquired in infants during natural sleep to characterize functional brain connectivity. In a prospective study of 59 HR infants, a recent IBIS study found that fcMRI at 6 months could accurately identify which infants would receive an ASD diagnosis at 24 months.Citation55 First, functional connections at 6 months were identified on the basis of their association with ASD-related behaviors at 24 months (ie, scores on social behavior, language, motor development, and repetitive behavior). Then, a machine-learning prediction algorithm was conducted on these functional connections, utilizing a fully cross-validated scheme (ie, subjects who were tested in the “leave-N-out” folds of the classifier were then left out of the feature identification and reduction procedures to avoid contaminating the testing with the training information). The prediction algorithm using fcMRI data from 6-month-olds achieved 100% PPV, 82% sensitivity (correctly predicting 9 of the 11 infants who received an ASD diagnosis at 24 months), and 100% specificity (correctly predicting 48 of 48 infants who were negative for ASD at 24 months of age).Citation55 This study provided the first evidence that functional neuroimaging in 6-month-old infants could accurately predict which individuals would receive a diagnosis of ASD at 24 months of age.
These recent fcMRI results, coupled with electrophysiological evidence for differences in EEG and event-related potential (ERP) response,Citation56,Citation57 add to the increasing evidence that there are alterations in both brain function and brain structure (as described above) that are present in the first year of life in infants who later received an ASD diagnosis.
Conclusions and future directions
Infant sibling studies have shown that ASD-specific behaviors emerge in the latter part of the first and during the second year of life,Citation4 with diagnosis typically occurring around 4 years of age.Citation1 Before 12 months of age, behavioral differences between those who develop ASD vs those who do not, have only been identified at the group-average level (eg, see Estes et alCitation18). Behavioral markers in the first year of life have not been shown to be sensitive or specific enough at the individual level for clinically useful prediction of later ASD diagnosis.
Thus, it is current practice in child psychiatry to not initiate treatment until after behavioral diagnosis of ASD. However, there are recent data that indicate that earlier behavioral intervention for autism is more effective than later treatment.Citation58-Citation61 Thus, there is a need for both early and biologically derived markers for ASD in infancy to reliably detect which children need treatment, what type of treatment, and when to initiate treatment.
The potential for an early biological marker for ASD
The promise of a biological marker for ASD is a lofty and elusive goal, and the benchmarks for success are justifiably high. The US National Institutes of Health (NIH) working group established the definition of a biological marker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to therapeutic intervention” (NIH Biomarkers Definitions Working Group,Citation62 2001). Biological markers should have valuable applications, which may include the following (see ref 62, p 91):
“Use as a diagnostic tool for the identification of those patients with a disease or abnormal condition”
“Use as a tool for staging of disease...or classification of the extent of disease”
“Use as an indicator of disease prognosis”
“Use for prediction and monitoring of clinical response to an intervention.”
We have made considerable progress in pursuing these benchmarks for autism, particularly in the following areas (see ):
Early markers to predict ASD. The first year of life is a period of tremendous neural plasticity, as the brain doubles in size from birth to 1 year.Citation63 Thus, it is not surprising that intervention during this time has been shown to produce better outcomes for children with ASD. This underscores the critical need for early predictive markers for early detection of autism. In the last year alone, two studies have shown predictive accuracy (PPV over 80%) that holds promise for early prediction of autism.Citation35,Citation55
Age-specific treatment windows. Longitudinal studies of infants who develop autism have identified specific brain changes during specific time periods (eg, cortical surface area expansion from 6 to 12 monthsCitation35), which may point to specific windows of opportunity for treatment when the brain is most plastic and malleable to treatment.
Stratification markers to address heterogeneity. There is tremendous phenotypic heterogeneity in ASD, which has hampered efforts toward targeted treatments. Parsing the heterogeneity in ASD and identifying biological subtypes have become major initiatives in child psychiatry, as evidenced by the strategic objectives of the US National Institute of Mental Health.Citation64 Thus, there is a need for stratification biomarkers that can separate children into subtypes that share a common pathophysiology (eg, increased EA-CSFCitation34-Citation38). Such stratification biomarkers have the potential to carve up the etiological heterogeneity and map on to specific, mechanistically targeted treatments.
Clinical and ethical implications of early detection
The potential for presymptomatic detection of ASD in infancy has important clinical, ethical, and social implications that warrant consideration. First, it is unlikely that neuroimaging in infancy will replace expert clinical diagnosis, but rather that objective quantifiable biological markers (eg, measures generated from infant brain scans) may serve as an additional screening tool for clinicians to flag infants at very high risk for developing autism.
Second, although the studies reviewed herein have shown that it is possible for brain features at 6 to 12 months of age to predict ASD diagnosis at 24 months, there is ample research demonstrating that some ASD children will not fully manifest the diagnostic features of ASD until later in childhood.Citation65-Citation67 Thus, the studies to date on early prediction of autism have not attempted to detect all children who might later receive a diagnosis of autism, but those who receive an autism diagnosis at the typical age of diagnosis. For this reason, the focus of early prediction should be on positive predictive value (PPV: the probability that a positive test will identify children with a high likelihood of developing the disorder) and not on negative predictive value (NPV: the probability that a negative test will rule out children with a low likelihood of developing the disorder). In other words, an early predictor with high PPV could appropriately flag a child who is likely to develop ASD, and then route them to early treatment; whereas an early predictor with high NPV could not confidently rule out the possibility that the child will eventually develop ASD later in childhood, because a negative test in this case would inappropriately give parents a false sense of security.
Lastly, careful consideration must be devoted to the parental and societal effects of a positive predictive result. Studies of predictive testing and early detection in other conditions have suggested there are potential benefits to families and society. For example, predictive testing or presymptomatic screening has been shown to improve family coping.Citation68 Furthermore, most studies of predictive genetic testing show limited or no adverse psychological impact after a positive result.Citation69-Citation72 Similarly, most studies of newborn screening report minimal psychological distress for parents after early diagnosis.Citation68,Citation73,Citation74 However, there are still concerns about the psychological impact on parents who receive results from pediatric predictive testing,Citation75 and additional research should be focused on identifying and assisting those parents who may be more vulnerable to adverse outcomes after receiving results.Citation74,Citation76 Furthermore, additional research and supports need to address the challenge of managing the child's condition,Citation73 impact on family dynamics,Citation77 potential for discrimination and stigma,Citation78 and ever-changing societal perceptions.Citation79
Although considerable work lies ahead of the autism field, the development and validation of biomarkers for ASD will help move the field of psychiatry toward its aspiration of precision medicine,Citation80 in order to determine the best treatment, at the optimal time, for specific subtypes. The goal might be to combine biomarkers with existing clinical expertise in behavioral assessments to detect autism earlier and more reliably, with the goal to improve the long-term outcomes for children affected by autism.
MDS is supported by a US National Institutes of Health (NIH) career development award (K12-HD001441). The coauthors' research and the Infant Brain Imaging Study (IBIS) Network are supported by grants from the US National Institutes of Health (R01-HD055741, R01-HD05571-S1, R01-HD059854, T32-HD040127, U54HD086984), Autism Speaks, and the Simons Foundation (#140209). All coauthors report no financial interests or potential conflicts of interest.
REFERENCES
- Centers for Disease Control and Prevention (CDC). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 sites. United States, 2010.MMWR Surveill Summ.2014632121
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association;2013
- RogersSJ.What are infant siblings teaching us about autism in infancy?Autism Res.20092312513719582867
- OzonoffS.losifAM.BaguioF.et al.A prospective study of the emergence of early behavioral signs of autism.J Am Acad Child Adolesc Psychiatry.20104932566620410715
- LandaRJ.GrossAL.StuartEA.FahertyA.Developmental trajectories in children with and without autism spectrum disorders: the first 3 years.Child Dev.201384242944223110514
- ZwaigenbaumL.BrysonS.RogersT.RobertsW.BrianJ.SzatmariP.Behavioral manifestations of autism in the first year of life.Int J Dev Neurosci.2005232-314315215749241
- DawsonG.RogersS.MunsonJ.et al.Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model.Pediatrics.20101251e17e2319948568
- RogersSJ.VismaraLA.Evidence-based comprehensive treatments for early autism J Clin Child Adolesc Psychol.200837183818444052
- ClarkMLE.VinenZ.BarbaroJ.DissanayakeC.School age outcomes of children diagnosed early and later with autism spectrum disorder.J Autism Dev Disord. 2017 Sep 14. doi:10.1007/s10803-017-3279-x.
- OzonoffS.YoungGS.CarterA.et al.Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study.Pediatrics.20111283e488e49521844053
- ConstantinoJN.ZhangY.FrazierT.AbbacchiAM.LawP.Sibling recurrence and the genetic epidemiology of autism.Am J Psychiatry.2010167111349135620889652
- GamlielI.YirmiyaN.SigmanM.The development of young siblings of children with autism from 4 to 54 months.J Autism Dev Disord.200737117118317203244
- GeorgiadesS.SzatmariP.ZwaigenbaumL.et al.A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder.JAMA Psychiatry.2013701424722945359
- OzonoffS.HeungK.ByrdR.HansenR.Hertz-PicciottoI.The onset of autism: patterns of symptom emergence in the first years of life.Autism Res.20081632032819360687
- ElisonJT.WolffJJ.ReznickJS.et al.Repetitive behavior in 12-month-olds later classified with autism spectrum disorder.J Am Acad Child Adolesc Psychiatry.201453111216122425440311
- WolffJJ.BotteronKN.DagerSR.et al.Longitudinal patterns of repetitive behavior in toddlers with autism.J Child Psychol Psychiatry.201455894595324552513
- FilliterJH.LongardJ.LawrenceMA.et al.Positive affect in infant siblings of children diagnosed with autism spectrum disorder.J Abnorm Child Psychol.2014433567575
- EstesA.ZwaigenbaumL.GuH.et al.Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life.J Neurodev Disord.2015712426203305
- FlanaganJE.LandaR.BhatA.BaumanM.Head lag in infants at risk for autism: a preliminary study.Am J Occup Ther.201266557758522917124
- SwansonMR.ShenMD.WolffJJ.et al.Subcortical brain and behavior phenotypes differentiate infants with autism versus language delay.Biol Psychiatry Cogn Neurosci Neuroimaging.201728664672
- ChawarskaK.MacariS.ShicF.Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders.Biol Psychiatry.201374319520323313640
- JonesW.KlinA.Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism.Nature.20145047480427431
- ShicF.MacariS.ChawarskaK.Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder.Biol Psychiatry.201475323123723954107
- OzonoffS.YoungGS.SteinfeldMB.et al.How early do parent concerns predict later autism diagnosis?J Dev Behav Pediatr.200930536737519827218
- ChawarskaK.ShicF.MacariS.et al.18-Month predictors of later outcomes in younger siblings of children with autism spectrum disorder: a Baby Siblings Research Consortium Study.J Am Acad Child Adolesc Psychiatry.2014531213171327.e125457930
- RobinsDL.FeinD.BartonML.GreenJA.The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders.J Autism Dev Disord.200131213114411450812
- PandeyJ.VerbalisA.RobinsDL.et al.Screening for autism in older and younger toddlers with the Modified Checklist for Autism in Toddlers.Autism.200812551353518805945
- ZwaigenbaumL.BrysonS.LordC.et al.Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants.Pediatrics.200912351383139119403506
- HazlettHC.PoeMD.GerigG.et al.Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years.Arch Gen Psychiatry.201168546721536976
- HazlettHC.PoeM.GerigG.et al.Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years.Arch Gen Psychiatry.200562121366137616330725
- CourchesneE.KarnsCM.DavisHR.et al.Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study.Neurology.200157224525411468308
- NordahlCW.LangeN.LiDD.et al.Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders.Proc Natl Acad Sci U S A.201110850201952020022123952
- SparksBF.FriedmanSD.ShawDW.et al.Brain structural abnormalities in young children with autism spectrum disorder.Neurology.200259218412136055
- ShenMD.NordahlCW.YoungGS.et al.Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder.Brain.2013136pt 92825283523838695
- HazlettHC.GuH.MunsellBC.et al.Early brain development in infants at high risk for autism spectrum disorder.Nature.2017542764134835128202961
- PanizzonMS.Fennema-NotestineC.EylerLT.et al.Distinct genetic influences on cortical surface area and cortical thickness.Cerebral Cortex.200919112728273519299253
- OhtaH.NordahlCW.IosifAM.LeeA.RogersS.AmaralDG.Increased surface area, but not cortical thickness, in a subset of young boys with autism spectrum disorder.Autism Res.20169223224826184828
- ShenMD.KimSH.McKinstryRC.et al.Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism.Biol Psychiatry.201782318619328392081
- ElisonJT.PatersonSJ.WolffJJ.et al.White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism.Am J Psychiatry.2013170889990823511344
- JohansonCE.DuncanJA.KlingePM.BrinkerT.StopaEG.SilverbergGD.Multiplicity of cerebrospinal fluid functions: new challenges in health and disease.Cerebrospinal Fluid Res.200851018479516
- XieL.KangH.XuQ.et al.Sleep drives metabolite clearance from the adult brain.Science.2013342615637337724136970
- Del BigioMR.Neuropathology and structural changes in hydrocephalus.Dev Disabil Res Rev.2010161162220419767
- IliffJJ.WangM.LiaoY.et al.A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β.Sci Transl Med.20124147147ra111
- LehtinenMK.WalshCA.Neurogenesis at the brain-cerebrospinal fluid interface.Annu Rev Cell Dev Biol.20112765367921801012
- SwansonMR.PivenJ.Neurodevelopment of autism: the first three years of life. In:Autism Imaging and Devices. Casanova MF, El-Baz AS, Suri JS, eds. Boca Raton, FL: Taylor & Francis Group;2016
- LehtinenMK.ZappaterraMW.ChenX.et al.The cerebrospinal fluid provides a proliferative niche for neural progenitor cells.Neuron.201169589390521382550
- MashayekhiF.DraperCE.BannisterCM.PourghasemM.Owen-LynchPJ.MiyanJA.Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF.Brain.2002125pt 81859187412135976
- ChazalJ.TanguyA.IrthumB.JannyP.VanneuvilleG.Dilatation of the subarachnoid pericerebral space and absorption of cerebrospinal fluid in the infant.Anat Clin.19857161663994855
- BrinerS.BodensteinerJ.Benign subdural collections of infancy.Pediatrics.19816768028046972029
- WolffJJ.GuH.GerigG.et al.Differences in white matter fiber tract development present from 6 to 24 months in infants with autism.Am J Psychiatry.2012169658960022362397
- WolffJJ.GerigG.LewisJD.et al.Altered corpus callosum morphology associated with autism over the first 2 years of life.Brain.2015138pt 72046205825937563
- WolffJJ.SwansonMR.ElisonJT.et al.Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism.Mol Autism.20178828316772
- BashatDB.Kronfeld-DueniasV.ZachorDA.et al.Accelerated maturation of white matter in young children with autism: a high b value DWI study.Neuroimage.2007371404717566764
- SolsoS.XuR.ProudfootJ.et al.Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers.Biol Psychiatry.201579867668426300272
- EmersonRW.AdamsC.NishinoT.et al.Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age.Sci Transl Med.20179393eaag288228592562
- ElsabbaghM.MercureE.HudryK.et al.Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism.Curr Biol.201222433834222285033
- JonesEJ.VenemaK.EarlR.et al.Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: a longitudinal prospective study of infants at high familial risk.J Neurodev Disord.20168726981158
- VivantiG.DissanayakeC.Outcome for children receiving the early start Denver model before and after 48 months.J Autism Dev Disord.20164672441244927020055
- RogersSJ.VismaraL.WagnerAL.McCormickC.YoungG.OzonoffS.Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants.J Autism Dev Disord.201444122981299525212413
- GreenJ.CharmanT.PicklesA.WanMW.Parent-mediated intervention versus no intervention for infants at high risk of autism: a parallel, single-blind, randomised trial.Lancet Psychiatry.20152213314026359749
- JonesEJH.DawsonG.KellyJ.EstesA.Jane WebbS.Parent-delivered early intervention in infants at risk for ASD: effects on electrophysiological and habituation measures of social attention.Autism Res.201710596197228244271
- NIH Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework.Clin Pharmacol Ther.2001693899511240971
- KnickmeyerRC.GouttardS.KangC.et al.A structural MRI study of human brain development from birth to 2 years.J Neurosci.20082847121761218219020011
- InselTR.Translating scientific opportunity into public health impact: a strategic plan for research on mental illness.Arch Gen Psychiatry.200966212813319188534
- OzonoffS.YoungGS.LandaRJ.et al.Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study.J Child Psychol Psychiatry.201556998899825921776
- ZwaigenbaumL.BrysonSE.BrianJ.et al.Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort.Autism Res.20169779080026613202
- BrianJ.BrysonSE.SmithIM.et al.Stability and change in autism spectrum disorder diagnosis from age 3 to middle childhood in a high-risk sibling cohort.Autism.201620788889226685198
- ChungJ.SmithAL.HughesSC.et al.Twenty-year follow-up of newborn screening for patients with muscular dystrophy.Muscle Nerve.201653457057826260293
- MacLeodR.BeachA.HenriquesS.KnoppJ.NelsonK.Kerzin-StorrarL.Experiences of predictive testing in young people at risk of Huntington's disease, familial cardiomyopathy or hereditary breast and ovarian cancer.Eur J Hum Genet.201422339640123860040
- CrozierS.RobertsonN.DaleM.The psychological impact of predictive genetic testing for Huntington's disease: a systematic review of the literature.J Genet Couns.2015241293925236481
- BroadstockM.MichieS.MarteauT.Psychological consequences of predictive genetic testing: a systematic review.Eur J Hum Genet.200081073173811039571
- MichieS.BobrowM.MarteauTM.Predictive genetic testing in children and adults: a study of emotional impact.J Med Genet.200138851952611483640
- SparbelKJ.TluczekA.Patient and family issues regarding genetic testing for cystic fibrosis: a review of prenatal carrier testing and newborn screening.Annu Rev Nurs Res.20112930332922891510
- BaileyDB.WheelerA.Berry-KravisE.et al.Maternal consequences of the detection of fragile X carriers in newborn screening.Pediatrics.20151362e433e44026169437
- HendriksKS.GrosfeldFJ.WildeAA.et al.High distress in parents whose children undergo predictive testing for long QT syndrome.Community Genet.20058210311315925886
- LermanC.CroyleRT.TercyakKP.HamannH.Genetic testing: psychological aspects and implications.J Consult Clin Psychol.200270378479712090383
- BaileyDB.SkinnerD.DavisAM.WhitmarshI.PowellC.Ethical, legal, and social concerns about expanded newborn screening: fragile X syndrome as a prototype for emerging issues.Pediatrics.20081213e693e70418310190
- GreenRC.RobertsJS.CupplesLA.et al.Disclosure of APOE genotype for risk of Alzheimer's disease.N Engl J Med.2009361324525419605829
- WalshP.ElsabbaghM.BoltonP.SinghI.In search of biomarkers for autism: scientific, social and ethical challenges.Nat Rev Neurosci.2011121060361221931335
- InselTR.CuthbertBN.Medicine. Brain disorders? Precisely.Science.2015348623449950025931539
