Abstract
Microvesicles were isolated from the conditioned media of 3 cell lines (MDA-MB-231, AsPC-1 and A375) by ultracentrifugation at a range of relative centrifugal forces, and the tissue factor (TF) protein and activity, microvesicle number, size distribution and relative density compared. Also, by expressing TF-tGFP in cells and isolating the microvesicles, the relative density of TF-containing microvesicles was established. Nanoparticle tracking analysis (NTA) indicated that the larger-diameter microvesicles (>200 nm) were primarily sedimented at 100,000g and possessed TF-dependent thrombin and factor Xa generation potential, while in the absence of factor VII, all microvesicles possessed some thrombin generation capacity. Immuno-precipitation of TF-containing microvesicles followed by NTA also indicated the range of these microvesicles to be 200–400 nm. Analysis of the microvesicles by gradient density centrifugation showed that lower-density (<1.1 g/ml) microvesicles were mainly present in the samples recovered at 100,000g and were associated with TF antigen and activity. Analysis of these fractions by NTA confirmed that these fractions were principally composed of the larger-diameter microvesicles. Similar analysis of microvesicles from healthy or patient plasma supported those obtained from conditioned media indicating that TF activity was mainly associated with lower-density microvesicles. Furthermore, centrifugation of healthy plasma, supplemented with TF-tGFP-containing microvesicles, resulted in 67% retrieval of the fluorescent microvesicles at 100,000g, but only 26% could be recovered at 20,000g. Pre-centrifugation of conditioned media or plasma at 10,000g improved the speed and yield of recovered TF-containing microvesicles by subsequent centrifugation at either 20,000g or 100,000g. In conclusion, TF appears to be associated with low-density (1.03–1.08 g/ml), larger-diameter (200–350 nm) microvesicles.
Microvesicles are phospholipid vesicles which range in diameter from below 100 nm to around 700 nm, although a recent study has reported these to be at the lower end of this range (Citation1). Microvesicles are released from cells following cellular activation and contain proteins, lipids and nucleic acids derived from the parent cell. Increased levels of tissue factor (TF)–positive microvesicles have been detected in the plasma of patients with various chronic diseases compared to healthy individuals (Citation2, Citation3). Elevation in procoagulant microvesicles is linked with hypercoagulability and increased risk of thrombosis (Citation3). As such, the relevance of measurement and examination of procoagulant microvesicles to the understanding of the mechanisms of regulation of haemostasis and the induction of coagulation during pathological condition is conspicuous. Whereas the measurement of microvesicles is often carried out while still within plasma, enrichment of the microvesicles permits the examination of physical and biochemical properties of the microvesicles which otherwise cannot be determined accurately due to contamination. Moreover, although there are guidelines for the pre-analytical variables recommended for the preparation of microvesicle samples (Citation4, Citation5), currently there are no clear protocols for the preparation of microvesicles (Citation5) for the purpose of examination of the associated procoagulant activity. It is clear that even microvesicles derived from 1 type of cell do not constitute a homogeneous population and differ widely in their source of origin within the cell and, consequently, protein and lipid composition as well as physical properties (Citation6). It has been suggested that discrepancies in the reported properties of microvesicles may arise as a consequence of laboratory practices during the preparation of microvesicles (Citation7). A common means of microvesicle purification and enrichment involves the sedimentation of microvesicles by ultracentrifugation. However, since procedures described by various laboratories use different centrifugal forces to sediment the microvesicles, it is also evident that the optimal centrifugation speed for the sedimentation of all populations of microvesicles has not yet been determined (Citation5). Previously, we reported that the majority of TF antigen appeared to be associated with microvesicles of larger diameter which were recoverable at higher centrifugal forces (Citation8). In this study, we have further examined the properties of the TF-containing microvesicles isolated from conditioned media of 3 cell lines. In addition, we have assessed the influence of centrifugal force on the efficiency of the recovery of microvesicles and particularly TF-containing microvesicles. Furthermore, gradient density ultracentrifugation techniques have previously been used to determine the relative density of exosomes (Citation9, Citation10). Similar techniques were used here to establish the relative density of microvesicle fractions recovered from conditioned media, as well as from samples of healthy and patient plasma. In addition, by expressing a TF-tGFP hybrid protein in the cells prior to microvesicle purification, we have established the relative density and size of TF-containing microvesicles.
Material and methods
Cell culture, transfection and microvesicle purification
Human breast cancer cell line MDA-MB-231 and human melanoma cells A375 (ATCC, Teddington, UK) were cultured in DMEM (Lonza, Cambridge, UK) containing foetal calf serum 10% (v/v; Source Bioscience plc, Nottingham, UK). Human pancreatic (ascites meta) cell line AsPC-1 (ATCC) was cultured in RPMI-1640 (Lonza) containing foetal calf serum 10% (v/v). These 3 cell lines are capable of expressing and releasing bursts of high levels of TF as microvesicles following activation. Cells were cultured in 75 cm2 flasks until 80% confluent prior to microvesicle preparation. Cells were washed and pre-adapted to respective serum-free medium prior to activation and harvesting of conditioned media. To induce microvesicle release, the cells were stimulated with PAR2-activating peptide (PAR2-AP); SLIGRL (20 µM) (Sigma Chemical Company, Poole, UK) for 30 min according to published procedures (Citation11–Citation13). The conditioned media were cleared of any cell debris by centrifuging for 10 min at 5,400g on a microcentrifuge. The samples (1 ml aliquots) were then placed in 11×34 mm polycarbonate centrifuge tubes (Beckman Coulter, High Wycombe, UK), and the cell-derived microvesicles were sedimented at various relative centrifugal forces (RCF) ranging from 20,000 to 150,000g on a TL-100 ultracentrifuge at 20°C, using a TLA 100.2 rotor (Beckman) for 1 h, as described in Ref. (Citation11). In some experiments, the denser microvesicles were first removed by centrifugation at 10,000g for 1 h prior to sedimentation at the above RCF. All isolated microvesicles were then resuspended in pre-filtered (0.1 µm) PBS (200 µl). Previous analysis of the microvesicles for the exosome markers Tsg101 and CD9 by western blot examination revealed that exosomes were absent, which was likely to be due to the short incubation times (Citation8, Citation11). To demonstrate this further, exosomes were immunoprecipitated using either anti-Tsg101 antibody or CD9 (Gentex/Source Bioscience, Nottingham, UK), followed by protein A-magnetic beads. The samples were tested for TF antigen by enzyme immunoassay (EIA), as below. In some experiments, MDA-MB-231 cells were cultured in 25 cm2 flasks and transfected with pCMV-AC-TF-tGFP (Origene, Rockville, MD, USA), which encodes for turbo-GFP (tGFP) in tandem, on the C-terminal of TF. DNA transfection was performed using Lipofectin (Invitrogen, Paisley, UK), as previously described (Citation11–Citation14). Transfection of MDA-MB-231 cells resulted in a marginal increase in TF antigen and activity. However, due to the nature of these cells, the amount of TF within the cells is moderated (Citation15). Cells were permitted to express TF-tGFP for 48 h, and microvesicles were isolated from the conditioned media at 20,000g and 100,000g, as described in this article. The transfection efficiency into MDA-MB-231 cells was typically 63%±12, and the TF-tGFP possessed similar TF activity to unconjugated TF, in agreement with our previous data (Citation8). Microvesicles were also recovered from the plasma of diseased (colorectal cancer and cardiovascular patients) or normal subjects (Innovative Research, Novi, MI, USA) (Citation11) and used in some experiments. Finally, citrated normal human plasma was supplemented with isolated microvesicles containing TF-tGFP, in PBS (50 µl), prepared as above, without clot formation. The plasma and the fluorescent microvesicles were centrifuged at 20,000g and 100,000g at 20°C for 1 h, as described in this article, and the microvesicles resuspended in the original volume of 50 µl of PBS. The fluorescence emissions of the recovered samples were measured at 510 nm and compared to the original samples before centrifugation.
Analysis of microvesicle numbers and size distribution
The numbers and size distributions of the isolated microvesicles were determined by nanoparticle tracking analysis (NTA) using NanoSight LM10 and NTA software (NanoSight Ltd, Amesbury, UK). The NanoSight instrument was calibrated using FluoSpheres® carboxylate-modified microspheres with diameters of 0.02, 0.1 and 1.0 µm (Invitrogen). The lower limit of detection was determined to be between 0.02 and 0.1 µm. MV samples were diluted 1:10 in 0.1 µm-filtered PBS and analysed by tracking particles over 90s using a camera level of 12 and shutter speed of 23.22 frames/s, as described in Ref. (Citation14). Data were analysed using the NTA software to determine the concentration and size distribution of the microvesicles. In order to quantify the distribution of the microvesicles, the traces obtained from NTA were separated (according to the main peaks observed) into 3 classes with diameters of <100 nm (small), 100–250 nm (medium) and >250 nm (large size), and the number of microvesicles within each group determined.
Analysis of microvesicle-associated TF antigen and activity
TF antigen content of the microvesicles was determined using a TF-antigen EIA kit (Enzyme Research Laboratories Ltd, Swansea, UK), as described in Refs. (Citation11–Citation15). Microvesicle-associated thrombin generation potential of the samples was measured using a chromogenic thrombin generation assay, as described in Refs. (Citation11–Citation15), and the absorptions were measured at 410 nm on a spectrophotometer. In order to distinguish between TF-mediated and TF-independent thrombin generation, thrombin generation activities were also compared in the presence of normal and factor VII–deficient plasma and also in the presence of an inhibitory anti-TF antibody (HTF-1; Affymatrix eBioscience, Hatfield, UK). The potential for factor Xa generation was assessed using the Actichrome TF activity assay kit (American Diagnostica/Invitech Ltd, Molesworth, UK) in the presence and absence of factor VII as described before (Citation15), and the absorptions were measured at 410 nm on a spectrophotometer.
Examination of the relative density of the microvesicles by density gradient centrifugation
Microvesicles were analysed by density gradient ultracentrifugation using a sucrose-OptiPrep (PROGEN Biotechnik GmbH, Heidelberg, Germany) gradient according to the manufacturer's instructions. A 50% (w/v) working solution (WS) was prepared by mixing a solution of 0.25 M sucrose, 6 mM EDTA and 60 mM Tris-HCl pH7.4 with OptiPrep at a ratio of 1:5 (v:v). Prior to the experiments, the stability of the microvesicles in EDTA solutions was examined by analysing the NTA profiles, and was shown to remain unaffected at least up to 5 mM EDTA (Supplementary Fig. 1). Two solutions representing the top and the bottom concentration of sucrose–OptiPrep were prepared by mixing the WS with dilution buffer (DB) (0.25 M sucrose, 1 mM EDTA and 10 mM Tris-HCl pH7.4) at WS:DB ratios of 1:15.7 and 24:1 (v:v), respectively. The microvesicle samples (100 µl in PBS) were mixed with the Optiprep at a ratio of 1:5 (v/v) and were in turn placed at the bottom of 14×89 mm Ultra-clear centrifuge tubes (Beckman Coulter). A density gradient was then prepared using the top and bottom sucrose–Optiprep solutions covering an approximate density range of 1.02–1.22 g/ml. To ensure accuracy, 2 separate sets of DensityMarkerBeads (Amersham Pharmacia Biotech, Uppsala, Sweden) were loaded in 2 separate tubes and centrifuged alongside. The samples and markers were placed in a SW41Ti swing-out rotor and centrifuged at 52,000g for 90 min at 20°C on a Beckman L8-M ultracentrifuge (Beckman Coulter). Following centrifugation, aliquots (0.5 ml) were sequentially removed from the top of the tube, ensuring that the formed gradient was not disrupted. As a guide, the distribution of the microvesicles within the samples was analysed by measuring the protein content by Bradford assay and subtracted from a similar set but without any sample loaded. A density standard curve was then prepared using the 2 sets of markers from which the density of the various sample fractions was then determined. In some experiments, the amount of TF protein and the thrombin generation potential of each fraction were assessed using the chromogenic thrombin generation assay. Additionally, the presence of TF-tGFP-containing microvesicles was determined by measuring the fluorescence emission of GFP at 510 nm and subtracting the emission of similar fractions prepared from microvesicles from untransfected MDA-MB-231 cells. In order to analyse the TF content of fractions prepared from microvesicle samples derived from healthy and diseased plasma, the fractions (200 µl) were adsorbed onto annexin v–coated plates (Hyphen Biomed/Quadratech Ltd, Epsom, UK), washed and the thrombin generation potential measured in the presence and the absence of factor VII. Finally, fractions containing significant amounts of TF were pooled and the size of the microvesicle population analysed by NTA.
TF-containing microvesicles were also isolated directly from conditioned media of MDA-MB-231 and A375 cell lines, by immuno-precipitation using a monoclonal antibody against TF (10H10) (Santa Cruz Biotechnology, Heidelberg, Germany), followed by protein A magnetic beads (Miltenyi Biotec, Bisley, UK). The microvesicles were released in phosphate buffer (10 mM) pH 7.4, containing NaCl (500 mM). Controls without the antibody were included. The size distribution of the microvesicles was then assessed by NTA.
Determination of the density of the media and plasma
Since sedimentation of microvesicles is also dependent on the density of the supporting media, the density of the media or plasma prior to centrifugation was measured by accurately measuring the weight of a precise volume (1 ml) of each fluid. Subsequent to centrifugation, the density of the top and bottom 100 µl fractions were also determined by weighing.
Results
Analysis of the TF protein and activity in microvesicles purified by sedimentation at various RCF
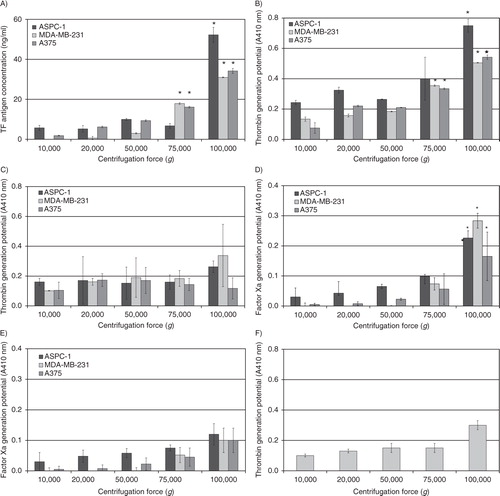
Analysis of the microvesicle samples prepared by ultracentrifugation at 100,000g showed at least a 2-fold increase in the amount of TF antigen content compared to samples sedimented at lower centrifugal forces (A), and it was highest in the sample isolated from AsPC-1 cells. In agreement with the amount of TF antigen, the thrombin generation activity was highest in samples recovered at 100,000g. However, the values for the thrombin generation (measured as absorption at 410 nm) did not reflect the magnitude of the differences in TF antigen, observed between the procedures of ultracentrifugation (B). In fact, analysis of the thrombin generation associated with the microvesicles, in the absence of factor VII, indicated the presence of some TF-independent thrombin generation potential in all of the samples (C). Analysis of the samples using a factor Xa generation assay, in the presence and absence of factor VIIa, produced a better representation of the measured levels of TF antigen associated with the microvesicles (D and E). These data were also confirmed by the inhibition of TF activity using the HTF-1 antibody (F). None of the isolated samples contained detectable amounts of CD9 or Tsg101 (Supplementary Fig. 2), as demonstrated in Refs. (Citation8, Citation11) (Citation14). Furthermore, depletion of the samples by immunoprecipitation with anti-Tsg101 or CD9 did not precipitate any detectable microvesicles, or TF antigen (Supplementary Fig. 3), and did not alter the amount of TF antigen in the supernatant. Furthermore, adsorption of the microvesicles onto annexin v resulted in the retention of all of the TF activity on the plate with no further activity detected in the supernatant, indicating that the TF was associated with microvesicles only (Supplementary Fig. 4).
Fig. 1. Analysis of TF antigen and activity associated with microvesicles isolated at various RCF. Cell lines (AsPC-1, MDA-MB-231 and A375) were washed and pre-adapted to respective serum-free media prior to activation with PAR2-AP (SLIGRL; 20 µM). The conditioned media were collected and cleared of any cell debris by centrifuging at 5,400g for 10 min on a microcentrifuge. Cell-derived microvesicles were then sedimented at various RCF ranging from 20,000 to 100,000g at 20°C for 1 h. (A) TF antigen content of the recovered microvesicles was determined using a TF-specific EIA assay (n=3, =p<0.05 vs. respective values recovered at 20,000g). TF-dependent and TF-independent thrombin generation potential of the fractions was assessed using a chromogenic thrombin generation assay in the presence (B) and absence of factor VII (C), respectively, by measuring the absorption at 410 nm (n=3, *=p<0.05 vs. respective values recovered at 20,000g). In addition, TF-dependent and TF-independent factor Xa generation potential of the samples was analysed using the Actichrome TF-activity assay in the presence (D) and absence of factor VII (E), respectively, by measuring the absorption at 410 nm (n=3, *=p<0.05 vs. respective values recovered at 20,000g). (F) Samples of microvesicles were isolated at various RCF ranging from 20,000 to 100,000g at 20°C for 1 h from the media of the MDA-MB-231 cell line and were pre-incubated with an inhibitory anti-TF antibody (HTF-1) prior to analysing the thrombin generation potential (n=3).
Analysis of the number and size distribution of microvesicles purified by sedimentation at various RCF
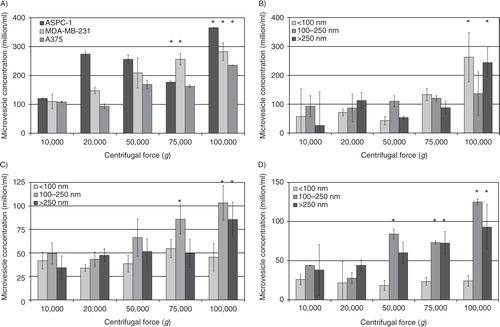
Analysis of the concentration of microvesicles by NTA showed enhancement of the efficiency of total microvesicle recovery with increasing centrifugal forces up to 100,000g (A), but no significant advantage was detected at 150,000g (not shown). Moreover, in this study (Supplementary Fig. 5) and also in Ref. (Citation14), we have shown that both the number and the size distribution of the microvesicles recovered at 100,000g were closely comparable to those observed in pre-centrifuged conditioned media. Therefore, the possibilities of the production of artefacts through microvesicle break-up, or alternatively microvesicle merger, as a consequence of ultracentrifugation may be discounted. Additionally, the use of fresh serum-free medium, the short incubation time (30 min), and the initial sedimentation using the microcentrifuge explain the lack of any observable membrane debris or protein aggregates. Analysis of the size distribution of the microvesicles, recovered at various centrifugal forces, showed a narrower range at 20,000g which became wider with the increasing centrifugal force used (Supplementary Fig. 5). In order to quantify the distribution of the microvesicles, the traces obtained from the NTA were separated into 3 classes according to the main peaks observed. These 3 groups were designated as smaller-sized (diameters of <100 nm), medium-sized (100–250 nm) and larger-sized (>250 nm) microvesicles. Subsequently, the number of microvesicles in each size group, recovered at each centrifugal force, was determined for each of the 3 cell lines (B–D). Analysis of these distributions of MDA-MB-231-derived and A375-derived microvesicles showed progressive enhancement of the recovery of medium-sized microvesicles with increasing centrifugal force. Some enrichment of the population of smaller-sized microvesicles was achievable at centrifugal forces of 75,000g or higher, although the media of A375 cells did not contain a large proportion of this group of microvesicles. Separation of the larger-sized microvesicles from MDA-MB-231 and AsPC-1 cells required centrifugation at 100,000g, but limited recovery from A375 cells was possible at 75,000g. The number of APSC-1-derived microvesicles was significantly higher in all 3 size groups.
Fig. 2. Analysis of the number of microvesicles isolated at various RCF. Cell lines (AsPC-1, MDA-MB-231 and A375) were washed and pre-adapted to respective serum-free media prior to activation with PAR2-AP (SLIGRL; 20 µM). The conditioned media were collected and cleared of any cell debris by centrifuging at 5,400g for 10 min on a microcentrifuge. Cell-derived microvesicles were then sedimented at various RCF ranging from 20,000 to 100,000g. (A) Total number of microvesicles was determined using NTA (n=6, *=p<0.05 vs. respective values recovered at 20,000g). The traces from each analysis (samples shown in Supplementary Fig. 5) were then divided into 3 sections representing microvesicles with diameters of <100 nm, 100–250 nm and >250 nm. The number of microvesicles within each group were then determined for (B) AsPC-1, (C) MDA-MB-231 and (D) A375 cells (n=9, *=p<0.05 vs. respective values recovered at 20,000g).
Density gradient centrifugation of the purified microvesicles and analysis of the protein distribution
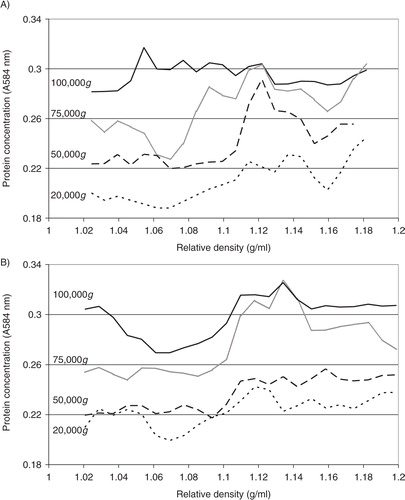
To analyse the distribution of the relative density of the microvesicles recovered at different centrifugal forces, the samples prepared from the conditioned media of MDA-MB-231 and A375 cells were fractionated by sucrose–OptiPrep density gradient centrifugation. Fractions (0.5 ml) were in turn removed from the top of the tube, and the relative amount of protein in the fractions estimated by Bradford assay and subtracted from a similar set without loading the microvesicles. Analysis of total protein concentration, in the fractions from both MDA-MB-231 and A375 cell lines, indicated the presence of microvesicles in all fractions with relative densities of >1.1 g/ml (A and B). Furthermore, the amount of protein in these fractions increased with increasing RCF used during the preparative step. In contrast, lower protein concentrations were detected in fractions with lower relative density (<1.1 g/ml) following preparation at 20,000g. Increasing the RCF during the recovery of the microvesicles, particularly when performed at 100,000g, enhanced the yield of lower-density microvesicles derived from both MDA-MB-231 and A375 cells.
Fig. 3. Analysis of the relative density of microvesicles by density gradient centrifugation. Cells lines (MDA-MB-231 and A375) were washed and pre-adapted to respective serum-free media prior to activation with PAR2-AP (SLIGRL; 20 µM). The conditioned media were collected and cleared of any cell debris by centrifuging at 5,400g for 10 min on a microcentrifuge. Cell-derived microvesicles were then sedimented at various RCF ranging from 20,000 to 100,000g at 20°C for 1 h. Microvesicles recovered at various centrifugal forces were analysed by density gradient ultracentrifugation using a sucrose–OptiPrep gradient covering an approximate range of 1.02–1.22 g/ml against 2 sets of DensityMarkerBeads as density markers. The samples and markers were centrifuged at 52,000g for 90 min at 20°C. The distribution of the protein content of the microvesicles derived from (A) MDA-MB-231 and (B) A375 cells was analysed using the Bradford assay, by measuring the absorption at 584 nm and subtracting the values obtained from a similar set without loading the microvesicles. Traces were obtained by calculating the moving-average values calculated from the data.
Analysis of the relative density of TF-containing microvesicles
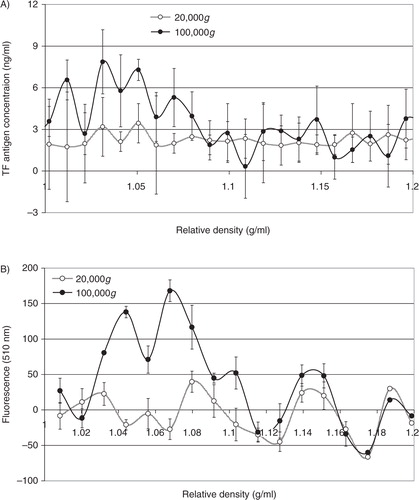
Analysis of the distribution of TF antigen (A) and thrombin generation potential (B) in the fractions prepared from MDA-MB-231 cells alluded to the presence of TF within the lower-density fractions (1.03–1.1 g/ml), in samples prepared at 100,000g but not 20,000g. To confirm the relative density of TF-bearing microvesicles, MDA-MB-231 cells were transfected to overexpress TF-tGFP hybrid protein and activated to release TF-tGFP-containing microvesicles. Microvesicles were prepared from the conditioned media of PAR2-activated cells at 20,000g and 100,000g, and the presence of TF-tGFP within the microvesicles was confirmed by measuring the fluorescence emission of the samples and compared to those isolated from the conditioned media of non-transfected MDA-MB-231 cells. The samples were then fractionated by sucrose–OptiPrep density gradient centrifugation. Fractions (0.5 ml) were in turn removed from the top of the tube, and the presence of TF was examined by measuring the amount of TF antigen (A). The presence of TF-tGFP in the fractions was also assessed by measuring the fluorescence emission at 510 nm on a BMG plate-reader (BMG Labtech, Aylesbury, UK) against microvesicle fractions from non-transfected cells as respective controls. Analysis of these microvesicle fractions indicated the presence of TF-tGFP in lower-density fractions (1.03–1.08 g/ml) in the microvesicles isolated at 100,000g, while the samples prepared at 20,000g did not exhibit significant levels of fluorescence (B). Analysis of similar microvesicles from MDA-MB-231 cells, transfected to overexpress tGFP alone, indicated the presence of higher-density (>1.1 g/ml) fluorescent microvesicles in the samples prepared at 20,000g and at 100,000g, but lower-density fluorescent microvesicles were mainly present at 100,000g (not shown).
Fig. 4. Analysis of the TF antigen content and thrombin generation potential of microvesicle fractions separated by density gradient centrifugation. MDA-MB-231 cells were washed and pre-adapted to respective serum-free media prior to activation with PAR2-AP (SLIGRL; 20 µM). The conditioned media were collected and cleared of any cell debris by centrifuging at 5,400g for 10 min on a microcentrifuge. Cell-derived microvesicles were then sedimented at 20,000g and 100,000g at 20°C for 1 h. Microvesicles were analysed by density gradient ultracentrifugation using a sucrose–OptiPrep gradient covering an approximate range of 1.02–1.22 g/ml against 2 sets of DensityMarkerBeads as density markers. The samples and markers were centrifuged at 52,000g for 90 min at 20°C. Following centrifugation, aliquots (0.5 ml) were sequentially removed. The TF antigen content of the samples (A) was determined using the TF-EIA procedure, and the thrombin generation potential of the fractions (B) was measured using a thrombin generation assay and quantified by measuring the absorption at 410 nm (n=3).
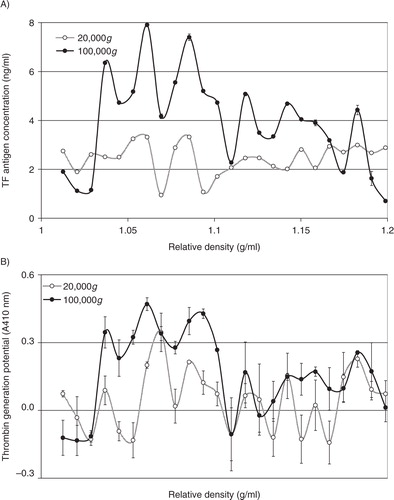
Fig. 5. Analysis of the TF antigen content and fluorescence of TF-tGFP-containing microvesicle fractions separated by density gradient centrifugation. MDA-MB-231 cells were transfected to express the TF-tGFP hybrid protein. Cells were washed and pre-adapted to respective serum-free media prior to activation with PAR2-AP (SLIGRL; 20 µM). The conditioned media were collected and cleared of any cell debris by centrifuging at 5,400g for 10 min on a microcentrifuge. Cell-derived microvesicles were then sedimented at 20,000g and 100,000g at 20°C for 1 h. Microvesicles were analysed by density gradient ultracentrifugation using a sucrose–OptiPrep gradient covering an approximate range of 1.02–1.22 g/ml against 2 sets of DensityMarkerBeads as density markers. The samples and markers were centrifuged at 52,000g for 90 min at 20°C. Following centrifugation, aliquots (0.5 ml) were sequentially removed. The TF antigen content of the samples was determined using the TF-EIA procedure (A). The presence of TF-tGFP protein was assessed by measuring the fluorescence at 510 nm and subtracting the values obtained from a similar set of microvesicles but from untransfected cells (B) (n=3).
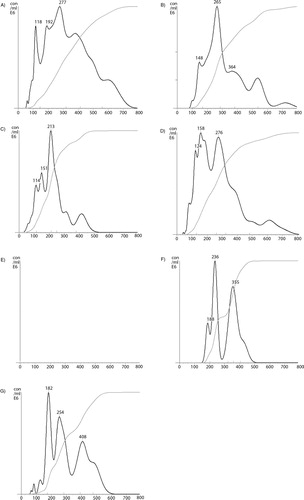
In addition to cell-derived conditioned media, microvesicles were also prepared from the plasma of cancer patients, or from pooled healthy plasma. In each case, the samples were fractioned using density gradient centrifugation, and the thrombin generation potential was assessed in the presence and absence of factor VII. Overall, the thrombin generation potential was higher in samples prepared from cancer patients (the average of 3 samples is shown in A) than the fractions from healthy plasma (B). Moreover, the majority of TF-dependent thrombin generation potential was associated with the lower-density fractions in plasma from cancer patients, while this was almost exclusively associated with lower-density fractions in healthy plasma. To highlight the TF-dependent activity, the difference in thrombin generation, in the presence and absence of factor VII, was calculated from all samples (cancer+healthy) and plotted against the measured relative density of the fractions. Consequently, C shows significant TF-derived thrombin generation in samples with lower relative density (1.02–1.06 g/ml). Examination of pooled fractions identified to contain TF antigen (1.03–1.08 g/ml), prepared from the media of MDA-MB-231 cells, from the media of transfected MDA-MB-231 expressing TF-tGFP or from plasma, confirmed that these samples contained higher proportions of medium to larger-sized microvesicles (77, 78 and 78%, respectively) (A–C) compared to 49% prior to separation (D), although none of the samples were completely free of smaller-sized microvesicles. Moreover, immuno-purified microvesicles from MDA-MB-231 and A375 cells, isolated using a monoclonal anti-TF antibody, exhibited a size distribution range (190–400 nm) in agreement with the above data (F and G).
Fig. 6. Analysis of the thrombin generation potential of microvesicle fractions separated by density gradient centrifugation. Samples of plasma from cancer patients (n=3) or pooled healthy plasma were cleared of any cell debris by centrifuging at 5,400g for 10 min on a microcentrifuge, and microvesicles were then sedimented at 20,000g and 100,000g at 20°C for 1 h. Microvesicles were then analysed by density gradient ultracentrifugation using a sucrose–OptiPrep gradient covering an approximate range of 1.02–1.22 g/ml against 2 sets of DensityMarkerBeads as density markers. The samples and markers were centrifuged at 52,000g for 90 min at 20°C. Following centrifugation, aliquots (0.5 ml) were sequentially removed. Thrombin generation activity in the fractions from cancer patients (A) and healthy plasma (B) was measured in the presence (circle) and absence of factor VII (square). (C) To highlight the TF-dependent activity, the average of the differences between thrombin generation in the presence and absence of factor VII was calculated (n=3).
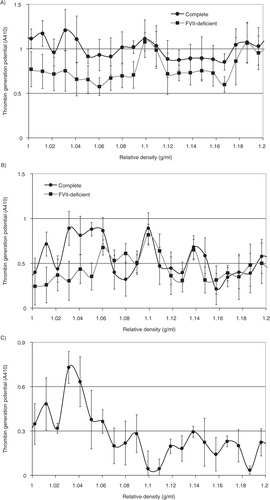
Fig. 7. (Continued). Fig. 7. Nanoparticle tracking analysis (NTA) of TF-containing microvesicles (1.03–1.08 g/ml) separated by density gradient centrifugation. Microvesicles were prepared from normal human plasma (A), conditioned media from MDA-MB-231 cells (B), and conditioned media from MDA-MB-231 cells expressing TF-tGFP protein (C). The plasma and conditioned media were collected and cleared of any cell debris by centrifuging at 5,400g on a microcentrifuge, and microvesicles sedimented at 100,000g. The microvesicles were resuspended in PBS and were fractionated by density gradient ultracentrifugation using a sucrose–OptiPrep gradient covering an approximate density range of 1.02–1.22 g/ml alongside 2 sets of DensityMarkerBeads. The samples were centrifuged at 52,000g for 90 min at 20°C in a SW41Ti rotor on a Beckman L8-M ultracentrifuge. Following centrifugation, aliquots (0.5 ml) were sequentially removed and assessed for TF antigen. Samples containing TF antigen were then pooled (1.03–1.08 g/ml) and diluted 1:10 in PBS, and the size of the microvesicle population was analysed by NTA using a NanoSight LM10 instrument. A control sample was prepared by adding MDA-MB-231-derived microvesicles to the same pooled fractions from a blank density gradient centrifugation (D). A negative control made of the pooled fractions from a blank density gradient centrifugation showed no detectable trace (E). The illustrations are typical (n=3) of the size distributions which were determined using NTA software. The total amounts of microvesicles in the samples are not comparable. TF-containing microvesicles were immuno-purified from conditioned media of MDA-MB-231 (F) and A375 cell lines (G). The samples were incubated with a monoclonal antibody against TF (10H10; 4 µg/ml) followed by protein A-magnetic beads. The samples were washed with PBS and eluted in phosphate buffer containing NaCl (500 mM). The samples were analysed by NTA against a sample treated similarly but without the antibody.
Determination of the duration of centrifugation required for the sedimentation of TF-containing microvesicles
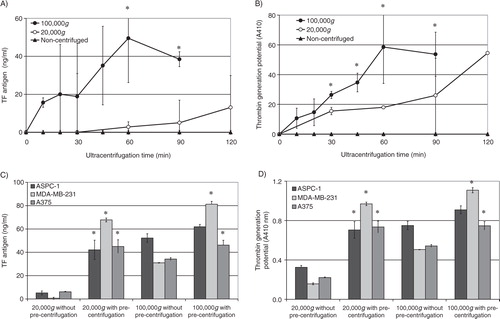
In order to determine the centrifugation time required for the sedimentation of TF-containing microvesicles, conditioned media from PAR2-activated MDA-MB-231 cells were centrifuged at 20,000g and 100,000g for intervals ranging from 10 to 120 min. The microvesicles were then resuspended in PBS, and the TF antigen levels measured. Analysis of the data indicates the progressive increase in the recovery of TF-containing microvesicles at 100,000g up to 60 min (A) but not at 20,000g. In contrast, thrombin generation potential of the recovered microvesicles increased with duration of centrifugation at 20,000g but was significantly higher at 100,000g (B). In addition, centrifugation of normal plasma supplemented with TF-tGFP-containing microvesicles, at 20,000g and 100,000g for 1 h, resulted in the recovery of 26±5.4 and 67±1.1%, respectively, of the original fluorescence prior to centrifugation. This indicates that a large proportion of the microvesicles may be recovered from plasma by centrifugation at 100,000g for 1 h.
Fig. 8. Analysis of the influence of the duration of centrifugation and pre-centrifugation on the recovery of TF-containing microvesicles. Conditioned media from PAR2-activated MDA-MB-231 cells were centrifuged at 20,000g and 100,000g for intervals ranging from 10 to 120 min. The microvesicles were then resuspended in PBS, and (A) the TF antigen levels and (B) the thrombin generation potential of the recovered microvesicles were measured (n=3, *=p<0.05 vs. the amounts detected in non-centrifuged samples). The conditioned media from AsPC-1, MDA-MB-231 and A375 cells were pre-centrifuged at 10,000g before attempting to recover the microvesicles by centrifuging at 20,000g or 100,000g for 60 min. Similar samples were prepared from microvesicles but without pre-centrifuging. The microvesicles were then resuspended in PBS, and (C) the TF antigen levels and (D) the thrombin generation potential of the recovered microvesicles were measured (n=3, *=p<0.05 vs. the amounts detected in respective samples without pre-centrifuging).
Influence of pre-centrifugation on the sedimentation rate of lower-density microvesicles
The conditioned media from MDA-MB-231, A375 and AsPC-1 cells were pre-centrifuged at 10,000g before attempting to recover the microvesicles at 20,000g and 100,000g. Pre-centrifugation of the conditioned media at 10,000g significantly improved the recovery of TF-containing microvesicles at 20,000g and 100,000g (C). Furthermore, while the thrombin generation potential of the recovered microvesicles was higher in samples centrifuged at 100,000g compared to those isolated at 20,000g (D), the differences between these 2 sets were less significant compared to those observed with TF antigen levels (C). Interestingly, pre-centrifugation also improved the recovery of the TF-tGFP microvesicles from plasma from 26±5.4 (see above) to 58±4.1%.
The density of the media prior to centrifugation was calculated to be 1.028±0.005 g/ml at 20°C, and that of plasma was measured to be 1.030±0.005 g/ml (distilled water=1.000±0.003 g/ml). Subsequent to centrifugation, the density of the top and bottom 100 µl fractions were determined to be 1.010±0.032 g/ml and 1.050±0.019 g/ml, respectively for conditioned media and 1.025±0.005 g/ml and 1.057±0.006 g/ml, respectively, for plasma.
The sedimentation of any particulate matter by gravitational force may be calculated from the equation , where ν is the velocity of sedimentation (m/s), Δρ is the difference between the relative density of microvesicles and the surrounding fluid (g/ml), g is equal to 9.8 m/s2, d is the diameter of the microvesicles (m), and η is the viscosity of the surrounding fluid and was assumed to be 0.001 Pas. Therefore, the sedimentation rate is dependent on both the size and the density of the microvesicles, as well as the density of conditioned media or plasma. Using median values for the diameter and density of TF-containing microvesicles, we estimated the rate of sedimentation of this range of microvesicles in pre- and post-centrifuged (10,000g) media and plasma. Using these data, the rate of sedimentation of TF-containing microvesicles from conditioned media or plasma, at 100,000g, was calculated to be in range with the experimental value (50±18 min for a 2.5 cm tall centrifuge tube). Moreover, in agreement with our experimental data, the rate of sedimentation at 20,000g was too slow, and the required centrifugation time was estimated to be >120 min. However, pre-centrifugation of conditioned media or plasma at 10,000g removed the denser microvesicles, consequently reducing the overall relative density of the fluid and increasing the difference between the remaining microvesicles and the surrounding fluid (Δρ) from 0.002 to 0.020 g/ml. As a result, the time needed for the recovery of TF-containing microvesicles was determined to be within the order of magnitude using subsequent centrifugation at 20,000g for the majority, if not all, of the microvesicle populations, and it explains the observations in C and D.
Discussion
The procoagulant activity of microvesicles is mainly derived from their ability to harbour TF, as well as the high ratio of negatively charged phosphatidylserine on the surface of the microvesicles (Citation1). However, it is also acknowledged that not all types of microvesicles contain TF (Citation16), or at least not at sufficient amounts to be detectable using common measurements (Citation17). Therefore, discrepancies can arise as a consequence of differences in sub-populations of microvesicles (Citation4), and preparative procedures used for the purification of microvesicles may discriminate between subpopulations of microvesicles (Citation5). Because, during the sedimentation of microvesicles by ultracentrifugation, the microvesicles are isolated on the basis of size and relative density, this method is crucially influenced by the centrifugation force, the duration of centrifugation and the density of the supporting medium.
In this study, we employed 3 cell lines on the basis of high levels of TF expression, as well as the ability to release measurable amounts of TF-containing microvesicles. In agreement with published data (Citation4), all recovered microvesicles regardless of the centrifugal forces used possessed some thrombin generation potential (B), but this was not always dependent on the presence of FVII (C) and could not be inhibited using an inhibitory anti-TF antibody (F). However, assessment of TF antigen content of the recovered microvesicles indicates that high RCF values (ideally, 100,000g) improve the sedimentation of TF-containing microvesicles (). In agreement, these microvesicles also possessed the highest factor VIIa–dependent thrombin and factor Xa generation potential (B–F). Analysis of the size of the recovered microvesicles indicated a general pattern in which medium-sized microvesicles were sedimented at lower centrifugal forces (20,000g), followed by smaller-sized microvesicles at >50,000–75,000g, and the larger microvesicles were mostly recoverable at 100,000g (). These data are in agreement with previously published studies on determination of size of platelet-derived microvesicles which show 4 separate sub-populations of microvesicles (Citation6). The study by Dean et al. (Citation6) indicates that larger microvesicles contain membrane proteins, whereas the contents of the smaller-sized microvesicle classes originated from within the cell compartments. In addition, filtration of the microvesicle samples has been shown to remove the major proportion of TF antigen and activity in the samples (Citation18). However, the authors reported a larger drop in TF activity compared to TF antigen, which may suggest an exchange of lipids. Therefore, as a parallel, the association of TF with the larger-sized microvesicles is in line with the notion that the formation of these microvesicles is initiated from the lipid rafts on the cell surface (Citation19, Citation20).
Since the sedimentation rate is dependent on both the size and relative density of the microvesicles, it would then be logical to assume that the larger-sized microvesicles prepared in our study ought to have lower relative density compared to the medium- and smaller-sized microvesicles. Interestingly, the lower-density fractions obtained at 100,000g were shown to contain measurable levels of TF antigen (A and 5A) and TF activity (B), as well as possessing fluorescence intensity arising from the TF-tGFP hybrid protein (B). Finally, examination of the relative density of microvesicle samples obtained by ultracentrifugation of patient and normal plasma also indicated the presence of TF in the lower-density fractions (A and B). Moreover, the lower density fractions containing the TF also exhibited higher proportions of larger-diameter microvesicles (Supplementary Fig. 5). Previous analysis of the protein and lipid content of the platelet-derived microvesicles also indicates that the highest lipid–protein ratio may be associated with the larger microvesicles, with the medium-sized microvesicles having the lowest ratio (Citation6). This in turn suggests that the larger-sized microvesicles have the lowest, while the medium-sized microvesicles possess the highest, relative densities, and it is in agreement with our findings. Furthermore, lipid rafts have been shown to be regions of low relative density, arising from the higher lipid–protein ratios compared to the rest of the cell membrane (Citation21, Citation22). Therefore, this is also in agreement with the concept of formation of microvesicles from distinct regions within the surface of the cells. However, future morphological (e.g. electron microscopy) studies are required to fully characterize the vesicular nature of the low-density TF-positive microvesicles.
A time course of sedimentation of TF-containing microvesicles showed that the sedimentation of these microvesicles was time dependent as well as RCF dependent (A and B). Moreover, the removal of the denser microvesicles by pre-centrifugation at 10,000g permitted the partial recovery of TF-containing microvesicles at 20,000g but did not present a huge advantage in the recovery at 100,000g in most cases (C and D).
The understanding of physical and compositional properties of procoagulant microvesicles is crucial for the understanding of the biological functions of these particles. As far as we are aware, this is the first study in which an attempt has been made to characterize the physical properties of TF-containing microvesicles and to discern these from the population of microvesicles released by cells. In summary, our studies indicate that TF mainly resides within lower-density microvesicles (1.03–1.08 g/ml) with diameters ranging between 200 and 350 nm. Furthermore, we have established some guidelines for the recovery of these microvesicles from both conditioned media and plasma, but further refinement would be essential.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Supplemental Data
Download PDF (321.1 KB)Supplementary Figures
Download PDF (252.5 KB)Acknowledgements
The support from the Hull and East Riding Cardiac Trust Fund is acknowledged.
References
- Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011; 9: 2251–61.
- Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis?. J Thromb Haemost. 2007; 5: 520–7.
- Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BCet al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009; 15: 6830–40.
- Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DAet al. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012; 129: 80–5.
- Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011; 105: 396–408.
- Dean WL, Lee MJ, Cummins TD, Schultz DJ, Powell DW. Proteomic and functional characterisation of platelet microparticle size classes. Thromb Haemost. 2009; 102: 711–18.
- Lechner D, Weltermann A. Circulating tissue factor-exposing microparticles. Thromb Res. 2008; 122: S47–54.
- Collier ME, Mah PM, Xiao Y, Maraveyas A, Ettelaie C. Microparticle-associated tissue factor is recycled by endothelial cells resulting in enhanced surface tissue factor activity. Thromb Haemost. 2013; 110: 966–76.
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AMet al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012; 56: 293–304.
- Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJet al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013; 13: 3354–64.
- Collier ME, Ettelaie C. Regulation of the incorporation of tissue factor into microparticles by serine phosphorylation of the cytoplasmic domain of tissue factor. J Biol Chem. 2011; 286: 11977–84.
- Collier ME, Ettelaie C. Induction of endothelial cell proliferation by recombinant and microparticle-tissue factor involves beta1-integrin and extracellular signal regulated kinase activation. Arterioscler Thromb Vasc Biol. 2010; 30: 1810–17.
- Ettelaie C, Su S, Li C, Collier ME. Tissue factor-containing microparticles released from mesangial cells in response to high glucose and AGE induce tube formation in microvascular cells. Microvasc Res. 2008; 76: 152–60.
- Collier ME, Maraveyas A, Ettelaie C. Filamin-A is required for the incorporation of tissue factor into cell-derived microvesicles. Thromb Haemost. 2014; 111: 647–55.
- Ettelaie C, Fountain D, Collier MEW, ElKeeb A, Xiao YP, Maraveyas A. Low molecular weight heparin downregulates tissue factor expression and activity by modulating growth factor receptor-mediated induction of nuclear factor-κB. Biochim Biophys Acta Mol Basis Dis. 2011; 1812: 1591–600.
- Sturk-Maquelin KN, Nieuwland R, Romijn FP, Eijsman L, Hack CE, Sturk A. Pro- and non-coagulant forms of non-cell-bound tissue factor in vivo. J Thromb Haemost. 2003; 1: 1920–6.
- van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PWet al. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thromb Haemost. 2012; 108: 160–5.
- Davila M, Robles-Carrillo L, Unruh D, Huo Q, Gardiner C, Sargent ILet al. Microparticle association and heterogeneity of tumor-derived tissue factor in plasma: is it important for coagulation activation?. J Thromb Haemost. 2014; 12: 186–96.
- Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microparticles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005; 106: 1604–11.
- Davizon P, Munday AD, López JA. Tissue factor, lipid rafts, and microparticles. Semin Thromb Hemost. 2010; 36: 857–64.
- Sonnino S, Prinetti A. Membrane domains and the “lipid raft” concept. Curr Med Chem. 2013; 20: 4–21.
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992; 68: 533–44.
