Abstract
Extracellular vesicles (EV) in breast milk carry immune relevant proteins and could play an important role in the instruction of the neonatal immune system. To further analyze these EV and to elucidate their function it is important that native populations of EV can be recovered from (stored) breast milk samples in a reproducible fashion. However, the impact of isolation and storage procedures on recovery of breast milk EV has remained underexposed. Here, we aimed to define parameters important for EV recovery from fresh and stored breast milk. To compare various protocols across different donors, breast milk was spiked with a well-defined murine EV population. We found that centrifugation of EV down into density gradients largely improved density-based separation and isolation of EV, compared to floatation up into gradients after high-force pelleting of EV. Using cryo-electron microscopy, we identified different subpopulations of human breast milk EV and a not previously described population of lipid tubules. Additionally, the impact of cold storage on breast milk EV was investigated. We determined that storing unprocessed breast milk at −80°C or 4°C caused death of cells present in breast milk, leading to contamination of the breast milk EV population with storage-induced EV. Here, an alternative method is proposed to store breast milk samples for EV analysis at later time points. The proposed adaptations to the breast milk storage and EV isolation procedures can be applied for EV-based biomarker profiling of breast milk and functional analysis of the role of breast milk EV in the development of the neonatal immune system.
To access the supplementary material to this article, please see Supplementary files under Article Tools online.
Breast milk not only functions as a source of nutrition but also delivers immune modulatory factors to neonates. Breast milk components relevant to immunity include cytokines, antibodies, multiple types of immune cells, antimicrobial peptides, microbes, and unique lipids (reviewed in (Citation1–Citation4)). Components such as IgA and IgG have been shown to modulate immune reactivity of the child to antigens present in the environment (Citation5, Citation6). Breast milk is therefore thought to play an important role in the development of the immune system of the child. Direct evidence that the composition of breast milk can influence immune responses in neonates was provided in mouse models of allergic asthma and food allergy (Citation7, Citation8). In these studies, offspring was breastfed by mothers exposed to inhaled or food antigens. It was shown that this offspring exhibited profoundly reduced immune reactions to these antigens. The mechanism behind this type of immune modulation via breast milk is not understood, but preliminary data indicated a key role for induction of tolerance and regulatory T cells.
Recently, extracellular vesicles (EV) have been identified in human breast milk (Citation9–Citation11) and in bovine milk (Citation12, Citation13). EV are released by many different cell types and are recognized as potent vehicles for intercellular communication, capable of transferring messages encoded in proteins, lipids and RNA. The majority of EV are <200 nm in size and typically consist of lipid bilayer enclosed vesicles. The cargo of EV is regulated by the EV producing cells and depends on activation- and stress-stimuli imposed on these cells (Citation14, Citation15). EV derived from different cell types can influence various biological processes, with EV from leukocytes playing an active role in the regulation of innate and adaptive immune responses (Citation15). It is currently unknown whether the EV in breast milk originate from cells present in milk, from epithelial cells lining the mammary gland or from cells elsewhere in the body. A potential role for milk-derived EV in immune modulation was suggested based on their protein and miRNA contents (Citation9, Citation11–Citation13). Moreover, human breast milk–derived EV were found to facilitate regulatory T cell induction (Citation9). This has led to the hypothesis that EV in breast milk could be involved in the instruction of the neonatal immune system.
General protocols to isolate EV from cell culture supernatants or body fluids involve steps of differential (ultra)centrifugation and further purification on a density gradient. However, since different body fluids have a highly variable composition, each of the isolation protocols to recover EV from these body fluids may require specific optimization steps (Citation16). Factors that could complicate isolation of EV from milk are the high fat and protein content of this body fluid. Most of the fat is stored in milk fat globules (MFG), which are lipid droplets that are formed and secreted by the epithelial cells lining the mammary gland (Citation17). Major milk proteins are casein and whey (Citation18). The tertiary structures of these proteins add to the complexity of the milk matrix, as casein is organized into micelles (Citation19), while whey consists of globular proteins known for their emulsifying capacities (Citation20). Besides the complex milk matrix, storage protocols could also affect recovery of EV from breast milk. Previous studies used refrigerated or frozen milk as a source for EV (Citation9–Citation12, Citation21–Citation24) . Cold storage of milk is common practice and effects on nutritional components and soluble immunological factors have been described (Citation25–Citation27). However, storage effects on cells and EV populations in breast milk have mostly been ignored (Citation28). Since disintegration of dead cells leads to the formation of membrane-enclosed vesicles (Citation29), cell death can lead to contamination of the pool of naturally present EV with vesicles induced upon sample storage.
Here, we assessed and optimized the efficiency and reproducibility with which EV can be recovered from breast milk samples. To this end, milk samples were spiked with a well-characterized mouse EV population as an internal standard. The commonly used differential centrifugation and density gradient ultracentrifugation protocol was modified, in order to achieve efficient and quantitative recovery of EV from breast milk. Additionally, we show that cells in refrigerated or frozen breast milk samples die rapidly, leading to contamination of the native milk EV population with vesicles derived from dying or dead cells. Thus, we provide an alternative method for the storage of breast milk samples for efficient and reliable recovery of EV at later time points.
Materials and methods
Breast milk collection
Fresh, mature milk samples were collected by 17 healthy mothers (at<8 months (15 donors) or 11–20 months (2 donors) after delivery), who were not actively terminating breast feeding. Donors were instructed to collect fore and hind milk of 1 breast using an electric breast pump, and to gently mix and aliquot 10–50 ml of milk in sterile containers. Milk was transported to the lab and used for experiments within 20 minutes of collection. Milk was prevented from cooling down by transportation in a Styrofoam box, insulated with tissues and containing a heat-generating package. All donors gave their signed informed consent to participate and the study was approved by the local ethics committee.
Preparation of BMDC for spiking of milk samples
Murine bone marrow–derived dendritic cells (BMDC) used for spiking of breast milk samples were generated from C57BL/6 mice as described previously (Citation30, Citation31). Cells were cultured in EV-depleted culture medium, prepared by centrifuging solutions of 30% FCS in IMDM and conditioned medium from GM-CSF producing R1 cells for 15 hours at 100,000 g. On day 14, cells were harvested, counted, washed twice in PBS containing 1% vesicle-depleted FCS, and finally resuspended in PBS/1% FCS at a concentration of 15×106 cells/ml. Vesicle-depleted FCS was obtained by 65 minutes centrifugation at 100,000 g (Beckman Coulter Optima L-90K using a SW28 rotor).
Preparation of murine reference EV for spiking of milk samples
Murine EV were obtained by cognate co-culture of the immature DC-line D1 with p53-specific CD4+-T cells derived from a C57BL/6 p53−/− mouse, as described previously (Citation32). Briefly, supernatant was harvested after 20 hours of co-culture and centrifuged 2×200 g and 2×500 g for 10 minutes, 10,000 g for 30 minutes (Beckman Coulter Avanti J-26 XP using a JA-12 rotor), and 100,000 g for 65 minutes (Beckman Coulter Optima L-90K ultracentrifuge using a SW28 rotor). All centrifugation steps were performed at 4°C. The 100,000 g pellet was resuspended in PBS supplemented with 0.2% BSA which had been ultracentrifuged O/N at 100,000 g. This EV suspension was used immediately for spiking of breast milk samples. For spiking of 1 ml of breast milk, reference EV derived from co-cultures of 1.75×106 DC with 1.75×106 T cells were used. Recovery of these reference EV was calculated as a percentage of the total number of reference EV in control conditions in each individual experiment.
Breast milk EV isolation
Fresh breast milk samples were spiked with murine reference EV and gently inverted to mix or were left unspiked. Milk was centrifuged at RT for 10 minutes at 3,000 g (Beckman Coulter Allegra X-12R) to remove cells and cream layer (Supplementary Fig. 1). Supernatant was then transferred to new tubes and centrifuged again at 3,000 g at RT to remove remaining cells and cream. The supernatant was then either immediately processed or frozen at −80°C. If frozen milk samples were used, they were thawed in a 37°C water bath until a small clump of ice remained and used immediately. The fresh or thawed milk supernatants were subsequently transferred to polyallomer SW40 tubes (Beckman Coulter) and centrifuged at 5,000 g for 30 minutes at 4°C to remove larger cell debris and residual cream, and subsequently at 10,000 g for 30 minutes at 4°C to remove smaller cell debris. For top-down ultracentrifugation (Supplementary Fig. 1, left side), 6.5 ml of the cleared 10,000 g milk supernatant was loaded on top of a sucrose density gradient in an SW40 tube. This density gradient was prepared by layering successive sucrose solutions (15 fractions of 350 µl) of decreasing density (2.0 M–0.4 M) on top of 700 µl 2.5 M sucrose. For bottom-up density gradient ultracentrifugation (Supplementary Fig. 1, right side), 9.0 ml of the cleared 10,000 g milk supernatant was layered on top of a 2-step cushion of 2.0 M and 0.74 M sucrose in an SW40 tube and centrifuged at 100,000 g for 125 minutes. The interphase between the sucrose layers was harvested using a 21G syringe, diluted with PBS/0.1% O/N ultracentrifuged BSA, and pelleted at 100,000 g for 65 minutes. The pellet was resuspended in PBS/0.2% ultracentrifuged BSA, mixed with 1.5 ml 2.5 M sucrose, and overlaid with sucrose solutions (15 fractions of 750 µl) of decreasing density (2.0 M–0.4 M). Both top-down and bottom-up density gradients were ultracentrifuged for 14–21 hours at 192,000 g (Beckman Coulter Optima L-90K with a SW40 rotor). Density fractions of 0.5 ml (top-down) or 1 ml (bottom-up) were collected from the bottom of the tube in Eppendorf tubes. Densities were determined by refractometry.
Cryo-electron microscopy
For cryo-electron microscopy (cryo-EM), fractions of indicated densities were pooled and diluted with Annexin V-binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4), supplemented with 0.1% ultracentrifuged BSA. Material was pelleted at 100,000 g for 65 minutes using an SW40 rotor and pellets were resuspended in 50 µl Annexin V-binding buffer/0.1% ultracentrifuged BSA and kept at 4°C prior to Annexin V labeling and processing. For cryo-EM Annexin V labeling, a 7 µL aliquot was mixed with 1 µl Annexin V-conjugated gold nanoparticles at 2×1016 particles/l (Citation33) and 1 µl 2 mM CaCl2 and incubated for 15 minutes at RT. Then, a 4 µL aliquot was deposited onto an EM grid coated with a perforated carbon film (Ted Pella, Redding, CA, USA), the excess liquid was blotted off with filter paper, and the grid was quickly plunged into liquid ethane using a Leica EM-CPC cryo-chamber. EM grids were stored in cryo-boxes under liquid nitrogen until use, then mounted in a Gatan 626 cryo-holder and transferred in a Tecnai F20 microscope operated at 200 kV. Images were recorded with an USC1000-SSCCD Gatan camera.
SDS-PAGE, Coomassie staining, and western blotting
Density fractions were either pooled or processed individually, as indicated. Fractions were diluted with PBS/0.1% ultracentrifuged BSA and pelleted at 100,000 g for 65 minutes in a Beckman Coulter Optima L-90K, using a SW60 rotor for individual fractions and a SW40 rotor for pools. Pellets were resuspended in non-reducing SDS-PAGE sample buffer, heated at 100°C for 3 minutes and run on a 4–20% TGX-Criterion gel (Bio-Rad, Hercules, CA, USA). Gels were stained with Coomassie brilliant blue solution or transferred to PVDF membranes and blocked in PBS containing 0.2% fish skin gelatin and 0.1% Tween-20. Proteins were detected by immunoblotting using rat anti-mouse CD9 (KMC8, eBioscience, Vienna, Austria), rat anti-mouse CD63 (R5G2, MBL, Woburn, MA, USA), rabbit anti-mouse MHC class II (polyclonal, Genscript, Piscataway, NJ, USA), mouse anti-human CD9 (HI9a, BioLegend, Fell, Germany), mouse anti-human flotillin-1 (clone 18, BD Biosciences, San Jose, CA, USA), mouse anti-HLA-DP/DQ/DR (CR3/43, Dako, Heverlee, Belgium), mouse anti-human CD63 (TS63, Abcam, Cambridge, UK), rabbit anti-human β-casein (polyclonal, Abcam), rabbit anti-human lactoferrin (polyclonal, Abcam), and rabbit anti-human α-lactalbumin (EPR12460, Abcam). Secondary antibodies goat anti-mouse-HRP (Jackson Immuno Research, Suffolk, UK), rabbit anti-rat-HRP, and goat anti-rabbit-HRP (both from Dako) were used. Labeled antibodies were detected using SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific, Landsmeer, Netherlands). Protein levels were quantified by densitometry using a ChemiDoc XRS and Quantity One Basic software V4.6.9 (Bio-Rad).
Cellular viability assessment
Fresh breast milk was spiked with murine BMDC at a final concentration of 0.75×106 cells/ml or was left unspiked. Milk was divided into equal volumes and was either processed immediately or stored at −80°C, 4°C, RT or 37°C for the indicated time periods. Breast milk was centrifuged at 3,000 g for 10 minutes. Cell pellets were resuspended and washed twice in IMDM containing 5% FCS and centrifuged at 500 g for 10 minutes. Cell pellets were resuspended in PBS/1% FCS. Cellular viability was determined by trypan blue exclusion.
Alternatively, cells were incubated with APC-conjugated anti-mouse MHC class II (M5/114, eBioscience) or the corresponding isotype control, or Alexa488-conjugated Annexin V (Life Technologies, Bleiswijk, Netherlands) and propidium iodide (PI; eBioscience) as indicated by manufacturer. Cells were analyzed using a FACS Canto II and FACS Diva software (BD Biosciences), and FCS Express software (De Novo software, Los Angeles, CA, USA). Murine cell viability was determined by gating on MHC class II positive cells, and determining the percentage of Annexin V and/or PI positive cells.
Storage of milk supernatant cleared from cells and cream
Fresh breast milk was spiked with murine reference EV, as indicated above, and gently inverted to mix. Milk was centrifuged at 3,000 g for 10 minutes at RT. Subsequently, the cream layer was removed and the supernatant was transferred to a new tube. Supernatant was then centrifuged again at 3,000 g for 10 minutes at RT, after which milk was divided into equal volumes and stored for 2–8 weeks at −80°C.
Statistics
Normal distribution of data was determined using the Shapiro-Wilk normality test. Normally distributed data were analyzed by 1 sample t-test, relative to control values, which were set to 100%, otherwise a Wilcoxon signed-rank test was used. Normally distributed paired data were analyzed using a paired t-test. If multiple parameters were compared, 1-way ANOVA was applied, followed by Tukey's post hoc test. Significance was defined as *p<0.05 and **p<0.01, and calculated with GraphPad Prism software V6.02.
Results
Differential centrifugation followed by top-down density gradient ultracentrifugation allows efficient density separation of EV from breast milk
Differential (ultra)centrifugation followed by density gradient ultracentrifugation is a common protocol used for EV isolation from cell culture supernatant (Citation34, Citation35). Although this procedure has been used in previous studies on breast milk EV, the efficiency with which EV can be isolated from milk using this procedure has not been assessed. Determining such efficiency, however, is hampered by the lack of knowledge on markers characteristic for milk EV, and by the large differences in milk composition observed between individuals and even between feeds of the same individual (Citation36–Citation38). In order to optimize EV isolation from breast milk, fresh human milk was spiked with a known population of murine EV (hereafter referred to as ref-EV) as an internal standard, prior to the centrifugation procedure. These ref-EV consisted of EV released during in vitro co-culture of murine DC with T cells, which were previously found to be enriched in CD9 and CD63 (32,39 and data not shown). Based on earlier observations that characteristics such as size (~50–150 nm) and density (1.12–1.18 g/ml) appear common to EV throughout species and body fluids, we assumed that the ref-EV and human breast milk EV behaved similarly with regard to sedimentation and density separation (Citation40).
First, the spiked breast milk was subjected to the conventional protocol of differential centrifugation, in which unwanted components are removed in low/intermediate speed centrifugation steps prior to pelleting EV at 100,000 g (Citation34, Citation35). An initial centrifugation step of 3,000 g was applied to clear the milk from cells and cream layer. In comparison to the more conventional initial centrifugation at lower speed (500 g), this higher g-force led to more efficient separation of the cream layer from the milk supernatant, whereas the efficiency of cell pelleting and the viability of these cells were similar (data not shown). The milk supernatant was additionally centrifuged at 5,000 g and 10,000 g to further remove fat and cellular debris. Following 100,000 g centrifugation, we observed that the EV-containing pellet was too solid to resuspend in PBS or SDS sample buffer, which prevented further processing and reliable analysis of these EV. The inability to resuspend the 100,000 g pellet could be due to highly abundant milk proteins, such as whey and casein, which can complex and sediment at 100,000 g, forming a compact protein matrix (Citation19). In order to remove such large protein complexes prior to 100,000 g pelleting, the 10,000 g milk supernatant was centrifuged into a 0.74 M/2.0 M sucrose cushion (Supplementary Fig. 1). The EV-containing interphase was harvested, and, after centrifugation at 100,000 g, the EV-containing pellet could be resuspended. This material was overlaid with a sucrose gradient, after which EV were separated based on buoyant density in a “bottom-up” approach (Supplementary Fig. 1). Next, the distribution and quantity of recovered ref-EV was assessed by western blotting for mouse CD9 (mCD9) and CD63 (mCD63). Based on previous studies in our lab, the murine ref-EV were expected to equilibrate at densities of 1.12–1.18 g/ml (Citation32, Citation39, Citation41). The antibodies used to detect mCD9 and mCD63 did not cross-react with proteins in human milk (data not shown). Although mCD9 was observed in the expected density fractions (1.12–1.18 g/ml), a substantial amount of the murine EV-associated protein stayed behind in the high density fractions (1.25–1.28 and 1.20–1.23 g/ml; a). This indicates that the bottom-up approach for density-based purification of EV from milk is not efficient.
Fig. 1 Efficient isolation of reference EV from breast milk by top-down density gradient ultracentrifugation. (a) Fresh human breast milk was spiked with murine ref-EV, after which EV were recovered via bottom-up or top-down density gradient ultracentrifugation. The distribution of recovered ref-EV over the different density fractions was analyzed by western blotting for murine CD9 (mCD9). Indicated are the sizes for monomeric mCD9 (*, ~23 kD), N-glycosylated monomeric mCD9 (**, ~27 kD), and high molecular weight complex mCD9 (***, ~150 kD). This result is representative of 4 independent experiments in 3 different donors. (b) Fresh human breast milk (☐) or PBS/BSA control solution (■) were spiked with murine ref-EV, after which EV were recovered via top-down density gradient ultracentrifugation. Fractions of the density gradient and the overlying milk supernatant were analyzed for the presence of mCD9 and mCD63 by western blotting. Indicated are the quantified western blot signals (mean values of 2 independent experiments in 2 different donors) normalized to the values detected in the 1.13–1.18 g/ml fractions of the PBS/BSA control (set to 100%).
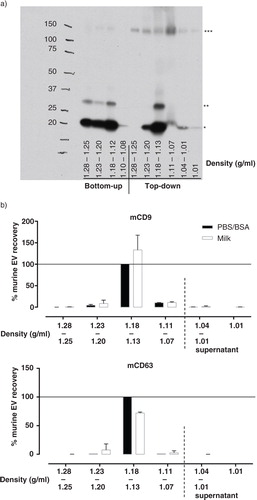
Next, we tested whether the ref-EV could be efficiently purified and recovered from milk using top-down density gradient ultracentrifugation (Supplementary Fig. 1). In this approach, the EV-containing 10,000 g supernatant was applied directly on top of a density gradient, thereby circumventing the 100,000 g EV pelleting step. Following top-down density gradient ultracentrifugation, most of the mCD9 was present in density fractions 1.13–1.18 g/ml to which the ref-EV were expected to float (a). In addition to the 23 and 27 kD monomeric mCD9, high molecular weight complexes (~150 kD) of mCD9 were detected after top-down density gradient ultracentrifugation. These complexes could correspond to CD9 coupled to the β1-integrin subunit (Citation42).
Next, we investigated to what extent the complex composition of breast milk affected the efficiency with which murine ref-EV could be recovered. The ref-EV were spiked in either milk or PBS/0.1% BSA, after which differential centrifugation and top-down density gradient ultracentrifugation were used to isolate EV. The quantity of ref-EV recovered from the 2 different fluids was determined by western blotting for mCD9 and mCD63. A similar distribution of mCD9 and mCD63 was observed over the different density fractions for EV recovered from breast milk and PBS/BSA, with the majority of signal detected in the 1.13–1.18 g/ml density fraction (b). The quantity of mCD9 recovered from both solutions was similar, whereas slightly less CD63 was recovered from milk compared to PBS/BSA (b). This could be explained by the disparate presence of CD9 and CD63 on different subsets of the ref-EV sample. Based on these data, we conclude that top-down gradient ultracentrifugation is an efficient method for isolation of EV from breast milk.
Native breast milk EV are heterogeneous in size and composition
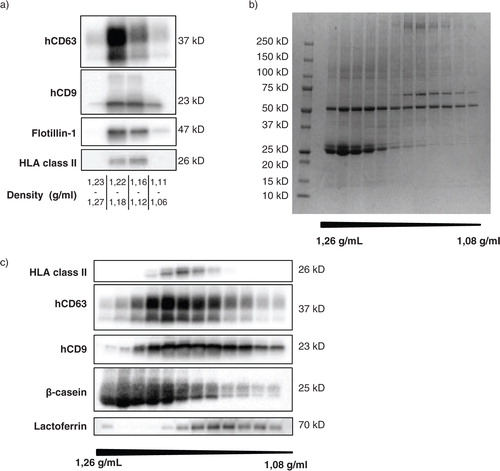
To confirm that the top-down procedure described above allowed isolation of EV naturally present in human breast milk, unspiked milk samples were subjected to top-down gradient ultracentrifugation and analyzed for the presence of human EV-associated proteins. Western blot analysis of human CD63 (hCD63), human CD9 (hCD9), flotillin-1, and HLA class II showed that these proteins were present in the same density fractions as the mouse reference EV (a). Next, we investigated in more detail whether our top-down procedure separated milk EV from milk-specific proteins known to be abundantly present in breast milk. For these experiments, individual gradient fractions rather than pools of fractions were tested. Coomassie blue staining of proteins in the different gradient fractions showed that differently sized proteins distributed unequally over the gradient fractions, with some proteins being predominantly present in the high density bottom fractions and some in the EV containing fractions at lower densities (b). Western blot analysis was performed to investigate the presence of the 3 major milk proteins β-casein, lactoferrin, and α-lactalbumin in the different gradient fractions (c). To visualize the location of EV within the gradient, the fractions were also stained for the EV-associated markers HLA class II, hCD63, and hCD9. We observed that β-casein was predominantly present in the high-density fractions of the gradient. In contrast, α-lactalbumin did not migrate into the density gradient, but was abundantly present in the supernatant on top of the gradient (data not shown). The majority of lactoferrin was also present in this supernatant (data not shown). Interestingly, a small amount of lactoferrin was detected in the EV-containing density fractions (c). These data indicate that milk proteins that easily form aggregates or micelles, such as β-casein, are high in density and sediment towards the bottom of the gradient. Smaller soluble proteins, such as α-lactalbumin and lactoferrin, do not migrate into the density gradient, although some lactoferrin appeared to be associated to EV.
Fig. 2 Unequal distribution of native breast milk EV and major milk proteins over density gradient fractions. Fresh, unspiked human breast milk was subjected to top-down density gradient ultracentrifugation, after which the material in the different density fractions was harvested and analyzed. (a) Density fractions were pooled as indicated and analyzed for the recovery of native human breast milk EV by western blotting for human CD63 (hCD63), CD9 (hCD9), flotillin-1, and HLA class II. Results for hCD9 and HLA class II are representative of>10 independent experiments in>8 different donors; results for hCD63 and flotillin-1 represent 2 independent experiments in 2 different donors. (b, c) The protein content of individual density fractions was assessed by Coomassie blue staining (representative of 2 different donors) (b) and by western blotting for HLA class II, hCD63, hCD9, β-casein, and lactoferrin (representative of 3 different donors) (c).
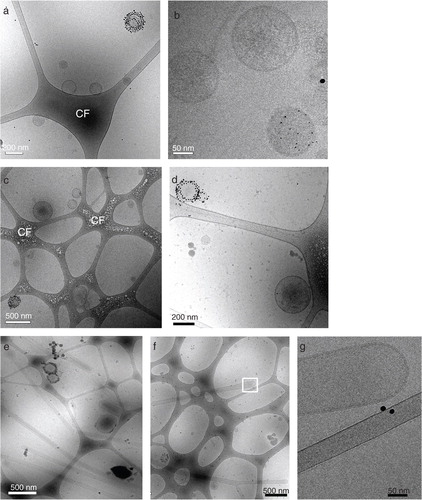
To visualize and further characterize the size and morphology of breast milk EV in the low-density fractions, EV were labeled with Annexin V-conjugated gold nanoparticles and analyzed by cryo-EM. Both the 1.12–1.18 and 1.18–1.21 g/ml density fractions contained EV, which appeared as spherical structures within the porous carbon film (). The ratios between Annexin V-positive and -negative EV populations were similar in both densities (). Furthermore, the ratio between small (<200 nm) and large (>200 nm) EV was ~ 4:1 in the 1.12–1.18 g/ml fraction, and ~2:1 in the 1.18–1.21 g/ml fraction. In addition to EV, the 1.12–1.18 g/ml fractions contained tubular structures (). These tubules did not stain with Annexin V, were enclosed by a lipid bilayer, and had a smooth appearance. The tubular structures did not contain striations typical for the presence of actin filaments and were approximately 5 times more prominent in the 1.12–1.18 g/ml as compared to the 1.18–1.21 g/ml fraction. However, it should be noted that quantitative analysis of EM samples is difficult due to potential bias for larger objects, which are more easily retained within the perforated carbon film. The data presented here are based on assessing 50–100 EV per density fraction. Results were comparable in 2 different donors. Taken together, these data suggest that a heterogeneous population of native breast milk EV can be isolated using top-down gradient ultracentrifugation.
Fig. 3 Native breast milk EV are heterogeneous in size and composition. Fresh, unspiked human breast milk was subjected to top-down density gradient ultracentrifugation, after which the material from density fractions 1.12–1.18 and 1.18–1.21 g/ml was labeled with Annexin V-conjugated gold nanoparticles and analyzed by cryo-EM. (a, b, c, d) Representative and close up images of Annexin V positive and negative EV in the (a, b) 1.12–1.18 g/ml and (c, d) 1.18–1.21 g/ml density fraction. (e, f) Representative images of smooth tubular structures predominantly present in the 1.12–1.18 g/ml fraction, with (g) close up image of indicated field in (f). CF=carbon film. Data are representative of 3 independent experiments and 2 different milk donors.
Storage of breast milk samples induces milk cell death and contamination of the naturally present EV population
Breast milk is often stored in the refrigerator (4°C) or freezer (−20 or −80°C) for the purpose of feeding the infant, as well as for research. Here, the effects of breast milk storage on milk-derived cells and EV were investigated.
Fresh breast milk was either processed immediately (within 20 minutes after collection) or stored for 2 hours at −80°C or 4°C (a and ) prior to processing. First, the number of viable human breast milk cells in fresh versus stored milk was assessed. The number of viable cells present in breast milk immediately after collection was largely donor-dependent with a mean cell count of 2.2±2.1×105 cells/ml (mean±SD). As expected, the number of viable cells was strongly decreased in milk stored at −80°C (a). Remarkably, however, the number of viable cells also declined significantly after storage for 2 hours at 4°C (b). Although the strength of the effect varied between milk samples of different donors, the reduction in milk cell viability upon cold storage was observed in all milk samples tested. The decrease in cell viability during storage at above zero temperatures was not specific for 4°C, since storage for 2 hours at room temperature or 37°C induced similar levels of cell death (Supplementary Fig. 2).
Fig. 4 Cell death in stored breast milk samples leads to contamination of the milk EV population. (a, b) Cells were isolated from fresh or stored breast milk and their viability was assessed by trypan blue exclusion. Number of viable cells determined in fresh milk samples and after storage (a) for 2 hours at −80°C (n=6 different donors) or (b) for 2 hours at 4°C (n=9 different donors). (c, d, e) Fresh breast milk was spiked with murine cells, after which cells or EV were recovered immediately or after storage at −80°C for ≥2 weeks. (c) Flow cytometric analysis of the percentage of dead murine cells in culture medium, or in fresh or stored breast milk samples. Box plots show mean±SD of 3 independent experiments in 5 donors. (d, e) EV were recovered from breast milk samples by top-down density gradient ultracentrifugation. Contamination of the breast milk EV population with vesicles induced upon sample storage was assessed by western blot detection of murine EV markers. Indicated are the quantified western blot signals for (d) mCD9 and (e) mMHC class II (mCD9 n=7; mMHC class II n=6). *p<0.05; **p<0.01.
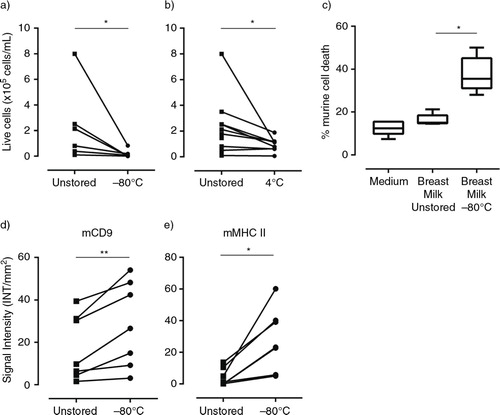
Cell death and consequent formation of cell debris can affect the composition of the total pool of milk EV. However, unique markers to distinguish (apoptotic) vesicles released by dying cells from EV actively released by viable cells are lacking. To determine whether cell death induced by cold storage led to contamination of the milk EV population, fresh breast milk samples were spiked with in vitro cultured dendritic cells of murine origin (hereafter referred to as ref-cells). The milk was then either processed immediately or stored at −80°C. First, the percentage of ref-cell death in breast milk in response to freezing was determined by analyzing the number of propidium iodide and/or Annexin V positive cells by flow cytometry. Although the murine ref-cells appeared more robust than the human milk cells, 28–50% of the spiked cells died or underwent apoptosis due to freezing (c). Next, we investigated whether this storage method led to contamination of the pool of actively released milk EV with vesicles induced upon cold storage of the murine cells in milk. EV were isolated from immediately processed or frozen spiked milk samples and tested for the presence of mCD9 and mMHC class II. Although immediately processed control samples were typically not negative for the murine EV markers, a significant increase in mCD9 (22–225%) and mMHC class II (186–2,222%) signals was observed in the milk EV population after storage at −80°C compared to immediately processed milk samples (d and ). These data indicate that a newly formed pool of vesicles, released by cells in milk during cold storage, contaminated the pool of EV naturally present in breast milk.
Freezing milk supernatant cleared of cream and cells is an alternative method for efficient and reliable EV recovery
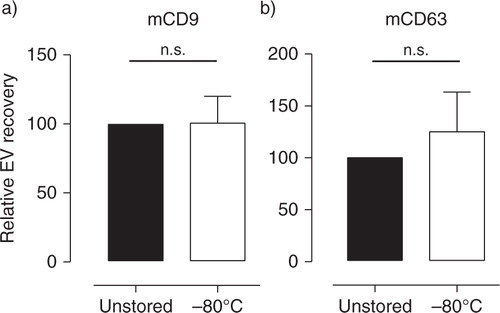
The release of storage-induced vesicles may be circumvented by clearing milk from cells as soon as possible after milk collection and prior to cold storage. Fresh breast milk spiked with murine ref-EV was cleared from cells and cream layer by 2 centrifugation steps at 3,000 g, after which EV were isolated from the milk supernatant either immediately or after storage for at least 2 weeks at −80°C. The quantity of recovered ref-EV was assessed by western blot analysis of mCD9 and mCD63. No significant differences were found in the amount of mCD9 and mCD63 recovered from freshly processed and stored milk supernatant (a and ). These data indicate that freezing milk supernatant devoid of cells and cream layer can be used to store breast milk samples for efficient recovery and reliable analysis of EV at later time points.
Fig. 5 Milk supernatant cleared from cells and cream can be stored for efficient and reliable recovery of EV. Fresh breast milk was spiked with murine reference EV and centrifuged twice to remove cells and cream. EV were recovered from milk supernatant before (■) and after (☐) storage at −80°C for 2–8 weeks and analyzed by western blotting for the presence of (a) mCD9 and (b) mCD63. Indicated are the quantified western blot signals (mean±SD) normalized to the values detected in unstored milk supernatant (set to 100%). mCD9 n = 5; mCD63 n = 4. n.s.=not significant.
Discussion
We here gained insight into parameters affecting the recovery of EV from (stored) human breast milk samples. Differential centrifugation followed by top-down density gradient ultracentrifugation resulted in efficient and reliable isolation of EV from breast milk. In addition, we showed that freezing of unprocessed milk samples, comprising a variety of milk cell types and cream-forming MFG, led to contamination of the natural milk EV population with EV induced by the storage process. An alternative storage protocol was proposed in which milk supernatant cleared of cells and most of the MFG was frozen for efficient and reproducible recovery of EV in breast milk at later time points.
EV isolation
Marker proteins for the discrimination of EV, MFG, and cellular debris in human milk are currently lacking. An additional complicating factor in the field of milk research is the high inter- and intra-donor variation with regard to the protein, fat, and cellular content of milk. Hence, the yield of breast milk EV using different EV isolation protocols could not be compared based on detection of human EV-associated markers. To overcome this problem, we spiked breast milk with a well-characterized murine EV population. By profiling defined murine EV-associated proteins, different protocols could be compared for the recovery of EV from human breast milk samples derived from a range of different donors.
A major difference between the 2 EV isolation protocols tested here was the presence or absence of a high-speed centrifugation step (100,000 g) to sediment EV. In our hands, the 100,000 g sedimented material formed a gelatinous pellet that could not be resuspended, as was also observed in several other milk EV studies (Citation13, Citation43). This gelatinous pellet is most likely caused by condensing of casein micelles and the globular nature of the abundantly present whey proteins, which can form gel-like matrices under high mechanical pressure (Citation44). Others have applied a filtration step of the milk supernatant prior to the EV pelleting step, and a subsequent overnight incubation step to desolidify the pellet (Citation9). However, using filtration to remove large protein complexes from the milk supernatant entails a high risk for selective loss of EV subpopulations. As an alternative approach, we have cleared the 10,000 g breast milk supernatant of abundant protein complexes using a sucrose cushion. This step was followed by a 100,000 g EV pelleting step and bottom-up floatation of EV into a density gradient. Using this protocol, however, a substantial amount of EV still did not efficiently reach their expected buoyant density of 1.12–1.18 g/ml. This could be explained by trapping of EV within residual protein complexes that still contaminated the EV pellet. This bottom-up protocol, including the sucrose cushion, was compared to top-down density gradient ultracentrifugation. In this case, the 10,000 g milk supernatant was applied directly on top of a density gradient, thereby circumventing the 100,000 g pelleting step. Using this procedure, the majority of spiked murine EV reached their expected buoyant density. Moreover, recovery of the spiked EV from breast milk samples was as effective as from PBS, indicating that the complexity of milk did not hamper quantitative recovery of EV from milk.
The use of spiked reference murine EV allowed close monitoring of the efficiency of different protocols for isolation of EV from milk. However, we cannot fully exclude that spiked murine EV and naturally occurring human breast milk EV behaved differently in these procedures. Nevertheless, we confirmed the presence of native human breast milk EV in the same density fractions as the spiked reference murine EV using western blot and cryo-EM ( and ). Interestingly, the breast milk EV floating to the different densities contained relatively different amounts of HLA class II, hCD63 and hCD9. The different distributions of these EV-associated proteins over the gradient, which has also been observed for EV derived from tumour cells (Citation45), indicate that the protein composition of breast milk EV is heterogeneous. Most of the major milk proteins separated from EV, either by sedimentation towards the bottom of the gradient, or because these proteins did not migrate into the gradient. A minor proportion of beta-casein and lactoferrin co-localized with EV markers. Whether this denotes small-scale contamination of the EV fractions with these proteins or whether a small amount of these proteins is physically associated with EV is currently unknown.
By using cryo-EM analysis, we demonstrated that the human breast milk EV population was very heterogeneous with regard to size, electron density, and Annexin V labeling. With regard to Annexin V labeling, which detects exposed phosphatidylserine (PS), EV were either highly positive or fully negative. This difference in apparent loss of phospholipid asymmetry in the lipid bilayer of these 2 subtypes of EV might reflect a difference in their biogenesis. Besides EV, lipid bilayer enclosed tubules were identified, which were markedly enriched in the 1.12–1.18 g/ml density fraction (), while being almost absent in the higher density (1.18–1.21 g/ml) EV-containing fractions and the lowest density fractions (1.10–1.04 g/ml, data not shown). These tubules were homogenous in diameter (~100 nm) and negative for Annexin V staining. Their smooth appearance suggests a lipid core, as striations typical for actin-like filaments were lacking. The tubes observed in human breast milk are distinct from tubular structures observed in platelet-free plasma (Citation46) and are currently under further investigation.
Storage of milk samples
Until now, most studies on human and bovine milk EV have utilized frozen or refrigerated unprocessed milk (Citation9–Citation12, Citation21–Citation24). Although the effects of sample storage on recovery of EV from blood plasma has been well documented (reviewed in 16), storage effects on EV in milk have not been studied in detail. We here showed that storage of unprocessed milk, especially at sub-zero temperature, led to massive death of milk cells. Spiking human breast milk with cells of murine origin allowed us to monitor the induction of storage-induced EV. Upon storage of spiked milk samples we found a large increase in the recovery of murine EV corresponding to an increase in murine cell death. These data indicated that storage-induced stress or death of cells in milk induced EV formation, thereby contaminating the pool of native milk EV. Despite thorough washing of the murine cell preparations used to spike milk samples, low levels of murine EV could also be recovered from fresh spiked milk samples. This could be explained by rapid stimulation-induced release of EV (Citation47) caused by mechanical stress imposed on cells during harvesting from the in vitro cultures. Alternatively, dying or dead cells present in the murine cell population at the time of spiking could have led to detectable contamination in the EV population.
In contrast to the predictable death of cells caused by freezing of milk samples, the large-scale cell death detected in milk stored at RT and 4°C was unexpected (a and b, Supplementary Fig. 2). This cell death may be caused by the action of multiple proteases and lipases, with bile-salt-stimulated lipase (BSSL) being one of the most prominent lipases in milk. It has been described that BSSL loses its dependency on bile salt at low temperature, rendering it constitutively active (Citation48, Citation49). This could cause cells in milk to lose their membrane integrity and die. Besides cells being a source of storage-induced EV, contamination of the EV population might also occur by storage-induced MFG fragmentation. Although the withdrawal, processing and storage of body fluids will always affect the fluid composition to a certain extent, these effects should be kept to a minimum to allow for accurate analysis of EV naturally present in body fluids. Previous studies demonstrated that it is difficult to discriminate EV from apoptotic bodies and cell debris based on protein profile. Although some proteins (e.g. histones) are more abundant in apoptotic bodies, a large number of proteins abundantly present in EV from healthy cells, such as MHC class II, also occur in apoptotic bodies and cell debris (Citation29). Together with our results, this emphasizes the necessity to remove potential sources of storage-induced EV, such as cells and MFG present in milk, as soon as possible after collection of the body fluid. The impact of our findings on the conclusions drawn in previous studies on stored breast milk samples is unknown. Some of the observed effects in these studies could have been due to cell debris, while other EV-specific effects could have been missed. In conclusion, the data presented here provide new insights into storage and isolation methods for breast milk EV. This knowledge can be used to further elucidate the role of breast milk EV in development of the infant immune system and for EV-based biomarker studies in breast milk.
Conflict of interest and funding
This research was performed as part of project 11676 within the framework of a partnership program jointly funded by Nutricia Research and the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research (NWO), and is partly funded by the Ministry of Economic Affairs.
Supplementary figure 1
Download PDF (163.4 KB)Supplementary figure 2
Download PDF (121.4 KB)Acknowledgements
We thank H. de Boer-Brouwer and M. Kleinjan for technical assistance, and Dr. P. Vos, Prof. Dr. W. Stoorvogel, and Dr. R. Wubbolts for valuable discussions.
Notes
To access the supplementary material to this article, please see Supplementary files under Article Tools online.
References
- Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol. 2004; 4: 565–72.
- Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr. 2005; 135: 1–4.
- Lawrence RM, Pane CA. Human breast milk: current concepts of immunology and infectious diseases. Curr Probl Pediatr Adolesc Health Care. 2007; 37: 7–36.
- M'Rabet L, Vos AP, Boehm G, Garssen J. Breast-feeding and its role in early development of the immune system in infants: consequences for health later in life. J Nutr. 2008; 138: 1782S–90.
- Mosconi E, Rekima A, Seitz-Polski B, Kanda A, Fleury S, Tissandie E et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 2010; 3: 461–74.
- Brandtzaeg P. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine. 2003; 21: 3382–8.
- Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008; 14: 170–5.
- Yamamoto T, Tsubota Y, Kodama T, Kageyama-Yahara N, Kadowaki M. Oral tolerance induced by transfer of food antigens via breast milk of allergic mothers prevents offspring from developing allergic symptoms in a mouse food allergy model. Clin Dev Immunol. 2012; 2012: 721085.
- Admyre C, Johansson SM, Qazi KR, Filen J-J, Lahesmaa R, Norman M et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007; 179: 1969–78.
- Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011; 9: 9.
- Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q et al. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. 2012; 8: 118–23.
- Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun. 2010; 396: 528–33.
- Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE. Bovine milk exosome proteome. J Proteomics. 2012; 75: 1486–92.
- Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011; 12: 1659–68.
- Nolte-‘t Hoen ENM, Wauben MHM. Immune cell-derived vesicles: modulators and mediators of inflammation. Curr Pharm Des. 2012; 18: 2357–68.
- Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013; 2 20360, doi: http://dx.doi.org/10.3402/jev.v2i0.20360.
- Heid HW, Keenan TW. Intracellular origin and secretion of milk fat globules. Eur J Cell Biol. 2005; 84: 245–58.
- Lonnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003; 77: 1537S–43.
- Nissen A, Bendixen E, Ingvartsen KL, Røntved CM. In-depth analysis of low abundant proteins in bovine colostrum using different fractionation techniques. Proteomics. 2012; 12: 2866–78.
- Sarkar A, Goh KKT, Singh RP, Singh H. Behaviour of an oil-in-water emulsion stabilized by β-lactoglobulin in an in vitro gastric model. Food Hydrocoll. 2009; 23: 1563–9.
- Torregrosa Paredes P, Gutzeit C, Johansson S, Admyre C, Stenius F, Alm J et al. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy. 2014; 69: 463–71.
- Näslund TI, Paquin-Proulx D, Paredes PT, Vallhov H, Sandberg JK, Gabrielsson S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS. 2014; 28: 171–80.
- Chen T, Xi Q-Y, Ye R-S, Cheng X, Qi Q-E, Wang S-B et al. Exploration of microRNAs in porcine milk exosomes. BMC Genomics. 2014; 15: 100.
- Gu Y, Li M, Wang T, Liang Y, Zhong Z, Wang X et al. Lactation-related microRNA expression profiles of porcine breast milk exosomes. PLoS One. 2012; 7: e43691.
- Lawrence R. Storage of human milk and the influence of procedures on immunological components of human milk. Acta Paediatr. 2007; 88: 14–18.
- Ogundele MO. Techniques for the storage of human breast milk: implications for anti-microbial functions and safety of stored milk. Eur J Pediatr. 2000; 159: 793–7.
- Garza C, Johnson CA, Harrist R, Nichols BL. Effects of methods of collection and storage on nutrients in human milk. Early Hum Dev. 1982; 6: 295–303.
- Pittard WB, Bill K. Human milk banking: effect of refrigeration on cellular components. Clin Pediatr (Phila). 1981; 20: 31–3.
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001; 166: 7309–18.
- Lutz MB, Kukutsch N, Ogilvie AL, Rößner S, Koch F, Romani N et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999; 223: 77–92.
- Nolte-'t Hoen ENM, van der Vlist EJ, de Boer-Brouwer M, Arkesteijn GJA, Stoorvogel W, Wauben MHM. Dynamics of dendritic cell-derived vesicles: high-resolution flow cytometric analysis of extracellular vesicle quantity and quality. J Leukoc Biol. 2013; 93: 395–402.
- Nolte-'t Hoen ENM, Buschow SI, Anderton SM, Stoorvogel W, Wauben MHM. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009; 113: 1977–81.
- Brisson A, Mornet S. Functionalization of gold nanoparticles with oriented proteins. Application to the high-density labelling of cell membranes. 2007. WO/2007/122259.
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200: 373–83.
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002; 2: 569–79.
- Khan S, Prime DK, Hepworth AR, Lai CT, Trengove NJ, Hartmann PE. Investigation of short-term variations in term breast milk composition during repeated breast expression sessions. J Hum Lact. 2013; 29: 196–204.
- Urwin HJ, Miles EA, Noakes PS, Kremmyda L-S, Vlachava M, Diaper ND et al. Salmon consumption during pregnancy alters fatty acid composition and secretory IgA concentration in human breast milk. J Nutr. 2012; 142: 1603–10.
- Yuhas R, Pramuk K, Lien EL. Human milk fatty acid composition from nine countries varies most in DHA. Lipids. 2006; 41: 851–8.
- Buschow SI, Nolte-'t Hoen ENM, van Niel G, Pols MS, ten Broeke T, Lauwen M et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009; 10: 1528–42.
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009; 9: 581–93.
- Van der Vlist EJ, Arkesteijn GJA, van de Lest CHA, Stoorvogel W, Nolte-'t Hoen ENM, Wauben MHM. CD4(+) T cell activation promotes the differential release of distinct populations of nanosized vesicles. J Extracell Vesicles. 2012; 1 18364, doi: http://dx.doi.org/10.3402/jev.v1i0.18364.
- Serru V, Le Naour F, Billard M, Azorsa DO, Lanza F, Boucheix C et al. Selective tetraspan-integrin complexes (CD81/alpha4beta1, CD151/alpha3beta1, CD151/alpha6beta1) under conditions disrupting tetraspan interactions. Biochem J. 1999; 340: 103–11.
- Yamada T, Inoshima Y, Matsuda T, Ishiguro N. Comparison of methods for isolating exosomes from bovine milk. J Vet Med Sci. 2012; 74: 1523–5.
- Famelart M-H, Chapron L, Piot M, Brulé G, Durier C. High pressure-induced gel formation of milk and whey concentrates. J Food Eng. 1998; 36: 149–64.
- Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012; 1 18397, doi: http://dx.doi.org/10.3402/jev.v1i0.18397.
- Arraud N, Linares R, Tan S, Gounou C, Pasquet J-M, Mornet S et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014; 12: 614–27.
- Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999; 163: 4564–73.
- Mehta NR, Jones JB, Hamosh M. Lipases in preterm human milk: ontogeny and physiologic significance. J Pediatr Gastroenterol Nutr. 1982; 1: 317–26.
- Gillin FD, Reiner DS, Gault MJ. Cholate-dependent killing of Giardia lamblia by human milk. Infect Immun. 1985; 47: 619–22.
