Abstract
Extracellular vesicles (EVs) are small vesicles released by donor cells that can be taken up by recipient cells. Despite their discovery decades ago, it has only recently become apparent that EVs play an important role in cell-to-cell communication. EVs can carry a range of nucleic acids and proteins which can have a significant impact on the phenotype of the recipient. For this phenotypic effect to occur, EVs need to fuse with target cell membranes, either directly with the plasma membrane or with the endosomal membrane after endocytic uptake. EVs are of therapeutic interest because they are deregulated in diseases such as cancer and they could be harnessed to deliver drugs to target cells. It is therefore important to understand the molecular mechanisms by which EVs are taken up into cells. This comprehensive review summarizes current knowledge of EV uptake mechanisms. Cells appear to take up EVs by a variety of endocytic pathways, including clathrin-dependent endocytosis, and clathrin-independent pathways such as caveolin-mediated uptake, macropinocytosis, phagocytosis, and lipid raft–mediated internalization. Indeed, it seems likely that a heterogeneous population of EVs may gain entry into a cell via more than one route. The uptake mechanism used by a given EV may depend on proteins and glycoproteins found on the surface of both the vesicle and the target cell. Further research is needed to understand the precise rules that underpin EV entry into cells.
Extracellular vesicles (EVs) are small spherical packages that are released by cells into the extracellular environment (Citation1). EVs consist of a lipid bilayer membrane that encases a small organelle-free cytosol. Suspended in the aqueous core, or associated with the lipid casing, are proteins and nucleic acids derived from the cell of origin (Citation2, Citation3). EVs can be categorized further depending upon where in the cell they originate; for example, vesicles that are derived from multi-vesicular bodies (MVBs) are referred to as exosomes and those from the plasma membrane as microvesicles (Citation1). It has become evident that EVs are important factors involved in a range of physiological processes including intercellular exchange of proteins and RNA (Citation4, Citation5), induction of angiogenesis (Citation6), bystander effect (Citation7) and immune regulation (Citation2, Citation8) (Citation9).
EVs protect their cargo from enzymatic degradation during transit through the extracellular environment (Citation10–Citation12). Upon release of their functionally active mRNA and microRNA load inside the recipient cell, EV contents can regulate gene expression through de-novo translation and post-translational regulation of target mRNAs (Citation3). Changes in miRNA levels are particularly important during development (Citation13) and stress response (Citation14), and EVs may play a role in their exchange between cells (Citation7). EVs can also exert effects on cells by stimulating specific signalling pathways (Citation15). The ability of EVs to alter the transcriptome and signalling activity within recipient cells allows them to induce specific phenotypic changes (Citation16–Citation18). Indeed, alterations in EV activity may be a feature of certain pathologies, including cancer (Citation19). There is also interest in EVs as potential therapeutics. By harnessing the capability of EVs to transfer their contents into target cells it may be possible to convert these vesicles into vehicles for the delivery of therapeutic proteins, RNA molecules and drugs (Citation20, Citation21). Given their emerging roles in normal physiological processes and in disease, and their therapeutic potential, it is important to understand the molecular mechanisms of EV release by donor cells and the processes by which they are taken up by recipient cells. In this review we will outline the ways in which EV uptake can be studied and review the current understanding of how EVs enter target cells.
Evidence for EV uptake
Both direct and indirect evidence exists to suggest that EVs are internalized into recipient cells. EVs have been shown to transfer functional mRNA and miRNA from mouse to human mast cells where mouse proteins were identified in the recipient human cells (Citation3). EV-mediated siRNA delivery has been shown to knockdown target gene expression (Citation20), and administration of EVs laden with luciferin substrate to luciferase expressing cells resulted in production of bioluminescence (Citation22). These results imply that merging of the EV cytosol and the cytoplasmic compartment had occurred through membrane fusion at the plasma membrane or by uptake through other pathways followed by fusion with the endosomal membrane (Citation22).
EV uptake can also be visualized directly. The most common method for detecting EV uptake involves the use of fluorescent lipid membrane dyes to stain EV membranes. Examples of such dyes include PKH67 (Citation23–Citation29), PKH26 (Citation28),Citation30–(Citation32), rhodamine B (also known as R18) (Citation22),Citation33–(Citation38), DiI (Citation30, Citation33) (Citation39) and DiD (Citation40) which are lipophilic dyes. Membrane permeable chemical compounds are also used to stain EVs. These include carboxyfluorescein succinimidyl ester (CFSE) (Citation39–Citation46) and 5Citation6-carboxyfluorescein diacetate (CFDA) (Citation46). These compounds become confined to the cytosolic lumen and fluoresce as a consequence of esterification. Subsequent entry of EVs into recipient cells can be measured using methods such as flow cytometry and confocal microscopy. To distinguish between internalized and surface-bound fluorescent EVs, the surface of the cell can be stripped by treatment with acid (Citation27) or trypsin (Citation32). Such experiments suggest that many cells do indeed internalize EVs.
One potential issue with membrane-binding dyes is that the presence of the fluorescent molecules could affect the normal behaviour of EVs. Uptake of EVs has been observed with many different lipid-binding dyes, suggesting that such molecules do not affect the coarse internalization of vesicles; nevertheless, further experimentation is needed to verify whether the precise biological behaviour of EVs is affected by dyes. Another consideration is that the distinct types of dyes may lead to different patterns of cellular staining following uptake. Lipophilic dyes associate with lipids, whereas molecules that become esterified in the lumen remain in solution; their fate is thus bound with membrane and cytosol, respectively. Another potential limitation of the use of such lipophilic dyes is the leaching of the fluorescent molecules from EV onto cellular membranes, leading to a pattern of internalization that is due to normal membrane recycling rather than EV uptake. However, this seems unlikely given the numerous reports of molecular inhibitors that appear to prevent EV uptake (Table ). Other control experiments, such as incubation of cells with excess unlabelled EVs and direct measurement of the rate of transfer of fluorescence between EVs also support the idea that the increased fluorescence in recipient cells is due to specific uptake of EVs rather than non-specific dye leaching (Citation25).
Table I. Compounds, chemicals and peptides used to inhibit EV uptake
It should also be appreciated, however, that almost all studies have relied on fluorescence microscopy, which has limited resolution because the wavelength of visible light is approximately 390–700 nm; therefore single EVs or clusters of vesicles that are less than 390 nm in diameter cannot be distinguished. This should not affect the assessment of EV uptake in general but may affect the visualization and dynamic localization analysis of individual EVs. EVs can be visualized in a potentially more specific way via the use of fluorescent proteins fused with vesicular proteins. For example, CD9 and CD63 are tetraspanin proteins found enriched in EVs which, when tagged with GFP, can be used to show uptake and processing of vesicles in cells (Citation10, Citation47) (Citation48). A caveat of such experiments is the assumption the fluorescent protein tag does not affect the normal function or trafficking of the tetraspanin protein, and does not therefore potentially alter the behaviour of the EV during uptake.
The evidence that EVs can enter cells and deliver their cargo is overwhelming. The mechanism responsible for EV internalization into cells, however, has raised great debate in the literature. Various mechanisms for EV uptake have been proposed (), including clathrin-mediated endocytosis (CME), phagocytosis, macropinocytosis and plasma or endosomal membrane fusion. The roles of lipid rafts and specific protein–protein interactions have also been studied. A range of techniques can be used in conjunction with EV uptake assays to tease out these molecular mechanisms. This includes the use of antibodies to test the role of specific ligands or receptors, and the use of chemical inhibitors to block specific uptake pathways. The results of such studies have shed much light on the routes by which EVs enter cells.
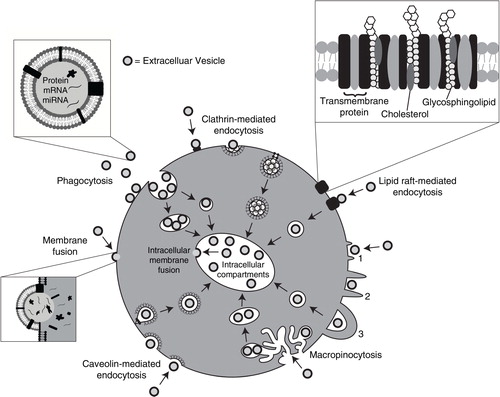
Fig. 1. Pathways shown to participate in EV uptake by target cells. EVs transport signals between cells. EVs have been shown to be internalized by cells through phagocytosis, clathrin- and caveolin-mediated endocytosis. There is also evidence to support their interaction with lipid rafts resulting in EV uptake. Lipid rafts are involved in both clathrin- and caveolin-mediated endocytosis. EVs can be internalized by macropinocytosis where membrane protrusions or blebs extend from the cell, fold backwards around the EVs and enclose them into the lumen of a macropinosome; alternatively EVs are macropinocytosed after becoming caught in membrane ruffles. EVs may also deliver their protein, mRNA and miRNA cargo by fusion with the plasma membrane. Alternatively, intraluminal EVs may fuse with the endosomal limiting membrane following endocytosis to enable their EV contents to elicit a phenotypic response.
Protein interactions
The EV uptake mechanisms involve protein interactions that facilitate subsequent endocytosis (Citation9, Citation25) (Citation26, Citation30) (Citation49). Proteinase K treatment of EVs was shown to significantly reduce their uptake by ovarian cancer cells which strongly supports the role of proteins in the EV uptake pathway (Citation41). Many EV proteins have been shown to interact with membrane receptors on target cells (Citation50, Citation51). Hence, EV uptake is most likely dependent upon the signalling status of recipient cells and of the protein complement of the vesicle. In the literature there is a growing list of specific protein–protein interactions that mediate EV attachment and uptake into cells. Many of these interactions have been elucidated by the use of specific antibodies that recognize ligands or receptors, leading to a steric block that prevents their interaction. Here we review some of the proteins shown to participate in EV uptake. It should be noted that in some cases the phenotypic effects of EVs do not require internalization of the vesicle (see conclusions and future directions); therefore in our discussion we focus on examples where the involvement of specific protein–protein interactions is evidenced by the effect on direct EV uptake or binding, rather than by functional outputs.
Tetraspanins
Tetraspanins are membrane proteins which have numerous functions including cell adhesion, motility, activation and proliferation (reviewed in (Citation52)). Tetraspanins are highly abundant on the EV surface which suggests they may have a role in EV function (Citation53, Citation54). CD63, CD9 and CD81 are well-established markers of EVs (Citation2, Citation54–Citation58). CD9 and CD81 are tetraspanins involved in oocyte-spermatozoa and phagocyte fusion (Citation56, Citation57) (Citation59, Citation60); in addition, numerous viruses and parasites require interaction with tetraspanins in order to enter the cell and replicate (Citation61). Due to the high abundance of tetraspanins and their roles in cell adhesion it is possible that EV uptake could occur through similar processes (Citation37). Tetraspanin-enriched microdomains (TEMs) are clusters of tetraspanins, adhesion molecules and transmembrane receptor proteins located in raft-like structures in the plasma membrane (Citation52). TEMs have been shown to be involved in a number of processes, including vesicular and cellular fusion (Citation52, Citation62) (Citation63), leading to the hypothesis that they have a role in EV-cell binding (Citation64–Citation67).
Treatment of recipient cells with antibodies against the tetraspanins CD81 or CD9 can reduce uptake of EVs by dendritic cells (Citation30). Tspan8 is a tetraspanin known to complex with integrins (Citation35). Cells over-expressing Tspan8 released EVs bearing a Tspan8-CD49d complex, the presence of which contributed to EV uptake by rat aortic endothelial cells (Citation35). Antagonistic antibody treatment suggests that CD106 strengthens this interaction (Citation35). In addition, it was discovered that EVs that presented Tspan8-CD49d complex on their surface were readily internalized by endothelial cells and pancreatic cells where intercellular adhesion molecule 1 (ICAM-1, also known as CD54) was the major ligand (Citation37). These data point to a role for tetraspanins in the internalization of EVs.
Integrins and immunoglobulins
The observation that EVs play a role in the immune response has garnered much interest in the roles of integrins and immunoglobulins in the interaction between vesicles and cells. These proteins are involved in a range of functions, including cell-to-cell adhesion, cell signalling, leukocyte transendothelial transmigration and antigen presentation (Citation68). Indeed, several reports in the literature suggest they may also be involved in EV uptake. Antibodies that mask the binding sites of CD11a or its ligand ICAM-1 can reduce dendritic cell uptake of EVs (Citation30). Similar results were observed after blocking the integrins αv (CD51) and β3 (CD61) on the dendritic cell surface (Citation30). CD11a is a subunit of the lymphocyte function-associated antigen 1 (LFA-1), which interacts with ICAM-1 to regulate critical pathways in the immune response (Citation69). Inducing a high-affinity state of LFA-1 on resting T-cells using manganese chloride treatment was sufficient to increase EV binding in a dose-dependent manner (Citation70). Antagonistic antibody treatment inhibited this process (Citation44, Citation45) (Citation70). Naïve T-cells have been shown to internalize EVs through a mechanism requiring the participation of T-cell receptor (TCR), CD28 and LFA-1 (Citation71). It was also shown that dendritic cell–derived EVs were internalized via TCR-major histocompatibility complex (MHC) and LFA-1–ICAM-1 interactions in CD4+ cells (Citation44). Similarly dendritic cells take up CD8+T-cell EVs in an endocytic pathway that requires pMHC I/TCR and LFA-1-ICAM-1 interactions (Citation43). The protein milk fat globule-epidermal growth factor 8 (MFG-E8) is thought to enhance the phagocytic uptake of apoptotic cells by binding phosphatidylserine (PS) via 1 domain and with cell surface integrin proteins CD51 and CD61 via a second domain (Citation72). Perturbation of MFG-E8 leads to alterations in the rate of EV uptake (Citation30). These results highlight the emerging roles of protein–protein interactions in vesicle uptake, particularly in cells of the immune system.
A role for proteoglycans
Proteoglycans are proteins with significant carbohydrate components. For example, the heparin sulphate proteoglycans (HSPGs) are proteins with sulphated glycosaminoglycan polysaccharides attached. Various complexes, including viral particles and lipoproteins, use HSPGs to help gain entry into cells (Citation73). Fluorescently labelled EVs co-localize with internal vesicles containing GFP-linked syndecan or glypican (the 2 main types of HSPG) inside recipient cells (Citation25). Treatment of cells with a heparin sulphate mimetic reduced EV uptake in a dose-dependent manner (Citation25, Citation32). Cells whose ability to produce normal levels and structures of HSPGs, either because of genetic defects or chemical inhibition, showed a reduced ability to internalize EVs (Citation25). These results are consistent with a role for proteoglycans in the uptake of EVs. Interestingly, treatment of EVs with heparinase to remove surface proteoglycans had no effect on uptake, suggesting that it is the presence of HSPGs on the cell surface that are important for mediating vesicular entry (Citation25).
Lectins
DC-SIGN is a C-type lectin receptor (a receptor able to recognize and internalize glycoprotein ligands) that can trigger phagocytic entry for a range of molecules, including viruses and bacteria (Citation74). One potential ligand is the MUC1 protein found on epithelial cells and on the surface of EVs derived from breast milk. The recruitment of these EVs by monocyte-derived dendritic cells was blocked by antibodies specific to DC-SIGN on the recipient cell surface (Citation29). Another C-type lectin, DEC-205, also appears to mediate entry of EVs into dendritic cells; vesicle uptake was inhibited by treatment with DEC-205-specific antibodies or by incubation with excess mannose (a sugar recognized by DEC-205) (Citation44). Chelation of calcium with ethylenediaminetetraacetic acid (EDTA) significantly reduced EV uptake by dendritic cells (Citation44), and also in macrophages (Citation24); supporting the hypothesis that the EV uptake is facilitated by C-type lectin/C-type lectin receptor interactions. Interestingly, ovarian cancer–derived EVs were found to be enriched in specific mannose- and sialic acid containing glycoproteins (Citation41). Sialic acid removal caused a small but non-significant increase in uptake (Citation41). Galectin-5, a lectin with binding specificity towards certain glycoproteins, can be found associated with EVs (Citation24). Incubating EVs with cells in the presence of excess galectin-5 significantly reduced vesicular internalization (Citation24). When asialofetuin (a glycoprotein that can bind galectin-5) was included in the uptake assay the entry of EVs was abrogated (Citation24). These results are all consistent with a role for glycoproteins and proteoglycans in the uptake of EVs.
Endocytosis
Most experimental evidence suggests that EVs are usually taken up into endosomal compartments via endocytosis (Citation22, Citation30) (Citation41). Uptake can be extremely rapid, with EVs being identified inside cells from as early as 15 minutes after initial introduction (Citation27, Citation48). A number of research groups have shown that when cells are incubated at 4°C their capacity to internalize EVs is dramatically reduced suggesting that uptake is an energy-requiring process (Citation25, Citation30) (Citation33, Citation38) (Citation41, Citation46). Further evidence that internalization is not a passive process is provided by observations that EVs are not taken up by cells fixed in paraformaldehyde (Citation28, Citation36). Cytochalasin D is a metabolite known to depolymerize the actin filament network resulting in inhibition of endocytic pathways (Citation75, Citation76). Cytochalasin D treatment has been shown, on several occasions in various cell types, to significantly reduce, but not completely prevent, EV uptake in a dose dependent manner (Citation22, Citation23) (Citation26, Citation27) (Citation28, Citation30) (Citation41, Citation44) (Citation77) . Taken together these results suggest that EV uptake is an energy-dependent process that requires a functioning cytoskeleton, both of which are indicative of endocytic pathways. However, one implication of the frequent failure to completely abrogate internalization following treatment with any given inhibitor is that EV uptake occurs through more than one mechanism (Citation24, Citation27) (Citation30, Citation40) (Citation41, Citation44) (Citation77).
Endocytosis is an umbrella term for a range of molecular internalization pathways (Citation78). By using a range of inhibitors to block specific pathways (Table ), antibodies to prevent receptor–ligand interactions (Table ) and other experimental techniques such as RNAi to knockdown certain genes the role of the endocytic processes responsible for EV uptake are being elucidated. Roles for many of these subdivisions have been shown, including macropinocytosis (Citation28, Citation41), phagocytosis (Citation22, Citation27) (Citation41, Citation82) and CME (Citation41) ().
Table II. Antibodies used to block EV uptake
Clathrin-mediated endocytosis
CME involves cellular internalization of molecules through progressive and sequential assembly of clathrin-coated vesicles that contain a range of transmembrane receptors and their ligands. The clathrin-coated vesicles strategically deform the membrane which collapses into a vesicular bud, matures and pinches off. The subsequent intracellular vesicle undergoes clathrin un-coating and then fuses with the endosome where it deposits its contents (Citation83). Various studies implicate CME in the uptake of EVs. Chlorpromazine prevents formation of clathrin-coated pits at the plasma membrane (Citation84). CME inhibition by chlorpromazine decreased uptake of EVs by ovarian cancer recipient cells (Citation41) and phagocytic recipient cells (Citation27). Dynamin2 is a GTPase required for the CME process (Citation85, Citation86). Dynamin2 is recruited to nascent clathrin-coated pits where it forms a collar-like structure at the neck of deeply invaginated clathrin-coated pits (Citation87–Citation89). GTP-hydrolysis mediated changes in dynamin2 conformation lead to membrane fission and clathrin-coated vesicle release (Citation86, Citation90–Citation92). Dynamin2 also facilitates membrane binding (Citation93–Citation95) and membrane curvature (Citation96) during CME. In phagocytic cells, inhibition of dynamin2 (Citation85, Citation86) prevented almost all EV internalization activity (Citation24, Citation27) (Citation28). A small percentage of EVs were also shown to co-localize with clathrin during uptake in macrophages (Citation27). Epidermal growth factor receptor pathway substrate clone 15 (EPS15) is a component of clathrin-coated pits that is ubiquitously associated with AP-2 adaptor complex which is an integral component of the clathrin coat (Citation97). Expression of a dominant-negative mutant of EPS15 inhibits CME and leads to a reduction in EV uptake (Citation27). These results suggest that CME plays at least some part in EV uptake.
Caveolin-dependent endocytosis
CME has been extensively studied for many years, but it is becoming increasingly apparent that a plethora of clathrin-independent endocytotic pathways exist in eukaryotic cells (Citation78). One such mechanism is caveolin-dependent endocytosis (CDE). Caveolae are small cave-like invaginations in the plasma membrane which, like clathrin-coated pits, can become internalized into the cell. Caveolae are sub-domains of glycolipid rafts of the plasma membrane that are rich in cholesterol, sphingolipids and caveolins; hence, CDE is sensitive to cholesterol depletion agents such as filipin, and methyl-β-cyclodextrin (MβCD) (Citation98–Citation100). Caveolin-1 is a protein that is required and sufficient for the formation of caveolae (Citation78) and can be found clustered within such membrane invaginations. Oligomerization of caveolins (facilitated by caveolin oligomerization domains) mediates formation of caveolin-rich rafts in the plasma membrane. The increased levels of cholesterol accompanied by attachment of caveolin scaffolding domains to the plasma membrane and dynamin2 (also required in CME) activity enable assembly and expansion of caveolar endocytic vesicles (Citation78, Citation100) (Citation101). Dynamin2, can be blocked by the specific inhibitor dynasore (Citation102). Blocking dynamin2 leads to significantly reduced internalization of exosomes (Citation103) or larger microvesicles (Citation24, Citation104), suggesting a role for caveolae-mediated endocytosis in vesicular uptake. However, because dynamin2 is also required for CME it is not possible to rule out a role for clathrin-coated vesicles in these experiments (Citation105). Specific knockdown of the CAV1 gene leads to reduced caveolin-1 protein and significantly impaired uptake of EVs (Citation103). Paradoxically, knockout of CAV1 in mouse embryonic fibroblast cells leads to increased EV uptake (Citation26). CAV1 null mice show phenotypic changes in vasculature but are viable (Citation106), suggesting that either CAV1 is not essential for full EV uptake or that the ability to internalize vesicles via CDE is not essential for viability. Nevertheless, taken together the results described above imply some kind of function for caveolae-mediated endocytosis in EV uptake, though the precise role of this pathway may vary between cell and EV types.
Macropinocytosis
Macropinocytosis is an endocytic uptake pathway that involves the formation of invaginated membrane ruffles that then pinch off into the intracellular compartment. The vesicles carry extracellular fluid and components sampled from the region around the membrane ruffles (Citation78). Ruffled extensions of the plasma membrane protrude from the cell surface and encompass an area of extracellular fluid, subsequently this area of extracellular fluid is internalized entirely as a result of fusion of the membrane protrusions with themselves or back with the plasma membrane () (Citation107). The mechanism is similar to that of phagocytosis, however, direct contact with the internalized material is not required. This mechanism is rac1-, actin- and cholesterol-dependent and requires Na +/H+ exchanger activity (Citation108). Cholesterol is required for the recruitment of activated rac1 to sites of macropinocytosis (Citation109). Phosphatidylinositol-3-kinase (PI3K), ras, and src activities have also been shown to stimulate macropinocytosis (Citation78). Rac1 is a GTPase which not only has a major role in macropinocytosis (Citation110), but also in regulation of cell growth, cytoskeletal reorganization, protein kinase activation (Citation111) and epithelial-to-mesenchymal transition (EMT) in cancer (Citation112). Abrogation of macropinocytosis by inhibiting the Na+/H+ exchanger results in significantly reduced oligodendrocyte-derived EV uptake by microglia (Citation28). A small molecule inhibitor of rac1, NSC23766, also inhibited EV uptake by microglia (Citation28). The alkalinizing drugs bafilomycin A, monensin and chloroquine all inhibited microglial internalization of EVs, consistent with a role for the acidification of vacuoles in macropincytosis (Citation28). However, other studies using inhibitors do not find a role for macropinocytosis in the uptake of EVs (Citation25, Citation27) (Citation103). These findings suggest that macropinocytosis is either a minor pathway used by cells to internalize EVs, or a mechanism used in specific cell types.
Phagocytosis
The process of phagocytosis involves the internalization of opsonized particulate matter, including bacteria and fragments of apoptotic cells. This function is often performed by specialized cells such as macrophages (Citation78). Phagocytosis is a receptor-mediated event that involves the progressive formation of invaginations surrounding the material destined for internalization, with or without the participation of enveloping membrane extensions (as required for macropinocytosis) (Citation78, Citation107). Generally, phagocytosis is employed to internalize larger particles. However, it has been shown that particles as small as 85 nm in diameter have been internalized by phagocytosis; therefore it is possible that EVs could be internalized via this route (Citation113). In one study the EVs released by leukaemia cells were shown to be taken up efficiently by macrophages but were not internalized by other cell types (Citation27). PI3Ks play an important role in phagocytic processes, particularly in enabling membrane insertion into forming phagosomes (Citation114). The PI3K inhibitors wortmannin and LY294002 were used to assess the necessity of functional PI3Ks in EV uptake. Both drugs inhibited EV uptake in a dose dependant manner (Citation27). Furthermore, the EVs co-localized with fluorescent phagosome tracers (Citation27). Dendritic cell–derived EVs were labelled with pHrodo, a dye that becomes fluorescent red at the phagosome pH. Acceptor dendritic cells treated with pHrodo-EVs emitted red fluorescence confirming that dendritic cells can phagocytose EVs (Citation22). Taken together these results implicate phagocytosis in EV uptake.
Phosphatidylserine (PS) is essential in initiating the removal of apoptotic bodies by phagocytosis (Citation115) and is used by some viruses to enter cells by macropinocytosis (Citation116). PS is typically located on the inner leaflet of the plasma membrane; however, EVs are enriched for PS on their outer-membrane (Citation117) which may facilitate entry into cells. Incubation of macrophages with an antibody that masks TIM4, a receptor involved in PS-dependent phagocytosis (Citation27, Citation81), leads to reduced uptake of EVs (Citation27). Treatment of dendritic cells with a competitive soluble PS analogue also reduced EV uptake (Citation30). Treatment of EVs with annexin-V (a protein that binds PS) reduces the uptake of EVs into macrophages (Citation31) and natural killer cells (Citation70). Phagocytic and macropinocytic uptake of EVs may therefore be, at least in part, triggered by the PS found on the outer leaflet of EV membranes.
Involvement of lipid rafts
Lipid rafts are microdomains within the plasma membrane with altered phospholipid compositions. They are rich in protein receptors and sphingolipids such as sphingomyelin. They act as organizing centres for the assembly of signalling molecules, they affect membrane fluidity and mediate membrane protein trafficking (Citation100). Components of lipid rafts are highly ordered and more tightly packed than the surrounding bilayer, consequently they are less fluid but float freely in the plasma membrane (Citation118). Endocytosis that is clathrin-independent largely requires cholesterol, which is found enriched in lipid rafts. Lipid rafts are known to contribute to viral particle uptake by mediating glycoprotein binding and adjusting the physicochemical and mechanical properties of the membrane (Citation119). These rafts can be found in the invaginations formed by caveolin-1 or in planar regions of the plasma membrane marked by another family of proteins called flotillins (Citation120). Flotillins associate with lipid rafts and mediate endocytosis independent of clathrin and caveolin (Citation121–Citation125). Flotillins have been found to bind to GPI-anchored proteins during their internalization (Citation121, Citation122) (Citation125). It is possible that EVs are internalized by cells through lipid raft domains.
To test the role of lipid rafts in EV uptake, a range of inhibitors have been employed. EV uptake was significantly reduced in dendritic cells when EV-producing cells were pre-treated with fumonisin B1 and N-butyldeoxynojirimycin hydrochloride (Citation80), compounds which decrease glycosphingolipid composition in the plasma membrane by preventing its biosynthesis (Citation126, Citation127). This suggests that sphingolipids within the EV have an important role in binding and endocytosis, possibly through cholesterol-rich microdomains in dendritic cells (Citation80). EV uptake was prevented following pre-treatment of recipient cells with the cholesterol reducing agents MβCD (Citation26, Citation27) (Citation41, Citation47), filipin (Citation22, Citation26) (Citation34) and simvastatin (Citation26). These treatments disrupt lipid raft–mediated endocytosis but may also affect EV membrane integrity, causing the reduced EV uptake effects observed. Co-localization can be observed between fluorescently labelled EVs and cholera toxin B (a protein known to be internalized via lipid rafts) in recipient cells (Citation26, Citation47). Poor co-localization was observed between caveolin-1 and labelled EVs, suggesting that the lipid rafts used by EVs may be caveolae-independent (Citation26, Citation41). The potential roles of some proteins involved in this process are also being identified. For example, annexin II may have a role in anchoring of EVs to lipid raft domains of the plasma membrane whilst annexin-VI may contribute to the trafficking of EVs to the late endosomal compartment (Citation47). These findings support the hypothesis that lipid rafts are involved in the EV uptake mechanism; the scale and precise mechanisms of this route into cells remains to be elucidated.
Cell surface membrane fusion
Another possible entry mechanism is via direct fusion of the EV membrane with the cell plasma membrane (Citation34). Fusion of lipid bilayers in an aqueous environment is a process whereby 2 initially distinct membranes merge. The lipid bilayers are brought into close proximity and the outer-leaflets come into direct contact which leads to formation of a hemi-fusion stalk with fused outer-leaflets. Following this, stalk expansion produces the hemi-fusion diaphragm bilayer from which a fusion pore opens (Citation128–Citation130). As a result, the two hydrophobic cores mix forming one consistent structure. Several protein families participate in this process including SNAREs, Rab proteins, and Sec1/Munc-18 related proteins (SM-proteins) (Citation131).
Fusion of membranes can be observed in various ways, including via fluorescent lipid dequenching. This technique was applied to study the uptake of EVs from melanoma cells, the results suggested that at least some of the vesicles are able to fuse with the recipient cell Citation 34; this fusion was enhanced under acidic conditions. Similarly, when the dequenching method was used to demonstrate fusion of R18-labelled EVs with the plasma membrane of bone marrow-derived dendritic cells (Citation22). The delivery of miRNAs and luciferin to the cytosol of the recipient cells provides further evidence of fusion of EVs with either the plasma or endosomal membrane (Citation22). The lipid raft-like membrane composition of EVs may aid their fusion with recipient cell membranes (Citation132). EV-cell fusion may be limited to acidic pH conditions which are present in endosomes, perhaps owing to differences in lipid content or overall ionic charge of the EV surface following release (Citation34). The body of research supporting a primarily endocytic mechanism for EV uptake makes it unlikely that fusion is the main entry route, but a fusion-based pathway cannot be ruled out. Future work will be needed to ascertain the extent to which fusion-based EV entry occurs under physiological conditions.
Cell-specific EV uptake
One question currently vexing the EV field is whether or not EV uptake is a cell type–specific process. Results from some studies show that fluorescently labelled EVs can be taken up by virtually every cell type tested (Citation26, Citation38), whereas others suggest that vesicular uptake is a highly specific process which can only occur if cell and EV share the right combination of ligand and receptor. Heterogeneity in the donor/recipient cells, EVs, experimental setup and the context of experiments will all affect the outcome, and may thus account for the observed discrepancies. However, there are certainly examples of cell-type specific uptake. Pancreatic adenocarcinoma–derived EVs were shown to be internalized most efficiently by peritoneal exudate cells and less proficiently in granulocytes and T-cells (Citation38). Tspan8 containing lymph node stroma–derived EVs were most effectively internalized by endothelial cells and pancreatic cells but to a lower degree by parental lymph node stroma cells (Citation37). In some cases, the basis for a specific interaction may have been elucidated. For example, milk EVs can be taken up via monocyte-derived dendritic cells thanks to the interaction between DC-SIGN and MUC1, whereas EVs derived from other sources and lacking MUC1 were unable to enter these cells (Citation29). Treatment with an RGD peptide (which can block integrin-mediated receptor internalization) reduced EV uptake in dendritic cells (Citation30) and macrophages (Citation23), but did not inhibit EV uptake by microglia (Citation28). This indicates that multiple mechanisms are responsible for EV-cell communication and different combinations of EV communication strategies are used by different cell types.
Conclusions and future directions
A growing body of evidence suggests that EVs are involved in normal homeostasis (Citation133) and are deregulated in disease (Citation51). EVs, which are released in greater numbers by cancer cells (Citation134), can promote tumour development and are involved in mediating intercellular communication within the tumour microenvironment (Citation135). Further advancing our understanding of both the EV uptake mechanism and characterization of disease-promoting EVs will enable development of therapeutic strategies to inhibit interactions between such EVs and healthy recipient cells (Citation136). EVs are also being explored as natural vectors for therapeutic delivery, with some early studies showing great potential (Citation20, Citation137) (Citation138). Improved understanding of the EV uptake mechanism will therefore benefit design of novel and sophisticated drug delivery systems.
To this end, we have reviewed here the mechanisms by which EVs are internalized by cells. Endocytosis, in its various guises, appears to be the primary method of entry by EVs. There appears to be little agreement in the literature as to which type of endocytic mechanisms are most important, with clathrin-dependent, caveolae-dependent, macropinocytosis, phagocytosis and lipid raft–mediated uptake variously described as being prevalent. These differences reflect the heterogeneity both in EV populations and in the cell types being used. It is possible that a population of EVs can simultaneously trigger a number of different gateways into a cell, with the primary entry points depending on the cell type and EV constituents (Citation29, Citation37) (Citation38). This would also explain why inhibition of any given pathway rarely leads to a complete abrogation of EV entry (Citation23, Citation24) (Citation25, Citation27) (Citation34, Citation38) (Citation40, Citation41) (Citation42, Citation44) (Citation45, Citation81) . There are also potential problems with the use of some inhibitors that have known (or potentially unknown) cross-reactivity with multiple pathways. For example, cytochalasin D inhibits actin polymerization and so the finding that it reduces EV uptake has been used to support an endocytic uptake pathway for vesicles (Citation139). However, the global cellular effects of disrupting the actin cytoskeleton are profound. The reduced EV uptake caused by cytochalasin D could therefore be caused indirectly via perturbation of other cellular processes, such as cellular polarization (Citation140), migration or cell cycle (Citation141). Similarly, PI3K has a multitude of roles within various cell-signalling networks (Citation142). PI3K signalling has been implicated in phagocytic uptake (Citation143). The reduced EV uptake observed following treatment of recipient cells with the PI3K inhibitors wortmannin and LY294002 could be independent of any effects on phagocytosis, and instead caused indirectly by the deregulation of other processes such as migration, cell growth, or motility (Citation144, Citation145). There is also overlap in the pathways that such inhibitors can affect. For example, inhibiting PI3K can perturb both phagocytosis and macropinocytosis (Citation146). Dynamin2 is involved in both CME and CDE, so its inhibition cannot easily distinguish between these 2 processes (Citation147, Citation148). MβCD causes depletion of cholesterol and can cause a decrease in both lipid raft–mediated uptake as well as caveolin-dependent internalization. Furthermore, MβCD has substantial effects across a range of cellular functions, including effects that are independent of cholesterol chelation (Citation149). The results of studies using small molecular inhibitors are important contributions towards understanding the EV uptake puzzle, but the pleiotropic nature of these compounds means that interpretation of such data must be tempered with extreme caution.
Large areas of plasma membrane are naturally recycled as part of normal cellular maintenance (Citation150). Indeed, an area equivalent to the entire surface of the cell can be internalized and replenished every few hours (Citation151). It could be expected that EVs bound at the cell surface would eventually be internalized as part of this normal membrane recycling. In such a scenario, it would stand to reason that inhibiting any of the processes that regulate membrane recycling would also reduce EV uptake. The results of the small molecule inhibitor experiments described above (Citation28) could therefore be explained by their effects on membrane recycling rather than by their proposed ability to affect a direct EV uptake pathway. However, the rapidity of EV uptake would argue against this model of “passive endocytosis.” The ability to inhibit uptake with antibodies that block specific protein–protein interactions also suggest that internalization is an active process. Nevertheless, these arguments all highlight the complexities in studying vesicular uptake. It is also worth mentioning that there are several other clathrin-independent endocytic pathways, such as the recently described CLIC/GLEEC pathway (Citation78, Citation152). The extent to which these other pathways may be involved remains to be determined.
Whilst EV uptake leads to the delivery of nucleic acids and protein, internalization is not always necessary to elicit a phenotypic response. Receptor–ligand interactions which take place on the cell surface may be sufficient; for example, interaction of soluble ligands (produced by proteolytic cleavage of EV membrane proteins) with cell receptors may successfully permit signal transduction and subsequent downstream signalling effects in the recipient cell (Citation153–Citation155). In another study, uptake of EVs by phagocytosis was not actually essential for the induction of cytokine IL-1β secretion suggesting that EV-associated fibronectin surface receptor interaction is sufficient to direct this activity (Citation23). Interestingly, EVs may stimulate MAPK signalling leading to altered activity within the recipient cell (Citation25, Citation104). Indeed, pharmacological inhibition of ERK1/2 actually inhibited EV uptake, suggesting that these signalling pathways may also be involved in EV uptake (Citation25).
In this review, we have focused on the mechanisms of exosomal uptake. However, there exists a range of types and sizes of EV. Many preparations of vesicles used in studies contain heterogeneous collections of such vesicles. This heterogeneity probably contributes to the differences in apparent internalization mechanisms observed in various studies, as well as the lack of a single clear uptake route in any given study. Our ability to pinpoint the uptake route for different vesicles of similar sizes is still limited by a lack of biochemical markers to characterize and isolate them. As this knowledge increases the means by which they enter cells will be unravelled.
Understanding of EV internalization is a key goal of the fledgling EV field. Despite the EV research field still being in its infancy and the limited number of relevant studies performed to date, the discoveries concerning EV uptake made so far are promising for future research. The potential that EVs have shown as therapeutic agents means that it is imperative that the EV uptake mechanism is understood to aid prospective therapeutic design. Excitingly, EV research is continually expanding and developing, therefore greater understanding of the EV uptake pathway is certainly achievable in the foreseeable future.
Conflict of interest and funding
The authors declare that they have no relevant conflicts of interest. LAM is supported by funding from Oxford Brookes University. DRFC is supported by grants from The Royal Society and the Cancer and Polio Research Fund.
Acknowledgements
We thank members of the lab for critical reading of the manuscript. We also thank the anonymous reviewers for their suggestions that have helped to improve this article.
References
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200: 373–83.
- Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999; 147: 599–610.
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9: 654–9.
- Jung T, Castellana D, Klingbeil P, Cuesta Hernández I, Vitacolonna M, Orlicky DJ et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009; 11: 1093–105. [PubMed Abstract] [PubMed CentralFull Text].
- Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y, Hou KZ et al. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis. 2009; 41: 875–80.
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005; 113: 752–60.
- Al-Mayah AH, Irons SL, Pink RC, Carter DR, Kadhim MA. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat Res. 2012; 177: 539–45.
- Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003; 33: 522–31.
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996; 183: 1161–72.
- Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011; 9: 86.
- Silverman JM, Clos J, de'Oliveira CC, Shirvani O, Fang Y, Wang C et al. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 2010; 123: 842–52.
- Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009; 113: 1957–66.
- Tang F, Kaneda M, O'Carroll D, Hajkova P, Barton SC, Sun YA et al. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007; 21: 644–8.
- Jacobs LA, Bewicke-Copley F, Poolman MG, Pink RC, Mulcahy LA, Baker I et al. Meta-analysis using a novel database, miRStress, reveals miRNAs that are frequently associated with the radiation and hypoxia stress-responses. PLoS One. 2013; 8: e80844.
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010; 73: 1907–20.
- Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010; 78: 838–48.
- Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010; 38: 233–45.
- Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009; 81: 717–29.
- Ogorevc E, Kralj-Iglic V, Veranic P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol Oncol. 2013; 47: 197–205.
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011; 29: 341–5.
- Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012; 3: 1282.
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012; 119: 756–66.
- Atay S, Gercel-Taylor C, Taylor DD. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1β production by macrophages. Am J Reprod Immunol. 2011; 66: 259–69.
- Barrès C, Blanc L, Bette-Bobillo P, André S, Mamoun R, Gabius HJ et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010; 115: 696–705.
- Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013; 110: 17380–5.
- Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signalling and lipid raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013; 288: 17713–24.
- Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010; 11: 675–87.
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011; 124: 447–58.
- Näslund TI, Paquin-Proulx D, Paredes PT, Vallhov H, Sandberg JK, Gabrielsson S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+T cells. AIDS. 2014; 28: 171–80.
- Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004; 104: 3257–66.
- Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J Biol Chem. 2012; 287: 10977–89.
- Franzen CA, Simms PE, Van Huis AF, Foreman KE, Kuo PC, Gupta GN. Characterization of uptake and internalization of exosomes by bladder cancer cells. Biomed Res Int. 2014; 2014: 619829.
- Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2012; 228: 1487–95.
- Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009; 284: 34211–22.
- Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010; 70: 1668–78.
- Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi). Gut. 2012; 61: 1330–9.
- Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012; 44: 1574–84.
- Zech D, Rana S, Büchler MW, Zöller M. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2012; 10: 37.
- Obregon C, Rothen-Rutishauser B, Gitahi SK, Gehr P, Nicod LP. Exovesicles from human activated dendritic cells fuse with resting dendritic cells, allowing them to present alloantigens. Am J Pathol. 2006; 169: 2127–36.
- Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010; 111: 488–96.
- Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011; 11: 108.
- Keller S, König AK, Marmé F, Runz S, Wolterink S, Koensgen D et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009; 278: 73–81.
- Xie Y, Zhang H, Li W, Deng Y, Munegowda MA, Chibbar R et al. Dendritic cells recruit T cell exosomes via exosomal LFA-1 leading to inhibition of CD8+CTL responses through downregulation of peptide/MHC class I and Fas ligand-mediated cytotoxicity. J Immunol. 2010; 185: 5268–78.
- Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007; 120: 90–102.
- Nanjundappa RH, Wang R, Xie Y, Umeshappa CS, Chibbar R, Wei Y et al. GP120-specific exosome-targeted T cell-based vaccine capable of stimulating DC- and CD4(+) T-independent CTL responses. Vaccine. 2011; 29: 3538–47.
- Temchura VV, Tenbusch M, Nchinda G, Nabi G, Tippler B, Zelenyuk M et al. Enhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virus. Vaccine. 2008; 26: 3662–72.
- Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS One. 2011; 6: e24234.
- Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012; 109: E2110–16.
- Tumne A, Prasad VS, Chen Y, Stolz DB, Saha K, Ratner DM et al. Noncytotoxic suppression of human immunodeficiency virus type 1 transcription by exosomes secreted from CD8+ T cells. J Virol. 2009; 83: 4354–64.
- Rana S, Zöller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011; 39: 559–62.
- Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014; 1841: 108–20.
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005; 6: 801–11.
- Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009; 9: 40–55.
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998; 273: 20121–7.
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999; 94: 3791–9. [PubMed Abstract].
- Rubinstein E, Ziyyat A, Prenant M, Wrobel E, Wolf JP, Levy S et al. Reduced fertility of female mice lacking CD81. Dev Biol. 2006; 290: 351–8.
- Zhu GZ, Miller BJ, Boucheix C, Rubinstein E, Liu CC, Hynes RO et al. Residues SFQ (173–175) in the large extracellular loop of CD9 are required for gamete fusion. Development. 2002; 129: 1995–2002. [PubMed Abstract].
- Helming L, Gordon S. The molecular basis of macrophage fusion. Immunobiology. 2007; 212: 785–93.
- Rubinstein E, Ziyyat A, Wolf JP, Le Naour F, Boucheix C. The molecular players of sperm-egg fusion in mammals. Semin Cell Dev Biol. 2006; 17: 254–63.
- Takeda Y, Tachibana I, Miyado K, Kobayashi M, Miyazaki T, Funakoshi T et al. Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol. 2003; 161: 945–56.
- Thali M. The roles of tetraspanins in HIV-1 replication. Curr Top Microbiol Immunol. 2009; 339: 85–102. [PubMed Abstract] [PubMed CentralFull Text].
- Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005; 5: 136–48.
- Vjugina U, Evans JP. New insights into the molecular basis of mammalian sperm-egg membrane interactions. Front Biosci. 2008; 13: 462–76.
- Johnstone RM. Exosomes biological significance: a concise review. Blood Cells Mol Dis. 2006; 36: 315–21.
- Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008; 18: 199–209.
- Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008; 9: 871–81.
- Lebreton A, Séraphin B. Exosome-mediated quality control: substrate recruitment and molecular activity. Biochim Biophys Acta. 2008; 1779: 558–65.
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998; 50: 197–263. [PubMed Abstract].
- Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987; 51: 813–19.
- Nolte-‘t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009; 113: 1977–81.
- Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci U S A. 2003; 100: 6670–5.
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002; 417: 182–7.
- Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999; 99: 13–22.
- Garcia-Vallejo JJ, van Kooyk Y. The physiological role of DC-SIGN: a tale of mice and men. Trends Immunol. 2013; 34: 482–6.
- Flanagan MD, Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem. 1980; 255: 835–8. [PubMed Abstract].
- Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997; 272: 20332–5.
- Obregon C, Rothen-Rutishauser B, Gerber P, Gehr P, Nicod LP. Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway. Am J Pathol. 2009; 175: 696–705.
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009; 78: 857–902.
- Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003; 170: 3037–45.
- Izquierdo-Useros N, Naranjo-Gómez M, Archer J, Hatch SC, Erkizia I, Blanco J et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009; 113: 2732–41.
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007; 450: 435–9.
- Pasquetto MV, Vecchia L, Covini D, Digilio R, Scotti C. Targeted drug delivery using immunoconjugates: principles and applications. J Immunother. 2011; 34: 611–28.
- Kirchhausen T. Clathrin. Annu Rev Biochem. 2000; 69: 699–727.
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993; 123: 1107–17.
- Vallee RB, Herskovits JS, Aghajanian JG, Burgess CC, Shpetner HS. Dynamin, a GTPase involved in the initial stages of endocytosis. Ciba Found Symp. 1993; 176: 185–93. [PubMed Abstract] discussion 93–7.
- Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993; 122: 565–78.
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004; 118: 591–605.
- Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002; 4: 691–8.
- Taylor MJ, Lampe M, Merrifield CJ. A feedback loop between dynamin and actin recruitment during clathrin-mediated endocytosis. PLoS Biol. 2012; 10: e1001302.
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994; 127: 915–34.
- Marks B, Stowell MH, Vallis Y, Mills IG, Gibson A, Hopkins CR et al. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001; 410: 231–5.
- Chappie JS, Acharya S, Leonard M, Schmid SL, Dyda F. G domain dimerization controls dynamin's assembly-stimulated GTPase activity. Nature. 2010; 465: 435–40.
- Achiriloaie M, Barylko B, Albanesi JP. Essential role of the dynamin pleckstrin homology domain in receptor-mediated endocytosis. Mol Cell Biol. 1999; 19: 1410–15. [PubMed Abstract] [PubMed CentralFull Text].
- Lee A, Frank DW, Marks MS, Lemmon MA. Dominant-negative inhibition of receptor-mediated endocytosis by a dynamin-1 mutant with a defective pleckstrin homology domain. Curr Biol. 1999; 9: 261–4.
- Vallis Y, Wigge P, Marks B, Evans PR, McMahon HT. Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis. Curr Biol. 1999; 9: 257–60.
- Ramachandran R, Pucadyil TJ, Liu YW, Acharya S, Leonard M, Lukiyanchuk V et al. Membrane insertion of the pleckstrin homology domain variable loop 1 is critical for dynamin-catalyzed vesicle scission. Mol Biol Cell. 2009; 20: 4630–9.
- Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci. 1999; 112: 1303–11. [PubMed Abstract].
- Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998; 67: 199–225.
- Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999; 11: 424–31.
- Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003; 161: 673–7.
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007; 8: 185–94.
- Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2006; 103: 17955–60.
- Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013; 87: 10334–47.
- Menck K, Klemm F, Gross JC, Pukrop T, Wenzel D, Binder C. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget. 2013; 4: 2057–66. [PubMed Abstract] [PubMed CentralFull Text].
- Orth JD, Krueger EW, Cao H, McNiven MA. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc Natl Acad Sci U S A. 2002; 99: 167–72.
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001; 276: 38121–38.
- Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008; 9: 639–49.
- Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009; 10: 364–71.
- Grimmer S, van Deurs B, Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002; 115: 2953–62. [PubMed Abstract].
- Ahram M, Sameni M, Qiu RG, Linebaugh B, Kirn D, Sloane BF. Rac1-induced endocytosis is associated with intracellular proteolysis during migration through a three-dimensional matrix. Exp Cell Res. 2000; 260: 292–303.
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006; 16: 522–9.
- Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008; 135: 510–23.
- Rudt S, Müller RH. In vitro phagocytosis assay of nano- and microparticles by chemiluminescence. III. Uptake of differently sized surface-modified particles, and its correlation to particle properties and in vivo distribution. Eur J Pharm Sci. 1993; 1: 31–9.
- Stephens L, Ellson C, Hawkins P. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol. 2002; 14: 203–13.
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992; 148: 2207–16. [PubMed Abstract].
- Shiratsuchi A, Kaido M, Takizawa T, Nakanishi Y. Phosphatidylserine-mediated phagocytosis of influenza A virus-infected cells by mouse peritoneal macrophages. J Virol. 2000; 74: 9240–4.
- Fomina AF, Deerinck TJ, Ellisman MH, Cahalan MD. Regulation of membrane trafficking and subcellular organization of endocytic compartments revealed with FM1-43 in resting and activated human T cells. Exp Cell Res. 2003; 291: 150–66.
- Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002; 110: 597–603.
- Teissier E, Pécheur EI. Lipids as modulators of membrane fusion mediated by viral fusion proteins. Eur Biophys J. 2007; 36: 887–99.
- Palecek SP, Schmidt CE, Lauffenburger DA, Horwitz AF. Integrin dynamics on the tail region of migrating fibroblasts. J Cell Sci. 1996; 109: 941–52. [PubMed Abstract].
- Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006; 8: 46–54.
- Frick M, Bright NA, Riento K, Bray A, Merrified C, Nichols BJ. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol. 2007; 17: 1151–6.
- Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem. 1999; 274: 12702–9.
- Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem. 1997; 272: 13793–802.
- Otto GP, Nichols BJ. The roles of flotillin microdomains – endocytosis and beyond. J Cell Sci. 2011; 124: 3933–40.
- Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991; 266: 14486–90. [PubMed Abstract].
- Platt FM, Neises GR, Dwek RA, Butters TD. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J Biol Chem. 1994; 269: 8362–5. [PubMed Abstract].
- Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003; 112: 519–33.
- Chernomordik LV, Melikyan GB, Chizmadzhev YA. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987; 906: 309–52.
- Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008; 15: 675–83.
- Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999; 68: 863–911.
- Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem. 2011; 286: 30911–25.
- Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013; 27: 31–9.
- Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009; 4: e5219.
- Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta. 2012; 1826: 103–11. [PubMed Abstract].
- Marcus ME, Leonard JN. FedExosomes: engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals (Basel). 2013; 6: 659–80.
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010; 107: 6328–33.
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998; 4: 594–600.
- Casella JF, Flanagan MD, Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981; 293: 302–5.
- Brawley SH, Robinson KR. Cytochalasin treatment disrupts the endogenous currents associated with cell polarization in fucoid zygotes: studies of the role of F-actin in embryogenesis. J Cell Biol. 1985; 100: 1173–84.
- Selden SC, Rabinovitch PS, Schwartz SM. Effects of cytoskeletal disrupting agents on replication of bovine endothelium. J Cell Physiol. 1981; 108: 195–211.
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002; 296: 1655–7.
- Greenberg S. Signal transduction of phagocytosis. Trends Cell Biol. 1995; 5: 93–9.
- Hazeki O, Hazeki K, Katada T, Ui M. Inhibitory effect of wortmannin on phosphatidylinositol 3-kinase-mediated cellular events. J Lipid Mediat Cell Signal. 1996; 14: 259–61.
- Knight ZA. Small molecule inhibitors of the PI3-kinase family. Curr Top Microbiol Immunol. 2010; 347: 263–78. [PubMed Abstract].
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996; 135: 1249–60.
- Prieto-Sánchez RM, Berenjeno IM, Bustelo XR. Involvement of the Rho/Rac family member RhoG in caveolar endocytosis. Oncogene. 2006; 25: 2961–73.
- Rappoport JZ, Heyman KP, Kemal S, Simon SM. Dynamics of dynamin during clathrin mediated endocytosis in PC12 cells. PLoS One. 2008; 3: e2416.
- Ormerod KG, Rogasevskaia TP, Coorssen JR, Mercier AJ. Cholesterol-independent effects of methyl-β-cyclodextrin on chemical synapses. PLoS One. 2012; 7: e36395.
- Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983; 96: 1–27.
- McNeil PL, Steinhardt RA. Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol. 1997; 137: 1–4.
- Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, Parton RG et al. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr Biol. 2008; 18: 1802–8.
- Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, Le Naour F et al. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J. 2006; 393: 609–18.
- Hakulinen J, Junnikkala S, Sorsa T, Meri S. Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. Eur J Immunol. 2004; 34: 2620–9.
- Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M et al. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci U S A. 2004; 101: 1297–302.
