Abstract
Exosomes are nano-sized vesicles of endocytic origin released into the extracellular space upon fusion of multivesicular bodies with the plasma membrane. Exosomes represent a novel mechanism of cell–cell communication allowing direct transfer of proteins, lipids and RNAs. In the nervous system, both glial and neuronal cells secrete exosomes in a way regulated by glutamate. It has been hypothesized that exosomes can be used for interneuronal communication implying that neuronal exosomes should bind to other neurons with some kind of specificity. Here, dissociated hippocampal cells were used to compare the specificity of binding of exosomes secreted by neuroblastoma cells to that of exosomes secreted by cortical neurons. We found that exosomes from neuroblastoma cells bind indiscriminately to neurons and glial cells and could be endocytosed preferentially by glial cells. In contrast, exosomes secreted from stimulated cortical neurons bound to and were endocytosed only by neurons. Thus, our results demonstrate for the first time that exosomes released upon synaptic activation do not bind to glial cells but selectively to other neurons suggesting that they can underlie a novel aspect of interneuronal communication.
For the sake of clarity here, we define exosomes as extracellular vesicles having a diameter of 40–100 nm, which are released upon fusion of endosomes called multivesicular bodies (MVBs) to the plasma membrane. Exosomes were first described as a way for cells to discard unwanted material (Citation1). It is now widely accepted that they also represent a novel mechanism of cell–cell communication by allowing intercellular transfer of proteins, lipids and RNAs (Citation2). Many cell types can secrete exosomes, including cells from the central nervous system (CNS): microglia (Citation3), oligodendrocytes (Citation4, Citation5), astrocytes (Citation6) and neurons (Citation7). Exosomes are found in cerebrospinal fluid, underlining the possibility of a secretion by nerve cells in vivo (Citation8). In neurons, MVBs are found mainly in somato-dendritic compartments (Citation9). Fusion of endosomes to the plasma membrane is a fundamental mechanism of synaptic plasticity allowing insertion of post-synaptic receptors and thereby reinforcing synaptic efficacy (Citation10). We previously showed that neurons secrete exosomes. The endocytic origin of neuronal exosomes was demonstrated using a soluble recombinant protein, made of GFP fused with the C-terminal part of tetanus toxin (GFP–TTC), which was specifically endocytosed by neurons, passed through MVBs and constitutively released bound to exosomes. Exosome release was triggered by glutamatergic synaptic activation through calcium entering via N-methyl-D-aspartic acid (NMDA) receptors (Citation11). The regulated secretion of neuronal exosomes might be a way for synapses undergoing plasticity to locally eliminate obsolete proteins and RNAs, without the need for dendritic transport to lysosomes mainly found in soma and proximal dendrites. It is tempting to speculate that exosomes released by neurons might also represent a novel way of intercellular communication within the nervous system (Citation12). This idea has been reinforced by the finding that exosomes might mediate trans-synaptic transfer of Wnt1/Wingless proteins at the Drosophila neuromuscular junction to allow maturation of synapses (Citation13, Citation14). It was also recently shown, both in vivo and in vitro, that exosomes secreted by mouse oligodendrocytes can enter both microglial cells and neurons, and modify gene transcription of the latter (Citation5, Citation15) (Citation16). Exosomes have also been hypothesized to allow trans-synaptic communication and mediate spreading of pathogenic proteins like tetanus toxin, PrPsc, Tau, the amyloid Aβ or synuclein within the nervous system (Citation13, Citation17) (Citation18, Citation19) . The possible role of exosomes in interneuronal communication awaited the demonstration that exosomes secreted by neurons can be taken up by other neurons.
Here we have studied the binding of exosomes, secreted by N2a neuroblastoma cells and by primary cortical neurons, to dissociated hippocampal cells. We found that fluorescent exosomes released constitutively by N2a cells expressing GFP–CD63, bind both to neurons and glial cells and endocytosis could mainly be detected in glial cells. In contrast, GFP–TTC- labelled exosomes released by cortical neurons upon activation of glutamatergic synapses bound to and were internalized only by neurons but not by glial cells. Thus, our work showing that exosomes released by neurons upon synaptic activation are specifically transferred to neurons gives ground to the hypothesis that they might mediate interneuronal communication within the CNS.
Material and methods
Antibodies and reagents
Polyclonal anti-Alix has been previously described in Chatellard-Causse et al. (Citation20). Monoclonal anti-LBPA (lyso-bis phosphatidic acid) was a kind gift from J. Gruenberg (University of Geneva, Switzerland). Monoclonal antibodies against EEA1 and against flotillin-1 were from Santa Cruz Biotechnology (sc6415) and BD Transduction Laboratories (610820), respectively. Monoclonal antibody against GFAP (Glial Fibrillary Acidic Protein) was from Cell Signaling (#3670), polyclonal anti-Lamp-1 from Abcam (ab24170), monoclonal anti-MAP2 from Millipore (ab5622) and monoclonal anti-O4 was a kind gift from J.C. Deloulme (Grenoble Institute of Neuroscience, France). Monoclonal anti-PSD95 and anti-synaptophysin were purchased from Abcam (ab2723) and Sigma (S5768), respectively. Monoclonal anti-GFP for immunoblotting was from Covance (MMS-118R). For immuno-electron microscopy N2a exosomes were labelled with monoclonal anti-GFP (MMS-118R) and anti-CD63 from BD Pharmingen (556019). Polyclonal anti-GFP used to label exosomes carrying GFP–TTC was from Life Technologies (A11122), Protein-A gold was from CMC Utrecht. Bicuculline and 4-aminopyridine (4-AP) were purchased from Sigma. Wheat germ agglutinin (WGA) Alexa Fluor 594 conjugate was from Life Technologies.
pGEX plasmid coding for GST–GFP–TTC has been described in Bordet et al. (Citation21). From this construct, TTC was amplified by PCR and cloned in a pGEX vector without the GFP. Both proteins, GST–GFP–TTC and GST–TTC were expressed in BL21 E. coli and purified on a glutathion-Sepharose4b column (Amersham) as described (Citation21). After dialysis against phosphate-buffered saline (PBS) containing 0.5 M NaCl and 5% glycerol, proteins were centrifuged at 100,000×g for 1 h to remove insoluble proteins.
GFP-CD63 N2a cell line
N2a cells were cultured in DMEM (Dulbecco's Modified Eagle Medium, Gibco) supplemented with 10% foetal calf serum (FCS, Gibco), 2 mM L-glutamine and antibiotics (10 µg/ml streptomycin, 10 U/ml penicillin). N2a cells were transfected with a plasmid encoding GFP fused to CD63 (GFP-CD63) containing a G418 resistance gene (nptII). Cells expressing CD63 were selected using G418 at 0.4 mg/ml (Gibco). Several clones were isolated and one expressing GFP-CD63 was selected (N2aGFP-CD63).
Primary cultures of cortical and hippocampal neurons
Procedures involving animals and their care were conducted in conformity with the French decree n°118 of 1st February 2013. Every effort was made to minimize the number of animals used.
Cells were prepared from embryonic rat embryos (E18 or E19) as described in Fauré et al. (Citation7). Dissociated cortical neurons were seeded at 4.5×104 cells/cm2 onto 100 mm dishes precoated with poly-d-lysine (50 µg/ml, Sigma). Hippocampal neurons were seeded at 1.2×104 cells/cm2 on glass coverslips (14 mm diameter, Marienfeld, Germany) coated with 50-µg/ml poly-d-lysine. Cortical and hippocampal neurons were cultured in Neurobasal (Gibco) supplemented with 2% B27 (Gibco), 1 mM sodium pyruvate, and 2 mM L-glutamine, in a humidified incubator (37°C and 5% CO2). Twenty-five per cent freshly made medium was added every 4 days. In the case of cortical neurons, 5 µM cytosine β-d-arabinoside (Ara-C, Sigma) was added to avoid glial cell proliferation. Immunostaining of cortical cultures after 15 days in vitro (DIV) with a monoclonal antibody against GFAP, revealed staining of only 1–2% of cells, demonstrating minimal contamination by astrocytes. In some cases, 5 µM Ara-C was also used for hippocampal neurons.
GST–GFP–TTC (GFP–TTC) fusion protein was diluted to 36 nM in culture medium and incubated on 15 DIV cortical neurons for 2 h. After extensive washes, the medium was replaced with K5 medium (5 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 110 mM NaCl, 26 mM NaHCO3, 1 mM NaH2PO4, 40 mM d-glucose, 15 mM HEPES, pH 7.4) containing 40 µM bicuculline and 100 µM 4-aminopyridine. The medium was harvested after 15 min to collect exosomes.
For WGA staining, hippocampal cells were incubated for 10 min at 37°C in K5 medium containing 5 µg/ml WGA-Alexa Fluor 594 before incubation with exosomes.
Purification of exosomes
Cell culture media were collected and a cocktail of protease inhibitors added (25X, tablets Complete EDTA, Roche). Media were cleared of debris by 2 successive centrifugation steps (2,000×g for 10 min, 20,000×g for 20 min) and filtration through a 0.22 µm filter (Millex GV PVDF, Millipore). Exosomes were recovered by centrifugation for 2 h at 100,000×g (average speed, 29,000 rpm, SW32Ti). For density separation, 100,000×g pellets were resuspended in 0.211 M sucrose, 3 mM imidazole pH 7.4, and loaded onto a continuous sucrose gradient (0.3–1.4 M).
Gradients were centrifuged for 18 h at 200,000×g (bottom speed, 35,000 rpm, SW41Ti), and 10 fractions (1 ml) were collected. The sucrose density of each fraction was determined by refractometry. Fractions were divided into 2, diluted to 10 ml in 3 mM imidazole, pH 7.4, and centrifuged for 2 h at 200,000×g (bottom speed, 35,000 rpm, SW41Ti). For one half, exosome pellets from fractions 1.1, 1.12 and 1.15 g/ml sucrose were resuspended in conditioned medium, pooled and used for incubation on hippocampal neurons (see below). Pellets obtained from the other half were resuspended in Laemmli buffer and used for Western blot analysis. Input corresponds to 10% of the solution before gradient separation; total cell lysates (TCL) were prepared using RIPA buffer and 10 µg of proteins loaded per lane.
GFP-CD63 containing exosomes were isolated from the cell culture supernatants of N2aGFP-CD63 cell line. Cells were washed twice in PBS and cultured in DMEM medium containing 10% foetal calf serum from which exosomes had been eliminated by ultracentrifugation (18 h, average speed, 35,000 rpm, 45Ti). Cell culture supernatants were collected after 24 h and exosomes isolated by 100,000×g ultracentrifugation as described above.
Exosome incubation on hippocampal neurons
Hippocampal neurons (2×105) cultured on coverslips were incubated with exosomes secreted from 6.5×106 cortical neurons in a final volume of 200 µl. Incubation was done for the indicated times in a humidified incubator at 37°C and 5% CO2. Cells were then extensively washed in medium before further processing for immunofluorescence or electron microscopy. GFP-CD63-exosomes secreted from 9×106 N2aGFP-CD63 cells were also incubated for the indicated times on 2×105 hippocampal neurons and then washed in medium.
Competition of GFP–TTC, or GFP–TTC-exosome binding with soluble TTC
Purified GST–TTC (referred to as TTC in the text) was used to compete with TTC–GFP or GFP–TTC-exosomes. TTC was diluted to 100 nM in culture medium and pre-incubated on 16 DIV hippocampal neurons for 20 min. GFP–TTC (0.36 nM) mixed with 100 nM TTC was then incubated on hippocampal neurons for 1 h. In the case of exosomes, GFP–TTC-exosomes were resuspended in incubation medium containing 100 nM TTC and incubated for 1 h on TTC-preincubated hippocampal cultures. Cells were then extensively washed in medium before further processing for immunofluorescence.
Immunofluorescence
N2a stably overexpressing GFP-CD63 were fixed at room temperature for 20 min using 4% paraformaldehyde (PFA) in PBS, permeabilized 30 min with 0.05% saponin in PBS-3% BSA (Bovine Serum Albumin, Sigma). Labelling with mouse anti-LBPA antibody was revealed using Cyanin 5-labelled anti-mouse antibody (Jackson Immunoresearch 0.2 µg/ml). Primary and secondary antibodies were diluted in PBS-3% BSA.
After incubation with exosomes, neurons were fixed using 4% PFA, 4% sucrose in PBS. After extensive washes in PBS, cells were permeabilized for 10 min at 4°C using PBS containing 0.1% Triton X-100. Cells were incubated in PBS containing 3% goat pre-immune serum (GPI, Sigma-Aldrich) and stained with the indicated primary antibodies and with secondary antibodies conjugated to either Alexa Fluor 488, Alexa Fluor 594 or Cyanine 5 (Jackson Immunoresearch 0.2 µg/ml). Primary and secondary antibodies were diluted in PBS 1% GPI. Nuclei were stained with Hoechst (Sigma).
Coverslips were mounted in Mowiol (Calbiochem) and observed using epifluorescence (Axiovert 200 M, Zeiss) or confocal microscopy (LAS AF – TCS SPE, Leica or LSM 710, Zeiss using Zen software). Some figures show acquisition in one given plan, others present maximal intensity projections (cf. figure legends). Image files were processed with ImageJ.
Immunoelectron microscopy
In order to confirm the presence of GFP and CD63 on the exosomes secreted by the N2aGFP-CD63 cell line, the 100,000×g pellet was resuspended in 2% PFA in PBS. Four microlitres were spotted onto Formvar-carbon-coated grids for 20 min, treated with PBS-glycine (50 mM) then PBS alone, before proceeding with immunolabelling according to the protocols described by Théry et al. (Citation22). To reveal GFP inside exosomes, exosomal membranes were permeabilized by 0.05% saponin before consecutive incubation with a mouse anti-GFP (45 min), a rabbit anti-mouse (30 min as a bridge) and finally a goat anti-rabbit 1.4 nm gold conjugated -Fab’ (Nanoprobes, 1 h). The exosomes were then fixed for 5 min with 1% glutaraldehyde and finally treated with the HQ silver intensification kit (Nanoprobes) for 6 min before negative staining with uranyl acetate. To reveal CD63, grids were incubated successively with anti-CD63 (45 min), rabbit anti-mouse (as above) and protein-A gold conjugates (10 nm, 30 min). The exosomes were then fixed with 1% glutaraldehyde for 5 min and negatively stained.
In the case of GFP–TTC labelled exosomes, the exosomal pellet was resuspended in 50 µl PBS, and 4 µl were incubated on Formvar-carbon-coated grids for 20 min before fixation in 2% PFA in 0.1 M phosphate buffer, pH 7.2 also for 20 min. The presence of GFP on the exosomes was then revealed using rabbit anti-GFP, for 30 min and protein-A gold, 10 nm, as described above.
To visualize by EM the binding of GFP–TTC exosomes on receiving neurons, exosomes were purified by 100,000×g centrifugation from 5×107 cortical neurons (15 DIV) preincubated for 2 h with either GFP–TTC or GFP alone as a control. Pellets were resuspended in 300 µl, and exosomes incubated 1 h on hippocampal neurons before extensive washing in neurobasal medium. GFP was revealed by immunolabelling as follows: receiving neurons were fixed at room temperature for 90 min in pre-warmed 0.1 M phosphate buffered 2% glutaraldehyde, washed, treated with PBS glycine (50 nM), washed again and blocked with BSA (10 mg/ml). Anti-GFP (A11122) was then applied at 1/500 overnight, and revealed with protein-A gold (15 nm). The cells were fixed with 2% glutaraldehyde in phosphate buffer for 45 min, post-fixed with 1% osmic acid for 1 h before en bloc uranyl acetate treatment and embedding in Epon. Sections were stained with uranyl acetate and lead citrate before observation with a Jeol JEM 1200EX transmission electron microscope.
Results
Exosome characterization
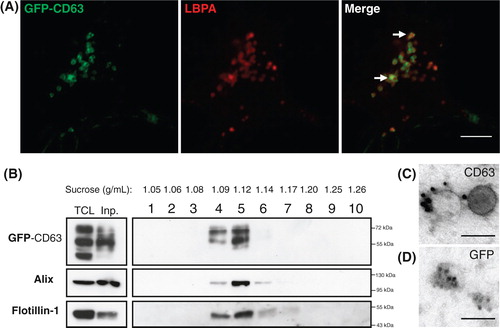
We used 2 approaches to fluorescently label exosomes in order to test their binding properties on hippocampal neurons. First, we developed a neuroblastoma cell line (N2a) expressing GFP-CD63 (N2aGFP-CD63). CD63 is a tetraspanin strongly enriched in MVBs and in exosomes (Citation23). As expected GFP labelling was massively concentrated inside LBPA-containing compartments (Citation24) (A). Vesicles were pelleted from cell culture supernatant of N2aGFP-CD63 cells and gradient separation demonstrated the presence of GFP-CD63 in fractions having the density (1.09–1.14 g/ml) of exosomes and containing Alix and flotillin-1 (B) (Citation25). Immunogold labelling with an antibody against CD63 confirmed the presence of the tetraspanin on vesicles having the size and shape of exosomes (C). After permeabilization, anti-GFP immunogold labelling was seen inside exosomes showing that, as expected from the known conformation of the protein, the N-terminal part of CD63 points into the exosomal lumen (D).
Fig. 1. N2a cells constitutively expressing GFP-CD63 (N2aGFP-CD63) secrete exosomes containing the fusion protein. (A) Co-staining of N2aGFP-CD63 with anti-LBPA (red) shows that GFP-CD63 (green) is concentrated inside LBPA-containing multivesicular bodies (arrows). (B) Density separation of extracellular vesicles released by N2aGFP-CD63 cells; Western blot analysis using anti-GFP, anti-flotillin-1 and anti-Alix, shows GFP immunoreactivity in fractions containing exosomes. TCL: total cell lysates, Inp: input. (C, D) Immunogold labelling of vesicles secreted by N2aGFP-CD63 cells pelleted at 100,000×g. Anti-CD63 labels the surface of intact exosomes (C), while anti-GFP stains the lumen of permeabilized exosomes (D). Scale bars: (A) 5 µm, (C, D) 100 nm.
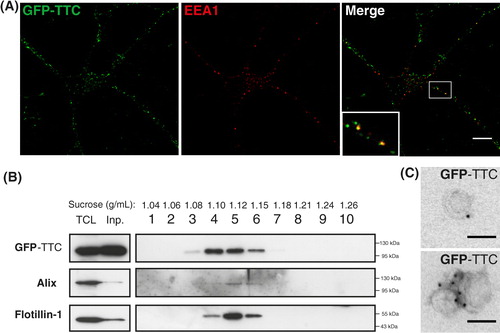
Based on our previous work, we also used GFP–TTC to label neuronal exosomes. After 2 h incubation on cortical cells, the soluble bacterial recombinant GFP–TTC labelled neurons exclusively. Labelling was seen both on the surface and inside endosomes, some of which were labelled with the early endosomal marker EEA1 (A). GFP–TTC pre-labelled neurons were then extensively washed and incubated for 15 min in a medium containing bicuculline and 4-aminopyridine to trigger exosome release. Vesicles secreted during the 15 min incubation were harvested by ultracentrifugation and separated on a sucrose gradient. As seen in B, GFP–TTC protein could be detected in the exosome fractions containing flotillin-1 and Alix demonstrating that the soluble protein is bound to vesicles floating in sucrose at the density of exosomes. Immunoelectron microscopy using anti-GFP demonstrated that GFP–TTC was indeed bound to the surface of exosomes (C).
Fig. 2. Soluble GFP–TTC is endocytosed before being secreted via neuronal exosomes. Cortical neurons were incubated at 37°C with GFP–TTC for 2 h (36 nM). (A) Confocal microscopy of neurons stained with anti-EEA1 (red), shows that GFP–TTC proteins (green) bind to and are endocytosed by neurons. (B) Density separation of extracellular vesicles secreted during a 15 min treatment with bicuculline and 4-AP; Western blot analysis using anti-GFP, anti-flotillin-1 and anti-Alix, shows GFP immunoreactivity in fractions containing exosomes. TCL: total cell lysates, Inp: input. (C) Immunogold labelling with anti-GFP of vesicles pelleted at 100,000×g demonstrates the presence of GFP–TTC on the exosomal surface. Scale bars: (A) 10 µm (C) 100 nm.
We then went on to compare binding to dissociated hippocampal cells, of GFP-CD63-exosomes secreted by neuroblastoma cells and GFP–TTC-exosomes secreted from cortical neurons.
Exosomes from neuroblastoma cells bind indifferently to neurons and glial cells
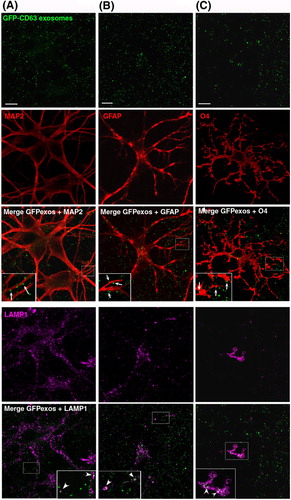
GFP-CD63 exosomes purified from N2aGFP-CD63 cell culture supernatant were incubated on mixed cultures of hippocampal cells (14 DIV) which contain neurons, astrocytes and oligodendrocytes as detected using antibodies against MAP2, GFAP and O4, respectively (). GFP-CD63 exosomes were bound to the surface of all cells with no apparent preference for any cell types. Co-staining with anti-Lamp-1 demonstrated the presence of GFP-CD63 inside Lamp-1-positive late endosomes of astrocytes or oligodendrocytes. In contrast, GFP-CD63 was very rarely seen inside Lamp-1-positive compartments of neurons. Thus, exosomes released by neuroblastoma cells indiscriminately bind to neurons and glial cells, and are mainly, but not exclusively, taken up by the latter.
Fig. 3. Neuroblastoma exosomes carrying GFP-CD63 bind to and are endocytosed by neurons and glial cells. Exosomes released by N2aGFP-CD63 cells were resuspended in conditioned medium and incubated for 1 h on mixed primary culture of hippocampal neurons (14 DIV). After washing, cells were co-stained with antibodies against MAP2 (A), or GFAP (B), or O4 (C), to label neurons, astrocytes and oligodendrocytes, respectively, and with antibodies against Lamp-1 (A, B, C) to stain late endosomes and lysosomes. Arrows point to exosomes bound on the cell surface. Arrowheads point to colocalization of exosomes with Lamp-1. Stacks of 3 confocal sections inside cells are shown for all conditions. Scale bars: 10 µm.
Exosomes from cortical neurons bind to neurons only
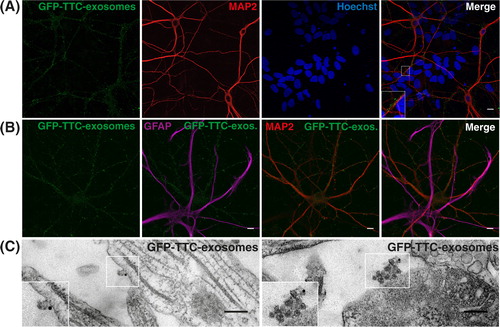
We next tested the binding on mixed cultures of hippocampal cells (14 DIV) of GFP–TTC exosomes released by cortical neurons upon synaptic activation. Exosomes secreted from cortical neurons, which had endocytosed GFP–TTC, were separated on sucrose gradients (pooled fractions 4, 5, 6 in B). This purification step made sure that all GFP–TTC was bound to exosomes and precluded any contamination with soluble GFP–TTC. As seen in A, GFP–TTC-exosomes exclusively decorated MAP2-positive soma and dendrites and did not bind to MAP2 negative cells of the same field. Co-staining with anti-GFAP antibodies further demonstrated that GFP–TTC exosomes bind only to MAP2 positive neurons and not to GFAP positive astrocytes (B). EM observation using immunogold against GFP of hippocampal neurons incubated with exosomes, confirmed the presence of single (C, left panel) or of aggregated exosomes (C, right panel) bound to the neuronal surface.
Fig. 4. Neuronal exosomes bearing GFP–TTC bind specifically to neurons. (A) Exosomes released by cortical neurons pre-incubated with GFP–TTC were harvested, pelleted at 100,000×g and separated on a sucrose gradient. Three GFP–TTC containing fractions (sucrose density of 1.1–1.15 g/ml) were pooled, pelleted, resuspended in incubation medium and incubated for 1 h on hippocampal cell cultures (16 DIV) (A, B, C). A) After washing, cells were immunostained with anti-MAP2 antibody (red) to label neurons. Hoechst nuclear staining shows the presence of numerous MAP2 negative cells which are not stained with GFP–TTC exosomes (maxima intensity). (B) After washing, cells were immunostained with anti-MAP2 antibody (red) to label neurons and anti-GFAP to stain astrocytes (magenta). (C) Cells were washed and processed for immunogold labelling using anti-GFP and processed for EM observation. Single or aggregated exosomes carrying gold-labelled GFP–TTC can be seen on the surface of neurons. Scale bars: (A, B) 10 µm, (C) 500 nm.
GFP–TTC exosomes bound to neurons are often concentrated in patches, reminiscent of synapses. Immuno-labelling of neurons decorated by GFP–TTC-exosomes with antibodies against synaptophysin and PSD95 was used to label pre- and post-synaptic sites, respectively. As illustrated in , some fluorescent patches corresponding to GFP–TTC exosomes co-localized with synaptophysin (A) and juxtaposed with PSD95 (B), demonstrating that neuronal exosomes bind to pre-synaptic parts. Noteworthy is that GFP–TTC-exosomes bound only to a small fraction of the immunolabelled synapses.
Fig. 5. Neuronal exosomes bearing GFP–TTC preferentially bind to pre-synaptic terminals and can be internalized. Exosomes secreted by cortical neurons preincubated with GFP–TTC (green) were incubated for 1 h on hippocampal neurons (22 DIV), which were then immunostained with (A) anti-synaptophysin (magenta) or (B) anti-PSD95 (magenta). Confocal microscopy sections show patches of GFP–TTC exosomes (green) perfectly colocalized with some synaptophysin-positive presynaptic sites and juxtaposed with some PSD95-positive post-synaptic sites. Scale bars: (A, B, C) 10 µm. (C) GFP–TTC exosomes were incubated for 1 h on hippocampal neurons (21 DIV) preincubated with Alexa594-WGA for 10 min at 37°C to label endosomes. (A), (B) (C) are confocal sections except for the photo on the right of the (C) panel, representing the projection of maximal intensities. Thin and bold arrows show labelling on the surface and within the cytoplasm respectively.
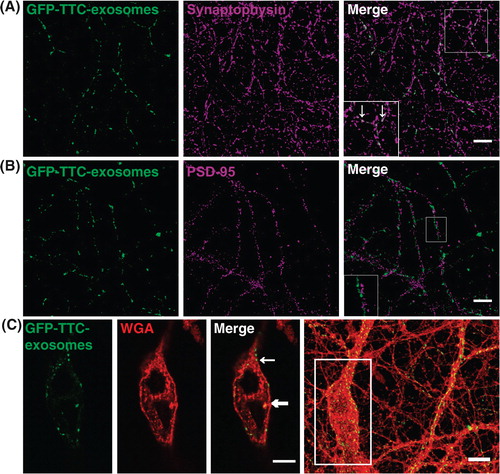
In another set of experiments, we used hippocampal neurons preincubated for 10 min with WGA coupled to Alexa-Fluor 594 in order to allow endocytosis of the fluorescent lectin and labelling of endosomes. After 1 h incubation with GFP–TTC exosomes, fluorescence accumulated in WGA-stained intracellular puncta of some neurons (C). Thus, GFP–TTC-exosomes bind to presynaptic terminal parts of some neurons and can be endocytosed.
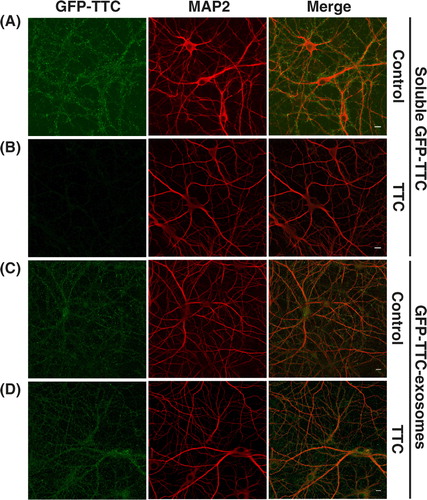
With GFP–TTC being on the surface of neuronal exosomes, it was conceivable that it might mediate the binding of exosomes to TTC receptors of the neuronal surface. In order to test whether this is the case, we used unlabelled purified TTC to compete for the binding of GFP–TTC-exosomes. As seen in , purified TTC abolished the binding of soluble GFP–TTC to hippocampal neurons (A and B), whereas it had no apparent effect on the binding of GFP–TTC exosomes (C and D). This result confirmed that the fluorescence detected on the surface of neurons incubated with GFP–TTC-exosomes is not due to soluble GFP–TTC and that GFP–TTC present on the exosomes does not mediate binding of the exosomes to the surface of hippocampal neurons.
Fig. 6. GFP–TTC exosomes do not bind to the neuronal surface because of their TTC cargo. (A, B) Incubation with TTC abolishes GFP–TTC staining of neurons: GFP–TTC was diluted to 0.36 nM in culture medium and incubated for 1 h on 16 DIV hippocampal neurons in absence (A) or in presence (B) of 100 nM TTC. In B) cells were pre-incubated for 20 min with 100 nM TTC. (C, D) TTC does not impair binding of GFP–TTC-exosomes to neurons: GFP–TTC-exosomes were incubated for 1 h on 16 DIV hippocampal neurons in absence (C) or in presence (D) of 100 nM TTC. In D) cells were pre-incubated for 20 min with 100 nM TTC. After incubation, cells were washed, fixed and immunolabelled with anti-MAP2 (red) (A, B, C and D). Scale bars: 10 µm.
Discussion
In this work, we have compared the binding to dissociated rat hippocampal cells, of fluorescent exosomes released by neuroblastoma cells and of exosomes secreted by neurons. We found that GFP-CD63-exosomes from N2a cells bound to both neurons and glial cells. In sharp contrast, GFP-TTC labelled neuronal exosomes released upon synaptic activation bound only to neurons, preferentially presynaptically.
GFP-CD63 labelled exosomes have already been used to demonstrate the transfer of exosomes between Schwann cells or mesenchymal stromal cells and neurons (Citation26). Immunoelectron microscopy allowed the authors to show that Schwann cell-derived GFP-CD63-exosomes accumulated inside endocytic vesicles of axons. In our case, GFP-CD63-exosomes from neuroblastoma cells bound to the surface of hippocampal neurons and were sometimes internalized. However, endocytosis mainly occurred in astrocytes and oligodendrocytes, where exosomes or their cargoes accumulated in late endosomes or even lysosomes.
The fate of neuroblastoma exosomes was very different from that of exosomes released by neurons upon synaptic activation, which bound only to neurons, preferentially at their synapses. Based on our previous work, we made use of GFP–TTC to label neuronal exosomes and follow their binding on hippocampal cells. TTC corresponds to the C-terminal 451-amino-acid fragment of the Tetanus toxin (TeNT) heavy chain, which allows specific binding and endocytosis of the holotoxin by neurons (Citation27). Binding of TTC to the neuronal surface requires interaction of 2 carbohydrate binding sites to gangliosides and with a protein receptor, the identity of which remains a matter of debate (Citation28, Citation29). We already reported that endocytosed GFP–TTC can be detected in neuronal MVBs and is released by way of exosomes during long-term depolarization. Since GFP–TTC binds to and is endocytosed exclusively by neurons, the use of this soluble fluorescent protein is a unique tool to specifically label exosomes released by neurons and follow their binding to other cells. Here, we stimulated fast release of exosomes using bicuculline, which indirectly activates glutamatergic synapses and thereby massively triggers exosomal release (Citation11). GFP–TTC labelled exosomes were harvested during a 15 min stimulation of cortical neurons having endocytosed GFP–TTC and further purified by density centrifugation. When incubated on dissociated hippocampal cells these GFP–TTC-exosomes stained dendrites and soma of neurons but did not bind to glial cells present in the same cultures. Fluorescent labelling of neurons by the exosomes was not due to GFP–TTC, either free or bound to exosomes, interacting with its neuronal surface receptor. Indeed labelling was not abolished when GFP–TTC-exosomes were co-incubated with unlabelled TTC. Thus, GFP–TTC on the exosomal surface, probably bound by gangliosides known to be enriched in exosomes, does not bind to the surface of hippocampal neurons and therefore does not underlie the neuron specific binding of exosomes. Interestingly, binding of exosomes occurred mainly at presynaptic parts of synapses and GFP fluorescence was also detected in the soma of some neurons, implying that endocytosis of the exosomes or of their cargoes had occurred. Hence, our results show that exosomes released post-synaptically by cortical neurons upon activation of synaptic NMDA receptors can bind presynaptically to hippocampal neurons. Future work will be required to examine whether exosomes can be endocytosed at these sites and transported back to the soma. Noteworthy, was the heterogeneity of staining of the MAP2 positive soma and dendrites with about 20% being strongly labelled while on the opposite, some were barely decorated by exosomes. Hippocampal neurons are heterogeneous by nature and future work is now needed to try to understand which type of neurons are the specific targets of cortical exosomes and how such a specificity of binding can be achieved.
Using GFP-CD63- and especially GFP–TTC-containing exosomes, punctate fluorescent staining could be seen in the neuronal cytoplasm, suggesting uptake of exosomes. Noteworthy is the fact that the intracellular fluorescent staining of neurons was scarce compared to the heavy surface labelling. Increasing the incubation time of the exosomes to 3 h slightly reduced the surface labelling but did not significantly increase the intraneuronal staining (not shown). This scarcity of internalization by neurons precluded the identification, by immunocytochemistry or immunoelectron microscopy, of the endosomal compartments containing labelled exosomes or their cargoes. In contrast, massive endocytosis by some glial cells, including astrocytes, was observed using GFP-CD63-exosomes. In these cells, accumulation of fluorescence was detected in Lamp-1 positive late endosomes and lysosomes suggesting that the exosomes or their cargoes are targeted towards degradation. It has recently been shown that exosomes secreted by an oligodendrocyte precursor cell line are preferentially taken up and degraded by microglial cells, whereas exosomes secreted from differentiated oligodendrocytes can be taken up by cortical neurons, microglial cells but not by astrocytes. Using transduction of oligodendrocytes with recombinant Adeno-Associated Virus/Myelin BasicPprotein (AAV/MBP-Cre), Frühbeis et al. found that the recombinase was active in receiving neurons, suggesting that exosome cargoes escape degradation inside neurons (Citation15). These results, combined with ours using neuronal exosomes, suggest that exosomes secreted by different cell types of the nervous system have different cell-binding specificities and fates once internalized by receiving cells.
Our results showing that exosomes released upon activation of glutamatergic synapses preferentially bind to other neurons strengthen the hypothesis that exosomes might represent a novel way for interneuronal communication. Trans-synaptic transfer of exosomes suggested by our observation that neuronal exosomes mainly bind presynaptically, could lead to the entry of exosomal membrane proteins into the endosomal protein pool, and possible local modifications of the cell surface properties of afferent neurons. Release into the cytosol of the exosome content, including signal transduction molecules and miRNAs could modulate local synaptic transmission. More work is now required to test how trans-synaptic transfer of exosomes might affect synaptic plasticity and participate in the spreading of pathogenic proteins across the nervous system in the course of neurodegenerative pathologies such as Alzheimer or Parkinson's diseases.
Conflict of interest and funding
The authors declare no conflict of interest. M.C. and C.J. were supported by the Ministère de l'Enseignement Supérieur et de la Recherche. K.L. was supported by the Foundation Plan Alzheimer. This work was funded by Institut National de la Santé et de la Recherche Médicale (Inserm), Université Joseph Fourier, Foundation France Alzheimer and ANR MALZ 2011 and ANR Blanc 2008 programs.
Acknowledgements
All members of the laboratory are acknowledged for ideas, support and comments on the manuscript.
References
- Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci. 1997; 110: 1867–77. [PubMed Abstract].
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200: 373–83.
- Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ et al. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005; 175: 2237–43.
- Krämer-Albers E-M, Bretz N, Tenzer S, Winterstein C, Möbius W, Berger H et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons?. Proteomics Clin Appl. 2007; 1: 1446–61.
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011; 124: 447–58.
- Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol. 2007; 67: 1815–29.
- Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006; 31: 642–8.
- Vella LJ, Greenwood DL, Cappai R, Scheerlinck JP, Hill AF. Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid. Vet Immunol Immunopathol. 2008; 124: 385–93.
- Von Bartheld CS, Altick AL. Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog Neurobiol. 2011; 93: 313–40.
- Wang Z, Edwards JG, Riley N, Provance DW, Karcher R, Li X-D et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008; 135: 535–48.
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011; 46: 409–18.
- Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet S et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013; 41: 241–4.
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009; 139: 393–404.
- Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012; 287: 16820–34.
- Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013; 11: e1001604.
- Fröhlich D, Kuo WP, Frühbeis C, Sun J, Zehendner CM, Heiko J et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci. 2014; 369: 20130510.
- Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases?. Front Physiol. 2012; 3: 124.
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010; 30: 6838–51.
- Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M et al. Cells release prions in association with exosomes. Proc Natl Acad Sci USA. 2004; 101: 9683–8.
- Chatellard-Causse C, Blot B, Cristina N, Torch S, Missotten M, Sadoul R. Alix (ALG-2-interacting protein X), a protein involved in apoptosis, binds to endophilins and induces cytoplasmic vacuolization. J Biol Chem. 2002; 277: 29108–15.
- Bordet T, Castelnau-Ptakhine L, Fauchereau F, Friocourt G, Kahn A, Haase G. Neuronal targeting of cardiotrophin-1 by coupling with tetanus toxin C fragment. Mol Cell Neurosci. 2001; 17: 842–54.
- Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006; Chapter 3: Unit 3.22.1–3.22.29.
- Escola J, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998; 273: 20121–7.
- Kobayashi T, Vischer UM, Rosnoblet C, Lindsay M, Parton RG, Kruithof EKO et al. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol Biol Cell. 2000; 11: 1829–43.
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012; 1820: 940–8.
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012; 30: 1556–64.
- Rossetto O, Scorzeto M, Megighian A, Montecucco C. Tetanus neurotoxin. Toxicon. 2013; 66: 59–63.
- Chen S, Hall C, Barbieri JT. Substrate recognition of VAMP-2 by botulinum neurotoxin B and tetanus neurotoxin. J Biol Chem. 2008; 283: 21153–9.
- Rummel A, Bade S, Alves J, Bigalke H, Binz T. Two carbohydrate binding sites in the HCC-domain of tetanus neurotoxin are required for toxicity. J Mol Biol. 2003; 326: 835–47.
