Abstract
Extracellular vesicles (EVs), including the nano-sized exosomes, have the capacity to transfer multiple functional molecules between cells. In cell culture experiments, fetal bovine serum (FBS) is often used to supplement cell culture medium as a nutrient, but it is important to know that the FBS also contain significant quantities of EVs. The aim of the current study was to determine whether the FBS EVs can influence cultured cell phenotype, and secondly to determine the efficiency of FBS-EV elimination protocols. Firstly, FBS that had not been depleted of EVs induced a migratory phenotype in a lung cancer epithelial cell line (A549 cells), an effect that could be mimicked by isolated FBS EVs alone. FBS-derived EVs also contained RNA, which was protected from consecutive proteinase K and RNase A treatment. Comparison of common isolation protocols suggested that an 18-hour centrifugation period eliminates approximately 95% of RNA-containing FBS EVs, whereas a 1.5-hour protocol is insufficient. In conclusion, this study shows that FBS EVs substantially influence cultured cell behaviour, but also that they can be virtually removed by an 18-hour ultracentrifugation protocol.
To access the supplementary material to this article, please see Supplementary files under Article Tools online.
Exosomes are small extracellular vesicles (EVs) that are 40–100 nm in size, and are considered to be released by most cells via an intracellular origin involving multivesicular bodies (Citation1, Citation2). However, several other subgroups of EVs also exist, including larger microvesicles thought to be released directly from the cell surface (Citation3). EVs are thought to take part in cell-to-cell communication, as they can shuttle proteins, lipids, and RNA between cells (Citation4–Citation6), and the status of the producing cells determines the signal conveyed (Citation6, Citation7). EVs are also found in all body fluids, such as serum, saliva, breast milk, and urine (Citation8, Citation9). As cell culture experiments often involve the presence of fetal bovine serum (FBS), it is possible that vesicles present in the FBS can influence experimental results. Indeed, it has been suggested that FBS-derived EVs may influence results in cell biology, such as growth of breast cancer cell lines (Citation10). Also FBS-derived EVs are a major cause of concern as these vesicles could contaminate EVs derived from cell cultures (Citation11, Citation12). Therefore, EVs are often removed from the FBS prior to use in cell culture experiments, by the use of different depletion protocols (Citation13, Citation14).
The aims of this study were firstly to determine whether EVs present in FBS can influence cultured cells phenotype such as the migration of an airway epithelial cancer cells, and secondly to determine the efficiency of elimination of EVs by different ultracentrifugation protocols.
Material and methods
Cell cultures
The human lung epithelial cell line, A549 (ATCC), was cultured in DMEM/F12 (1:1) medium (HyClone laboratories, Inc., Logan, UT), the human mast cell, HMC-1 (Dr. Joseph Butterfield, Mayo Clinic, Rochester, MN, and a kind gift from professor Gunnar Nilsson at Karolinska Institute, Stockholm, Sweden), was cultured in IMDM (Sigma Aldrich, St. Louis, MO) and the mouse fibroblast cell line, NIH3T3 (ATCC), was cultured in DMEM (with Glutamine; HyClone laboratories, Inc.). All media was supplemented with 10% FBS, 100 units/ml penicillin and 100 µg/ml streptomycin (HyClone laboratories, Inc.). Additional, 2 mM l-glutamine (HyClone laboratories, Inc.) and 1.2 mM alpha-thioglycerol (Sigma Aldrich) were added to the HMC-1 cell media. All supplement FBS was depleted of EVs by ultracentrifugation for 18 hours at 120,000×g (Type 45 Ti rotor, 38, 800 rpm, k-factor 178.6, Beckman coulter, Brea, CA) unless it is specifically mentioned as non-depleted. All cells were cultured at 37°C and 5% CO2.
Isolation of FBS-derived extracellular vesicles for characterization
FBS was purchased from Sigma Aldrich and had been filtered (0.1 µm) by the manufacturer prior to purchase. The FBS was subjected to 18 hours (overnight) centrifugation at 120,000×g (Type 45 Ti rotor, Beckman Coulter). The pellet was washed by adding PBS, and placing the sample under gentle agitation until the pellet was dissolved, before filtering it (0.2 µm) and re-pelleting it with an ultracentrifugation at 120,000×g for 70 minutes.
Analysis of FBS vesicles depletion protocols
In order to test EVs depletion protocols, FBS was either kept undiluted or were diluted with PBS in a ratio of 3:7 (FBS:PBS). These preparations were then subjected to 1.5 hours or 18 hours (overnight) centrifugation at 120,000×g (Type 45 Ti rotor, Beckman Coulter). The supernatants were then used to prepare 10% FBS-containing medium that was never in contact with cells. The 10% FBS-containing medium was later subjected to a standard exosome isolation protocol using differential centrifugation previously described (Citation14). Briefly the FBS-containing medium was centrifuged sequentially at 300×g (10 minutes) to remove debris and at 16,500×g (20 minutes) to remove large vesicles. Furthermore the supernatant was filtered with 0.2 µm filter before the samples were centrifuged at 120,000×g (70 minutes) to pellet exosomes.
Reverse migration assay
To evaluate the migratory phenotype of lung epithelial cells (A549), reverse migration assay was performed with a 46-well Boyden chamber (Neuroprobe Inc., Gaithersburg, MD) as reported (Citation15). Briefly, 20,000 cells/well were added to the lower chamber and were allowed to adhere onto the gelatin coated (0.1%) polycarbonate membrane by inverting the chamber assembly upside down for 3 hours. Later the chamber was placed in the correct orientation and incubated for 12 hours with various FBS-EV dosages in the upper chamber, which is the opposite side of the membrane in relation to the cells. The membrane was then carefully removed, and cells on the migrated side were fixed in ethanol, and stained with Giemsa (Histolab, Gothenburg, Sweden). Cells from the non-migrated side were wiped out completely before acquiring images with a light microscope (Zeiss Axioplan, Gottingen, Germany).
RNA isolation and detection
RNA was isolated from the different pellets described in the “Isolation of FBS-derived extracellular vesicles for characterization” and “Analysis of FBS vesicles depletion protocols” sections, using miRCURY™ RNA Isolation Kit for Cell and Plant (Exiqon, Vedbaek, Denmark) according to manufacturer's protocol and as described previously (Citation16). One microliter of isolated RNA was analyzed for its quality, yield and nucleotide length with capillary electrophoresis using Agilent RNA 6000 Pico chip on an Agilent 2100 Bioanalyzer® (Agilent Technologies, Santa Clara, CA).
Proteinase and RNase protection assay
For this assay, washed pellets obtained according to the method section “Isolation of FBS-derived extracellular vesicles for characterization” was used. Isolated vesicles were incubated with proteinase K (0.05 µg/µl; Sigma Aldrich) for 10 minutes at 37°C, before 5 mM phenylmethylsulfonyl fluoride (PMSF; Sigma Aldrich) was added for 10 minutes at room temperature to inhibit the proteinase K activity. Proteinase K activity was further inhibited by incubating the solution at 90°C for 5 minutes. After the inactivation of proteinase K, the samples were incubated with RNase A (final concentration 0.5 µg/µl; Thermo Fischer Scientific, Waltham, MA) for 20 minutes at 37°C to degrade unprotected RNA. Finally RNA was extracted and profiled as described earlier. Control samples were treated equally, but with PBS added instead of proteinase K, PMSF and/or RNase A. Furthermore, one control was kept at 4°C.
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed on the EV pellet isolated as described in section “Isolation of FBS-derived extracellular vesicles for characterization” as well as on the EV pellet isolated from the 10% FBS media using the 18 hours depletion protocol described in “Analysis of FBS vesicles depletion protocols.” Fifteen microgram of sample was loaded onto UV treated (5 minutes) formvar/carbon-coated nickel grids (Ted Pella Inc., Redding, CA) and incubated for 10 minutes before adding 2% paraformaldehyde for fixation. Later the samples were further fixed in 2.5% glutaraldehyde and contrasted in 2% uranyl acetate. Prepared samples were observed under a LEO 912AB Omega electron microscope (Carl Zeiss NTS, Jena, Germany).
Uptake of FBS-derived extracellular vesicles
EV pellet obtained as described in the “Isolation of FBS-derived extracellular vesicles for characterization” method section was labelled with PKH67 Green Fluorescent Cell Linker Kit (Sigma Aldrich) as per manufacturer's protocol, with minor modifications in the washing process as previously described (Citation8). Briefly, vesicles in PBS corresponding to 25 µg were added to 1 ml of diluent C. As a control equivalent 1 ml of diluent C was mixed with the same volume of PBS as was taken for the 25 µg exosomes. To this 4 µl of PKH67 dye each was added. After gentle mixing of samples for 4 minutes, 2 ml of 1% BSA was added to reduce the unbound dye. Both the samples were then transferred to 300 kDa Vivaspin filters (Sartorius Stedim Biotech GmbH, Goettingen, Germany) and centrifuged at 4,000×g. Proper washing was performed with 5 ml of PBS three times before PKH67-labelled vesicles were incubated with the lung epithelial cell line A549 (~70,000 cells) for 6 or 20 hours. Cells were washed with PBS, before being fixed in 4% paraformaldehyde. Samples were imaged under a fluorescence light microscope (Zeiss Axioplan, Germany).
Western Blot analysis
For immuno-blotting, the vesicle used was isolated as described in “Isolation of FBS-derived extracellular vesicles for characterization” method section. Furthermore, cell lysate and HMC-1 exosomes were used as positive control. All samples were lysed in 1X RIPA buffer (Cell Signaling Technology ST, Danvers, MA) with 15 minutes of sonication with intermittent vortex-mixing, followed by centrifugation at 20,000×g for 12 minutes at 4°C. Protein estimation was performed with BCA assay as per product manual (Pierce™ BCA Protein Assay Kit, Rockford, IL). One hundred micrograms of protein lysate were subjected to SDS-PAGE and proteins were transferred onto nitrocellulose membranes. Membranes were blocked with 5% Blotting Grade Blocker Non-Fat Dry Milk in TBS containing 0.05% Tween-20 and probed with the following antibodies: CD63 (1:1,000; clone H-193; Santa Cruz Biotechnology, Santa Cruz, CA), TSG101 (1:1,000; clone 4A10; Abcam, Cambridge, UK), and CD81 (1:800; clone H-121; Santa Cruz Biotechnology). The membranes were then incubated for one hour with the secondary antibodies diluted in 0.25% Blotting Grade Blocker Non-Fat Dry Milk in TBST. The secondary antibodies used were; donkey anti-rabbit IgG HRP-linked F(ab')2 fragment (1:10,000) and sheep anti-mouse IgG HRP-linked F(ab')2 fragment (1:2,000), both from GE Healthcare, Buckinghamshire, UK. The probed proteins were detected with the ECL Prime Western Detection (GE Healthcare, Amersham Buckinghamshire, UK) and a VersaDoc 4000 MP (Bio-Rad Laboratories) according to the manufacturer's protocol.
Statistical methods
Statistical analyses were performed using Student's t-test and Kruskal-Wallis test, and p<0.05 was considered significant. All scores in dose response studies are Spearman Rank Correlations. All experiments were performed on at least three different occasions.
Result
Effect of fetal bovine serum on epithelial cell migration
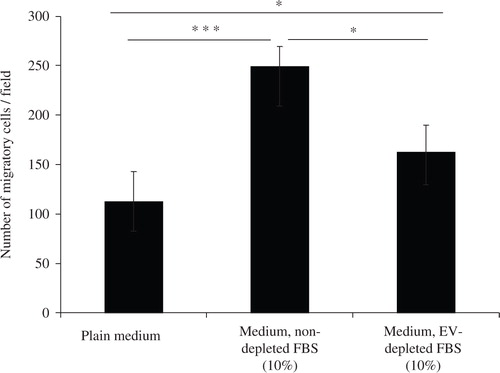
FBS has previously been shown to induce cell migration (Citation17) and has been used as positive control in migration assays (Citation18). We hypothesized that EVs present in the FBS could in whole or partially contribute to this phenotype change in cells. The effect of FBS, depleted or non-depleted of EVs, on reversed migration of the airway epithelial cell line A549, is shown in . Medium supplemented with FBS not depleted of EVs resulted in the transmigration of ~250 cells per field. Medium supplemented with FBS depleted of EVs resulted in a significantly reduced number of transmigrated cells, only somewhat higher than medium alone.
Fig. 1. Depletion of extracellular vesicles reduces FBS-induced cell migration. Medium was either supplemented with 10% FBS (Medium, non-depleted FBS) or 10% FBS that had been centrifuged for 18 hours to eliminate extracellular vesicle (Medium, EV-depleted FBS). As a control, FBS-free media was used (Plain medium). A549 cells were seeded on one side of a gelatin coated membrane of a Boyden chamber to evaluate cellular transmigration towards the various stimuli on the other side of the membrane. Student's t-test was used to determine significant differences, p<0.05, ***p<0.001. n=3.
Isolation and characterization of fetal bovine serum-derived extracellular vesicles
We hypothesized that the migratory effects observed by the FBS that had not been depleted of EVs indeed could be explained by their EVs. Although the presence of EVs in FBS has been known for over 40 years (Citation19) and has been discussed previously within the exosome field (Citation11, Citation12), to our knowledge these vesicles has not been characterized in depth previously. Therefore, we isolated FBS-derived EVs and performed additional characterization and functional experiments. The presence of vesicular structures was determined by TEM in the EV-enriched pellet from FBS (a). The presence of the “exosome markers” TSG101 using anti-human antibodies was detected in cell lysates from both mouse NIH3T3 cells and human HMC-1 cells, but also in the FBS EVs pellet from two separate batches of FBS (b, uncropped images of the Western blot membranes and background are provided in Supplementary Figure 1). An anti-human CD81 antibody signal was also detected in all FBS EVs preparation but not in equivalent cellular protein lysate showing the enrichment of CD81 in FBS-derived EVs. Another “exosome marker” CD63 was also detected in the FBS-derived EV pellet of one of the FBS batches (b). The positive binding of anti-human antibodies to mouse cell lysates and FBS-derived EVs could most likely be explained by the very high homology among species for the epitopes studied, which is up to 95% (CLUSTALW-multiple sequence alignment tool: www.ebi.ac.uk).
Fig. 2. Characterization of FBS-derived extracellular vesicles. FBS-derived vesicles were collected at 120,000×g for 18 hours, dissolved in PBS and re-pelleted at 120,000×g for 70 minutes. a) To visualize if FBS had vesicular structures present, TEM was performed on the EV-enriched pellet. The scale bar represents 200 nm. b) Immuno-blotting for the exosome enriched proteins; TSG101, CD81, and CD63. Pellet of EVs were obtained from different batch of FBS. Cell lysate from human mast cells (HMC-1) and mouse fibroblast (NIH-3T3), as well as HMC-1 derived exosomes were used as positive controls. One hundred microgram of protein was loaded per well for all samples. c) RNA was isolated using the miRCURY total RNA isolation kit and quantified with a Bioanalyzer. d) The FBS-derived EV-enriched pellet was incubated with RNase A (0.5 µg/µl) alone (i) or was incubated with proteinase K (0.05 µg/µl) prior to the RNase treatment (ii) to study mode of RNA protection. Cellular RNA was used as control for the RNase activity (iii). Graphs are representative of the RNA profile obtained with a Bioanalyzer from two independent experiments. e) PKH67-labelled FBS-derived EVs were incubated with 7×104cells (A549) for 6 or 20 hours and visualized with a fluorescent microscope (40X). PBS was used as control for determining the PKH67 background labelling.
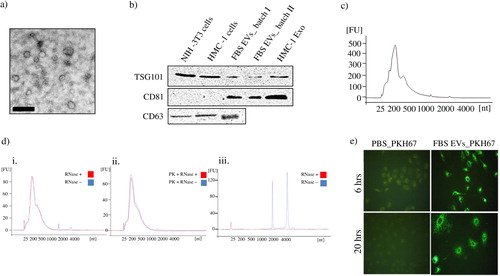
The pellet obtained after ultracentrifugation of FBS contains a typical exosomal RNA profile, as determined by Bioanalyzer (c). Hence, the RNA profile shows abundant RNAs of 60–500 bases, with very small or undetectable 18S and 28S ribosomal RNA peaks.
To determine whether the RNA obtained from the EV-enriched FBS pellets is present in vesicles or in protein–RNA complexes, the samples were treated with RNase, with or without pre-treatment with proteinase K. Proteinase K treatment of a cell lysate protein mixture (at the same enzyme concentration as for vesicles) resulted in total degradation of proteins, shown by an SDS gel (Supplementary Figure 2; lane 3). The effect of proteinase K was totally inhibited by the addition of a blocker molecule (PMSF; Supplementary Figure 2; lane 2). d shows that the RNA profiles from the FBS pellets were protected from RNase treatment, also after combined proteinase K and RNase treatment (d i and ii). RNase treatment of isolated cellular RNA resulted in total elimination of the unprotected RNA, showing that the RNase is active (d iii).
To determine whether EVs from FBS can be taken up by A549 cells, the isolated FBS-derived EVs were labelled using a lipid fluorescent dye (PKH67), and were incubated with the A549 cells for different periods of time. Fluorescent microscopy revealed the association of fluorescence lipid dye (PKH67) as punctate structures inside the cells. Similar fluorescence patterns are observed at 6 and 20 hours after addition of the vesicles to the cells (e).
Induced migratory effect by FBS-derived EVs
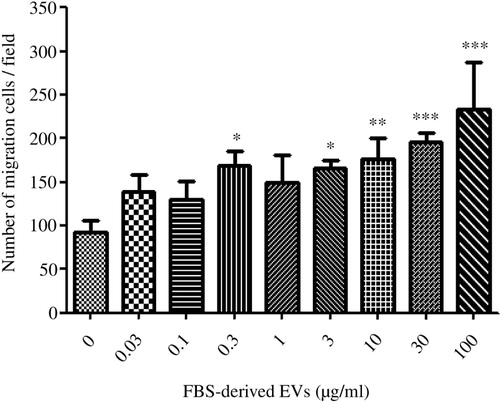
In order to evaluate whether the depleted EVs from FBS directly could induce a cell migratory phenotype, the washed FBS-derived EVs alone were used as a chemoattractant for A549 cells, and reversed transmigration was evaluated. Briefly, the A549 cells were allowed to attach to the membrane on the lower chamber side for 3 hours before the EVs were added at different concentrations to the upper chamber. After 12 hours the membrane was then carefully detached, and cells on the lower chamber side were removed before the cells on the upper chamber side were fixed and stained. Images were acquired with a light microscope (Supplementary Figure 3), and the number of migrating cells was counted. The addition of medium without FBS resulted in the transmigration of ~90 cells per field, whereas the addition of 0.03–100 µg/ml of FBS-derived EVs (protein weight) resulted in the transmigration of 138–234 cells per field, in a statistically significant (Kruskal-Wallis test: p<0.001) dose-dependent manner ().
Fig. 3. FBS-derived extracellular vesicles induce migratory phenotype in an epithelial cell line. FBS-derived EVs were collected at 120,000×g for 18 hours, washed in PBS and re-pelleted for 70 minutes at 120,000×g. Isolated vesicles were incubated with A549 cells, but were separated by a gelatin coated membrane in a Boyden chamber to evaluate cellular transmigration towards variable EVs concentrations. FBS-derived EVs showed a dose-dependent response in inducing migration in A549 cells. Kruskal-Wallis test was used to determine significant differences. Comparisons were only made against the control. *p<0.05, **p<0.01, ***p<0.001. n=3. Spearman Rank gave a significant correlation of Rs=0.82.
Efficiency of centrifugation-based depletion protocol for the removal of FBS EVs containing RNA
The recommended protocol for eliminating FBS EVs is to centrifuge the FBS for 18 hours at 100,000×g (Citation13). However, a protocol using a 1.5-hour centrifugation only is also used by some investigators. To our knowledge the efficiency of these established FBS-EV depletion protocols to reduce EVs has not been evaluated. We therefore centrifuged the FBS at 120,000×g for a long (18 hours) and short (1.5 hours) period, and obtained supernatant was used to supplement media at 10%. An exosome isolation protocol was then applied on this medium that had never been in contact with cultured cells, and EV RNA was quantified (a and b). The longer centrifugation period reduced the RNA by ~95%, whereas the short centrifugation only did so by approximately ~50%. We also continued to verify the efficiency of RNA depletion after reducing the viscosity of the FBS. To do this, we diluted the FBS in PBS at a 3:7 ratio, before the 1.5 and 18 hours depletion ultracentrifugation protocol was applied. EV RNA was evaluated similarly as mentioned above (Supplementary Figure 3) and importantly, there was no significant difference between the depletion protocols using diluted and undiluted FBS (142±37 ng/ml after 1.5 hours centrifugation of diluted FBS compared to 138±20 ng/ml after 1.5 hours of undiluted FBS, and 29±9 ng/ml after 18 hours centrifugation of diluted FBS compared to 17±8 ng/ml after 18 hours of undiluted FBS).
Fig. 4. Analysis of FBS vesicles depletion protocols. EV-depleted FBS was obtained from 1.5 and 18 hours of ultracentrifugation and was used to prepare complete media (10% FBS). The media was never in contact with cells, but used to isolate exosomes from. The RNA was extracted from the 120,000×g pellet and the concentration and nucleotide size was determined by a Bioanalyzer and the Pico chip. a) Data represent the overlay of RNA profile concentration of bovine RNA retained in complete media supernatant and pelleted during exosome isolation. b) Efficiency of exosome depletion with different centrifugation period is compared based on total RNA content in their exosome preparation in complete media. Results represent mean±SEM of sample obtained from 3–6 independent experiments. c) Presence of vesicular structures was determined by transmission electron microscopy. Vesicles are from the pellet preparation from EV-depleted FBS after 18 hours of ultracentrifugation (Bar represents 200 nm).
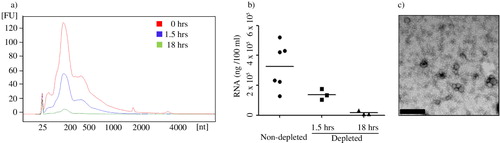
Despite reducing the EV-associated RNA with over 95% EVs could still be detected with electron microscopy in medium supplemented with FBS centrifuged for 18 hours prior to use in medium (c).
Discussion
FBS is often used to supplement culture medium in cell biology experiments, argued to provide “nutrients and growth factors.” Importantly, commercially available FBS contains many EVs, including exosomes, despite having been filtered. We here show that FBS-induced migration of an airway epithelial cancer cell line (A549) is reduced when the FBS EVs are removed, and that FBS EVs have a direct migratory effects on the same cells. We have characterized the FBS-derived EVs, showing the presence of exosome-like vesicles by electron microscopy, and a positive signal in Western blot using anti-human TSG101, CD81 and CD63, despite the vesicles originating from bovine blood. Further, typical exosome-like RNA profiles with primarily short RNA is observed in the FBS-derived EVs. The EVs from FBS are taken up by epithelial cells, in a similar way to CD34+ cells taking up mast cell–derived EVs (Citation20). A long centrifugation period (18 hours) is required to remove the majority of RNA-containing FBS EVs, while a 1.5-hour protocol is insufficient.
Here, we show that FBS induces a migratory effect in recipient A549 cells, and this effect is significantly reduced by extended centrifugation, which is commonly utilized to remove FBS EVs. Supplementing FBS in cell cultures is commonly used to promote proliferation of the cells studied, and to provide factors that improve viability, such as nutrients and growth factors. However, little is known about the role of the exosomes that are present in FBS, although one earlier study argues that they can promote proliferation of a breast cancer cell line (Citation10).
We therefore pursued with experiments to carefully characterize FBS-derived EVs, using multiple methodologies. Firstly, the EVs derived directly from FBS have a circular morphology, similar to exosomes as shown previously by electron microscopy (Citation1, Citation5). Importantly, there is no suggestion that FBS-derived EVs and EVs derived from cell culture experiments or human body fluids are different in morphology (Citation5, Citation8). Further, we tested whether the FBS EVs would express markers similar to human EVs, and Western blot showed the presence of multiple “exosome markers,” including TSG101, CD81 and CD63, using different antibodies. Importantly, all the antibodies used in the present study are considered to be directed towards multiple species, and therefore the observed positivity in the FBS-derived EVs is not surprising. Actually, the epitopes of the antibodies used here for TSG101, CD81 and CD63 share very high sequence similarity between bovine and human, reaching approximately 99, 95 and 89%, respectively. Importantly, the intention of this study was not to determine the bovine binding of the “anti-human” antibodies, but to emphasize that EVs in FBS can be positive for proteins that may be considered to be the human proteins and have functional significance. Notably, the RNA profiles of FBS-derived EVs are very similar to the RNA profiles seen in EVs isolated from different human cell lines and body fluids (Citation8, Citation14). These results obviously open the possibility that FBS EVs can be mistaken as cell-derived EVs. Therefore, it is absolutely crucial to consider any contamination of FBS-derived EVs in any studies of EVs produced in vitro in the presence of FBS.
The RNA in FBS-derived EVs is resistant to direct RNase A treatment, which shows that the RNA profiles observed are not representing free RNA molecules. In some experiments, however, the very small rRNA peaks observed in the FBS-derived EVs disappeared by RNase A treatment only, which could imply that the ribosomal RNA may be present on the outside of the lipid bilayer of FBS-derived EVs. When the FBS-derived EVs were exposed to proteinase K, which degrades all proteins but will not penetrate the lipid bilayer, followed by RNase A treatment (d ii), the small RNA profiles were largely preserved. This argues that the RNA captured in the FBS-EV pellet is not harboured inside protein structures, but rather protected by a lipid bilayer resistant to proteinase K. This is important, as it has been shown that several extracellular proteins, including AGO-2 and HDL, can protect RNA molecules from RNase degradation (Citation21, Citation22). Therefore, our data collectively argue that most of the RNA in FBS is present inside EVs, and is thus protected from the RNase-rich extracellular environment.
It is well known that EVs can be taken up by cells, and can deliver their cargo to recipient cells by unknown intracellular molecular pathways. In a separate experiment, we showed that FBS-derived EVs, stained with a lipid dye, are rapidly taken up by the recipient A549 cells. This shows a similar time line as the uptake of mast cell–derived EVs in human CD34+ cells (Citation20), and suggests that interspecies uptake of EVs can occur, supporting earlier reports (Citation23). Indeed, our original study of “exosomes” from a mouse mast cell line (MC/9) showed that these vesicles could be taken up by human mast cells (HMC-1) (Citation23). These data again argue that any research group pursuing experiments studying uptake of EVs in recipient cells needs to harvest those EVs from cells that are cultured without the presence of FBS EVs. However, the data also support the concept that EVs can transfer materials across species.
We could also show that the isolated FBS-derived EVs have a direct migratory dose-dependent effect on the airway epithelial cancer cell line (A549 cells), which again clarifies the importance to consider the role of FBS EVs on cell phenotype and behaviour. Some studies have suggested that there is large alteration in gene expression in cells when cultured in FBS compared to know growth factors supplements (Citation24). However, very few studies have looked at the direct effect of FBS-derived EVs on cell physiology. Nevertheless, in 2009, Ochieng et al. showed that anchorage-independent breast cancer growth was promoted by FBS vesicles (Citation10). The previous study and our current study thus show that it is crucial to consider the overall involvement and importance of FBS EVs in any cell culture studies, but it is possible that FBS EVs influence also other cellular processes in vitro.
The effect of FBS that has not undergone an “exosome depletion protocol” on cell behaviour in vitro has important implications for anyone performing cell cultures in the presence of FBS. Thus, we here show that overnight centrifugation removes most of the FBS-mediated effect on cell migration, and also removes most of the EV RNA (). Often, shorter protocols to remove FBS EVs are utilized, but clearly they are not sufficient to get virtually “EV-RNA free FBS.” With an 18-hour centrifugation, we remove more than 95% of EV RNA, but still some EVs can be visualized by electron microscopy. Therefore, it is clear that no “depletion protocol” leads to total FBS-EV elimination, even though most of them are eliminated. Importantly, also, any RNA analysis of primary or cell line cultures that include high concentrations of FBS, must consider the possible influence of the RNA added by the EVs from FBS. FBS EVs and their associated cargo, additional to growth factors and other small soluble molecules, are important for the influence of FBS on cell migration, and should be consider to influence also other cellular functions.
Overall, this study shows that RNA-containing EVs in FBS can be removed to almost 95% with an 18 hours centrifugation protocol, and this approach strongly reduce the functionality of FBS vesicles on epithelial cell migration. Shorter protocols only reduce the presence of FBS EVs in the cell culture medium to a smaller extent, which still can have significant effects of cell physiology and phenotype.
Conflict of interest and funding
This work was funded by the Swedish Research Council, VBG Group Herman Krefting Foundation for Asthma and Allergy Research, the Swedish Heart and Lung Foundation, and the Swedish Cancer Foundation. The authors have no conflicts of interest.
Supplementary Figures
Download PDF (718.8 KB)Notes
To access the supplementary material to this article, please see Supplementary files under Article Tools online.
References
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996; 183: 1161–72. [PubMed Abstract].
- Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000; 165: 1259–65. [PubMed Abstract].
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200: 373–83. [PubMed Abstract] [PubMed CentralFull Text].
- Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010; 51: 2105–20. [PubMed Abstract] [PubMed CentralFull Text].
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9: 654–9. [PubMed Abstract].
- de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012; 1 18396, http://dx.doi.org/10.3402/jev.v1i0.18396 [PubMed CentralFull Text].
- Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010; 5: e15353. [PubMed Abstract] [PubMed CentralFull Text].
- Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011; 9: 9.
- Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011; 9: 86. [PubMed Abstract] [PubMed CentralFull Text].
- Ochieng J, Pratap S, Khatua AK, Sakwe AM. Anchorage-independent growth of breast carcinoma cells is mediated by serum exosomes. Exp Cell Res. 2009; 315: 1875–8. [PubMed Abstract] [PubMed CentralFull Text].
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001; 166: 7309–18. [PubMed Abstract].
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999; 147: 599–610. [PubMed Abstract] [PubMed CentralFull Text].
- Thery C, Clayton A, Amigorena S, Raposo G. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006. Chapter 3:Unit 3.22.
- Crescitelli R, Lässer C, Szabo TG, Kittel A, Eldh M, Dianzani I. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013; 2 20677, http://dx.doi.org/10.3402/jev.v2i0.20677.
- Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002; 62: 6312–7. [PubMed Abstract].
- Cvjetkovic A, Lötvall J, Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles. 2014; 3 23111, http://dx.doi.org/10.3402/jev.v3.23111 [PubMed CentralFull Text].
- Lin MC, Huang MJ, Liu CH, Yang TL, Huang MC. GALNT2 enhances migration and invasion of oral squamous cell carcinoma by regulating EGFR glycosylation and activity. Oral Oncol. 2014; 50: 478–84. [PubMed Abstract].
- Akasaka K, Akasaka N, Di Luozzo G, Sasajima T, Sumpio BE. Homocysteine promotes p38-dependent chemotaxis in bovine aortic smooth muscle cells. J Vasc Surg. 2005; 41: 517–22. [PubMed Abstract].
- Benz EW Jr, Moses HL. Small, virus-like particles detected in bovine sera by electron microscopy. J Natl Canc Inst. 1974; 52: 1931–4.
- Ekström K, Valadi H, Sjöstrand M, Malmhäll C, Bossios A, Eldh M. Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J Extracell Vesicles. 2012; 1 18389, http://dx.doi.org/10.3402/jev.v1i0.18389.
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011; 108: 5003–8. [PubMed Abstract] [PubMed CentralFull Text].
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011; 13: 423–33. [PubMed Abstract] [PubMed CentralFull Text].
- Berleman J, Auer M. The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ Microbiol. 2013; 15: 347–54. [PubMed Abstract].
- Bieback K, Ha VA, Hecker A, Grassl M, Kinzebach S, Solz H et al. Altered gene expression in human adipose stem cells cultured with fetal bovine serum compared to human supplements. Tissue Eng. 2010; 16: 3467–84.
