Aled Clayton1, Charlotte Lawson2, Chris Gardiner3, Paul Harrison4 and David Carter5
1Division of Cancer and Genetics, School of Medicine, Cardiff University, Cardiff, UK; 2Cardiovascular and Inflammation Biology, Comparative Biomedical Sciences, Royal Veterinary College, London, UK; 3Haemostasis Research Unit, Research Department of Haematology, University College London, London, UK; 4Institute of Inflammation and Ageing, College of Medical and Dental Sciences, Birmingham University, Birmingham, UK; 5Department of Biological and Medical Sciences, Faculty of Health and Life Sciences, Oxford Brookes University, Oxford, UK
The UK Extracellular Vesicles (UKEV) Forum meetings were born of the realization that there were a number of UK laboratories studying extracellular vesicle biology and using similar techniques but without a regular national meeting dedicated to EVs at which to share their findings. This was compounded by the fact that many of these labs were working in different fields and thus networking and sharing of ideas and best practice was sometimes difficult. The first workshop was organized in 2013 by Dr Charlotte Lawson, under the auspices of the Society for Endocrinology, led to the founding of the UKEV Forum and the organization of a British Heart Foundation sponsored 1-day conference held in London in December 2014. Although growing in size every year, the central aims of these workshops have remained the same: to provide a forum for discussion and exchange of ideas, to allow young scientists to present their data in the form of short talks and poster presentations and to discuss their work with more established scientists in the field. Here we include the presented abstracts for the 2015 1-day conference hosted by Cardiff University. This meeting was attended by approximately 130 delegates throughout the United Kingdom, but also attended by delegates from Belgium, Netherlands, France, Ireland and other nations. The day composed of plenary presentations from Prof Matthias Belting, Lund University, Sweden and Dr Guillaume van Niel, Institut Curie, Paris together with 10 short presentations from submitted abstracts. The topics covered were broad, with sessions on Mechanisms of EV production, EVs in Infection, EVs in Cancer and in Blood and Characterizing EVs in Biological fluids. This hopefully gives a reflection of the range of EV-related studies being conducted currently in the UK. There were also 33 poster presentations equally broad in subject matter. The organizers are grateful to the Life Science Research Network Wales – a Welsh government-funding scheme that part-sponsored the conference. We are also grateful to commercial sponsors, and 3 paid-presentations are included in the abstracts. The UK EV Forum is expected to become an established annual event held at different Universities across the UK and continue to attract increasing delegate numbers and abstract submissions. We look forward to the next planned conference, which will be hosted by David Carter and his colleagues at Oxford Brookes University on 13th December 2016.
Short Talk 1
Extrusion of inside-out, phosphatidylserine-exposed, autophagic vesicles in the formation of human erythrocytes
Tosti J. Mankelow*, Rebecca E. Griffiths* and David J. Anstee
Bristol Institute for Transfusion Sciences, NHS Blood and Transplant, Filton, Bristol, UK
*These authors have been equally contributed to the work.
Background: During maturation to an erythrocyte, a reticulocyte must eliminate any residual organelles and reduce its surface area. We have shown that both are achieved through a novel form of exocytosis whereby large (~1.4 µm) intact, inside-out phosphatidylserine-exposed vesicles are expelled from the maturing reticulocyte (1,2). The exposed “eat me” phosphatidylserine signal ensures that released autophagic vesicles are rapidly removed from circulation by professional phagocytic cells within the spleen. Asplenic patients (by surgery or the pathological processes of haemoglobinopathies such as sickle cell disease) show elevated levels of circulating PS positive red cells as a result of inefficient release of these autophagic vesicles from their surface (2). Methods: Confocal microscopy was used to analyse the cellular location of proteins in reticulocytes produced from an in vitro culture system described in (1). Results: In reticulocytes, the autophagic vesicles contain organelle marker proteins and numerous erythroid membrane proteins, notably CD71 (Transferrin receptor), CD147 (Basigin) and stomatin. The presence of ubiquitin suggests a recognized mechanism for the targeting of proteins for extracellular export or degradation. Myosin motors are used to traffic autophagic vesicles around the maturing reticulocyte whereas other proteins involved in vesicle trafficking, SNARE (VAMP7) and ESCRT (CHMP4B), locate to defined positions at the point of vesicle extrusion. Conclusions: Our results show that autophagic vesicle release by maturing reticulocytes is a cellular process that although initiated and directed by the cell is facilitated by passage through the spleen. Their release ensures that the maturation, into erythrocytes, of the 2 million reticulocytes that the human body produces every second (3) occurs without the systemic release of potentially toxic material. Together our results describe a previously unrecognized mode of exocytosis which may have significance beyond erythropoiesis particularly with respect to apoptosis and autophagy.
The 2nd United Kingdom Extracellular Vesicle Forum Meeting Abstracts15 December 2015, Hadyn Ellis Building, Cardiff University
The 2nd United Kingdom Extracellular Vesicle Forum Meeting Abstracts
15 December 2015, Hadyn Ellis Building, Cardiff University
References
1. Griffiths RE, Kupzig S, Cogan N, Mankelow TJ, Betin VM, Trakarnsanga K, Massey EJ, Lane JD, Parsons SF, Anstee DJ. Maturing reticulocytes internalize plasma membrane in glycophorin A-containing vesicles that fuse with autophagosomes before exocytosis. Blood. 2012 Jun 28;119(26):6296–306. doi: http://dx.doi.org/10.1182/blood-2011-09-376475
2. Tosti J. Mankelow, Rebecca E. Griffiths, Sara Trompeter, Joanna F. Flatt, Nicola M. Cogan, Edwin J. Massey, David J. Anstee. Autophagic vesicles on mature human reticulocytes explain phosphatidylserine-positive red cells in sickle cell disease. Blood. 2015 126:1831–1834.
3. Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008 Aug 1;112(3):470–8. doi: http://dx.doi.org/10.1182/blood-2008-03-077883. Review.
Short Talk 2
Role of purinergic signalling in regulation of unconventional protein secretion
Rhiannon Griffiths, Magdalena Adamczyk, Sharon Dewitt Parr and Daniel Aeshlimann
School of Dentistry, Cardiff University, Cardiff, UK
Background: Transglutaminase 2 (TG2) is an early response gene with an extracellular function in tissue repair. It is secreted via an unconventional and enigmatic pathway. Microvesicle release and the exosome pathway have been implicated. Our group has shown that active TG2 export is controlled by purinergic signalling, and implicated P2X7 receptor activation. P2X7R has several activation states; ATP stimulation causes ion channel opening, allowing membrane depolarization and Ca2+ entry into the cell. Prolonged stimulation leads to “large membrane pore” activity; however, the identity of this pore is unknown and as there is conflicting evidence suggesting either dilation of the P2X7R channel itself or an interaction with an alternative plasma membrane channel. P2X7R activation can lead to extensive changes to membrane structure, potentially leading to vesicle shedding. We investigate how cells export TG2 and control its activation, potentially identifying a novel secretory pathway used by select proteins, including potent signals regulating inflammation. Objective: We aim to elucidate mechanistically the process by which cells export TG2 and control its activation. Methods: P2X7R variants were stably expressed in HEK293 cells. P2X7R pore formation was assessed using YO-PRO1 uptake by cells. TG2 externalization was assessed by western blotting of conditioned medium. Differential centrifugation and sucrose density gradients were used to assess the presence of TG2 in vesicles by western blotting. Myeloid precursors were isolated from human blood and differentiated using GM-CSF. Results: P2X7R activation induced membrane bleb formation and MV shedding. Pharmacological suppression of P2X7R ion channel function without affecting membrane pore formation, abrogated formation of flotillin-2 containing MV but not TG2 export. Separation of MV and analysis of associated proteins confirmed the absence of TG2. In contrast, introducing a gain-of-function mutation in P2X7R, that enhanced pore activity, resulted in accelerated TG2 export. To confirm the transferability of our findings to innate immune cells, human peripheral blood monocytes were differentiated into M1 macrophages and P2X7R mediated TG2 export was confirmed and was independent of inflammasome formation. Discussion: We show that P2X7R activation triggers shedding of specific MV. Unexpectedly, TG2 export was MV independent but linked to thioredoxin-1 secretion. Furthermore, P2X7R induced membrane pore activity directly correlated with TG2 export but was not associated with MV formation. Hence, the respective pathways are distinct. P2X7R polymorphisms affecting membrane pore formation may also affect extracellular levels of proteins secreted via this pathway. This begins to identify components of a mechanism for unconventional protein secretion crucial to innate immunity.
Short Talk 3
Excluded on request of the authors
Short Talk 4
The pathogenic blood fluke Schistosoma mansoni releases protein and small non-coding RNA-enriched extracellular vesicles, which could play a role in host–parasite interactions during schistosomiasis
Fanny Carole Nowacki1, Martin T. Swain1, Oleg I. Klychnikov2, Umar Niazi1, Alasdair Ivens3, Juan F. Quintana3, Paul J. Hensbergen2, Cornelis H. Hokke4, Amy H. Buck3 and Karl F. Hoffmann1
1Animal and Microbial Sciences (AMS), Institute of Biological, Environmental and Rural Sciences, Aberystwyth University, UK; 2Centre for Proteomics and Metabolomics, Leiden University Medical Center, Leiden, The Netherlands; 3CIIE, University of Edinburgh, Edinburgh, UK; 4Department of Parasitology, Leiden University Medical Center, Leiden, The Netherlands
Background: Upon skin penetration, larval blood fluke schistosomes (schistosomula) release excretory/secretory (E/S) products in an attempt to establish and maintain infection in mammalian hosts. Previous studies have postulated functions for these E/S products in initiating crucial host-modulatory events. However, the role of extracellular vesicles (EVs) has yet to be investigated. Here, we conducted the first characterization of Schistosoma mansoni schistosomula EVs and their potential host-regulatory cargos. Methods: After cultivating schistosomula for 72 h in culture medium lacking foetal calf serum, E/S products were harvested using preparatory ultracentrifugation. Collected EVs were analysed by transmission electron microscopy (TEM) and liquid chromatography tandem mass spectrometry proteomics (LC–MS/MS) and both EV-enriched as well as EV-depleted fractions were subjected to Illumina next generation sequencing (NGS) of small-noncoding RNA (sncRNA) libraries. Results: TEM analysis revealed the presence of numerous exosome-like EVs, the first observation for S. mansoni. The proteomic analysis of these vesicle cargos revealed a set of 109 proteins, including homologues of proteins found enriched in other eukaryotic EVs and highly abundant non-conserved proteins of unknown function. The characterization of potentially gene regulatory E/S sncRNA population within and outside of EVs revealed the presence of tRNA-derived small RNAs (tsRNAs: nineteen 5’ and fourteen 3’ tsRNAs; using an in-house script) and microRNAs (miRNAs: 35 known and 170 platyhelminth novel miRNAs found using miRDeep2 and miRBase). In silico target prediction of these miRNAs (analysed by 4 different software packages: miRanda, microTar, RNAhybrid and PITA) identified thousands of putative mRNA targets, which supports their gene regulatory potential within both mammalian and schistosome biology. Conclusion: The discovery of schistosome EVs and the characterization of their protein and sncRNA components identify a new participant in the complex biology underpinning schistosome/host interactions. Further work is ongoing to define the role of schistosomula EVs and the functional significance of the biological components (e.g. proteins, sncRNAs) found within EV-enriched and EV-depleted E/S fractions. These findings open up the way for developing novel schistosomiasis diagnostics or interventions.
Short Talk 5
Expression of Molluscum contagiosum virus gene mc162 leads to extracellular vesicle formation
Joachim Jakob Bugert1,5, Iona Ashworth1, Hana Elasifer1, Tom Hickin1, Megan Wadon1, Laura Farleigh2, Asif Nizam3 and Nadja Melquiot4
1Department of Medical Microbiology and Infectious Diseases Cardiff University, Cardiff, UK; 2Wellcome Trust-MRC Institute of Metabolic Science Cambridge University, Cambridge, UK; 3Department of Microbiology, Dhaka University, Dhaka, Bangladesh; 4Channing Laboratory, Brigham & Women’s Hospital, Boston, MA, USA; 5Institut für Mikrobiologie der Bundeswehr, Neuherbergstr. 11, D-80937 München, Germany
Background: Molluscum contagiosum Virus (MCV) is a human poxvirus causing benign epidermal tumours in children and immunosuppressed individuals. The early MCV type 1 membrane protein mc162 contains 2 proline rich motifs (P5Y and P3Y) and a dileucine motif in its c-terminal cytoplasmatic domain. Methods: Human HaCaT keratinocyte extracts were probed with mc162–GST fusion proteins and tested for coprecipitating proteins by MS and targeted western blot. A pIRESneo plasmid construct (p20) and a recombinant vaccinia virus expressing mc162-flag as a GFP-fusion protein (v354) were used to establish membrane domain patterns in infected cells. Lentiviral constructs with a tet regulated promoter (tetON) expressing mc162-flag were established to minimize gene toxicity (pHAGE/tetON; p496/502). Results: The c-terminal 110 amino acid residues of mc162 bind E3 protein-ubiquitin ligases AIP4 and Nedd4 and the hepatocyte growth factor tyrosine kinase substrate (Hgs/Hrs) derived from human HaCaT keratinocytes. Cells over-expressing mc162 acquire an aberrant early endosomal vesicle phenotype similar to Hrs negative mouse fibroblasts derived from Hrs−/− knockout mice, which is toxic for cells, preventing stable transfection of cell lines and generation of admc162. In v354 infected cells we have observed extrusion of extracellular DAPI negative vesicles carrying mc162 and AIP4. Conclusions: Involvement of PY motif carrying proteins in virus budding has been described (1). Possible roles of mc162 in surface receptor regulation, setting up of viral factories and the vegetative spread in human epidermis are discussed. We currently use a lentiviral expression system to further study the connection between mc162 expression and release of extracellular vesicles.
Reference
1. Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc Natl Acad Sci USA. 2000 Dec 5;97(25):13871–6.
Short Talk 6
The role for platelet-derived extracellular vesicles in recruiting neutrophils to vascular endothelium during inflammation
Sahithi Jyothsna Kuravi, George Ed Rainger and Gerard B. Nash
nstitute of Cardiovascular Sciences, University of Birmingham, Birmingham, UK
Background: Platelet extracellular vesicles (PEV) account for a large proportion of circulating extracellular vesicles and have been suggested to promote leukocyte recruitment to the vascular endothelium. The rate of PEV binding to endothelial cells (EC), their influence on neutrophil recruitment and mechanisms involved are not well understood. We aimed to determine the binding kinetics of PEV to EC and the resultant effects on neutrophil recruitment from flow. Methods: PEV were generated from CD41-labelled platelets, stimulated with collagen related peptide (CRP-XL, 1 µg/ml) and were incubated with EC. PEV-mediated stimulation of EC was assessed by flow cytometry for adhesion receptors. Flow-based adhesion assay assessed neutrophil recruitment on PEV-coated on glass capillaries or on EC grown in flow chambers and treated with combinations of PEV and different concentrations of TNF-α. Blocking studies were performed to assess the role for chemokine receptors. Chemokines in cell supernatants were measured using multiplex chemokine array. Results: PEV binding to EC was detected within 1 h and maximal by 4 h with >60% dual positivity for CD41 and VE-cadherin on EC. The PEV uptake resulted in upregulation of endothelial activation markers (E-selectin and VCAM-1). Neutrophils bound directly to PEV enabling frequent inflow capture and low levels of stable adhesion to a PEV-coated surface. Similar effects of PEV were observed on unstimulated or minimally stimulated (1U/ml TNF-α) EC. Blocking studies revealed roles for P-selectin, platelet activating factor and chemokine receptors in PEV-mediated neutrophil capture and adhesion. Furthermore, PEV supernatants contained platelet chemokines such as platelet factor 4 and RANTES along with Interleukin-8, GRO-α, ENA-78 and MCP-1. Conclusions: Surface-bound PEV can directly capture flowing neutrophils and also activate ECs. Thus PEV may promote neutrophil recruitment in inflammation, by potentiating effects of low levels of cytokines acting on EC.
Short Talk 7
The modulatory potential of Mesenchymal Stem Cells is mediated by the release of immunologically active extracellular vesicles
Monica Sofia Correia dos Reis, Lindsay Nicholson, Emily Mavin, Anne Dickinson and Xiao-nong Wang
cademic Haematology, ICM, Medical School, Newcastle University, Newcastle upon Tyne, UK
Background: Mesenchymal Stem Cells (MSCs) are widely used for the treatment of many diseases due to their differentiation potential and immunomodulatory capacity. Increasing evidence has shown that the therapeutic effects of MSCs are a result of their secretome, which includes paracrine factors and extracellular vesicles (EVs). EVs are membrane-derived particles that function as mediators of cell communication via horizontal transfer of functional proteins and genetic material. In this study we examine the immunosuppressive potential of BM-MSC-derived EVs by investigating their ability to modulate allogeneic T cell responses. Methods: MSCs were isolated from BM aspirates from healthy donors and characterized as per the ISCT criteria. EVs were isolated by ultracentrifugation and characterized by protein quantification, nanoparticle tracking analyses (NTA), transmission electron microscopy and flow cytometry. For functional characterization, titrated doses of EVs were added to mixed leukocyte reaction containing 1×105 responder T cells and 1×104 monocyte-derived dendritic cells (moDCs) and proliferation was assessed by 3H-thymidine incorporation. EV uptake was assessed by EV labelling with membrane-dye PKH26 and detected using immunofluorescence microscopy and flow cytometry. The effect of inhibition of EV secretion by MSCs on T cell proliferation was performed by pre-treating MSCs with GW4869, following co-culture with PHA stimulated T cells. Results: Sufficient amount of MSC-derived EVs were successfully collected from approximately 5×107 MSCs. Flow cytometry analyses of EV markers showed enrichment in CD63, CD9 and CD81. TEM exhibited characteristic cup-shaped morphology of EVs. In vitro experiments showed a dose-dependent capacity of MSC-EVs to inhibit T cell proliferation with ~30% inhibition at highest EV dose. Inhibition of EV secretion by MSCs indicated an abrogation of MSC capacity to inhibit T cell proliferation, showing a 3-fold increase in proliferation of T cells in the co-cultures with GW4869 pre-treated MSCs when compared to the DMSO-vehicle control. Immunofluorescence detection of MSC-EVs internalization by target cells showed a preferential association of EVs with monocyte-derived DCs and a small subset of CD3+ T cells. Further analysis of EV uptake by flow cytometry, showed a dose-dependent increase in EV internalization by CD3+ T cells, with a preferential uptake by CD4+ T cells, with ~11.3% PKH26+ cells in comparison with 6.1% of CD8+PKH26+ T cells. Conclusions: Our results show that MSC-derived EVs are immunologically active structures that have the ability to suppress allogeneic T cell responses. Further studies are ongoing to elucidate the mechanism by which MSC-EVs modulate DC and T cell functions.
Short Talk 8
The human pericardial fluid is enriched with exosomal microRNAs of cardiac origin that show a therapeutic potential
Cristina Beltrami, Saran Shantikumar, Marie Besnier, Andrew Shearn, Cha Rajakaruna, Gianni D. Angelini and Costanza Emanueli
ristol Royal Infirmary, University of Bristol, Bristol, UK
Background: Cells release functionally active microRNAs (miRs) into extracellular vesicles (EVs) as intercellular communication. The functional relevance of EVs in the context of human physiopathology is still debated. We hypothesize that the pericardial fluid (PF) mediates myocardium cell-to-cell communication through exchange of exosomes. We aimed to (a) characterize the PF miRs and exosomes content; (b) investigate the biological function of PF exosomes. PF, plasma, thoracic aorta (TA) and right atrium appendage (RAA) samples were collected as leftover material from aortic valve replacement surgery. The top expressed cardiovascular miRs in the PF were identified by miRs-array and then validated by RTqPCR in TA and RAA, PF and plasma, and in exosomes from PF or plasma. Methods: Exosomes were studied by nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM). The angiogenic capability of PF and plasma exosomes was investigated in hypoxic human endothelial cells (ECs) and in a mouse model of limb ischaemia (LI). The contribution of miRs to the functional properties of exosomes was assessed in EC with reduced miR biogenesis after knockdown (KD) of DICER (enzyme responsible for the final step of miR maturation), followed by PF exosomes treatment. Additionally, the pro-angiogenic role of let-7b-5p (an miR that was highly expressed in PF exosomes and it is known to be pro-angiogenic) was investigated by comparing the effect induced by either PF-derived exosomes or PF-derived exosomes depleted of let-7b-5p on ECs with Dicer KD. Results: A pool of cardiovascular miRs was expressed in patients’ TA and RAA and enriched in the PF vs plasma, suggesting the cardiac and vascular origin of these PF miRs. As expected, miR-122 that is not expressed in cardiovascular cells was undetectable in the PF. Exosomes were present in the PF. In vitro PF-exosome treatment of hypoxic ECs decreased cells apoptosis, increased cell proliferation and tube formation. The pro-angiogenic effect of PF-derived exosomes is partially miRs dependent. We found that PF exo-miR, let-7b-5p, contributes to restore angiogenesis in DICER-depleted ECs. Local delivery of PF exosomes increased post-ischaemic blood flow recovery and reparative angiogenesis, while plasma exosomes were not effective. Conclusions: We have shown the therapeutic potential of PF exosomes from cardiovascular patients. Future studies will be tailored to exploit the properties of PF-exosome in regenerative medicine.
Short Talk 9
Cerebrospinal fluid extracellular vesicles as a source of biomarkers for multiple sclerosis
Joanne L. Welton1,2, Sam Loveless3, Tim Stone4, Neil P. Robertson3 and Aled Clayton1
1Cancer and Genetics, School of Medicine, Cardiff University, Cardiff, UK; 2Biomedical Sciences, Cardiff School of Health Sciences, Cardiff Metropolitan University, Cardiff, UK; 3Institute of Psychological Medicine and Clinical Neurosciences, School of Medicine, Cardiff University, Cardiff, UK; 4Central Biotechnology Services, Cardiff University, Cardiff, UK
Background: Multiple sclerosis (MS) is one of the most common neurological disorders in young adults affecting over 100,000 people in the UK, with 5,000 new cases diagnosed each year. There are currently no widely available reliable biomarkers to aid in, the often lengthy, diagnosis or monitoring of the disease. In this pilot study we develop methods of isolation and proteomic analysis to examine the potential use of extracellular vesicles (EVs), in particular exosomes, as a novel source of biomarkers for the relapsing remitting form of multiple sclerosis. Methods: A workflow was developed for the isolation of EVs from cerebrospinal fluid (CSF), using a combination of precipitation and size exclusion chromatography (SEC) (Exo-spin™; Cell Guidance Systems). The EV-enriched fractions were selected based on characteristics including increase in EV-associated tetraspanins using an ELISA-like assay and particle-to-protein ratio (NanoSight™ and NanoDrop™). EVs were isolated from pooled relapsing remitting-MS patient (RRMS) (n=4) and control (n=3) cell-free CSF. These EVs along with their paired cell-free CSF were subsequently analysed using a novel aptamer-based protein array assay (SOMAscan™), providing relative levels for each of 1,128 proteins. Results: The use of precipitation and SEC allowed for the selection and pooling of EV-enriched fractions and the removal of ~75% of contaminating abundant protein. These EV isolates were confirmed to be compatible with the SOMAscan™ platform, even with the potential presence of remaining precipitant that would otherwise interfere with a mass spectrometry-based proteomics platforms. Around 350 and 580 proteins out of 1,128 were identified in CSF-derived exosomes and cell-free CSF respectively, of which 50 proteins were found significantly and exclusively enriched in RRMS-derived exosomes. Some of these EV-enriched proteins were further evaluated and some proteins, such as KLKB1 (Fletcher factor), were found to be enriched in EVs compared to cell-free CSF, by western blot. An interactive network based on these 50 proteins was created using Cytoscape, revealing strong associations with proteins related to the complement pathway, ionic binding, secreted proteins and extracellular regions. Conclusions: This study demonstrates that EVs can be isolated from MS patient CSF and highlights the potential to identify proteins of possible interest which are specifically enriched in the concentrated EV fraction. This proof of concept study shows the potential of CSF EVs as a novel source of biomarkers in MS and future work will examine EVs in MS in more depth.
Short Talk 10
Lipoprotein-associated phospholipase A2 is released by the placenta into the maternal circulation via syncytiotrophoblast extracellular vesicles: a potential role in pre-eclampsia
Chris Gardiner1, Emily Russell2, Sylvia Shahjahan2, Dionne Tannetta2, Ian Sargent2 and Manu Vatish2
1Haemostasis Research Unit, University College London, London, UK; 2Nuffield Department of Obstetrics and Gynaecology, University of Oxford, oxford, UK
Background: The maternal syndrome of pre-eclampsia is the result of vascular dysfunction caused by the release of soluble factors (e.g. endoglin and soluble VEGF receptor[s-flt-1]) and syncytiotrophoblast extracellular vesicles (STBEV) from the placenta into the maternal circulation. Lipoprotein-associated phospholipase A2 (Lp-PLA2) causes endothelial dysfunction via F2-isoprostane (e.g. 8-isoprostane) formation and lipid peroxidation. As Lp-PLA2 has been reported in trophoblasts we hypothesized a role for Lp-PLA2 in pre-eclampsia. Methods: STBEV were prepared by perfusing placentae from 6 normal pregnant women and 6 with pre-eclampsia. Lp-PLA2 was detected by immunofluorescence of placental sections and western blotting of STBEV. In addition, paired peripheral and uterine venous samples collected from 6 normal pregnant women were analysed for vesicular placental alkaline phosphatase (PlAP, a protein unique to the placenta) and malondialdehyde (MDA, a marker of lipid peroxidation). Results: Lp-PLA2 was demonstrated on syncytiotrophoblast in placental sections and STBEV from normal and pre-eclamptic women. PLAP and MDA were significantly higher in blood collected from the uterine vein (200 v 109 ng/ml and 1.76 v 1.28 µM, respectively) than in peripheral venous blood, and were positively correlated (r=0.51), indicating an association between placental Lp-PLA2 activity and release of STBEV. Discussion/conclusion: Lp-PLA2 expression in STBEV, combined with increased lipid peroxidation in uterine venous blood on PE STBEV, indicate a potential role for placental Lp-PLA2 in the maternal endothelial dysfunction which is characteristic of pre-eclampsia.
Sponsored presentation 1
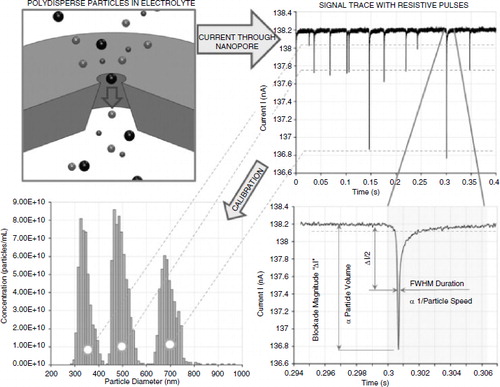
Combining size exclusion chromatography and tunable resistive pulse sensing technology for the characterization of extracellular vesicles
Julien Muzard1, Murray Broom2, Stephen Mann2 and Hans Van Der Voorn1
1Magdalen Centre, The Oxford Science Park, Izon Science, Oxford, United Kingdom; 2Bionanotechnology, Izon Science, Christchurch, New Zealand
Tuneable Resistive Pulse Sensing (TRPS) is a particle-by-particle technology that enables measurements of nano-sized particles with a very high-resolution. TRPS measures individual particle by analysing the durations, the frequencies and magnitudes of the resistive pulses under varying driving forces across a pore-based sensor and by using reference particles calibrated for size, surface charge and concentration. For the extracellular vesicles (EVs) field, the complete cycle of sample preparation, measurement and analysis of EVs from blood plasma using both size exclusion chromatography and TRPS is now by far the fastest option as well as the most reliable and accurate. In this communication, I will describe how TRPS offers significant and unique advantages over laser-based approaches for characterizing EVs (e.g. size and concentration). Furthermore, our latest developments on particle-by-particle charge analysis and phenotyping EVs population will be outlined and discussed.
Sponsored presentation 2
Exposure of cancer cells to EGFR-inhibitors changes protein composition of extracellular vesicles
Susan van Dommelen, Raymond Schiffelers, Roy van der Meel, Robbert Zuidema, Romy Verschoor and Pieter Vader
aboratory for Clinical Chemistry and Hematology, University Medical Center Utrecht, Utrecht, the Netherlands
Background: Tumour cell-derived extracellular vesicles (EVs) reflect the status of the parental cells. This makes EVs promising candidates for biomarkers, for example to monitor treatment. In this study, we exposed tumour cells to cetuximab (a monoclonal antibody that blocks the activation of epidermal growth factor receptor (EGFR) and erlotinib (a kinase inhibitor blocking EGFR-signal transduction) and examined the effect on extracellular vesicle composition. Methods: We exposed A431 human epidermoid carcinoma cells to 1 µM erlotinib or 100 µg/ml cetuximab. In one set-up we used ultracentrifugation to isolate extracellular vesicles followed by western blotting of EGFR and phospho-EGFR (pEGFR) and vesicles markers Alix, TSG101 and CD9. In another set-up we captured the extracellular vesicles with magnetic microbeads and isolated them in a magnetic stand. We used fluorescent antibodies to probe the vesicle surface for EGFR, CD147 and tetraspanins and analysed fluorescence on a flow cytometer. Results: Using the ultracentrifugation set-up, we could demonstrate that both EGFR and pEGFR on the EVs were reduced after cetuximab treatment, reflecting similar changes in the parental cells. Also the expression of the tetraspanin CD9 was reduced. Using density gradient ultracentrifugation, we could observe that cetuximab bound to EVs. EV-associated cetuximab retained its activity. For the magnetic beads conjugated with anti-CD9 antibody we could capture tumour-derived EVs directly from cell culture medium. Analysis by flow cytometry showed marked loss of CD147 and EGFR for EVs form cetuximab treated cells, which was not the case for cells treated with erlotinib. Discussion: Cetuximab treatment alters the composition of EVs, reflecting the parental cell status. This indicates that EVs could serve as biomarkers to monitor cetuximab treatment efficacy.
Sponsored presentation 3
Whole exosome fluorescent labelling measured with F-NTA
Pauline Carnell-Morris, Patrick Hole, Agnieszka Siupa and Andrew Malloy
alvern Instruments Ltd, Amesbury, UK
Background: NTA is a common technology for characterizing both size and number concentration of exosomes. It is often extended to fluorescence labelling in order to get better insight into the samples (F-NTA). However, the protocol for carrying out such a measurement can be challenging because there are multiple variables that are required to be investigated and optimized. This need not take a long time if carried out in a methodical approach. Membrane-labelling or cell-labelling dyes have been suggested as a means to positively identify exosomes from any contaminants that potential remain in the sample at the end of sample preparation. Here we review several proposed membrane-labelling dyes and share our results and advice on some of the factors that influence the effectiveness of the measurement. Methods: Three common and commercially available membrane- or cell-labelling dyes (PKH, DiI and CellMask™) were used to label human-derived exosomes. Titrations of dye: exosomes were carried out, incubation times were investigated as was the overall incubation volume in order to optimize labelling conditions. Various instrument configurations and settings were investigated to ensure optimum visualization of the sample under measurement. Sample measurements along with appropriate controls were taken under both scatter and fluorescence to measure the size distribution and concentration with NTA and to evaluate labelling efficiency. Results: All dyes were demonstrated to successfully label exosomes. Various issues were identified during the optimization process, such as disturbing the stability of the sample by the addition of dye formulation buffer, influence of dye preparation understanding the risk of labelling contaminants that may co-purify with exosomes and interactions with buffers, for example. For all dyes a constant flow of sample was required to ensure sufficient particles were included in the results due to the rate of photobleaching exhibited by the samples and to improve concentration measurements. Conclusions: F-NTA has been demonstrated to be an effective and rapid way to characterize and identify the presence of membrane-containing particles. From the dyes tested, we found CellMask™ orange to be the easiest reagent to use to label membrane-containing nanoparticles. The other dyes required more optimization steps and more complex protocols to label the exosome samples.
Poster 1
Host–parasite interactions: the Leishmania flagellum as a secretory organelle
Laura Makin and Eva Gluenz
ir William Dunn School of Pathology, University of Oxford, Oxford, UK
Leishmania are protozoan parasites which are transmitted between mammalian hosts by the Phlebotomine sand fly. The amastigote form resides in the hostile environment of the phagolysosome of mononuclear phagocytes where it must resist detection and degradation by the host cell. During differentiation upon entering the mammalian host the long promastigote flagellum shortens dramatically leaving only the tip exposed. It is unknown what becomes of the excess flagellar membrane during this process. To investigate whether flagellar-derived vesicles could provide a mechanism for delivery of virulence factors we used differential centrifugation to isolate vesicles from conditioned medium of Leishmania undergoing differentiation. This resulted in the isolation of vesicles which were further characterized by electron microscopy, protein western blotting and mass spectrometry. Putative vesicle-associated proteins and virulence factors have been identified in the isolated material and research is ongoing to determine the detailed composition and subcellular origin of the vesicles.
Poster 2
Urinary microRNAs are stabilized by association with exosomes and argonaute 2 protein
Kate Simpson1, Cristina Beltrami1, Aled Clayton2, Lucy J. Newbury1, Robert H. Jenkins1, Aled O. Phillips1, Donald J. Fraser1 and Timothy Bowen1
1Wales Kidney Unit, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK; 2Institute of Cancer & Genetics, School of medicine, College of Biomedical Life Sciences, Cardiff university, UK
Background: Chronic kidney disease (CKD) has been identified as a major global clinical problem, but despite extensive study prediction of injury progression remains elusive. MicroRNAs (miRNAs) have emerged as novel biomarkers for a variety of diseases. These transcripts are present in body fluids, and urinary miRNAs are accessible non-invasively. In this study we investigated the stability in control human urine samples of the ubiquitously expressed transcript miR-16, and of miR-192, which we have shown previously to be down-regulated in renal fibrosis. Methods: RNase A (0.1 mg/ml) and proteinase K (50 µg/ml) digestions were carried out using 2.5 ml urine aliquots in which endogenous miRNA expression was compared with spiked-in Caenorhabditis elegans miRNA cel-miR-39. Following incubation at 37 or 55°C, respectively, 250 µl aliquots were removed at appropriate time points, RNA was isolated, and then miR-16 and cel-miR-39 were detected by RT-qPCR. Extracellular vesicles were isolated using density gradient centrifugation protocols that we described previously. RNA-immunoprecipitation was used to analyse association of urinary miR-16 and miR-192 with argonaute 2 (AGO2) protein. Results: Endogenous urinary miR-16 was significantly more resistant to RNase-mediated degradation than exogenous, spiked-in cel-miR-39. MiR-16 and miR-192 were enriched in exosomal sucrose gradient fractions, but were also detected in all other fractions. This suggested association of urinary miRNAs with other urinary extracellular vesicles and/or pellet components, complicating previous estimates of miRNA: exosome stoichiometry. Proteinase K digestion destabilized urinary miR-16 and we showed, for the first time, RNA-immunoprecipitation of urinary miR-16:AGO2 and miR-192:AGO2 complexes. Conclusions: Association with exosomes and AGO2 stabilized urinary miR-16 and miR-192, suggesting quantitative urinary miRNA analysis has the potential to identify novel, non-invasive CKD biomarkers.
Poster 3
Evaluation of optimal extracellular vesicle small RNA isolation and qRT-PCR normalization for serum and urine samples
Rachel E. Crossland, Jean Norden, Louis Bibby, Joanna Davis and Anne M. Dickinson
aematological Sciences, Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, UK
Background: MicroRNAs are small regulatory molecules that demonstrate useful biomarker potential. They have recently been recognized in biofluids, where they are protected from degradation by encapsulation into extracellular vesicles (EVs). A number of commercial products are available for the isolation of EVs and their RNA content, however, extensive protocol comparisons are lacking. Furthermore, robust qRT-PCR assessment of microRNA expression within EVs is problematic, as endogenous controls previously used in cellular samples may not be present. This study compares EV isolation and RNA extraction methods (EV precipitation reagents, RNA isolation kits and ultracentrifugation) from serum or urine samples and evaluates suitable endogenous controls for incorporation into qRT-PCR analysis. Methods: Extracellular vesicles and associated RNA was isolated from serum and urine by comparing commercial precipitation reagents (Life Technologies & System Biosciences (SBI)), ultracentrifugation and popular RNA isolation kits (Norgen Biotek (NB) Total RNA Purification Kit, SBI SeraMir™ Exosome RNA Purification Column kit, Qiagen miRNeasy Micro kit, Ambion mirVana™ miRNA Isolation Kit, Invitrogen Total Exosome RNA & Protein Isolation Kit and NB Urine Exosome RNA Isolation Kit). Isolated EVs were assessed by electron microscopy (EM) and nanoparticle tracking analysis (NTA). Total RNA concentration was assessed using the Bioanalsyzer (RNA Pico Kit). Small RNA (HY3, RNU48, U6) expression was assessed by TaqMan qRT-PCR. The stability of 8 endogenous controls (EC) (HY3, RNU48, miR-320, RNU6B, RNU19, U6, RNU38B and RNU43) was compared for urine and serum EV RNA (BestKeeper and NormFinder) and retrospectively validated in independent cohorts (serum n=55, urine n=50). Results: The Life Technologies precipitation reagent gave superior serum EV recovery compared to SBI reagent, as assessed by NTA size distribution, increased RNA concentration, and lower small RNA Ct values. Similarly, the NB Urine Exosome RNA Isolation Kit gave improved results for urine EV isolation compared to ultracentrifugation, when determined by the same parameters. For serum EV RNA isolation, the Qiagen miRNeasy™ RNA Isolation Kit gave suitable EV RNA concentrations compared to other kits, as assessed by Bioanalyzer and small RNA qRT-PCR. Small RNAs HY3 (SD=1.77, CoV=6.2%) and U6 (SD=2.14, CoV=8.6%) were selected as optimal ECs for serum EV miRNA expression analysis, while HY3 (SD=1.67, CoV=6.5%) and RNU48 (SD=1.85, CoV=5.3%) were identified as suitable for urine studies. Conclusions: This study identifies optimal methods for isolation of serum and urine EV RNA, and suitable ECs for normalization of qRT-PCR studies. Such reports should aid in the standardization of EV microRNA data, particularly for biomarker studies.
Poster 4
Circulating microRNAs as biomarkers for acute graft versus host disease
Rachel E. Crossland1, Jean Norden1, Kile Green1, Mateja Kralj Juric2, Hildegard T. Greinix2 and Anne M. Dickinson1
1Haematological Sciences, Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, UK; 2Department of Internal Medicine, Bone Marrow Transplantation, Medical University of Vienna, Vienna, Austria
Background: Graft versus host disease (GvHD) is a major cause of morbidity and mortality in haematopoietic stem cell transplantation (HSCT) and despite recent advances, the incidence remains high (20–50%). Acute GvHD (aGvHD) can double the cost of a transplant and severe disease is associated with 40–60% mortality. A novel, non-invasive diagnostic test that predicts for the aGvHD incidence and severity would enable more timely prophylactic therapy, reducing morbidity and health care costs. Recently, microRNAs have been demonstrated as informative biomarkers in bodily fluids, where they are protected from degradation by extracellular vesicle (EV) encapsulation or protein binding. In this study we profiled patient serum (n=12) for microRNA (n=800) expression at aGvHD diagnosis, using nCounter technology. Potential diagnostic microRNAs were assessed for their prognostic potential prior to GvH onset, and the small extracellular vesicle (EV) fraction investigated. Materials and methods: A total of 12 serum samples were selected from patients undergoing allo-HSCT (2014). Total RNA was isolated from 1 ml serum (Norgen Biotek Total RNA kit) and assessed using the Bioanalyzer (RNA Pico Kit). RNA was profiled using the nCounter Human v3 miRNA Expression Assay Kit (NanoString Technologies). Individual microRNAs were evaluated in independent diagnostic (n=32) and prognostic (n=34, n=47) cohorts by TaqMan qRT-PCR. Small EVs were isolated using Total Exosome Isolation Reagent (Invitrogen) and total RNA isolated with the Total RNA kit (Norgen Biotek). Results: Sixty-one microRNAs were differentially expressed between aGvHD vs. noaGvHD patients by nCounter profiling (34/27 up/down-regulated in GvHD, p<0.05). Down-regulated microRNAs associated with GvHD/immunity (miR-146a, miR-30b & miR-374) were assessed by qRT-PCR in an independent cohort of diagnostic aGvHD serums, collected from a separate Institution (n=32). MiR-146a (p=0.005), miR-30b (p=0.004) & miR-374 (p<0.001) were significantly down-regulated in aGvHD at onset vs. no-GvHD, confirming their diagnostic potential. MiR-146a was assessed in patient serum samples taken prior to aGvHD onset (Day 14, n=34). Expression was significantly up-regulated in aGvHD vs. no-GvHD (p=0.02), significantly associated with disease grade (p=0.01) and validated in an independent cohort (n=47, p=0.05). Assessing D14 EVs, miR-146a was down-regulated in aGvHD vs. no-GvHD in a pilot (n=15, p=0.06) and validation (n=47, p=0.02) cohort. Work is ongoing to assess additional microRNAs of the nCounter signature. Conclusions: Results demonstrate the capacity for circulating microRNAs to act as diagnostic and prognostic biomarkers for aGvHD. Differential expression between whole serum and the EV compartment prior to aGvHD onset suggests a role for EV microRNAs in the biology of aGvHD, which warrants further investigation.
Poster 5
Differences in circulating extracellular vesicles between healthy volunteers and patients with established erectile dysfunction – do endothelial microvesicles play an ambivalent role?
Justyna Karolina Witczak1, Nicholas Burnley-Hall1, Fairoz Abdul1, Vitaliy Androshchuk1, Nicholas Ossei-Gerning2, Richard Anderson2, Aled Rees1and Philip James3
1Department of Endocrinology and Diabetes, Institute of Experimental and Molecular Medicine, School of medicine, Cardiff University, Cardiff, UK; 2Cardiovascular Biology and Medicine, University | Hospital of Wales, Cardiff, UK; 3Metabolic Health, Cardiff Metropolitan University, Cardiff, UK
Background: Plasma extracellular vesicles (EVs) may serve as biomarkers of cardiovascular disease (CVD). Erectile dysfunction (ED) and CVD share common pathophysiological mechanisms of which endothelial dysfunction is an early marker. Patients with ED may thus represent a risk group for CVD. We hypothesized that EVs might be elevated in patients with ED and/or display altered surface characteristics indicative of a heightened vascular risk. The aim of this project was to compare plasma EVs concentration and cellular origin in patients with ED±CVD with healthy volunteers (HV). Methods: Blood samples were obtained from patients with established ED (n=20, CVD n=14, no CVD n= 6) and healthy volunteers (n=21). EVs were isolated by ultracentrifugation as per local protocol. EVs size and concentration were determined by nanoparticle tracking analysis (Nanosight LM10); Time Resolved Fluorescence (TRF)-based ELISA was used to establish cellular origin using the following markers: CD41 platelets, CD11b leukocytes, CD235a erythrocytes, CD144 endothelial cells, CD9 exosomal marker. Results: There were no significant differences in EVs concentration between ED patients and healthy volunteers. However, analysis of cellular origin revealed significantly higher CD41 TRF signals in patients with ED vs. HV (12,090±754.7 vs. 8,210±941.7, p<0.005) and significantly lower CD144 TRF signals (1,185±203.5 vs. 5,511±1,072, p<0.001). There were no statistically significant differences in CD11b, CD235a and CD9 TRF signals (10122.7±4,042 vs 10737.8±12,459, p=ns, 2218.9±1643.6 vs. 3834.9±3899.3, p=0.096, 26,773±10,121 vs. 22,460±12,274, p=nsrespectively). In a sub-analysis of ED(+) CVD(−) compared to ED(+)CVD(+) samples, no further differences in EVs cellular origin were observed. Conclusions: A higher proportion of platelet-derived EVs in patients with ED is in keeping with ED as a disease state sharing a common vascular origin with CVD. The role of endothelium-derived EVs has recently been questioned. Traditionally, they were believed to be a marker of endothelial damage but recent data suggest their role in vascular homeostasis is much more complex and in fact, they might support endothelial regeneration and counteract coagulation.
Poster 6
Vascular smooth muscle cell exosomes thrombogenic activity is regulated by prothrombin recycling
Alexander N. Kapustin1, Leon J. Schurgers2, Michael Schoppet3, Paul Harrison4 and Catherine M. Shanahan1
1BHF Centre of Research Excellence, King's College London, London, UK; 2Cardiovascular Research Institute CARIM, University of Maastricht, Maastricht, the Netherlands; 3Department of Internal Medicine and Cardiology, Phillips University, Enid, UK; 4School of Immunity and Infection, University of Birmingham, Birmingham, USA
Background: Rupture of the fibrous cap in atherosclerotic lesions is associated with thrombus formation initiated by phosphatidylserine (PS)-enriched microparticles thought to originate from apoptotic cells. We recently showed that loss of contractile phenotype by vascular smooth muscle cells (VSMC) induces exosomes secretion and here we tested VSMC exosomes thrombogenicity. Methods: VSMCs exosomes and apoptotic bodies (AB) were isolated by differential ultracentrifugation. To study uptake human PT was labelled with Alexa488 and incubated with VSMCs. Thrombin generation was measured by fluorescence spectroscopy (Fluoroskan Ascent FL, Thermo Electron Corporation). Results: We found that VSMC exosomes but not apoptotic bodies were enriched with tissue factor (TF) and able to bind prothrombin (PT) and stimulate thrombin generation via the prethrombin-2 pathway in a fashion similar to activated platelets. Notably, PT was rapidly taken up by VSMCs in a calcium-dependent manner and loaded into exosomes along with the potent thrombin inhibitor, protease nexin-1 (PN-1). Prothrombin loaded into VSMC exosomes was resistant to activation by factor Xa and decreased exosome thrombogenic activity most likely by occupation of available PS sites. Conclusions: Taken together this data indicates that VSMC exosomes are novel potent activators of the extrinsic coagulation pathway in the vasculature acting via a platelet-like mechanism. Exosome coagulation activity is negatively regulated by a feed-back loop involving PT internalization and recycling via the exosome pathway, which acts to block further PT activation thereby preventing overwhelming vascular thrombogenesis.
Poster 7
Osteocyte-derived microvesicles modulate adipocyte and osteoclast differentiation: a role for microvesicles in cell–cell communication in bone?
Nicole E. E. Scully1,2*, Georgios Kremastiotis1,3*, Rob D. Young4, Michael Stone5 and Bronwen A. J. Evans1,2
1Institute of Molecular and Experimental Medicine, School of Medicine, Cardiff University, Cardiff, UK; 2Arthritis Research UK Biomechanics and Bioengineering Centre, Cardiff University, Cardiff, UK; 3Faculty of Pharmacy, National and Kapodistrian University of Athens, Athens, Greece; 4School of Optometry and Vision Sciences, Cardiff University, Cardiff, UK; 5Bone Research Unit, University Hospital Llandough, Penarth, UK. *Joint first authors.
Background: Osteocytes in vivo are derived from osteoblasts and are embedded in the mineralized matrix of bone. They contribute to the regulation of bone turnover by signalling to other cell types, but these pathways are not fully understood. It is possible that some of these pathways involve extracellular microvesicles (MVs), although there is very little published literature on the production and function of MVs by bone cells. This study investigated the effects of conditioned medium (CM) and MVs derived from osteocytes on (a) adipocyte, (b) osteoblast and (c) osteoclast differentiation in vitro. Methods: Mouse IDG-SW3 CM collected during osteoblast to osteocyte differentiation (3–41 days) in monolayer CM was stored at −80°C or ultracentrifuged (100,000 rpm, 4°C, 2 h) to pellet MVs. MVs were characterized by (a) TEM, (b) nanoparticle tracking analysis (NTA) or (c) assessment of total protein concentration. For adipogenesis, 7F2 cells were cultured in α-MEM, 10% FCS, 50 µM indomethacin, 50 µg/ml ascorbate-2-phosphate and 10−7 M dexamethasone for 3–6 days and stained with Oil Red O. For mineralization the same cells were maintained in α-MEM, 10% FCS, 50 µg/ml ascorbate-2-phosphate and 10 mM β-glycerophosphate for 12 days and stained with Alizarin Red. For osteoclastogenesis, RAW cells were cultured in α-MEM, 10% FCS and were primed with RANKL (2 ng/ml) for 3 days, prior to treatment for 3 days and TRAP staining. Treatments in each differentiation assay were either CM (10–60%), MV-depleted CM following ultracentrifugation, or MVs (20–150 µg). Results: IDG-SW3 osteocytes secrete particles that resemble MVs as shown by TEM. The particle concentration in CM collected during osteocyte differentiation (13–41 days) was 1.48+e08 – 3.8+e08/ml, with a mean size of 194.29 nm. The protein concentration in MV preparations ranged from 250 to 400 µg/150 µl. Adipogenesis was increased (p<0.05) by CM (day 7–30) and by MVs (20 or 40 µg) from day 14 (p<0.05, 40 µg) or day 17 (p<0.05, 20 µg) onwards. However, adipogenesis was reduced (p<0.01) by MV-depleted CM for days 7–21. Mineralization was not modulated by CM or MV-depleted CM collected on days 27 and 30. Following RANKL priming, MVs (100–150 µg) increased (p<0.01) osteoclastogenesis, whereas MV-depleted CM did not have any effect. Conclusion: Osteocytes secrete large numbers of protein-containing MVs. Both CM and MV preparations stimulated adipogenesis, but MV-depleted CM had a reduced effect when compared to non-depleted medium, indicating a role for these MVs in adipogenesis. The results also indicate that MVs have a potential role in osteoclastogenesis. This work supports the hypothesis that osteocyte-derived MVs play a role in osteocyte communication with other cell types.
Poster 8
Excluded on request of the authors
Poster 9
Excluded on request of the authors
Poster 10
Investigation of exosome miRNA content, stoichiometry and functionality in a human neural stem cell line
Lara Stevanato, Lavaniya Thanabalasundaram, Nickolai Vysokov and John Sinden
eNeuron, Surrey Research Park, Guildford, UK
Background: Exosomes (30–100 µm) are small membrane vesicles secreted by a variety of cell types and have only recently emerged as a new avenue for cell-to-cell communication. They are natural shuttles of RNA and protein cargo, making them attractive as potential therapeutic delivery vehicles. MicroRNAs (miRNA) are short non-coding RNAs and regulate biological processes. Here we characterized the miRNA contents of exosomes derived from human neural stem cells (hNSCs). Our studied hNSC is a clonal conditionally immortalized cell line suitable for banking according to quality controlled good manufacturing practice (GMP). HNSCs have shown multipotent capacity to ameliorate neurological vasculature deficits and blood flow in the cerebral ischaemia and in the limb ischaemia rodent model, respectively, and have been approved for stroke disabilities and critical limb ischaemia clinical trials. Methods: Identification and characterization of ultracentrifuged hNSC exosomes were performed using nanoparticle tracking analysis, qNano and western blot. Next generation sequencing (NGS) was utilized to assess exosomal miRNA content and compare it to cellular content. Real-time PCR was adopted to investigate stoichiometry. Validation of miRNA functionality was performed using a miRNA/mRNA 3’ untranslated target region (3'UTR) dual luciferase system. Results: By using NGS we identified the presence of a variety of miRNAs. Many of these miRNAs were enriched in exosomes indicating that cells specifically sort them for extracellular release. Although exosomes have been proven to contain miRNAs, the quantification in terms of copy number of a specific miRNA type per exosome is unclear. In here we quantified the copy number of an exosomal up-shuttled miRNA subtype (hsa-miR-1246) and calculated its stoichiometry per exosome. Furthermore, we developed an in vitro system to empirically investigate its functionality and confirmed that the exosome preparation could transfer functional moiety of miRNA in recipient cells. Conclusions: NGS analysis allowed the identification of a unique set of hNSC-derived exosomal miRNAs. Stoichiometry and biological functional analysis of one of the most abundant identified miRNA, hsa-miR-1246, was measured to support therapeutic potential of exosomal miRNA delivery.
Poster 11
Mining the sorting machinery of exosomal miRNAs in neural stem/precursor cells
Nunzio Iraci, Tommaso Leonardi, Anton J. Enright and Stefano Pluchino
MBL-EBI, University of Cambridge, Cambridge, UK
Background: Neural stem/precursor cell (NPC) transplantation protects the central nervous system from inflammatory damage via cell-to-cell communication mechanisms. Recent works suggest that the exosome-mediated transfer of molecules such as miRNAs might play an important role in mediating the protective effect of NPCs. Here we aim to identify the machinery that sorts miRNAs to exosomes in murine NPCs. Our hypothesis is that such a mechanism might work: (a) at the transcriptional level, with transcription factors (TFs) driving specifically the transcription of exosomal miRNAs; or (b) at the post-transcriptional level, with carrier proteins that recognize specific miRNAs, bind to them and export them to exosomes. Methods: We used RNA-Seq to identify miRNAs significantly more abundant in exosomes than parental cells. To address whether specific TFs drive the transcription of secreted miRNAs, we first identified their putative promoters and then we tested if any TF binding site is enriched in these regions. In parallel, we used a variety of motif enrichment tools available in R/Bioconductor (Cosmo, BCRANK, motifRG) to find short motifs enriched in secreted miRNAs. Results: We found that no specific TF binding site is enriched in the promoters of secreted miRNAs. However, we identified 2 short motifs over-represented in exosomal miRNAs, one of which matches the binding sequence of hnrnpa2b1, which previous works have shown to be involved in miRNA secretion. Using western blot we found that hnrnpa2b1 is present within NPC-derived exosomes, suggesting that even in our cellular context this RNA binding protein may be involved in miRNA secretion. Conclusion: The exosomal miRNAs do not seem to be regulated by specific TFs. At the moment we are validating the role of the 2 motifs in relation to the candidate carrier hnrnpa2b1 for miRNA secretion in NPCs. Altogether, this work will help to shed light on the molecular mechanism behind miRNA trafficking and on its implication on the therapeutic effect of transplanted NPCs.
Poster 12
miR-134 in extracellular vesicles reduces TNBC aggression and increases drug sensitivity
Michelle C. Lowry1, Keith O'Brien1, Claire Corcoran1, Vanesa G. Martinez1, Melissa Daly1, Sweta Rani1, William M. Gallagher2, Marek W. Radomski1, Roderick A. F. MacLeod3 and Lorraine O'Driscoll1
1School of Pharmacy and Pharmaceutical Sciences & Trinity Biomedical Sciences Institute, Trinity College, Dublin, Ireland; 2Cancer Biology and Therapeutics Laboratory, Conway Institute, UCD School of Biomolecular and Biomedical Science, Dublin, Ireland; 3Leibniz Institute DSMZ, German Collection of Human and Animal Cell Cultures, Braunschweig, Germany
Background: Exosomes (EVs) have relevance in cell-to-cell communication carrying pro-tumorigenic factors that participate in oncogenesis and drug resistance and are proposed to have potential as self-delivery systems. Advancing on our studies of EVs in triple-negative breast cancer (TNBC), here we comprehensively analysed isogenic cell line variants and their EV populations, as well as breast tumour and normal tissues. The aim of this project was to profile the miRNA contents of both TNBC-derived EVs and cells to identify miRNAs which may have therapeutic potential in TNBC and to exploit these EVs as therapeutic miRNA delivery vesicles to reduce TNBC aggression and increase TNBC drug sensitivity. Methods: Assessing miRNA content in TNBC isogenic cell line variants Hs578T and Hs578Ts(i)8 using low density arrays representing 384 miRNAs. Using GEO2R, assessing miR-134 levels in breast tumour vs. normal breast tissue using 2 publically available tumour datasets, GSE26659 and GSE40525. Investigating transcriptional silencing at chromosome 14q32.2 using qRT-PCR for the neighbouring MEG3/DLK1 loci. Investigating the effects of directly over-expressing miR-134 in TNBC cell lines to assess TNBC aggression (proliferation) and cisplatin drug sensitivity (Annexin/PI assay). Investigating the ability of EVs to transport miRNAs of choice to secondary cells, to determine cell aggression (migration and invasion assays) and sensitivity to anti-cancer drugs. Results: miRNAs profiling showed EV miRNA content to be highly representative of their cells of origin. miRNAs most substantially down-regulated in aggressive cells and their EVs originated from 14q32. Using 2publically-available tumour datasets, GSE26659 and GSE40525 analysis of miR-134, the most substantially down-regulated miRNA, supported its clinical relevance in breast tumours compared to matched normal breast tissue. Functional studies indicated that miR-134 controls STAT5B which, in turn, controls Hsp90. miR-134 delivered by direct transfection into Hs578Ts(i)8 cells (in which it was greatly down-regulated) reduced STAT5B, Hsp90, and Bcl-2 levels, reduced cellular proliferation, and enhanced cisplatin-induced apoptosis. Delivery via miR-134-enriched EVs also reduced STAT5B and Hsp90, reduced cellular migration and invasion, and enhanced sensitivity to anti-Hsp90 drugs. Conclusion: While the differing effects achieved by transfection or EV delivery are likely to be, at least partly, due to specific amounts of miR-134 delivered by these routes, these EV-based studies identified miRNA-134 as a potential biomarker and therapeutic for breast cancer.
Conflict of interest and funding
Irish Cancer Society's support of Breast-Predict [CCRC13GAL]; Marie Keating Foundation; HRB of Ireland [HRA_POR/2013/342]; HEA PRTLI Cycle 5 funding of TBSI; and EU H2020 funding of the Cooperation in Science and Technology ME-HAD [BM1202].
Poster 13
Excluded on request of the authors
Poster 14
Extracellular vesicle signalling in the oral cancer microenvironment
Mark Ofield, Daniel Lambert and Stuart Hunt
nit of Oral and Maxillofacial Pathology, The School of Clinical Dentistry, University of Sheffield, Sheffield, UK
Background: Oral cancer mortality rates have increased by 10% in the last decade. Efforts to reverse this are hampered by a limited understanding of the underlying molecular complexity of the disease. Recently, interest has grown in the contribution of extracellular vesicles (EVs) to cancer pathogenesis. EVs are produced by most cell types, but are produced in much higher quantities by cancer cells. Since the discovery of mRNA and miRNA in EVs they have been considered as an extracellular signalling system capable of exerting effects on local or distant cells. Developing tumours exist as a complex milieu comprising multiple cell types each capable of producing a range of different EVs with pleiotropic functions. The aim of this work is to explore the role of EVs and in particular their RNA cargo, in oral cancer progression. Methods: EVs were extracted from the culture media of oral cancer cell lines using ultracentrifugation or size exclusion chromatography and then characterized using a combination of transmission electron microscopy, tuneable resistive pulse sensing and western blotting. EVs were labelled with fluorescent markers and transferred to cells of a different line to examine the ability of EVs to transfer RNA. Results: We have successfully isolated EVs from a panel of cell lines representative of the stages of oral cancer development and confirmed their presence by western blot and transmission electron microscopy showing them to be between 50 and 200 nm and bearing common markers including CD63. Using fluorescent dyes, the horizontal transfer of RNA between oral cancer cells and normal stromal cells has been visualized. Conclusion: Using a combination of techniques the existence of an EV transfer network in the oral cancer microenvironment has been revealed. Future work will identify the RNA and protein cargo of the isolated EVs in order to reveal the roles they play in oral cancer progression.
Poster 15
Excluded on request of the authors
Poster 16
Identification of key components in exosome secretion and stromal activation in cancer
Vincent Yeung, Jason Webber and Aled Clayton
ivision of Cancer and genetics, School of Medicine, Cardiff University, Cardiff, UK
Background: Exosomes (50–100 nm) are secreted by many cell types and are formed within intraluminal vesicles of multivesicular bodies (MVBs), which are released upon plasma membrane fusion. Exosomes appear to contribute to the cross-talk between cancer and stromal cells generating a tumour-supporting stromal phenotype. Our group demonstrated that prostate cancer cell (Du145)-derived exosomes triggers myofibroblastic differentiation, resembling stromal cells isolated from cancerous prostate tissue. These cells supported angiogenesis in vitro and accelerated tumour growth in vivo. However, the molecular factors controlling exosome secretion are poorly understood. The most well-characterized factor-Rab27a modulates exosome secretion in prostate cancer cells, and its knockdown attenuates cancer to stroma communication blocking myofibroblast differentiation. Here, we examined the roles of some other putative exosome-regulating factors (CD9, Rab5a, Rab11b, Rab35, VAMP7 and VPS25) for their roles in exosome biogenesis/secretion and their importance in stromal activating capacity. Methods: The role of these 6 genes in exosome biogenesis/secretion was investigated using a short hairpin RNA (shRNA) lentiviral-based silencing approach in Du145 cells. Nanosight tracking analysis (NTA) and ELISAs were performed to examine exosome secretion levels in all Du145 knockdowns (Du145KDs). Cell viability and their functional capacity to drive fibroblast differentiation were studied in 2D cell models. To assess the functional impact of Rab27a silencing, a 3D-heterotypic cell spheroid model was established by mixing Du145 cells with primary stromal fibroblasts in cell-repellent surface plates. Tumour invasiveness was assessed by monitoring outgrowth from established spheroids cultured on matrigel-coated plates. Results: We show successful target knockdown at both the mRNA and protein level. Knockdown of the VPS25 gene led to some reduced cell viability, but morphologically all Du145KDs look normal. Interestingly, some of the Du145KDs (Rab35 and VPS25) appear to reduce exosome secretion levels as determined via NTA and ELISAs. Conditioned media from all Du145KDs cells appear to be deficient in factors driving fibroblast differentiation. In a 3D co-culture model, fibroblasts and exosome-competent tumour cells were required for robust tumour invasion into a matrix. Conclusion: We demonstrate that stromal cells are required for the invasive properties of tumours. However, this effect requires exosome secretion by cancer cells. Future work will investigate each target for its relevance in this model, and establish the importance of exosome secretion in stromally driven disease progression.
Poster 17
Characterization of the endocytic uptake of prostate-derived exosomes
Hope Roberts Dalton1, Edel Brown1,2, Jason Webber2, Peter Watson3, Aled Clayton2 and Arwyn Tomos Jones1
1Cardiff School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, UK; 2Institute of Cancer & Genetics, Velindre Cancer Centre, Cardiff University, Cardiff, UK; 3School of Biosciences, Cardiff University, Cardiff, UK
Background: Association of macromolecular therapeutics to vectors that increase cell uptake and protect from extracellular and intracellular degradation is a vital step in the creation of more efficient drug delivery systems for intracellular targets. Exosomes are natural, extracellularly expelled components of the endocytic system that are often loaded with materials such as nucleotides, peptides and proteins. These are the very entities that show promise as biopharmaceuticals. The fact that exosomes interact with and deliver macromolecules such as miRNA to cells to mediate a physiological effect suggests that they have potential as drug delivery vectors. However, the mechanisms by which exosome-cell interaction, route of cell entry and possible escape from the endolysosomal system occur, are unknown. The aim of our work is to characterize the cell uptake mechanism of exosomes in order to provide a greater understanding of how they may be able to deliver macromolecular cargo into the cytosol to mediate therapeutic effects. Methods: Prostate-derived exosomes were purified (1) and then conjugated to a fluorescent label to allow cell uptake analysis via live cell imaging confocal microscopy. To access the extent of involvement of different endocytic pathways, siRNA transfection targeting specific endocytic proteins and pharmacological inhibitors of endocytosis were utilized. Uptake was analysed in HeLa cells due to the fact that their endocytic profile has been extensively studied in the laboratory (2). Results and discussion: Exosomes were effectively internalized into HeLa cells and located in highly motile punctate structures scattered around the cytoplasm. Depletion of key endocytic proteins via siRNA indicated that proteins implicated in macropinocytosis (a growth factor-activated, actin-dependent form of constitutive fluid-phase endocytosis) may be involved in the uptake of these structures. Furthermore, the use of pharmacological inhibitors previously used to study macropinocytosis (EIPA, Rottlerin, IPA-3) also suggests the involvement of this pathway. These studies also extended to analysis of dextran, the fluid-phase endocytic probe that is also utilized to measure macropinocytosis. Currently it is difficult to distinguish fluid-phase uptake from constitutive and stimulated macropinocytosis. Complete characterization of the ways that exosomes gain intracellular access will help to unlock their potential for drug delivery, allowing their exploitation for therapeutic use. Ultimately, this work aids an overall aim to improve the in vitro characterization and in vivo delivery of macromolecular therapeutics across biological barriers.
References
1. Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302.
2. Al Soraj M, He L, Peynshaert K, Cousaert J, Vercauteren D, Braeckmans K, et al. siRNA and pharmacological inhibition of endocytic pathways to characterize the differential role of macropinocytosis and the actin cytoskeleton on cellular uptake of dextran and cationic cell penetrating peptides octaarginine (R8) and HIV-Tat. J Control Release. 2012;161:132–41.
Poster 18
Proteomics analysis of exosomes isolated from plasma and urine of prostate cancer patients
Joanne L. Welton1,2, Paul Brennan3, Mark Gurney1, Jason P. Webber1, David Gil4, Juan M. Falcón-Pérez4, Sean P. Walton5, Malcolm D. Mason1 and Aled Clayton1
1Division of Cancer and Genetics, School of Medicine, Cardiff University, Cardiff, UK; 2Biomedical Sciences, Cardiff School of Health Sciences, Cardiff Metropolitan University, Cardiff, UK; 3Division of Cancer and Genetics, School of Medicine, Cardiff University, Cardiff, UK; 4Metabolomics Unit, CIC bioGUNE, CIBERehd, Bizkaia Technology Park, Derio, Spain; 5College of Engineering, Swansea University, Swansea, UK
Background: There is an ever-increasing interest in biofluid exosomes as a biomarker source; however, a major challenge for proteomics is isolating sufficient high purity exosomes for analysis. When using mass spectrometry (MS) removal of abundant non-exosomal protein is imperative. The aims were to: develop biofluid exosome isolation methods, assess sample purity, and to test the workflow using prostate cancer (PCa) patient-derived material. Methods: Healthy donor plasma and urine was used as an exosome source to test isolation methods including: ultracentrifugation (UC), filtration, gradient UC, and size exclusion chromatography (SEC). Purity was tested using: ELISA-like assays, cryo-electron microscopy, western- blot and nanoparticle tracking. We used a novel aptamer-based protein array (SOMAscan™) for proteomics analysis. Results: Isolating exosomes from plasma using UC and gradient UC insufficiently removed plasma proteins and gave inadequate purity, measured by particle/protein (P/P) ratio. The use of SEC, however, removed >95% of contaminating protein and effectively separated EV-associated tetraspanins from serum albumin. Unfortunately, even with an increase in sample purity this was still insufficient to generate high quality datasets by LC/MALDI MS analysis (21 identifications, mostly plasma proteins). However, with the SOMAscan™ platform we were able to identify hundreds of proteins that were identified from urine- and plasma-exosome samples. Some abundant non-vesicular proteins were still identified, particularly in plasma, but this did not impede the identification of many vesicle-associated proteins. Subsequently we applied this workflow to paired urine and plasma samples from newly diagnosed metastatic PCa with patients with progressive disease. We discovered that over 60 ml of urine was required in order to obtain the minimum amount of protein required for the proteomic analysis, but 1.5 ml plasma was sufficient. Based on this small pilot dataset (plasma n=11; urine n=5) we used cluster analysis to examine the closest groups of patients, identifying numerous proteins that may be of interest in progressive PCa. These included immunological proteins, kinases and HSP cognate proteins. Discussion: The workflow developed shows the removal of >95% of contaminating protein and the enrichment of exosomes. Upon analysis of the samples by SOMAscan™ array it is possible to identify hundreds of proteins that discriminate plasma from urinary vesicles, and also a set of proteins of potential association with progressive PCa. This study has demonstrates the practical utility of SEC exosome isolation coupled with the SOMAscan™ assay for proteomics analysis of biofluid exosomes in clinical settings.
Poster 19
Utilizing stroma-derived exosomal-mRNAs as biomarkers for prostate cancer
Jason Paul Webber1, Alexandra Shephard1, Joanne Welton2, Lisa Spary1, Malcolm Mason1, Zsuzsanna Tabi1 and Aled Clayton1
1Division of Cancer and Genetics, School of Medicine, Cardiff University, Cardiff, UK; 2Biomedical Sciences, Cardiff School of Health Sciences, Cardiff Metropolitan University, Cardiff, UK
Background: Much of the research into biomarkers for prostate cancer has focused specifically on cancer cells. The tumour microenvironment is, however, very complex and we may be overlooking other potential sources of disease biomarkers. During disease the stromal tissue, surrounding glandular structures of the prostate, becomes aberrantly altered leading to the formation of a reactive stroma. This is often regarded as a rate limiting step in disease, and is typically associated with treatment-resistant disease and poor patient outcome. Such cells, present within the tumour microenvironment, secrete exosomes (nanometre-sized vesicles) that are detectable within the patient's circulatory system. Stroma-derived exosomes may therefore provide an attractive source of novel biomarkers, capable of distinguishing slow growing tumours from aggressive disease. The aim of the current study was to compare exosome-derived mRNA from normal, or tumour-associated, stromal cells in order to identify possible future biomarkers for aggressive prostate cancer. Methods: Patient-matched normal and tumour-associated stromal cells were cultured from needle biopsies of radical prostatectomy specimens, taken from disease-free or cancerous regions respectively. Exosomes were isolated by serial filtration and ultracentrifugation, prior to determining exosome size and concentration by nanoparticle tracking analysis (NanoSight; Malvern Instrument Ltd). Following phenol-based extraction, exosomal mRNA was analysed by quantitative RT-PCR using the RT2 PCR profiler array (Qiagen). Finally, mRNA expression of potential biomarkers was assessed in serum-derived exosomes, isolated by size exclusion chromatography (Exo-spin™; Cell Guidance Systems). Results: Tumour-associated stromal cells secrete twice as much exosome-associated mRNA compared to matched normal stromal cells. Interestingly, our data also suggests a selective enrichment of mRNA targets within exosomes compared to the cell of origin. From the 84 genes tested, we identified more than 30 (e.g. Gremlin-1, TGFbeta1, STAT1, HGF & VEGF) that were elevated in tumour stroma exosomes compared to exosomes from the normal stroma. We have demonstrated successful isolation of serum-derived exosomes, from which we can detect several mRNA targets. Conclusion: This study not only highlights the potential of using exosome-derived mRNA targets as potential markers of aggressive prostate cancer, but specifically mRNA targets present within stromal cell-derived exosomes. Future work shall continue to validate key mRNA targets, identified within the current study, and explore whether such biomarkers could distinguish indolent from aggressive forms of disease.
Poster 20
The role of heparan sulphate proteoglycans in exosome-induced stromal cell differentiation
Alexandra Shephard1, Joanne Welton2, Lisa Spary1, Malcolm Mason1, Zsuzsanna Tabi1, Aled Clayton1 and Jason Paul Webber1
1Division of Cancer and Genetics, School of Medicine, Cardiff University, Cardiff, UK; 2Biomedical Sciences, Cardiff School of Health Sciences, Cardiff Metropolitan University, Cardiff, UK
Background: The stromal tissue surrounding carcinomas is radically altered and consists of cells with a myofibroblasts-like phenotype. The presence of such cells is often regarded as a rate limiting step in prostate cancer, and is associated with both treatment-resistant disease and poor patient outcome. Whilst mounting evidence points to a role of transforming growth factor beta (TGFβ), the exact mechanism regulating the onset of this disease-associated stromal phenotype remains unclear. We have previously demonstrated that exosomes can deliver TGFβ and induce stromal cell differentiation to a disease-like phenotype. Furthermore, we have implicated heparan sulphate chains, present on the exosome surface, in the delivery of functional TGFβ. Here we characterize the expression of heparan sulphate proteoglycans (HSPGs) present on prostate cancer exosomes and explore the relationship between HSPGs and exosome-induced stromal cell differentiation. Methods: Prostate cancer exosomes, isolated by flotation within 30% sucrose/D2O, were characterized by immune-fluorescent analysis, nanoparticle tracking and western blot. A lentiviral-based shRNA approach was used to selectively knockdown HSPGs in prostate cancer (Du145) cells. Patient-matched normal or tumour-associated stromal cells were obtained from prostatectomy specimens. Myofibroblast differentiation was determined by the onset of α-smooth muscle actin (αSMA), and angiogenic function was assessed by co-culture with endothelial cells (HUVECs). Results: Exosomes from different prostate cancer cell lines express variable levels of TGFβ and we demonstrate a strong positive correlation between levels of TGFβ and HSPGs present on the exosome surface. TGFβ-high exosomes express syndecan 3, syndecan 4, glypican 1, glypican 6 and betaglycan. We have generated prostate cancer cell lines that secrete exosomes lacking specific HSPGs. These HSPG-deficient exosomes show a reduced ability to drive stromal cell differentiation. Conclusion: Exosomal, not soluble, delivery of TGFβ is essential for generating a disease-like stroma. This exosome function is dependent on heparan sulphate proteoglycans, such as betaglycan, present on the exosome surface. Exosomal-HSPGs may therefore represent novel targets for attenuating tumour growth.
Poster 21
Developing a novel means to detect prostate cancer exosomes from serum
Alexandra Shephard1, Joanne Welton2, Malcolm Mason1, Zsuzsanna Tabi1, Aled Clayton1 and Jason Paul Webber1
1Division of Cancer and Genetics, School of Medicine, Cardiff University, Cardiff, UK; 2Biomedical Sciences, Cardiff School of Health Sciences, Cardiff Metropolitan University, Cardiff, UK
Background: There is considerable interest in the use of exosomes as a potential source of biomarkers in a variety of diseases, including prostate cancer. The application of exosomes for this purpose is, however, hindered by the difficulty of detecting cancer-derived exosomes from other vesicles present within biofluids. In addition, the presence of highly abundant soluble proteins can interfere with downstream analysis of isolated exosomes, and even lead to erroneous data. We have previously demonstrated the use of Sepharose-based size exclusion chromatography columns as a means of isolating exosomes from plasma. Furthermore, we have identified a role of heparan sulphate proteoglycans (HSPGs), present on the surface of prostate cancer exosomes, in facilitating tumour growth in vivo. Here we demonstrate both the successful isolation of exosomes from serum and development of an assay capable of detecting HSPGs present on exosomes isolated from serum. Methods: Cell-free (donor) serum was spiked with cell culture-derived prostate cancer exosomes. Exosome isolation, from serum, was achieved using a Sepharose-based size exclusion chromatography column (Cell Guidance Systems). From each column we collected 30 fractions, each 500 µl, eluted from the column using 1.8 mg/ml EDTA in PBS. The presence of both exosome-associated and soluble proteins present within the fractions was examined by immuno-fluorescent analysis of fractions. Exosome concentration for each fraction was then determined by nanoparticle tracking analysis (NanoSight; Malvern Instruments Ltd), and total protein content of each fraction was measured by BCA assay (Pierce; ThermoFisher Scientific). The ratio of particles: protein was then used as a measure of exosome purity. Results: Positive detection of CD81 within distinct fractions confirmed the detection exosomes within the spiked serum samples. The exosome containing fractions were distinct from those containing human serum albumin (HAS), and the particle: protein ratios were elevated in the exosome containing fractions, suggesting successful separation of exosomes from the bulk of the soluble proteins present within serum. Finally, we were able to detect elevated levels of HSPGs, including syndecan 4 plus glypicans 1 and 6, within the exosome containing fractions. Conclusion: Column chromatography-based approaches offer a relatively fast and simple means of isolating exosomes from biological fluids. We have developed a method for the detection of HSPGs present on exosomes isolated from serum. Future studies shall explore whether exosomal-HSPGs can serve as markers capable of distinguishing patients with prostate cancer.
Poster 22
Tumour exosomes generate CD39+CD73+ double positive dendritic cells with impaired pro-inflammatory cytokine production
Josephine Chipo Salimu, Jason Paul Webber, Malcolm Mason, Aled Clayton and Zsuzsanna Tabi
ancer and Genetics, School of medicine, Cardiff University, Cardiff, UK
Background: Exosomes are a distinct population of extracellular vesicles of endocytic origin which express a phenotype and protein repertoire similar to the cell of origin. Tumour-derived exosomes harbour the immunosuppressive characteristics of tumour cells, such as inhibition of IL-2 production by T cells (1). However, there is relatively little known about the effect of exosomes on antigen presenting cells such as dendritic cells (DC), which was the subject of this work. Methods and results: We studied the effect of exosomes, isolated from prostate cancer cells (DU145 cell line), on DC function. For this, we used DU145 cells where Rab27a had been knocked down (DU145 Rab27aKD) or treated with an empty vector (DU145 Control). Rab27a is a molecule responsible for the release of exosomes from intracellular multivesicular endosomes. Knockdown of Rab27a drastically attenuated the secretion of exosomes. Cross-presentation of the tumour-associated antigen 5T4 from DU145 Rab27aKD cells generated significantly stronger anti-5T4 T cell responses compared to that from DU145 Control cells. These enhanced T cell responses were reduced again when tumour-derived exosomes were added back to the cross-presentation model incorporating DU145 Rab27aKD cells. We have excluded the direct effect of exosomes on T cell function in this model, so the results suggested that exosomes exert a negative effect on antigen presenting cells. When the phenotype of DC exposed to exosomes was studied, we found that a significant proportion of DC expressed CD73, an ecto-5-nucleotidase responsible for AMP to adenosine hydrolysis. DC and monocytes express CD39 (ectonucleotidase responsible for ATP-to-AMP hydrolysis), however CD73 expression on DC has not been demonstrated previously. Coexpression of CD39 and CD73 makes exosome-treated DC uniquely capable of catalysing pro-inflammatory ATP from the tumour microenvironment and replacing it with immunosuppressive adenosine. Functionally, exosome-treated DC produced significantly less IL-12 upon stimulation in the presence of ATP than control DC. This cytokine shift may have further consequences, such as suboptimal T cell activation. Conclusion: Taken together, the results reveal a hitherto unknown effect of tumour exosomes on DC, representing a significant immunosuppressive influence on tumour cell–immune cell cross-talk.
Reference
1. Mitchell JP, Court J, Mason MD, Tabi Z, Clayton A. Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. Journal of immunological methods. 2008;335(1):98–105.
Poster 23
Effect of extracellular vesicles on survival, proliferation, migration and chemoresistance of B-cells in chronic lymphocytic leukaemia
Emerence Crompot, Michael Van Damme, Karlien Pieters, Dominique Bron, Nathalie Meuleuman, Philippe Mineur, Laurence Lagneaux and Basile Stamatopoulos
ules Bordet Institute/ULB, Bruxelles, Belgium
Background: The interactions between chronic lymphocytic leukaemia (CLL) cells and the microenvironment (primarily composed by mesenchymal stromal cells – MSC) play an important role in promoting the increased survival of leukemic B cells. Extracellular vesicles (EVs) produced by leukemic cells and the microenvironment may be implicated in this cross-talk. EVs, including microparticles and exosomes, are small plasma membrane fragments with sizes ranging from 0.01 to 1 m, and contain products specific to the original cell, such as microRNA, mRNA and proteins. Our objective is to assess the role of EVs in the cross-talk between malignant cells and their microenvironment. Methods: Ultracentrifugation at 150,000×g during 1 h was applied to isolate EVs from supernatant of MSC culture. Protein concentration was measured by BCA kit and NanoDrop. Different concentrations of EVs were added to CLL-B-cells to evaluate their impact on cell survival. PKH67 labelling and qRT-PCR were performed to prove the inclusion of EVs in CLL B-cells (n=18). Results: We first demonstrated that EVs from MSCs are able to enter in CLL B-cells. By flow cytometry with PKH67-labelled EVs, we observed that 44.2, 93.8 and 100% of CLL B-cells had integrated fluorescent EVs after 1, 3 and 24 h respectively. Two highly expressed mRNA (collagen and fibronectin) in MSC, also detected in MSC-derived EVs by qRT-PCR, and were increased in CLL B-cells after 24 h of incubation with EVs confirming EV-mediated mRNA transfer to target cells. Further analysis of apoptosis in CLL cells was assessed by flow cytometry using an Annexin/7AAD staining: addition of increasing concentrations of EVs showed a protective effect on CLL B-cells from cell death (n=21/p<0.0001). We also studied the effect of EVs from MSC on proliferation (CFSE labelling), migration (Boyden chamber) and chemoresistance of CLL B-cells after 24 h of contact. Conclusion: We demonstrated, using 2 methods, that MSC-derived EVs enter into CLL B-cells. These vesicles protect CLL cells from spontaneous apoptosis and affect proliferation, migration and chemoresistance capacities of CLL B-cells. Further analyses will determine implication of EVs in several cell functions. This study provides evidence of the critical role played by EVs in the interactions between leukemic cells and their microenvironment.
Poster 24
Neutrophil microvesicles confer a chondrogenic, alternative activation phenotype in human macrophages via phosphatidylserine and annexin A1
Hefin Rhys Jones1, Sarah Headland2, Lucy Norling1 and Mauro Perretti1
1William Harvey Research Institute, Barts and the London School of Medicine, Queen Mary University of London, London, UK; 2Physiology, School of medicine, University of California, San Fransisco, USA
Background: Despite their propensity to promote inflammation, neutrophils are one of few cell types whose microvesicles can promote tissue protection. Activated neutrophils release large numbers of microparticles which promote the resolution of inflammation by affecting the function and phenotype of other inflammatory cells. Of these, tissue macrophages are central to the recovery of homeostasis after an inflammatory insult, although the effect of microvesicles on these cells is not fully understood, particularly in the context of rheumatoid arthritis. Methods: Neutrophils were stimulated for 20 min with 50 ng/ml TNF or diluted synovial fluid (vesicle-depleted) from rheumatoid arthritis patients and their microvesicles were enriched by differential centrifugation and enumerated using an ImageStream MkII as previously described (1). Macrophages were differentiated from human blood monocytes in the presence of M-CSF and phenotyped using flow cytometry and cytokine bead array. The C28/I2 chondrocyte 3D micromass culture system was used to quantify changes in chondrocyte cartilage deposition in vitro. Results: Neutrophil-derived microvesicles impaired the classical activation of macrophages by LPS and IFN (dependent on phosphatidylserine exposure) but did not seem to promote an alternative phenotype in the time studied (despite a phosphatidylserine-independent induction of TGFβ release from macrophages). Macrophages activated with IL-4 or neutrophil microvesicles (generated with different RA relevant stimuli) promoted proteoglycan deposition/retention by chondrocytes, whereas untreated and inflammatory mediator-activated macrophages reduced deposition/increased degradation. The chondrogenic properties of macrophages treated with neutrophil microvesicles was diminished upon blockade of TGFβ or microvesicle-associated annexin A1, the latter of which induced release of the former. Conclusions: Neutrophils release large numbers of annexin A1+ microvesicles upon activation with inflammatory stimuli, which can resist the pro-inflammatory, classical activation of macrophages. Microvesicle-associated annexin A1 induces TGFβ release from macrophages which induces proteoglycan deposition in chondrocytes. We propose the endogenous presence and/or pharmacological use of neutrophil microvesicles as an autologous therapeutic may promote resolution and improve cartilage integrity in rheumatoid arthritis.
Reference
1. Headland SE, Jones HR, D'Sa ASV, Perretti M, Norling LV. Cutting-edge analysis of extracellular microparticles using ImageStreamX imaging flow cytometry. Nat Sci Rep. 2014;4:5237.
Poster 25
The characterization and functional analysis of regulatory T cell exosomes
Sim Tung, Nicole Cianci, Robert Lechler, Lesley Smyth and Giovanna Lombardi
RC Centre for Transplantation, King's College London, London, UK
Background: Regulatory T cells (Tregs) are a subset of CD4+ CD25+ FoxP3+ T cells that are vital in suppressing excessive immune responses. Tregs can inhibit the function of cells such as dendritic cells (DCs) or T-effector cells via various different mechanisms. One recently discovered mechanism is through the release of immune modulatory exosomes. Exosomes are nano-sized extracellular vesicles of endocytic origin that carry cargo, such as protein and microRNA (miRNA), the delivery of which to cells alters their phenotype and function. Recently, Okoye et al. showed that Let-7d was present in Treg exosomes and this was transferred into T-helper 1 cells which suppressed their proliferation and prevented systemic disease. Tregs can downregulate the activation receptors of DCs and reduce their cytokine production, thus suppressing an immune response. Our hypothesis is that Treg exosomes suppress DC functions by transferring bioactive materials such as inhibitory miRNAs. Methods: Exosomes were isolated from culture supernatants of TCR activated mouse CD4+ CD25+ FoxP3+ and CD4+ CD25+ FoxP3- T cells using ExoQuick solution. Total RNA was extracted from exosomes using SeraMir columns. A complete miRNA content analysis was performed using QuantStudio 12K Flex Real-time PCR system and OpenArray plates. Individual real-time PCR validation assays were performed and normalized using RNU6. DCs were co-cultured with or without Tregs or Treg-derived exosomes for 24 h before DCs were FACS sorted or purified and total RNA extracted for miRNA content analysis. Results: A complete miRNA screen was performed on exosomes isolated from CD4+ CD25+ FoxP3+ and CD4+ CD25+ FoxP3- T cells. We identified several miRNAs, including miR-142-3p and miR-150, as being expressed significantly higher in exosomes isolated from Tregs compared to T cells. Given that both miR-142-3p and miR-150 play important roles in DC maturation and function such as phagocytosis and cytokine release, we investigated whether acquisition of these miRNAs via Treg exosomes facilitated modulation of DCs cytokine release. We observed that DCs cultured in the presence of Tregs or Treg exosomes expression levels of both miR-142-3p and miR-150 were higher than DCs alone. This correlated with the reduced capacity of DCs to produce IL-12 following TLR ligation compared to untreated DCs. Conclusions: Our data confirms the presence of miRNAs in Treg exosomes and suggests that intercellular transfer of Treg miRNAs via exosomes may be a novel mechanism by which these cells regulate DC activity. Further work is needed to understand the messenger RNA targets of these miRNAs and the protein levels affected.
Reference
1. Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014 Jul 17;41(1):89–103. http://dx.doi.org/10.1016/j.immuni.2014.05.019
Poster 26
Regulatory T cell-derived extracellular vesicles are able to modulate dendritic cell maturation and function
Emily Rose Mavin, Lindsay Nicholson, Monica Reis, Rachel Crossland, Anne Dickinson and Xiao-nong Wang
aematological Sciences, ICM – Medical School, Newcastle University, Newcastle upon Tyne, UK
Background: Graft versus host disease (GvHD) remains a significant cause of morbidity and mortality following haematopoietic stem cell transplantation, therefore developing novel therapeutic strategies is the target of significant research. Clinical trials using regulatory T cells (Treg) have shown promising results for both prevention and treatment of GvHD; however, their mechanisms of action remain to be fully clarified. We have previously shown Treg are able to modulate DC function and therefore reduce GvH reactions in vitro. We hypothesized this modulation could be through the release of extracellular vesicles (EVs) with immuno-modulatory properties, providing the opportunity to develop a new form of Treg-mediated treatment for GvHD. Methods: Treg were isolated from leukocyte reduction system cones, due to the rarity of Treg cells in peripheral blood, ex vivo expansion of Treg for 2–3 weeks was required. All Treg were phenotypically and functionally characterized prior to use. Before EV isolation, Treg were cultured in EV-depleted medium for 48 h. EVs were isolated by differential ultracentrifugation, collecting both the 10,000 g and 100,000 g pellets. The EVs were then characterized using flow cytometry, transmission electron microscopy (TEM), BCA protein quantification and nanoparticle tracking analysis. The functional properties of EVs were investigated by treating monocyte-derived dendritic cells (moDC) with both the 10,000 g and 100,000 g pellets and assessing their phenotype, antigen processing capacity and ability to stimulate T cells. Results: EVs were successfully isolated from 50 to 200×106 Treg following anti-CD3/CD28 stimulation. Flow cytometry analysis showed enrichment of CD63, CD9 and CD81 on both the 10,000 g and 100,000 g pellets, with the highest levels on the 100,000 g pellet. TEM revealed the presence of EV, with those from the 100,000 g pellet having the typical cup-shaped morphology. Preliminary data has shown treatment with both the 10,000 g and 100,000 g EV pellets, resulted in a dose-dependent reduction in expression of co-stimulatory and maturation markers on moDC. Expression of CD38, CD80, CD83, CD86 and HLA-DR were all decreased following LPS maturation when compared to untreated moDC. Additionally the EV-treated moDC showed a reduction in antigen processing capacity, measured by FITC-dextran uptake. Conclusions: Preliminary results suggest that treatment of moDC with Treg-derived EVs alters their ability to mature in response to the TLR-4 ligand LPS which is likely to result in a reduction in their capacity to stimulate T cells. Further work is required to investigate the impact of this on GvH reactions in vitro.
Poster 27
Stability of extracellular vesicles during lyophilization – implications for their pharmaceutical use
Gregor Fuhrmann and Molly M. Stevens
epartment of Materials, Department of Bioengineering, Imperial College London, London, UK
Background: Recently, extracellular vesicles (EVs) have attracted substantial attention as promising drug carriers for various applications (1,2). They were shown to possess natural composition and inherent stability under physiological conditions (3). When developing EVs as pharmaceutical delivery systems, lyophilization (freeze-drying) plays an important role for their storage and transportation but to date this has been poorly investigated. Here, we assessed the fundamental and important implications of freeze-drying and addition of different cryoprotectants on EV size and stability. Methods: EVs were isolated from human mesenchymal stem cells (average cell number 3–5 M) cultured in serum-free medium (TheraPEAK™ medium, Lonza) for 48 h. Conditioned medium was subjected to differential centrifugation (20 min at 300×g and 4,200×g, respectively) with a final spin-down of EVs at 120,000×g at 4°C for 2 h, and resuspended in PBS. Samples were lyophilized or stored at 4 or −80°C. EVs were lyophilized as is or upon addition of polyethylene glycol (PEG, Mw 400) or mannitol (both 0.5–20% w/v). For size determination, all samples were diluted to 100 µl and analysed by nanoparticle tracking analysis and electron microscopy upon negative stain with uranyl acetate. Results: Upon lyophilization of EVs without any additives their average size was 192±39 nm, compared to 184±21 and 181±22 nm during storage at 4 and −80°C, respectively. Electron microscopy analysis indicated that freeze-drying did not significantly alter the size and morphology of EVs. The normalized particle number did not significantly change for direct lyophilization of EVs compared to storage at 4 or −80°C but variations between samples were observed. Addition of PEG induced a significant increase in EVs’ average size and particle distribution, indicating that the polymer may induce aggregation of EVs during lyophilization. When freeze-drying with cryoprotecting sugars such as mannitol, the EVs’ size distribution, and particle number remained stable up to 4% (w/v) of sugar indicating that low concentrations of mannitol offer suitable cryoprotection for EVs. Conclusions: Our results indicate that EVs exhibit natural stability during lyophilization, a characteristic further underlined by the addition of cryoprotecting sugars. With this work we are establishing the important basis for the successful use of EVs in a pharmaceutical setting.
References
1. Gregor Fuhrmann, Inge K. Herrmann, Molly M. Stevens. Cell-derived vesicles for drug therapy and diagnostics: Opportunities and challenges. NanoToday 10(3):397–409.
2. Gregor Fuhrmann, Andrea Serio, Manuel Mazo, Rekha Nair, Molly M. Stevens. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 2015 205;35–44.
3. EL Andaloussi S, Mäger I, Breakefield XO, Wood Mathew. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013 May;12(5):347–57. doi: http://dx.doi.org/10.1038/nrd3978
Poster 28
Potential alternatives to gold standard ultracentrifugation for extracellular vesicle isolation
Melissa Daly1, Susan Breslin1, Norma O'Donovan2, John Crown3, Edna McDermott3 and Lorraine O'Driscoll1
1School of Pharmacy and Pharmaceutical Sciences & Trinity Biomedical Sciences Institute, Trinity College Dublin, Ireland; 2National Institute for Cellular Biotechnology, Dublin City University, Dublin; 3St Vincent’s University Hospital, Dublin, UK
Background: Exosomes/extracellular vesicles (EVs) are a class of membrane-bound vesicles released from a number of cell types. These nano-sized (30–130 nm) structures may specifically package certain nucleic acids and protein from their cell of origin and so represent a potential novel source of circulating biomarkers. However, studies of these EVs are impeded by a lack of standardization in methods of isolation and characterization. Here we compare standard ultracentrifugation (UC) methods and commercially available kits to determine if a more high throughput method for EV isolation exists and if it is comparable to the current gold standard in terms of EV purity. Methods: EVs were isolated from 1 ml aliquots of pooled serum specimens using 6 methods: filtration/UC, UC with sucrose cushion, UC with Optiprep density gradient, size exclusion chromatography (Izon Science), Total Exosome Isolation Reagent (TEI) (Invitrogen) and ExoSpin (Cell guidance systems). EV isolates were characterized by transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), immunoblot and protein quantification assay. Results: TEM analysis confirmed the presence of EVs of the expected size in all methods apart from the TEI and ExoSpin methods which either had far too much material to distinguish individual EVs or had EVs of a larger size respectively. NTA also confirmed the presence of larger particle sizes in the TEI and ExoSpin methods. Immunoblots show EV positive markers TSG101 and CD63 or CD81 present in EVs isolated using all methods. Four of the methods were negative for EV negative marker GRP94 (94 k Da); however, EV isolates from the TEI and ExoSpin methods were negative for GRP94 at 94 kDa but were positive for a GRP94 subunit at 24 kDa. Protein quantification shows discernibly higher amounts of protein in TEI and ExoSpin methods compared to other methods. Conclusion: Here we examine the capabilities of commercially available and ultracentrifugation-based EV isolation methods using serum. Standard UC and UC with sucrose cushion outperformed other methods on reproducibility and on purity of EVs. As of yet there seems to be no advantage in alternative methods to the gold standard method of ultracentrifugation.
Acknowledgements
Irish Cancer Society's support of Breast-Predict [CCRC13GAL].
Poster 29
Purification of extracellular vesicles using in-house size exclusion chromatography columns
Ben Peacock, Daniel W. Lambert, Keith Hunter and Stuart Hunt
nit of Oral and Maxillofacial Pathology, The School of Clinical Dentistry, The University of Sheffield, Sheffield, UK
Background: The purification of extracellular vesicles can be challenging. EVs isolated by ultracentrifugation have been shown to be contaminated with protein aggregates and large protein complexes. Similarly, density gradient ultracentrifugation preparations can be contaminated with high-density lipoproteins. This can have a confounding impact on downstream applications such as proteomics analysis. We sought to develop in-house size exclusion chromatography (SEC) columns capable of isolating EVs suitable for proteomics and next generation sequencing analysis. Methods: Components of conditioned medium from cancer cell lines were separated by SEC, using Sepharose CL-2B columns made in-house and eluted in 0.5 ml fractions. Soluble protein was measured by BCA assay. Size and concentration of EVs was measured by tuneable resistive pulse sensing (TRPS) using a qNano instrument. Transmission electron microscopy was used to validate size measurements made by TRPS. EV protein was solubilized in TEAB lysis buffer+0.1% SDS for subsequent iTRAQ proteomics analysis. EV RNA was extracted using either the Qiagen miRNeasy kit or Exiqon miRCURY kit and quantified using a NanoDrop spectrophotometer. Results: The in-house SEC columns successfully separated EVs from the soluble protein in the conditioned medium. Peak EV elution was typically at fraction 11, whilst significant amounts of soluble protein began eluting from the column from fraction 14 onwards. EV protein yields were typically ~10 µg from purified EVs released by 25–30 million cells over 72 h. RNA from similar EV quantities purified by the miRNeasy kit gave a comparatively low yield at 100–250 ng, whereas the miRCURY kit yielded >500 ng. Conclusion: Size exclusion chromatography using columns fabricated in-house is a promising alternative to other commonly used EV purification techniques. It removes the need for specialist ultracentrifugation equipment and is more cost effective than commercially available SEC alternatives.
Poster 30
Purification of salivary extracellular vesicles by size exclusion chromatography
Karima Alkasah, Ben Peacock, Mark Ofield, Lynne Bingle, Daniel W. Lambert and Stuart hunt
nit of Oral and Maxillofacial Pathology, The School of Clinical Dentistry, The University of Sheffield, Sheffield, UK
Background: Extracellular vesicles (EVs) are increasingly recognized as a source of biomarkers for disease diagnosis and also as novel therapeutic targets. They are nanoscale sized, membrane enclosed vesicles that contain molecular cargo including nucleic acids and proteins. They are produced by all cells of the body and are present in biological fluids including saliva. Cancer cells produce increased numbers of EVs with altered molecular cargo, and so EVs have attracted much interest as a diagnostic tool for many cancers. We have preliminary data showing that EVs are readily detectable in the whole saliva of healthy volunteers and that they can be purified from contaminating soluble factors by size exclusion chromatography (SEC). Methods: EVs were purified from 3 to 5 ml of whole saliva provided by healthy donors, using Sepharose CL-2B SEC columns that were generated in-house. EVs were characterized by Tuneable Resistive Pulse Sensing (TRPS) using a qNano instrument and by transmission electron microscopy (TEM). Soluble protein was measured using a micro-BCA kit. Results: EVs typically eluted in fractions 7–9 and soluble protein eluted from fraction 10 onwards. TRPS revealed a heterogeneous sized population of EVs with a mode diameter of ~100 nm, which was confirmed by TEM. Conclusion: SEC appears to be a suitable technique to separate salivary EVs from contaminating soluble protein ready for downstream biomarker analysis.
Poster 31
Purifying EV by size exclusion: give it a little longer
Thomas Whittaker, Gregor Fuhrmann and Molly M. Stevens
epartment of Materials, Imperial College London, London, UK
Background: Extracellular vesicles are increasingly appreciated as possessing functionally important biological activities. Size exclusion is growing in popularity as a method of isolating pure and functionally intact EVs. Here we present data concerning the effect of column length on the efficacy of separation of EV-associated and soluble protein. Methods: MDA-MB-231 cells were grown in a Corning HYPERFlask in 550 ml DMEM+FBS before serum-free conditioning for 48 h. Cells and debris were removed with a 0.45 µm bottle-top filter; 140 ml of media was concentrated to 9 ml using 100 kDa MWCO Amicon Ultra-15 concentrators, aliquoted, and stored at −80 °C until use; 10 mm diameter glass columns (Lenz Laborglas) were packed with Sepharose CL-2B to depths of 9.5 and 28 cm (7.5 and 22.0 cm3, respectively). They and qEV columns (iZON) were washed with 2–3 column volumes of fresh 0.22 µm-filtered PBS before each use. Concentrated conditioned media (500 µl) was loaded onto each column (n=3); 0.5 ml fractions were collected, excluding the void volume and those after 15.0 ml, for which 1 ml fractions were collected. Protein concentration was determined using a micro-BCA assay. Particle concentrations were measured on an LM10 Nanosight (Malvern) (3×30s videos per sample, camera level 15, detection threshold 10). Fractions were diluted to measure at <10×10^8 particles/ml. Results: Columns eluted both an initial low-concentration protein peak (<6 µg/ml and concurrent with particle elution) and a later high-concentration peak. The resolution of these peaks differed between columns. For the 9.5 cm column, >90% of particles eluted between 2.0 and 5.0 ml, with the associated protein appearing as a shoulder on a single continuous peak between 2.0 and 16.0 ml; >90% of particles eluted from the qEV column between 3.5 and 5.5 ml. The particle-associated and soluble protein peaks were distinct but adjacent, between 3.5–5.5 and 5.5–14.0 ml respectively, with fraction 5.5 containing >1 µg/ml protein; >90% of particles eluted from the 28 cm column between 7.0 and 11.0 ml, with the particle-associated protein peak between 7.0 and 9.5 ml. The major protein peak eluted between 15.0 and 30.0 ml. The intermediate fractions 10.0–14.0 contained <1 µg/ml protein. Conclusions: The 28-cm column separated soluble and particle-associated protein more effectively than the qEV and 9.5 cm columns. Increasing the length of size exclusion columns improves separation of vesicles from soluble protein contaminants with minor increases in processing time, allowing confident retrieval of the majority of the EV peak without concerns of contamination in later EV-containing fractions.
Poster 32
Cells release subpopulations of exosomes with distinct molecular and functional properties
Eduard Willms1, Henrik J. Johansson2, Imre Mäger1, Yi Lee1, K. Emelie M. Blomberg3, C.I. Edvard Smith3, Janne Lehtiö2, Samir EL Andaloussi1,3, Matthew J.A. Wood1 and Pieter Vader1,4
1Department of Physiology, Anatomy and Genetics, Medical Sciences Division, University of Oxford, Oxford, UK; 2Cancer Proteomics Mass Spectrometry, Science for Life Laboratory, Department of Oncology-Pathology, Karolinska Institutet, Stockholm, Sweden; 3Department of Laboratory Medicine, Karolinska Institutet, Stockholm, SE-141 57, Sweden; 4Department of Clinical Chemistry and Haematology, University Medical Center Utrecht, Utrecht, 3584 CX, The Netherlands
Background: Extracellular vesicles (EVs), including exosomes, are nano-sized membrane vesicles involved in intercellular communication by transferring biological information between cells. While numerous cellular functions are attributed to exosomes in health and disease, current dogma is that such vesicles are assumed to represent a homogenous population of EVs. Here, we identify, characterize and evaluate the biological effects of 2 distinct subpopulations of exosomes released by different cell types. Methods: Exosome subpopulations from various sources were isolated using differential ultracentrifugation followed by sucrose gradient separation. Vesicles were analysed by Nanosight analysis, western blotting and electron microscopy. Exosome subpopulations from melanoma cells were further characterized for protein and RNA composition using LC–MS/MS and Bioanalyzer analysis. Effects on gene expression in recipient H5V endothelial cells were assessed using an Affymetrix gene array. Results: Two distinct exosome subpopulations were identified with different sedimentation and flotation characteristics. Proteomic analysis allowed quantification of more than 2,000 proteins (1% FDR), with a significant number of proteins unique to each of the subpopulations. Subpopulations also differed in their RNA repertoires. We further demonstrate that exosome subpopulations have differential effects on gene expression in recipient endothelial cells. Conclusions: Cells release distinct subpopulations of exosomes that differ in composition and biological effects on recipient cells. More detailed analysis of exosome subpopulations will advance our understanding of exosome biogenesis and functional effects, and lead to a deeper insight into the biological basis for exosome-based diagnostic and therapeutic development.
Poster 33
Mie theory as a flow cytometer standardization tool for extracellular vesicles
Joshua Aden Welsh, Judith A. Holloway, David C. Smith and Nicola A. Englyst
aculty of Medicine, University of Southampton, Southampton, UK
Background: Extracellular vesicle (EV) interest continues to increase. Currently one of the main initiatives of the International Society of Extracellular Vesicles, the International Society of Thrombosis and Haemostasis and the International Society for Advancement of Cytometry is the standardization of EV studies between groups. One particular area of interest is the development of approximating EV size between flow cytometry platforms, which are mainly utilized for microvesicle analysis. Here we aim to show evidence of extracellular vesicles being correlated with predicted light scatter using Mie theory. Method: A total of 6 ml of blood was drawn into citrated tubes, following a 2 ml discard. Tubes were centrifuged at 140 g for 20 min to isolate platelet-rich plasma (PRP). Five microlitre of PRP was added to 200 µl HEPES buffered saline (HBS)+2 mM CaCl2 to stimulate platelet activation. Samples were incubated at 37°C before centrifugation at 1,500 g twice. Supernatants were stored at −80°C. Supernatants were thawed at 37°C. 100 µl of supernatant was added to 2 µl 500 µM glycine, N-[2-[(acetyloxy)methoxy]-2-oxoethyl]-N-[4-[[[3’,6’-bis(acetyloxy)-2’,7’-difluoro-3-oxospiro [isobenzofuran-1(3H),9’-[9H]xanthen]-5-yl]carbonyl]amino]-2-[2-[2-[bis[2-[(acetyloxy)methoxy]-2-oxoethyl]amino]phenoxy]ethoxy]phenyl]-, (acetyloxy)methyl ester (BAPTA-1-AM) and spun at 20,000 g×30 min. Supernatants were discarded and 100 µl HBS+2 mM CaCl2 was used to resuspend the microvesicle pellet. Five microlitre of 25 µg/ml Annexin V PerCP-Cy5.5 was added and incubated on ice for 20 min; 2 µl of 200 µM violet ratiometric asymmetry probe (VRD) was added and incubated for 5 min. Samples were analysed on a FACSAria and custom Attune NxT, as well as confocal microscopy. Results: Confocal images show that BATPA-1-AM staining was successful. The sample contains particles <1 µm, likely large EVs and also particles that are >1 µm likely to be apoptotic bodies, lipoproteins or residual platelets. The FACSAria showed that EVs had a stain index of 1.69 for BAPTA-1-AM, 1.31 for Annexin V and 1.75 for VRD. The population stained with these dyes were 13.3, 8.8 and 33.9%, respectively. The Attune NxT particles had a stain index of 0.07 for BAPTA-1-AM, 0.29 for Annexin V and 0.30 for VRD. The population stained with these dyes were 1.87, 1.99 and 34.4%, respectively. Discussion: While VRD had a consistent staining efficacy between flow cytometers, due to its universal applicability, BAPTA-1-AM and Annexin V had very different results on both cytometry platforms. It is not until the true resolution of the platforms is taken into account using Mie theory that a consistent view can be seen. That larger particles >1,000 nm are staining for BAPTA-1-AM only, with the majority of annexin V positive particles (<1,000 nm) are visible on the custom Attune NxT.