Abstract
Extracellular vesicles (EVs) represent an important mode of intercellular communication. Research in this field has grown rapidly in the last few years, and there is a plethora of techniques for the isolation and characterization of EVs, many of which are poorly standardized. EVs are heterogeneous in size, origin and molecular constituents, with considerable overlap in size and phenotype between different populations of EVs. Little is known about current practices for the isolation, purification and characterization of EVs. We report here the first large, detailed survey of current worldwide practices for the isolation and characterization of EVs. Conditioned cell culture media was the most widely used material (83%). Ultracentrifugation remains the most commonly used isolation method (81%) with 59% of respondents use a combination of methods. Only 9% of respondents used only 1 characterization method, with others using 2 or more methods. Sample volume, sample type and downstream application all influenced the isolation and characterization techniques employed.
To access the supplementary material to this article, please see Supplementary files under ‘Article Tools’.
Extracellular vesicles (EVs) are membrane-enclosed vesicles that are released from all cell types into the extracellular space. EVs represent an important mode of intercellular communication and play key roles in many physiological and pathological processes (Citation1, Citation2). Consequently, research in this field has grown rapidly in the last few years, and there has been a huge growth in the number of techniques for the isolation and characterization of EVs, many of which are poorly standardized. EVs are heterogeneous in size, origin and molecular constituents, with considerable overlap in size and phenotype between different populations of EVs (e.g. exosomes formed in multivesicular endosomes and vesicles released directly from the membrane such as microvesicles) (Citation3–Citation5). Pure isolations of EVs from tissue culture supernatant and body fluids are hampered by the presence of non-vesicular macromolecular structures that are present in variable extent in different (body)fluids. This makes comparison of data from different studies difficult. Besides “standard” differential (ultra)centrifugation, density gradients, polymer-based precipitation, microfiltration and size-exclusion-based methods have been developed for EV isolation. Importantly, these isolation methods all impact the amount, type and purity of EVs recovered (Citation6). The International Society for Extracellular Vesicles (ISEV) has attempted to address some of these issues through the publication of position papers, EV RNA analysis (Citation7) and EV-based therapeutics (Citation8), and the minimal experimental requirements for definition of EVs and their function (MISEV) (Citation5). However, little is known about current practices for the isolation, purification and characterization of EVs. We report here the first large, detailed survey of current worldwide practices for the study of EVs.
Methods
An online questionnaire was drafted and distributed via an emailed web link to the members of ISEV (Supplementary Table I) in October 2015. The questionnaire included questions about the number of EV samples analysed per month, starting material, starting volume, primary isolation technique, additional purification methods, characterization methods and downstream applications. Each question had multiple choice answers and/or an open-ended free text option, some with non-mutually exclusive answers. All questions were mandatory and at least one response was required for each question. All electronically completed questionnaires were collected by ISEV and converted to an Excel file. Data were expressed as percentages.
Results
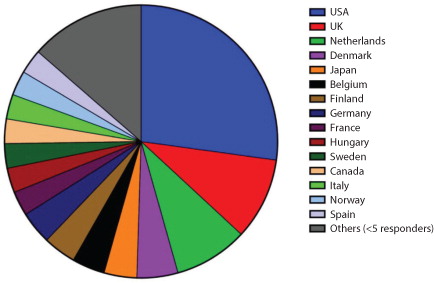
One hundred and ninety-six responses were collected from individual researchers in 30 countries belonging to 4 continents (). It was not possible to ascertain the country of origin for 2 responders. Workload varied widely: 38% of respondents isolated <10 EV samples per month, 49% processed 10–50 samples per month, 9% processed 50–100 samples per month and 4% processed over 100 samples per month. A wide range of starting sample volumes was also reported, with 23% using over 100 ml of starting material, 27% using 20–100 ml, 21% using 5–20 ml, 16% using 1–5 ml and 13% starting with <1 ml of the material.
Starting material
The most widely used starting material was conditioned cell culture media (83%), with 29% of researchers using both serum-enriched and serum-free culture conditions, 33% using only serum-added media and 37% using exclusively serum-free culture conditions. Several researchers indicated that EV-depleted serum was used but, as the questionnaire did not ask whether or how this depletion was performed, it is not possible to draw conclusions about the prevalence of this practice. Researchers using EVs for in vivo functional assays were most likely to use serum-free culture conditions (64%), while only 50% of those performing RNA analysis used serum-free culture conditions (Supplementary Table II).
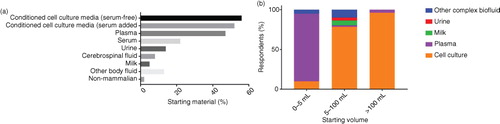
With respect to the isolation of EVs from biofluids, plasma (47%), serum (22%), urine (14%), cerebral spinal fluid (8%) and milk (5%) were the most common body fluids analysed (a). Only 4 researchers analysed EVs from non-mammalian sources (bacteria, n=2; Caenorhabditis elegans, n=2; parasites, n=1). Not surprisingly, the nature of the starting material had a major effect on the starting volume. Researchers using only conditioned media typically used much larger volumes than those using only complex biofluids (b). The majority of the respondents (96%) reporting a typical starting volume of >100 ml used conditioned cell culture media, whereas all of those reporting a typical sample volume of <1 ml studied complex biological/body fluids.
Fig. 2. (a) Starting material used by respondents by percentage. (b) Starting volume by sample type.Only responses indicating a single type of sample were included in the analysis (0–5 ml, n = 22; 5–100 ml, n = 51; >100 ml, n = 28).
There was an obvious relationship between the starting material and the laboratory workload. High-throughput laboratories (>50 samples per month) were more likely to use serum-free culture media (72%) than laboratories processing <50 samples per month (52%) and were more likely to be working with plasma samples (76% vs. 44%).
Isolation methods
Ultracentrifugation (including differential centrifugation) remains by far the most widely used primary isolation method (81%) across all applications (), with this figure rising to 85% for isolation of EVs from conditioned cell culture media. Over half (59%) of the respondents used a combination of isolation techniques. It was notable that researchers using exclusively conditioned culture media were least likely to use a combination of methods (44%), while those analysing complex biological fluids tended to use a combination of up to 6 methods for isolation and purification of EVs (65%). Density gradient centrifugation (20%), filtration (18%) and size-exclusion chromatography (SEC, 15%) were relatively well-used methods. This survey suggests that magnetic bead separation is rarely used for isolation of EVs from conditioned media (3%) but is more frequently used for isolating EVs from complex biological materials (13%), and this figure increased to 28% where the starting sample volume was <1 ml. Precipitation techniques were used by 14% of respondents but only 1 researcher reported using precipitation without further isolation/purification techniques. Of the researchers using precipitation techniques, 84% went on to perform RNA analysis. Less commonly used isolation techniques included field flow fractionation (n=2), fluorescence-activated cell sorting (FACS, n=2), high-throughput/high-pressure liquid chromatography (FPLC/HPLC, n=3) and affinity separation techniques (non-magnetic bead n=4). The most commonly used additional clean-up/purification methods (Supplementary Table IV) were washing by ultracentrifugation (64%), density gradient centrifugation (27%) and liquid chromatography techniques (20%). Liquid chromatographic methods were most commonly used by researchers performing proteomic analysis (20%) and in vitro functional testing (21%), while those performing proteomics were most likely to use density gradient centrifugation for EV purification (37%). By contrast, 37% of researchers performing proteomic analysis relied upon ultracentrifugation and an ultracentrifugation wash to prepare EV for analyses. It was notable that the use of liquid chromatography for EV purification was lowest among researchers performing in vivo functional analyses (9% vs. an average of 20%).
Fig. 3. (a) Primary isolation method (% of respondents) and (b) isolation method by starting volume of material.
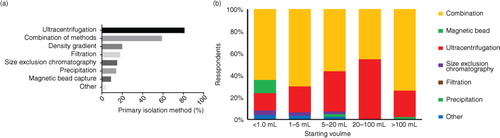
The starting sample volume had a substantial effect on the isolation techniques used. Ultracentrifugation was used as the primary isolation step by >80% of respondents, but only 64% of those with limited sample volume (e.g. <1 ml) used ultracentrifugation, 48% used an ultracentrifuge protocol with a washing step and only 4% employed density gradient centrifugation. Conversely, 28% of researchers using sample volumes of <1 ml used magnetic bead separation techniques, compared with a figure of 6% of researchers purifying EVs from larger starting volumes. It was notable that high-throughput laboratories (>50 samples per month) were much less likely to use precipitation techniques than laboratories processing <50 samples a month (4% vs. 15%).
Characterization methods
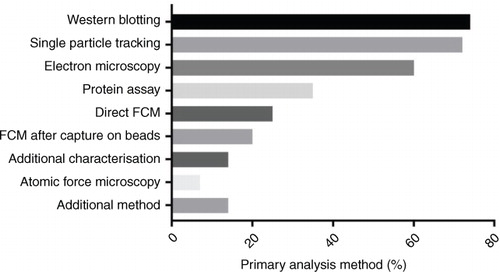
The 3 most widely used techniques for EV characterization were western blotting (74%), single-particle tracking (SPT, 72%) and electron microscopy (60%) (). Of the respondents who used SPT, 16 did not specify a method. Of the remainder, 80% used nanoparticle tracking analysis (NTA) (Nanosight or Zeta View), 18% used tunable resistive pulse sensing (TRPS) (Izon qNano) and 12% used dynamic light scattering (DLS) (various manufacturers). Flow cytometry remains a popular method for analysing EVs, with 41% of respondents using this method. Direct flow cytometry was employed by 61% of flow cytometry users, while 49% used flow cytometry following bead capture, with only 10% using both techniques. This division in technique seemed to be dictated by the cytometer used. It is likely that direct flow cytometry was largely performed by researchers interested in EVs larger than 200 nm in diameter or those with access to cytometers with small particle detection capability (e.g. Becton Dickinson Influx; Apogee A50-Micro; Beckman Coulter MoFlo Astrios and CytoFLEX; and Amnis ImageStream), whereas flow cytometry following bead capture was generally performed on more conventional cytometers (e.g. FACSCalibur, LSRFortessa and MACSQuant) using aldehyde/sulphate latex beads. Protein assays were performed by 35% of respondents, with most using BCA/micro BCA or Bradford assays, with a <5% using NanoDrop or Qubit measurements. The use of protein assays was highest amongst researchers performing proteomic analysis (41%) and lowest in those conducting in vivo investigations (32%).
w?>Atomic force microscopy was used by 9% of researchers, and several other techniques were used by a small number of respondents: antibody microarrays (n=5), procoagulant assays (n=5), non-flow-cytometric fluorescence methods (n=4), Raman spectroscopy (n=3), ELISA (n=2), acetylcholine/acetylcholinesterase activity assays (n=2), SP6800 spectral analyser (n=1), capillary electrophoresis (n=1), HPLC analysis (n=1), infrared spectroscopy (n=1) and zeta potential measurement (n=1). The number of methods used for EV characterization varied widely. Nine percent of respondents reported using only 1 characterization method, 23% used 2 methods, 33% 3 methods, 23% 4 methods and 12% used 5 or more characterization methods.
Downstream applications
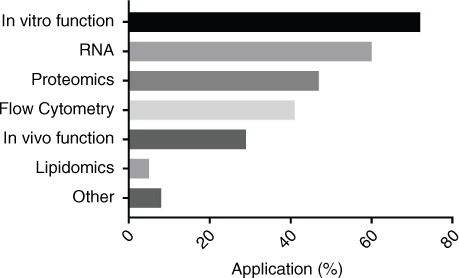
The most common downstream applications () were in vitro functional analyses (72%), RNA analysis (60%), proteomic analysis (47%), in vivo functional analyses (29%) and lipidomic analysis (5%). Investigation of EVs as therapeutic agents was performed by 6 researchers, 6 stated that novel biomarker discovery was their main application, 1 analysed EV cytokine/chemokine profiles and another used protein misfolding cyclic amplification for the detection of infectious prions. Detailed descriptions of functional assays were not recorded as this was beyond the scope of this survey. It was notable that while several respondents used serum-containing conditioned media for proteomic analysis, very few use serum-added media without extensive isolation/purification techniques.
The combinations of tests used by researchers were worthy of comment. Fifty-six percent of researchers performing RNA analysis also performed proteomic analysis, whereas 70% of those performing proteomics went on to analyse RNA. Similarly, while only 38% of researchers performing in vitro functional testing also investigated in vivo function, almost all of those investigating in vivo function also performed in vitro functional analysis (96%). High-throughput laboratories (>50 samples/month) were almost twice as likely to perform in vivo functional analysis as other laboratories (48% vs. 26%).
Discussion
Numerous methodologies have been used to isolate and analyse EVs, and it is clear that the different techniques influence the results of downstream analyses. We report on the first world-wide survey on techniques currently used for the isolation and characterization of EVs. At this time, there is no consensus on a “gold standard” method for EV isolation and purification (Citation5). It is clear from the results of this survey that the downstream application as well as the type and volume of starting material have a major influence on the selection of isolation method. As might be expected, researchers dealing with complex biological fluids and/or perform proteomic analysis tend to use more elaborate isolation/purification strategies than those who isolate EVs from conditioned cell culture media and/or use flow cytometry for EV analysis.
Ultracentrifugation remains by far the most popular primary isolation technique for EVs, irrespective of the starting material used. EVs isolated by ultracentrifugation are known to suffer from non-vesicular macromolecule contamination (Citation9). This is clearly an issue for omics, RNA and functional EV analysis (Citation6). In this survey, the majority of researchers isolating EVs by ultracentrifugation used an additional purification/clean-up technique. Furthermore, ultracentrifugation can cause aggregation of EVs (Citation10, Citation11), which might lead to artifacts during flow cytometric analysis and SPT analysis. Moreover, it has been reported that aggregation may affect the biodistribution of EVs in vivo (Citation12, Citation13). The application of SEC and other chromatography techniques (e.g. HPLC) to EV isolation was described in the early 1980s (Citation14) but was re-evaluated and validated only 2 years ago: since then, 15% of respondents already reported using these techniques. Although the purity of EVs isolated by these methods depends upon both the starting material and separation media used, it has been reported that EVs isolated by chromatographic methods suffer less contamination by non-vesicular proteins and macromolecule structures than after ultracentrifugation (Citation13, Citation15) (Citation16). Lipoprotein contamination had been identified as an important source of interference in EVs isolated from blood. Low-density lipoprotein contamination of blood-derived EVs interferes with flow cytometric analysis and is not entirely eliminated by ultracentrifugation (Citation17). Co-isolation of high-density lipoprotein with EVs isolated by density gradient centrifugation has also been reported (Citation18), resulting in non-vesicular miRNA contamination of EVs (Citation19). It is possible that different EV isolation methods may preferentially isolate different EV populations, with different size, protein, RNA and functional characteristics (Citation6, Citation13) (Citation20).
A considerable number of researchers (28%) used cell culture media supplemented with serum as their starting material. Serum is replete with EVs, which can influence the growth and the phenotype of cultured cells, and consequently, indirectly influence the quality and quantity of EVs secreted by those cells (Citation21). Additionally, EV-depleted serum has reduced capacity to support cell growth (Citation22), which may also impact on the recovery of EVs from conditioned media under these conditions. Further, the serum EVs carry a different subset of RNAs, proteins and lipids and can directly affect the experimental results involving proteomics, RNA analysis and functional analysis of the isolated EVs from the serum-added culture-conditioned media, if serum-derived EV depletion is not carried out efficiently. Conversely, switching cells grown in serum-containing medium to serum-free conditions during the EV-secretion time induces stress, which also may affect the nature and amount of secreted EVs.
To maintain quality and reproducibility in EV research, it is important to consider the impact of added EVs from serum to the culture media. Including EVs from a control cell type grown under similar culture conditions, EV-depleted bodyfluid/culture media, coupled with a transparent reporting of methods, may reveal EV-specific findings. Collectively, this necessitates suitable standards for EV isolation, characterization and analysis methods (Citation5).
It was notable that most respondents reported that they used 2 or more methods for EV characterization, as recommended in MISEV (Citation5). Single-particle analyses by NTA, TRPS and DLS are now widely used techniques for the quantification and sizing of EVs but currently do not provide much information regarding phenotype and are not ideal for measuring larger (>200 nm) vesicles. However, as most EVs released by living cells are <300 nm, this is rarely a problem but it should be noted that these methods perform poorly with EVs, which are highly polydisperse. A few researchers are beginning to combine these techniques with other methodologies, for example, TRPS with Raman spectroscopy and NTA with fluorescence labelling, but these combinations may not be commercially available for several years. Until recently, flow cytometry could analyse only large EVs or populations of smaller EVs captured on beads prior to analysis. This survey demonstrated the impact of next-generation flow cytometers equipped with small particle detectors on EV analysis, with several researchers performing direct flow cytometry of EVs using these advanced cytometers. However, proper validation of these analyses, especially to confirm that single vesicles rather than swarms of EVs are analysed, is not always easy to provide.
This survey had several limitations. As the survey was sent to all ISEV members, on several occasions the data included responses from more than 1 person from the same department. However, the responses from individuals within the same department frequently differed with respect to the type of sample and techniques used. Many respondents analysed several different types of sample, and as some of the survey questions (e.g. starting volume) allowed only a single response, it was not possible to record different responses for different types of sample. Several respondents stated that while they used large volumes of conditioned media, much smaller volumes of complex biological fluids (e.g. CSF and plasma) were typically used. Similarly, it is highly unlikely that the researchers who use many different techniques and applications use all techniques and applications on all sample types.
This survey offers a snapshot of the techniques for EV isolation and characterization used by ISEV members in late 2015. It is clear that the techniques used in the field are rapidly evolving, and this will create opportunities for future EV research. The rapidly evolving landscape will also pose challenges for future standardization. It is anticipated that the data from this survey will influence future ISEV position papers and that future surveys will identify trends and evolving practices in EV research.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Supplementary Tables
Download PDF (435.3 KB)Notes
To access the supplementary material to this article, please see Supplementary files under ‘Article Tools’.
References
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200: 373–83.
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, etal. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015; 4: 27066,. doi: http://dx.doi.org/10.3402/jev.v4.27066.
- van der Pol E, Böing AN, Gool EL, Nieuwland R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016; 14: 48–56.
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013; 2: 20389,. doi: http://dx.doi.org/10.3402/jev.v2i0.20389.
- Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, etal. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014; 3: 26913,. doi: http://dx.doi.org/10.3402/jev.v3.26913.
- Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, etal. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles. 2014; 3: 24858,. doi: http://dx.doi.org/10.3402/jev.v3.24858.
- Hill AF, Pegtel DM, Lambertz U, Leonardi T, O'Driscoll L, Pluchino S, etal. ISEV position paper: extracellular vesicle RNA analysis and bioinformatics. J Extracell Vesicles. 2013; 2: 22859,. doi: http://dx.doi.org/10.3402/jev.v2i0.22859.
- Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, etal. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles. 2015; 4: 30087,. doi: http://dx.doi.org/10.3402/jev.v4.30087.
- Webber J, Clayton A. How pure are your vesicles?. J Extracell Vesicles. 2013; 2: 19861,. doi: http://dx.doi.org/10.3402/jev.v2i0.19861.
- Erdbrügger U, Rudy CK, E. Etter M, Dryden KA, Yeager M, Klibanov AL, etal. Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytometry A. 2014; 85: 756–70.
- Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. 2015; 4: 29509,. doi: http://dx.doi.org/10.3402/jev.v4.29509.
- Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015; 199: 145–55.
- Nordin JZ, Lee Y, Vader P, Mager I, Johansson HJ, Heusermann W, etal. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed. 2015; 11: 879–83.
- Taylor DD, Chou IN, Black PH. Isolation of plasma membrane fragments from cultured murine melanoma cells. Biochem Biophys Res Commun. 1983; 113: 470–6.
- Böing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014; 3: 23430,. doi: http://dx.doi.org/10.3402/jev.v3.23430.
- de Menezes-Neto A, Saez MJ, Lozano-Ramos I, Segui-Barber J, Martin-Jaular L, Ullate JM, etal. Size-exclusion chromatography as a stand-alone methodology identifies novel markers in mass spectrometry analyses of plasma-derived vesicles from healthy individuals. J Extracell Vesicles. 2015; 4: 27378,. doi: http://dx.doi.org/10.3402/jev.v4.27378.
- Sodar BW, Kittel A, Paloczi K, Vukman KV, Osteikoetxea X, Szabo-Taylor K, etal. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep. 2016; 6: 24316.
- Yuana Y, Levels J, Grootemaat A, Sturk A, Nieuwland R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J Extracell Vesicles. 2014; 3: 23262,. doi: http://dx.doi.org/10.3402/jev.v3.23262.
- Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Roxe T, etal. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol. 2013; 33: 1392–400.
- Nawaz M, Camussi G, Valadi H, Nazarenko I, Ekstrom K, Wang X, etal. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat Rev Urol. 2014; 11: 688–701.
- Shelke GV, Lasser C, Gho YS, Lotvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014; 3: 24783,. doi: http://dx.doi.org/10.3402/jev.v3.24783.
- Eitan E, Zhang S, Witwer KW, Mattson MP. Extracellular vesicle-depleted fetal bovine and human sera have reduced capacity to support cell growth. J Extracell Vesicles. 2015; 4: 26373,. doi: http://dx.doi.org/10.3402/jev.v4.26373.