Abstract
Achnatherum inebrians, colloquially known as drunken horse grass, is associated with livestock toxicity in northern China. Epichloë gansuensis (Eg) was described from endophyte isolates from A. inebrians in Sunan County, Gansu Province, whereas a morphologically distinct variety, E. gansuensis var. inebrians (Ei), was described based on two isolates from A. inebrians seeds collected in Urumqi County, Xinjiang Province. Genome sequencing and alkaloid analyses also distinguish these taxa; the Ei isolates produce neurotropic lysergic acid amides (ergot alkaloids), and an Eg isolate produces paxilline (an indole-diterpene alkaloid). To better elucidate the taxonomic diversity of Epichloë spp. symbiotic with A. inebrians, we surveyed eight populations in Xinjiang, Gansu and Inner Mongolia provinces of China and analyzed their genotypes by multiplex PCR for alkaloid biosynthesis genes and mating-type genes. Genotypes consistent with Ei were present in all eight populations, of which they dominated seven. The Ei isolates were all mating type A and tested positive for the ergot alkaloid gene, dmaW. In contrast Eg isolates were all mating type B and had the indole-diterpene gene, idtG. The genome was sequenced from an Ei isolate from seeds collected in Xiahe County, Gansu, and compared to that of the varietal ex type isolate from Urumqi. Alkaloid genes and four different housekeeping genes were nearly identical between the two sequenced Ei isolates and were distinct from a sequenced Eg isolate. Phylogenetic analysis placed Ei, Eg and Epichloë sibirica into respective subclades of a clade that emanated from the base of the Epichloë phylogeny. Given its chemotypic, genotypic, morphological and phylogenetic distinctiveness, its widespread occurrence in rangelands of northern China, and its importance in livestock toxicity, we propose raising Ei to species rank as Epichloë inebrians.
Introduction
Drunken horse grass, Achnatherum inebrians ( in Chinese), a perennial bunchgrass native to northern China, is associated with livestock toxicosis (CitationMiles et al. 1996, CitationLi et al. 2007, CitationZhang et al. 2012). Toxicity of A. inebrians is thought to be caused by alkaloids produced by seed-transmitted symbiotic fungi (endophytes) (CitationRen 1959, CitationMiles et al. 1996, CitationShi 1997, CitationLi et al. 2007, CitationZhang et al. 2012). This grass species is a known host for two related taxa of seed-transmissible endophytes, Epichloë gansuensis (C.J. Li & Nan) Schardl (Eg) (CitationLi et al. 2004) and E. gansuensis var. inebrians (C.D. Moon & Schardl) Schardl (Ei) (CitationMoon et al. 2007).
in Chinese), a perennial bunchgrass native to northern China, is associated with livestock toxicosis (CitationMiles et al. 1996, CitationLi et al. 2007, CitationZhang et al. 2012). Toxicity of A. inebrians is thought to be caused by alkaloids produced by seed-transmitted symbiotic fungi (endophytes) (CitationRen 1959, CitationMiles et al. 1996, CitationShi 1997, CitationLi et al. 2007, CitationZhang et al. 2012). This grass species is a known host for two related taxa of seed-transmissible endophytes, Epichloë gansuensis (C.J. Li & Nan) Schardl (Eg) (CitationLi et al. 2004) and E. gansuensis var. inebrians (C.D. Moon & Schardl) Schardl (Ei) (CitationMoon et al. 2007).
Fungal endophytes belonging to Epichloë (Fr.) Tul. & C. Tul. (= Neotyphodium A.E. Glenn, C.W. Bacon & Hanlin, anamorph synonym) are important bioprotective symbionts of many cool season grasses (Poaceae subfamily Poöideae), including forage and turf grasses and wild grasses of meadows, woodlands and rangelands in temperate climatic zones. Epichloë species can benefit their host plants by enhancing tillering, root growth, nutrient acquisition, drought tolerance, resistance to root-parasitic nematodes and resistance to vertebrate and invertebrate herbivores (CitationMalinowski and Belesky 2000, CitationSchardl et al. 2004, CitationTimper et al. 2005, CitationSchardl et al. 2013b). However, livestock toxicosis is associated with alkaloids produced by symbiotic Epichloë species in several grasses worldwide, such as tall fescue (Lolium arundinaceum) (CitationLyons et al. 1986), perennial ryegrass (Lolium perenne) (CitationGallagher et al. 1981), sleepygrass (Achnatherum robustum) (CitationPetroski et al. 1992, Shymanovich et al. 2014), dronkgrass (Melica decumbens) (CitationGibbs-Russell and Ellis 1982), Poa huecu (CitationCabral et al. 1999) and drunken horse grass (CitationMiles et al. 1996).
Epichloë species are distinguished mainly by molecular phylogenetic relationships, host specificity, morphological variation, and alkaloid profiles (CitationSchardl et al. 2012, CitationLeuchtmann et al. 2014). Four distinct classes of bioprotective alkaloids are known products of Epichloë species—peramine, lolines, indole-diterpenes and ergot alkaloids—and there is considerable structural variation within the latter three classes. Different grass-Epichloë symbioses have different combinations of alkaloids mainly correlated with presence or absence of functional alkaloid biosynthesis genes in the endophyte genomes, but sometimes also reflecting differences in expression of those genes (CitationSchardl et al. 2013b). Lolines (CitationSchardl et al. 2007, CitationBacetty et al. 2009) and peramine (CitationTanaka et al. 2005) are active against invertebrates, whereas ergot alkaloids (CitationSchardl et al. 2006, CitationPotter et al. 2008) and indole-diterpenes (CitationSaikia et al. 2008) are active against both insects and mammals.
Horses and other livestock fed or grazed on A. inebrians can exhibit depression, narcosis, lachrymation, muscle tremors and increased respiration and heart rates (CitationDang et al. 1992, CitationMiles et al. 1996, CitationLi et al. 2007, CitationLi et al. 2009, CitationZhang et al. 2012). These symptoms are attributed to very high ergonovine and ergine (lysergic acid amide) in A. inebrians plants symbiotic with Ei (CitationMiles et al. 1996), and genes required for biosynthesis of these ergot alkaloids have been identified in the genome sequence of Ei isolate e818 (CitationSchardl et al. 2013b). In contrast the genome sequence of Eg strain e7080 (= CDM-2007b) includes genes for indole-diterpenes but not ergot alkaloids, and analysis of A. inebrians symbiotic with Eg e7080 confirms its production of the indole-diterpene, paxilline (CitationSchardl et al. 2013b). In addition to their chemotypic differences Eg e7080 and Ei e818 have distinct morphologies in culture. For example, the former is among the fastest growing Epichloë taxa (CitationLi et al. 2004) whereas the latter is among the slowest (CitationMoon et al. 2007).
To clarify diversity and investigate the population structures of endophytes in A. inebrians, we screened seed collections from eight populations in three provinces of China and characterized the endophytes based on alkaloid genes, mating types and in select cases assessed morphology in culture and determined phylogenetic relationships. Observing consistent differences between Eg and Ei isolates, we recognize Ei as a distinct species, Epichloë inebrians, below.
Materials and methods
Biological materials
All A. inebrians seed collections and endophyte isolates are listed (). Seeds originated from eight natural populations in northern and northwestern China: one at Murengaole Sumu, Alxa, Inner Mongolia Province (AIM), three in Sunan County, Gansu Province (SNA, SNB and SNC), three in Xiahe County, Gansu Province (XHA, XHB and XHC) and one previously reported from Urumqi County, Xinjiang Province (URQ) (CitationMiles et al. 1996). Collections from populations AIM, SNA, SNB, SNC and XHC were from individual plants, whereas the XHA, XHB and URQ populations were sampled in bulk seed collections from multiple plants. In total 48 seed samples were collected from the eight populations. In addition, two isolates from Japan were included for comparison, GR10140 (Eg) and GR10156 (Epichloë sylvatica). These were collected from native or naturalized grasses (CitationEnomoto et al. 1998) and were stored in NARO Livestock and Grassland Research Institute, Japan. The host plant of origin for GR10140 was Achnatherum pekinense (syn. A. extremorientale), collected in Hiroshima Prefecture. GR10156 was from Brachypodium sylvaticum collected in Shiga Prefecture.
Table I. Collections of Achnatherum spp. seeds and endophytesa
Grass plants were grown in a greenhouse at University of Kentucky, Lexington. Seeds were germinated, and seedlings were planted in 50% soil mix (three parts Pro-mix Bx (Premier Horticulture) to one part sterile Murray silt loam top soil) and 50% sterile sand, and grown at 26 C during the day and 22 C at night with supplemental lighting (high pressure sodium at 140 000 lumens) as needed to provide 8 h light in winter, slowly increasing to 14 h light in summer.
Fungal isolates were cultured from A. inebrians seeds or plants grown from the seed samples as follows. Individual seeds or pseudostems (leaf-sheath whorls at the base of vegetative tillers) were surface disinfested (CitationLeuchtmann and Clay 1990, CitationMoon et al. 2002), and the seeds or pseudostem sections (approx. 5 mm) were placed on fresh potato dextrose agar (PDA) amended with antibiotics (0.1 mg/mL penicillin G potassium salt and 0.1 mg/mL streptomycin sulfate), incubated at 23 C, and examined periodically by eye and by microscope for the emergence of mycelium. The sample plates were incubated until hyphae appeared from the cut ends of the host tissues or from the seeds. Isolates from seeds and plants were subcultured and maintained on PDA at 23 C.
Genome sequencing
All DNA and genome sequencing was conducted at the University of Kentucky Advanced Genetic Technologies Center. Genomic DNA was sequenced by 454-Titanium (Roche) pyrosequencing or Ion Torrent PGM (Life Technologies) as described by CitationSchardl et al. (2013b, Citation2013a). The genome of Ei isolate e818 (NCBI Bioproject PRJNA174039) was sequenced by a combination of pyrosequencing (approx. 780 nt read lengths, totaling 1590 million nt) and Ion Torrent sequencing (approx. 220 nt read lengths, totaling 1098 million nt) (CitationSchardl et al. 2013b), whereas the genome of another Ei isolate, e7478, was sequenced solely by Ion Torrent to a total of 965 million nt. Thus sequence coverage was approx. 90-fold for e818 and approx. 38-fold for e7478. Before assembly, Ion Torrent reads were trimmed of all base-calls after the first 230 bases. Genome sequences were assembled by Newbler Assembler 2.5.3 (Roche Diagnostics Corp./454 Life Sciences) or CLC Genome Workbench 8.0 (CLC Bio), with default parameters.
Gene sequences were manually annotated from available genome sequences (CitationSchardl et al. 2013a, Citation2013b) (www.endophyte.uky.edu) by comparison to homologs in related species. The GenBank accessions of the e818 ergot alkaloid biosynthesis gene clusters (EAS1 and EAS2) are JX072969 and JX273434 and the GenBank accession of the e7080 indole-diterpene gene cluster (IDT) is JN587271.
Phylogenetic analysis
Housekeeping genes that were chosen for phylogenetic analysis encode translation elongation factor 1-α (tefA), β-tubulin (tubB and tubP) and the second largest subunit of RNA polymerase II (rpbB). GenBank accession numbers for the housekeeping genes are listed (supplementary table I). Sequences of 5′-portions of tubB genes in Epichloë species are provided with GenBank accession numbers (CitationLeuchtmann et al. 2014 supplementary table I.)
Phylogenetic analysis was conducted on the Phylogeny.fr website (CitationDereeper et al. 2008) as follows: Sequences were aligned with MUSCLE (CitationEdgar 2004) without GBlocks curation, the phylogenetic trees were inferred with PhyML (CitationGuindon and Gascuel 2003), and branch support values were estimated by the approximate likelihood ratio (ALR) test (CitationAnisimova and Gascuel 2006) with the SH-like option. Alignments and trees are available in TreeBASE (purl.org/phylo/treebase/phylows/study/TB2:S16982).
DNA isolation from fungal isolates or symbiotic plant tissues
Genomic DNA was isolated from pure cultures or grass pseudostems and used as templates for PCR. Total genomic DNA was isolated from lyophilized mycelium using the ZR fungal/bacterial DNA miniprep kit (Zymo Research) and quantified by fluorometry with Hoechst dye staining with a DyNA Quant 200 Qubit® Fluorometer (Hoefer Inc.). Plant material (50–100 mg wet weight) was harvested from the tiller base, from which the outer leaf sheath was discarded. Each plant tissue sample was placed with a single bead (4.5 mm steel air-gun shot, Daisy 6000ct BB Ammo, Daisy Outdoor Products) into each well of a 96 deep-well plate. Tissue was disrupted with a TissueLyser II (QIAGEN, manufactured by Retsch Lab Equipment) by two 1 min pulses of 900–1320 strokes/min. DNA was isolated with a MagAttract 96 DNA Plant Core kit (QIAGEN) and was not quantified before PCR.
Multiplex PCR
Alkaloid biosynthesis gene fragments and housekeeping gene fragments were amplified from total genomic DNA extracted from fungal mycelia (20 ng DNA) or tillers (3 μL extract) by PCR with primer sets described (CitationTakach et al. 2012, CitationCharlton et al. 2014), plus the following pair of primers designed for the Ei e818 and Ei e7478 dmaW sequence: dmaW818(311+21)d (5′–AAC CCA TCA ACG GAG CAA CTG–3′) and dmaW818(1068+21)u (5′–GCC AAA CAC TGT GAA ATA CAC CTG–3′). Seeds and plant samples were screened with multiplex PCR (CitationTakach and Young 2014), and PCR mixtures included 1× Green GoTaq reaction buffer (Promega Corp.), 200 μM each deoxynucleoside triphosphate (dNTP), 200 nM each primer and 1 U GoTaq DNA polymerase (Promega Corp.). PCR amplifications were conducted under the following temperature conditions: an incubation at 94 C for 1 min; 30 cycles of 94 C for 15 s, 56 C for 30 s, and 72 C for 45 s; then a final incubation at 72 C for 10 min. Products were analyzed by agarose gel electrophoresis with ethidium bromide or GelGreen stain (Biotium Inc.).
Results and discussion
Genome sequence and alkaloid gene clusters
Genome sequences were reported for Ei isolate e818 from the URQ site in Xinjiang Province, and Eg isolate e7080 from the SNB site in Gansu Province (CitationSchardl et al. 2013b). We sequenced the genome of another Ei isolate, e7478, which was from seed collected in the XHA site, Xiahe County, Gansu Province, approx. 1650 km from the URQ site. The assembled genome size of e7478, 25.4 Mb, was comparable to the 29.8 Mb genome of e818. Both the e818 and e7478 assemblies produced complete contigs for two gene clusters, EAS1 and EAS2 (), which together contained all of the identifiable ergot alkaloid biosynthesis genes.
Fig. 1. Structures of alkaloid gene clusters and associated alkaloids of Achnatherum inebrians endophytes. A. The ergot alkaloid (EAS) biosynthesis gene clusters in Epichloë inebrians e818 and e7478, and structures of the most abundant ergot alkaloids detected in symbiota with E. inebrians (CitationMiles et al. 1996). B. The indole-diterpene (IDT) biosynthesis gene cluster in Epichloë gansuensis e7080 and the structure of paxilline, the most abundant indole-diterpene detected in symbiota with E. gansuensis e7080 (CitationSchardl et al. 2013b). Black boxes indicate coding sequences of alkaloid biosynthesis genes, gray boxes indicate coding sequences of other genes near the clusters and white boxes indicate pseudogenes. Arrows indicate directions of transcription. Filled circles indicate telomere repeat arrays at chromosome ends. Coordinates in kilobasepairs (kb) are from the ends of contigs in the genome assemblies.
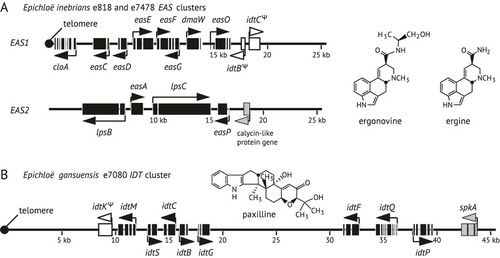
The two clusters of ergot alkaloid biosynthesis genes had 99.99% identity between the Ei e818 and Ei e7478 genome assemblies, exclusive of alignment gaps (). Most alignment gaps were in noncoding sequences, but some single-base indels in coding sequences (one each in cloA exon 3, easC exon 2, easD exon 2, easF exon 2, lpsC exon 2), and perhaps some indels in noncoding sequences, may reflect sequence errors of the type we experienced with the early releases of Ion Torrent technology. Considering the frequency of such errors we chose not to publish the e7478 genome assembly because the e818 genome assembly seemed to be of high quality and an appropriate reference for the new species. In both genome assemblies the EAS1 cluster was adjacent to the chromosome end with a telomere repeat array located only one base from the cloA stop codon (). Despite its location so close to the telomere, this gene was almost certainly functional considering that cloA is required for synthesis of lysergic acid (CitationHaarmann et al. 2006), which is the precursor of the lysergic acid amides that are the major ergot alkaloids produced by Ei (CitationOrtel and Keller 2009).
Both Ei e818 and Ei e7478 genome assemblies lacked IDT gene clusters sufficient to direct biosynthesis of indole-diterpenes. The IDT gene homologs in the two Ei isolates consisted of two pseudogenes, idtBΨ and idtCΨ, located proximal to the centromeric side of the EAS1 cluster (), and an idtP gene very similar to that of Eg e7080, which in the e818 assembly was located near the middle of a different scaffold (Supercontig70) amid genes on either side that were unrelated to known IDT genes. In contrast Eg isolate e7080 possessed a cluster of IDT genes sufficient for the production of the indole-diterpene, paxilline, which is produced in A. inebrians-e7080 symbiota (CitationSchardl et al. 2013b). Like the EAS1 cluster of the Ei isolates, the IDT gene cluster of Eg e7080 was also close to a chromosome end, located 5 kb from a telomere ().
Phylogenetic relationships
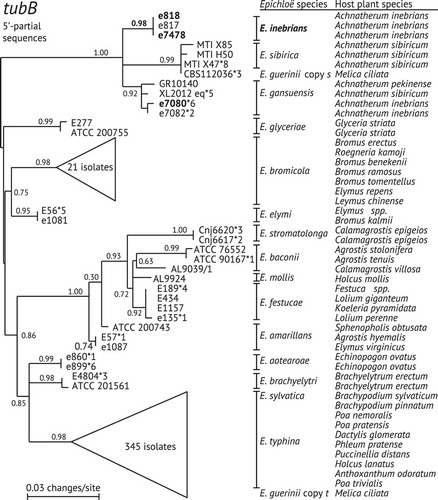
In genome assemblies of Ei isolate e818, Ei isolate e7478 and Eg isolate e7080 only single copies were identified for tubB, tubP, tefA and rpbB, indicating that these isolates were all nonhybrid. For phylogenetic analysis the available sequences of intron-rich portions of tubB were collated from these isolates and all other nonhybrid Epichloë species, including other isolates from A. inebrians, an isolate from Achnatherum pekinense (collected in Japan), and isolates from A. sibiricum (collected in Inner Mongolia) that previously were classified either as Eg or as Epichloë sibirica (CitationZhang et al. 2010, CitationLi et al. 2015). Also included were the two tubB gene copies in E. guerinii, which is an apparent hybrid of E. typhina and E. sibirica (CitationMoon et al. 2007, CitationZhang et al. 2009), and as such the only known hybrid with an ancestor closely related to Ei and Eg.
The tubB phylogeny (, supplementary fig. 1) indicated that the identical sequences from three Ei isolates constituted a well-supported clade that grouped in a trifurcation with two well-supported sister clades. One of its sister clades included only sequences from E. sibirica plus one of the tubB sequences from E. guerinii. The other sister clade grouped the isolate from A. pekinense with the Eg isolates from A. inebrians (CitationLi et al. 2004, CitationMoon et al. 2007) and A. sibiricum. Thus Eg appeared to be more diverse in genotype and host range than Ei and E. sibirica. This finding and the recent report that Eg can form presexual stromata on A. sibiricum (CitationLi et al. 2015) (mature stromata with perithecia have not been observed) suggest that Eg might be capable of horizontal transmission mediated by the conidia formed on those stromata (CitationLi et al. 2015).
Fig. 2. Phylogeny of Epichloë species based on alignment of tubB sequences. The sequences spanned the 5′–portion of tubB including introns 1, 2 and 3. The tree is unrooted, but the midpoint is placed at the left edge. ALR support scores are on branches. Each leaf is labeled with the strain identification number and, where additional isolates had identical sequences, an asterisk is followed by the number of additional isolates with that sequence. Strains indicated in boldface are endophytes of A. inebrians that have had their genomes sequenced. Clades corresponding to Epichloë bromicola and to species in the E. typhina complex are collapsed into triangles to save space; the full tree is provided (supplementary fig. 1). The two gene copies in the hybrid endophyte, Epichloë guerinii, are named with single letter abbreviations of ancestral Epichloë species, E. sibiricum (s), and E. typhina (t).
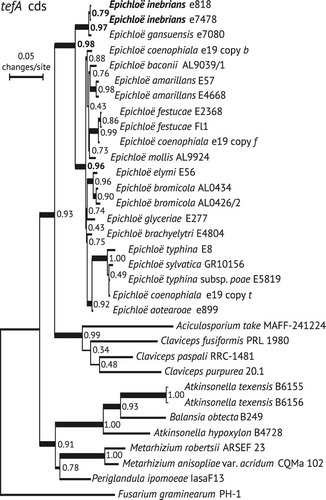
To identify the root of the Epichloë tree we conducted a molecular phylogenomic study that included all known sexual Epichloë species and other Clavicipitaceae for which genomes have been sequenced, representing the genera Aciculosporium I. Miyake, Atkinsonella Diehl, Balansia Speg., Claviceps Tul., Metarhizium Sorokīn and Periglandula U. Steiner, E. Leistner & Leuchtm. In addition to the genome sequences reported (CitationGao et al. 2011; CitationSchardl et al. 2013b, Citation2013a, Citation2014), that of E. sylvatica GR10156 and those of two E. bromicola strains were added (NCBI Bioprojects PRJNA275112, PRJNA274994, PRJNA274992). Outgroups were homologs from Hypocrealean fungi of other families. Coding sequences were aligned and phylogenies inferred for tubB, tubP, tefA and rpbB.
The tefA tree () and the others are available (supplementary figs. 2–4). The resulting trees differed in branching orders of clades corresponding to the various genera; In some trees, the three Claviceps species that were included did not group exclusively in a clade. However, a consistent characteristic of the trees was that the root of the Epichloë clade separated the Eg/Ei clade from the others. The same basal relationship of the Eg/Ei clade was reported in the phylogenies of genes for MCM7 and other housekeeping proteins, with the split between this clade and the other Epichloë spp. estimated at approximately 7.2 Ma before present (CitationAmbrose et al. 2014). Although the homologous E. sibirica sequences were unavailable for these analyses, it seems likely from the phylogeny of tubB intron regions () that they would group in the same clade as the Eg and Ei sequences. We conclude therefore that the three Epichloë species so far identified from Asian Achnatherum species represent a lineage that diverged from the other known Epichloë species early in evolution of the genus.
Fig. 3. Phylogeny of tefA coding sequences for Epichloë and related species. Gene-coding sequences were identified by manual annotation of sequenced genomes. The tree was inferred by maximum likelihood search using PhyML without GBlocks curation. The tree was rooted with Fusarium graminearum PH-1 as outgroup, and numbers on branches indicate ALR support. Boldface indicates isolates and branch support values relevant to the taxonomy and phylogenetic relationships of E. inebrians. The three gene copies in the hybrid endophyte, Epichloë coenophiala, are named with single-letter abbreviations of extant Epichloë species related to its three ancestors.
Diversity of endophytes of Achnatherum inebrians
A previous study indicated several differences among A. inebrians endophytes from host populations sampled in Xinjiang Province and in Sunan County, Gansu Province with respect to growth rate and colony morphology (CitationMoon et al. 2007), as well as alkaloid genes and alkaloid profiles (CitationSchardl et al. 2013b). Morphologically those from Sunan County fit the description of Neotyphodium gansuense (CitationLi et al. 2004), later renamed E. gansuensis (CitationLeuchtmann et al. 2014). Phylogenetic analysis indicated that the isolates from Xinjiang were closely related to the N. gansuense type, but were considered sufficiently different to be described as a distinct variety, N. gansuense var. inebrians (CitationMoon et al. 2007), later renamed E. gansuensis var. inebrians (CitationLeuchtmann et al. 2014). To address whether this variety represents a population genetically isolated from Eg, we sampled A. inebrians seeds from several populations in Gansu and Inner Mongolia and compared their endophytes with isolates e818 from Xinjiang and e7080 from Gansu.
We sampled A. inebrians from seven sites distributed across Xiahe and Sunan counties, Gansu Province and in Alxa County, Inner Mongolia (). Plants were sampled individually at five of those sites: Sunan sites SNA, SNB and SNC, Xiahe site XHC, and Alxa site AIM. From each of those sites 5–13 plants were sampled, giving 45 samples. In the other two locations (Xiahe sites XHA, XHB) seeds from multiple plants were bulked. For each of the samples from individual plants, six seeds were planted, of which between 4–6 seeds from each sample grew, producing a total of 242 progeny plants. An additional 35 progeny plants were grown from the bulk seed collections XHA and XHB. All of the progeny plants were screened by multiplex PCR (supplementary fig. 5). The endophyte infection frequency was remarkably high; 43 of the 45 samples gave rise to endophyte-symbiotic progeny plants and in 36 of those samples all progeny plants (n = 190) tested positive for endophytes. Also, in analysis of the bulked samples XHA and XHB, 30 of the 35 progeny plants were endophyte-symbiotic.
For each seed sample except one (sample SNC-2), progeny plants had endophytes of identical genotype. Overall only two distinct endophyte genotypes were detected (, Supplementary table II). One genotype was exemplified by the sequenced Ei isolate e818 and was the sole genotype identified in six of the eight populations. That genotype was mating type A, positive for the e818-related dmaW gene, and negative for idtG. The other genotype, exemplified by the sequenced Eg isolate e7080, was mating type B, negative for dmaW, and positive for idtG. This Eg e7080 genotype was rare outside the SNB population but also included isolate GR10140 from an A. pekinense plant sampled in Japan. Thus, although the Eg e7080 genotype was much less commonly sampled than the Ei e818 genotype, it was distributed nevertheless in populations separated by 2960 km. Although the negative PCR results cannot be considered a conclusive demonstration that all Eg isolates lacked dmaW and all Ei isolates lacked idtG, the consistency of the results within and across populations and in comparison with the sequenced genomes of e818, e7478 and e7080 suggest that every sampled A. inebrians endophyte fell into one of two distinct genotypes for which we designate Ei e818 and Eg e7080 as exemplars.
Table II. Endophyte detection and genotyping in progeny plants from seeds of Achnatherum inebrians collected at sites SNB and SNC
Sample SNC-2 was exceptional because both the Ei e818 and Eg e7080 genotypes were identified, the former in three progeny plants, and the latter in a single progeny plant. Finding multiple Epichloë genotypes in seeds from a single symbiotic plant is rare, probably because, whenever plants become co-infected, the endophytes efficiently segregate among the tillers (CitationWille et al. 1999, CitationChristensen et al. 2000). It is possible that the SNC-2 plant with the e7080 genotype was from contamination of that seed sample either during collection or during planting (A. inebrians seeds are fine).
The two distinct genotypes were confirmed in analysis of cultured endophytes (). The Eg e7080 genotype was associated with fast-growing isolates morphologically consistent with the description of E. gansuensis (CitationLi et al. 2004), whereas the Ei e818 genotype was associated with extremely slow-growing isolates morphologically consistent with the description of E. gansuensis var. inebrians (CitationMoon et al. 2007).
Table III. Characteristics of fungal isolates from Achnatherum inebrians and A. pekinensea
Extensive mating studies of Epichloë species indicate a bipolar mating system with two mating types that we designate A and B (previously mat-1 and mat-2, respectively; CitationLeuchtmann and Schardl 1998). Therefore an interbreeding sexual population should have both mating types. We observed only mating type A from six of the eight sampled populations. Furthermore, even in the other two populations SNB and SNC, all mating-type A endophytes possessed dmaW and lacked idtG, and all mating-type B endophytes lacked dmaW and possessed idtG, implying no recombination between mating types A and B in mixed populations. These results indicate that, at least in the host plant A. inebrians, Eg and Ei are asexual and clonal.
Conceivably Eg, Ei or both can complete a sexual cycle. However, this seems to be a remote possibility for Ei considering that no stromata were observed either for Ei or for any other slow-growing Epichloë species (CitationMoon et al. 2004). On the other hand expression of conidium-producing stromata, a prerequisite for the sexual cycle, has been observed occasionally on the related host species, A. sibiricum, when symbiotic with Eg (CitationLi et al. 2015). This and the identification of Eg from three different Achnatherum species suggest that some Eg strains may move plant to plant. Such mobility could be a function of conidia (CitationTadych et al. 2012; CitationLi et al. 2015; Li 2014 No. 6284) or ascospores (CitationChung and Schardl 1997) formed on the stromata.
Conclusions
This study of the diversity of endophytes in A. inebrians with their different alkaloid profiles might have important implications for management of grazing livestock on the grasslands of northern China. The moniker “drunken horse grass” is thought to be indicative of symptoms suffered by livestock after ingesting lysergic acid amides, the types of ergot alkaloids abundantly produced by Ei in symbio (CitationMiles et al. 1996). The consistent occurrence of dmaW genes in Ei isolates suggests that most or all produce ergot alkaloids. On the other hand the Eg genotypes indicate that they lack ergot alkaloids and instead produce indole-diterpenes such as paxilline, which have been observed in plants symbiotic with Eg e7080 (CitationSchardl et al. 2013b). Considering that indole-diterpenes also have neurotropic activity in mammals (CitationMunday-Finch et al. 1997, CitationSaikia et al. 2008), it remains to be determined whether the Eg-symbiotic plants also can cause toxicosis.
The close phylogenomic relationship of slow-growing isolates from two locations separated by 1650 km was in keeping with the distribution of morphologically and genetically similar isolates among widely separated populations of A. inebrians in China. These results and consideration of the importance of the A. inebrians-Ei symbiota in range-land degradation and livestock toxicosis motivated us to assign species rank to Epichloë inebrians.
Taxonomy
Epichloë inebrians (C.D. Moon & Schardl) L. Chen & C.J. Li, comb. & stat. nov.
MycoBank MB811807
≡ Neotyphodium gansuense var. inebrians C. D. Moon & Schardl, Mycologia 66: 899. 2007 (basionym).
Typification: CHINA, XINJIANG, Urumqi County, 43°35′N 87°01′E, 1800 m, infecting Achnatherum inebrians, 1993, K.F. Min, I. Fletcher, and P.S. Harris (holotype CUP 65636). Ex-type culture = e818 = ATCC MYA-1228. GenBank accession number PRJNA174039 (e818 genome).
Diagnosis: See diagnosis for Neotyphodium gansuense var. inebrians C.D. Moon & Schardl, CitationMoon et al. (2007).
Specimens examined: , .
tefA CDS
Download Text (46.7 KB)rpbB CDS
Download Text (92.3 KB)tubB CDS
Download Text (17.5 KB)tubP CDS
Download Text (44.3 KB)Supplementary material - Supplementary tables I, II; Supplementary figs. 1–5
Download PDF (1.2 MB)Acknowledgments
We gratefully acknowledge Walter Hollin for technical support, J. Douglas Brown for greenhouse management and Dr Juan Pan for preparation of E. bromicola genomic DNA for sequencing. This study was supported by the National Basic Research Program of China ( 2014CB138702), NSF EPSCoR grant EPS-0447479 and a scholarship to LC from the China Scholarship Council (CSC). KS expresses gratitude to the International Dispatch Program of NARO (National Agriculture & Food Research Organization, Japan) for letting him participate in this study and for advice from Prof Takashi Enomoto of Okayama University, Japan, concerning his collection of endophyte-infected grasses. This is publication No. 15-12-029 of the Kentucky Agricultural Experiment Station, published with the permission of the director.
Literature cited
- AmbroseKVKoppenhoferAMBelangerFC. 2014. Horizontal gene transfer of a bacterial insect toxin gene into the Epichloë fungal symbionts of grasses. Sci Rep 4:5562, doi:10.1038/srep05562
- AnisimovaMGascuelO. 2006. Approximate likelihood-ratio test for branches: a fast, accurate and powerful alternative. Syst Biol 55:539–552, doi:10.1080/10635150600755453
- BacettyAASnookMEGlennAENoeJPHillNCulbreathATimperPNagabhyruPBaconCW. 2009. Toxicity of endophyte-infected tall fescue alkaloids and grass metabolites on Pratylenchus scribneri. Phytopathology 99:1336–1345, doi:10.1094/PHYTO-99-12-1336
- CabralDCafaroMJSaidmanBLugoMReddyPVWhiteJFJr 1999. Evidence supporting the occurrence of a new species of endophyte in some South American grasses. Mycologia 91:315–325, doi:10.2307/3761376
- CharltonNDCravenKDAfkhamiMEHallBAGhimireSRYoungCA. 2014. Interspecific hybridization and bioactive alkaloid variation increases diversity in endophytic Epichloë species of Bromus laevipes. FEMS Microbiol Ecol 90:276–289, doi:10.1111/fem.2014.90.issue-1
- ChristensenMJSimpsonWRAl SamarraiT. 2000. Infection of tall fescue and perennial ryegrass plants by combinations of different Neotyphodium endophytes. Mycol Res 104:974–978, doi:10.1017/S0953756299002300
- ChungK-RSchardlCL. 1997. Sexual cycle and horizontal transmission of the grass symbiont, Epichloë typhina. Mycol Res 101:295–301, doi:10.1017/S0953756296002602
- DangXPCaoGRDuanDXLiSJ. 1992. Studies on the toxic constituent of Achnatherum inebrians. Xumu Shouyi Xuebao 23:366–371.
- DereeperAGuignonVBlancGAudicSBuffetSChevenetFDufayardJ-FGuindonSLefortVLescotMClaverieJ-MGascuelO. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36:W465–W469, doi:10.1093/nar/gkn180
- EdgarR. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:113, doi:10.1186/1471-2105-5-113
- EnomotoTShimanukiTTsukiboshiT. 1998. The gramineous plants in which Neotyphodium endopytes were found. J Weed Sci Technol 43(Suppl.):76–77 (in Japanese).
- GallagherRTWhiteEPMortimerPH. 1981. Ryegrass staggers: isolation of potent neurotoxins lolitrem A and lolitrem B from staggers-producing pastures. NZ Vet J 29:189–190, doi:10.1080/00480169.1981.34843
- GaoQJinKYingS-HZhangYXiaoGShangYDuanZHuXXieX-QZhouGPengGLuoZHuangWWangBFangWWangSZhongYMaL-JSt. LegerRJZhaoG-PPeiYFengM-GXiaYWangC. 2011. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet 7:e1001264, doi:10.1371/journal.pgen.1001264
- Gibbs-RussellGEEllisRP. 1982. The genus Melica L. (Poaceae) in southern Africa Melica racemosa, Melica decumbens, other species reduced to synonymy. Bothalia 14:37–44.
- GuindonSGascuelO. 2003. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704, doi:10.1080/10635150390235520
- HaarmannTOrtelITudzynskiPKellerU. 2006. Identification of the cytochrome P450 monooxygenase that bridges the clavine and ergoline alkaloid pathways. Chembiochem 7:645–652, doi:10.1002/cbic.v7:4
- LeuchtmannABaconCWSchardlCLWhiteJFTadychM. 2014. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 106:202–215, doi:10.3852/13-251
- LeuchtmannAClayK. 1990. Isozyme variation in the Acremonium/Epichloë fungal endophyte complex. Phytopathology 80:1133–1139, doi:10.1094/Phyto-80-1133
- LeuchtmannASchardlCL. 1998. Mating compatibility and phylogenetic relationships among two new species of Epichloë and other congeneric European species. Mycol Res 102:1169–1182, doi:10.1017/S0953756298006236
- LiCJGaoJHNanZB. 2007. Interactions of Neotyphodium gansuense, Achnatherum inebrians and plant-pathogenic fungi. Mycol Res 111:1220–1227, doi:10.1016/j.mycres.2007.08.012
- LiCJNanZBPaulVHDapprichPDLiuY. 2004. A new Neotyphodium species symbiotic with drunken horse grass (Achnatherum inebrians) in China. Mycotaxon 90: 141–147.
- LiCJNanZBZhangCJZhangCYZhangYH. 2009. Effects of drunken horse grass infected with endophyte on Chinese rabbit. J Agric Sci Technol 11:84–90.
- LiXZhouYZhuMQinJRenAGaoY. 2015. Stromabearing endophyte and its potential horizontal transmission ability in Achnatherum sibiricum. Mycologia 107:21–31, doi:10.3852/13-355
- LyonsPCPlattnerRDBaconCW. 1986. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 232:487–489, doi:10.1126/science.3008328
- MalinowskiDPBeleskyDP. 2000. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940, doi:10.2135/cropsci2000.404923x
- MilesCOLaneGADi MennaMEGarthwaiteIPiperELBallOJPLatchGCMAllenJMHuntMBBushLPMinFKFletcherIHarrisPS. 1996. High levels of ergonovine and lysergic acid amide in toxic Achnatherum inebrians accompany infection by an Acremonium-like endophytic fungus. J Agric Food Chem 44: 1285–1290, doi:10.1021/jf950410k
- MoonCDCravenKDLeuchtmannAClementSLSchardlCL. 2004. Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol Ecol 13: 1455–1467, doi:10.1111/mec.2004.13.issue-6
- MoonCDGuillauminJ-JRavelCLiCCravenKDSchardlCL. 2007. New Neotyphodium endophyte species from the grass tribes Stipeae and Meliceae. Mycologia 99: 895–905, doi:10.3852/mycologia.99.6.895
- MoonCDMilesCOJarlforsUSchardlCL. 2002. The evolutionary origins of three new Neotyphodium endophyte species from grasses indigenous to the southern hemisphere. Mycologia 94:694–711, doi:10.2307/3761720
- Munday-FinchSCWilkinsALMilesCOTomodaHOmuraS. 1997. Isolation and structure elucidation of lolilline, a possible biosynthetic precursor of the lolitrem family of tremorgenic mycotoxins. J Agric Food Chem 45:199–204, doi:10.1021/jf960396r
- OrtelIKellerU. 2009. Combinatorial assembly of simple and complex d-lysergic acid alkaloid peptide classes in the ergot fungus Claviceps purpurea. J Biol Chem 284:6650–6660, doi:10.1074/jbc.M807168200
- PetroskiRPowellRGClayK. 1992. Alkaloids of Stipa robusta (sleepygrass) infected with an Acremonium endophyte. Nat Tox 1:84–88, doi:10.1002/(ISSN)1056-9014
- PotterDAStokesJTRedmondCTSchardlCLPanaccioneDG. 2008. Contribution of ergot alkaloids to suppression of a grass-feeding caterpillar assessed with gene-knockout endophytes in perennial ryegrass. Entomol Experiment Appli 126: 138–147, doi:10.1111/eea.2008.126.issue-2
- RenJ. 1959. Common poisonous plants in north-western grasslands of China. J Gansu Agric Univ 1:9–16.
- SaikiaSNicholsonMJYoungCParkerEJScottB. 2008. The genetic basis for indole-diterpene chemical diversity in filamentous fungi. Mycol Res 112:184–199, doi:10.1016/j.mycres.2007.06.015
- SchardlCLGrossmanRBNagabhyruPFaulknerJRMallikUP. 2007. Loline alkaloids: currencies of mutualism. Phytochemistry 68:980–996, doi:10.1016/j.phytochem.2007.01.010
- SchardlCLLeuchtmannASpieringMJ. 2004. Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 55:315–340, doi:10.1146/annurev.arplant.55.031903.141735
- SchardlCLPanaccioneDGTudzynskiP. 2006. Ergot alkaloids—biology and molecular biology. Alkaloids: Chem Biol 63:45–86.
- SchardlCLYoungCAFaulknerJRFloreaSPanJ. 2012. Chemotypic diversity of epichloae, fungal symbionts of grasses. Fungal Ecol 5:331–344, doi:10.1016/j.funeco.2011.04.005
- SchardlCLYoungCAHesseUAmyotteSGAndreevaKCaliePJFleetwoodDJHawsDCMooreNOeserBPanaccioneDGSchweriKKVoiseyCRFarmanMLJaromczykJWRoeBAO’SullivanDMScottBTudzynskiPAnZArnaoudovaEGBullockCTCharltonNDChenLCoxMDinkinsRDFloreaSGlennAEGordonAGüldenerUHarrisDRHollinWJaromczykJJohnsonRDKhanAKLeistnerELeuchtmannALiCLiuJLiuJLiuMMaceWMachadoCNagabhyruPPanJSchmidJSugawaraKSteinerUTakachJTanakaEWebbJSWilsonEVWisemanJLYoshidaRZengZ. 2013b. Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet 9: e1003323, doi:10.1371/journal.pgen.1003323
- SchardlCLYoungCAMooreNKromNDupontP-YPanJFloreaSWebbJSJaromczykJJaromczykJWCoxMPFarmanML. 2014. Genomes of plant-associated Clavicipitaceae. Adv Bot Res 70:291–327.
- SchardlCLYoungCAPanJFloreaSTakachJEPanaccioneDGFarmanMLWebbJSJaromczykJCharltonNDNagabhyruPChenLShiCLeuchtmannA. 2013a. Currencies of mutualisms: sources of alkaloid genes in vertically transmitted epichloae. Toxins 5:1064–1088, doi:10.3390/toxins5061064
- ShiZC. 1997. Important poisonous plants of China grassland. Beijing: China Agric Press. p 49–50.
- ShymanovichTSaariSLovinMJarmuschAJarmuschSMussoACharltonNYoungCCechNFaethS. 2015. Alkaloid variation among epichloid endophytes of sleepygrass (Achnatherum robustum) and consequences for resistance to insect herbivores. J Chem Ecol 41:93–104, doi:10.1007/s10886-014-0534-x
- TadychMAmbroseKBergenMBelangerFWhiteJ. 2012. Taxonomic placement of Epichloë poae sp. nov. and horizontal dissemination to seedlings via conidia. Fungal Divers 54:117–131, doi:10.1007/s13225-012-0170-0
- TakachJEMittalSSwobodaGABrightSKTrammellMAHopkinsAAYoungCA. 2012. Genotypic and chemotypic diversity of Neotyphodium endophytes in tall fescue from Greece. Appl Environ Microbiol 78:5501–5510, doi:10.1128/AEM.01084-12
- TakachJEYoungCA. 2014. Alkaloid genotype diversity of tall fescue endophytes. Crop Sci 54:667–678, doi:10.2135/cropsci2013.06.0423
- TanakaATapperBAPopayAParkerEJScottB. 2005. A symbiosis expressed non-ribosomal peptide synthetase from a mutualistic fungal endophyte of perennial ryegrass confers protection to the symbiotum from insect herbivory. Mol Microbiol 57:1036–1050, doi:10.1111/j.1365-2958.2005.04747.x
- TimperPGatesRNBoutonJH. 2005. Response of Pratylenchus spp. in tall fescue infected with different strains of the fungal endophyte Neotyphodium coenophialum. Nematology 7:105–110, doi:10.1163/1568541054192216
- WillePAAeschbacherRABollerT. 1999. Distribution of fungal endophyte genotypes in doubly infected host grasses. Plant J 18:349–358, doi:10.1046/j.1365-313X.1999.00462.x
- ZhangXRenACiHGaoY. 2010. Genetic diversity and structure of Neotyphodium species and their host Achnatherum sibiricum in a natural grass–endophyte system. Microb Ecol 59:744–756, doi:10.1007/s00248-010-9652-3
- ZhangXRenA-ZWeiY-KLinFLiCLiuZ-JGaoY-B. 2009. Taxonomy, diversity and origins of symbiotic endophytes of Achnatherum sibiricum in the Inner Mongolia steppe of China. FEMS Microbiol Lett 301:12–20, doi:10.1111/fml.2009.301.issue-1
- ZhangXXLiCJNanZBMatthewC. 2012. Neotyphodium endophyte increases Achnatherum inebrians (drunken horse grass) resistance to herbivores and seed predators. Weed Res 52:70–78, doi:10.1111/wre.2011.52.issue-1
