Abstract
An experiment was conducted to study the effects of water-soluble DL-methionine supplied through water and feed on the performance and carcass yield of broilers housed at two constant temperatures from 21 to 42 days of age. Birds were housed in two rooms (240 birds per room) with temperatures set at 21±2 and 30±2°C, respectively. Birds were divided into five groups of equal number within each room and fed five different diets (G1-G5). A low-methionine basal diet without supplemental methionine was fed to group 1 (G1). The basal diet was fortified with 0.025% or 0.050% methionine, either in feed (G2 and G3, respectively), or in a water solution (G4 and G5, respectively). Mortality was not significantly altered by any dietary treatment. Neither feed nor water intake was affected adversely by DL-methionine inclusion in drinking water. Housing at high temperature showed deleterious effect on birds’ weight gain. Additional methionine intake both in feed and water was associated with significantly heavier body weight, weight gain and feed conversion ratio, than the basal diet at low and high environmental temperature. Carcass yields, as a percentage of live body weight, were not affected by dietary treatment. The results indicate that, under the experimental conditions, DL-methionine provided in drinking water can be effectively assimilated by broilers, at least from 21 to 42 days of age.
Introduction
High environmental temperatures have a negative effect on the performance of broilers during the finishing period, mainly through decreasing feed intake and growth rate or (Adams et al., Citation1962; Cowan and Michie, Citation1978; Cerniglia et al., Citation1983; Han and Baker, Citation1993; Cheng et al., Citation1997, Citation1999; Gu et al., Citation2008). A variety of experiments has been conducted to demonstrate the growth depression associated with heat stress, and several possible nutritional solutions have been recommended, but have given conflicting results when applied in practice (Balnave, Citation2004; Syafwan et al., Citation2011). One strategy for improving live performance of broilers suffering from heat stress was to limit the access to limiting nutrients. The optimum intake of the first-limiting amino acid will affect the requirements of all other amino acids. Methionine is the first limiting amino acid in the conventional corn-soybean and wheat-soybean diets (Leong and Mc Ginnis, Citation1952; Lemme et al., Citation2004; Hoehler et al., Citation2005; Payne et al., Citation2006), and synthetic methionine has been used for over six decades as a supplement to these diets. Birds under stressful conditions (hot temperature, rapid temperature changes, crowding, etc.) are often unable to eat but are able to drink (Nort and Bell, Citation1990) thus they would benefit from the delivering of methionine in the drinking water. The use of methionine in drinking water may allow the producers to supply this nutrient rapidly and efficiently, when birds have a reduced food intake. It has been shown that administering methionine in drinking water rather than feed does not result in adverse effects on water and feed intake and production or mortality of poults (Griggs et al., Citation1971), broilers (Damron and Goodson-Williams, Citation1987; Damron and Flunker, Citation1992) or layers (Cadirci, Citation2001). Both the DL form (Damron and Goodson-Williams, Citation1987) and the liquid analogue (Damron and Flunker, Citation1992) of methionine were found to be suitable for supplementation in water without adverse effects. It has also been demonstrated (Cadirci et al., Citation2009) that layers fed methionine deficient diet are able to select for water supplemented with methionine in preference to pure water, and the way of methionine delivery either in feed or water shows no difference from the point of view of satisfying requirements for normal egg production in laying hens (Cadirci, Citation2012).
There is no information regarding the performance of broilers receiving methionine in water under heat stress. Therefore, in the present study methionine was administered in water to broilers from 21 to 42 days of age at two different environmental temperatures. The effects were evaluated and a comparison has been made to the traditional way (feeding) of methionine supplying.
Materials and methods
A total of 480 mixed sex broiler chicks (Ross® 308) were used for the experiment. They were subjected to continuous light from day old to the end of the experiment (42 days of age). Birds were fed a crumbled starter diet (CP=23%, ME=3200 kcal/kg), the nutrient specifications were set to meet or exceed NRC (Citation1994) nutrient requirements. Rearing temperatures were 32°C at 1 day then 2°C at 1 week and 26°C at 2 weeks of age.
The experiment began when the chicks reached 21 days of age. All birds were wing tagged and placed into cages in two rooms, 240 birds per room. Birds were weighed and two male and two female birds were placed in each cage (50 × 50 × 50 cm). Weights were adjusted so that there were no significant differences in the weight of cages among the various treatments. Throughout the experiment, five different diets were given at both temperatures. For this, a low-methionine basal diet was prepared. Group 1 received basal diet and tap water and no additional methionine. For groups 2 and 3, basal diet fortified with 0.025% or 0.050% methionine from crystalline DL-methionine and tap water was given (G2 and G3, respectively). Groups 4 and 5 (G4 and G5) received basal diet and a water solution of 0.025 or 0.050% of methionine, respectively. For methionine treated water solution, commercially available water soluble crystalline DL-methionine [dl-2-amino-4(methylthio)-butanoic acid] was used (Weast, Citation1975). Treatments were repeated twelve times with four broilers (two male and two female) per replicate. Room temperatures were set at 21±2 and 30±2°C by controlling two air conditioners (White Westinghouse). Relative humidity (RH) in the rooms was not controlled but it was recorded daily. The mean values were 45±0.77% RH at the high temperature and 50±0.84% RH at the low temperature. The response of broilers at high temperatures differs with different relative humidity: high temperature accompanied by high humidity is more detrimental to broiler growth than high temperature with low humidity (Balnave, Citation2004). As relative humidity was low at both temperatures, only environmental temperature and the way of delivering methionine were the major factors influencing broiler performance.
Prior to the experiment, maize and soybean meal were analysed for crude protein using standard AOAC procedures (AOAC, Citation1980). Analysis of amino acids was carried out by using ion-exchange chromatography following the procedure of Llames and Fontaine (Citation1994). Diet was formulated based upon these values. The ingredients used and the calculated nutrient content of the basal diet formulations used in this study are shown in . Methionine solutions were batch-mixed weekly in tap water in large (60 L) plastic containers, and provided ad libitum according to the design of the experiment through plastic watering bottles (2000 mL) fitted with nipples at the base. One feed-trough and one water bottle were located at the front of each cage. During the course of the experiment all birds were fed ad libitum and every effort was made to ensure their welfare. Each day, the birds were allocated enough feed (1000 g) to exceed the expected daily food intake for broilers of this age and strain. Daily feed intake and water consumption were measured gravimetrically every 24 h and recorded for each replicate, then total and average FI and water consumption were calculated. The water remaining in the bottles was discarded weekly and replaced with fresh water. Methionine intake per replicate was also calculated from the amounts consumed via the feed and water.
Chickens were deprived of food for 8 h and individually weighed just before slaughter. At day 42, all birds were manually slaughtered and plucked then feet, head and the intestinal package were removed manually and the remaining carcass was weighed. All carcasses were chilled at approximately 2°C and the next day they were dissected in order to determine yields of breast meat (without bones and skin), thighs (without skin), drumsticks (without skin) and abdominal fat. Carcass yield data were expressed as a percentage of live body weight. Feed conversion ratio (feed intake/weight gain) was calculated to better evaluate overall bird performance. All results were analysed statistically by analysis of covariance using the GLM procedure of the SAS program (SAS, Citation2001) with temperature, diets and interaction between them as sources of variation. In addition, initial body weight was used as a covariable, in order to eliminate any effect of the initial body weight on the final performance parameters. Significant differences were tested further using a Tukey’s HSD multiple range test to determine the differences among treatments.
Results and discussion
The results for body weight gain, daily feed intake, water intake, methionine intake and feed conversion ratio of birds consuming methionine given either in feed or water at two environmental temperatures during the 21 days of the experiment are shown in . With the exception of feed conversion ratio, there was no significant interaction between environmental temperature and the way of methionine delivery. Therefore, primary attention is directed to the main effects of environmental temperature and the way of methionine delivery on body weight gain, daily feed, water, and methionine intake. A significant reduction (P<0.05) of body weight gain and feed intake was observed at 30°C compared to 21°C, whereas water intake significantly increased (P<0.05) at the high environmental temperature. The rate of feed intake reduction was greater than that of the increase of water intake, causing a decrease of mean methionine consumption (P<0.05). It has been shown that decreased rate of growth of broilers occurs when environmental temperature rises (Howlider and Rose, Citation1987). This negative effect of high temperature on performance may primarily be due to a reduced feed intake (Hurwitz et al., Citation1980). Geraert et al. (Citation1996) reported a greater reduction in growth than in feed intake resulting in a depressed feed efficiency. In this experiment, however, the negative effect of high environmental temperature resulted in similar rates of reduction in body weight gain and feed intake, thus feed conversion ratio was not significantly different at low and high environmental temperature. Thus it seems that, under the conditions of this study, reduced feed consumption and lower body weight gain associated with high temperature in broilers cannot be overcome by delivering methionine in drinking water.
In general, the addition of methionine (at both concentrations) positively affected the birds’ performance from 21 to 42 days of the experiment regardless of the way of delivery or the environmental temperature, the greatest effects being those caused by the high concentration delivered in water (G5). Body weight gain significantly increased with both concentrations and ways of delivery (P<0.05) in comparison to the basal diet. The increase was the smallest with the low level of methionine (0.025%) added to the feed (G2), a significant difference from the other groups. There was no significant difference amongst the other groups in body weight gain. The addition of DL-methionine in feed or water resulted in increased daily feed and water consumption. The increase was statistically significant in all groups except for the water consumption of the group receiving small amount of methionine in feed (G2). Amongst the groups, the only statistical difference in feed and water consumption occurred between birds receiving 0.025% additional methionine through feed (G2) and those getting 0.050% additional methionine in water (G5). Total daily methionine intake significantly increased with the addition of methionine. In turn, the increase of methionine intake resulted in a higher body weight gain. Methionine consumption significantly varied among the groups because of differing concentrations of supplementation in feed or water. It is notable that the addition of methionine at the highest (0.050%) level in feed (G3) and the lowest (0.025%) level in water (G4) resulted in almost the same methionine consumption (4.948 g/day and 4.937 g/day, respectively) and the same performance. Nevertheless, there is a trend of increasing body weight gain as methionine intake increases, the greatest body weight gain (1643.0 g) being associated with the highest waterborne and total methionine consumption (5.749 g/d). These results indicate that the way of delivering methionine (in feed or water) is indifferent from the point of view of satisfying requirements for the birds’ performance. The results obtained for performance traits during this study are similar to those reported by Damron and Goodson-Williams (Citation1987) and Damron and Flunker (Citation1992).
Significant differences (P<0.05) were found among the effects of dietary treatments on feed conversion ratio (). Consumed feed per unit of body weight gain significantly decreased with supplemental methionine (P<0.05), whether supplied via water or feed. The effect of 0.025% of methionine in feed was the lowest. The delivery of methionine in water (0.025 and 0.050%) had significantly greater effect on FCR (P<0.05).
shows the interaction of environmental temperature and dietary treatment in relation to feed conversion ratio. Feed conversion ratio significantly improved when the highest (0.050%) level of methionine was supplemented in water with the increase of environmental temperature from 21 to 30°C. In addition, the supplementation of 0.050% methionine via water significantly improved feed efficiency compared to 0.050% additional methionine supplied via feed (P<0.05) at high temperature. This might be the result of the consistent supply of methionine via water to the birds under heat stress, and consequently of the improved feed efficiency. The highest methionine consumption was observed when the additional 0.050% methionine was supplied via water. This result is in agreement with the observation by Balnave and Oliva (Citation1990). They found that improved feed conversions resulted when broilers were housed at hot temperatures and fed higher methionine levels than necessary for maximum weight gain at 21°C. In accordance with the works of Damron and Goodson-Williams (Citation1987) and Damron and Flunker (Citation1992), the mortality rate was not affected. On average 0.625% of the birds died during the experimental period with no appreciable differences between temperatures and among the treatments ().
shows live body weight, weight of eviscerated carcass and carcass parts (drums, thighs, de-boned breast and abdominal fat), and their ratio to live body weight of broiler slaughtered at 42 days of age. No significant interaction of temperature and diet was observed, therefore, only the main effects for temperatures and dietary treatments are presented. Austic (Citation1985), Leenstra and Cahaner (Citation1991) and Borges at al. (Citation2004) reported that broilers kept in high ambient temperature produce less absolute carcass weight, and this decrease is particularly evident in breast meat (Howlider and Rose, Citation1989). Our observations showed similarity with these findings: high environmental temperature significantly reduced live body weight and the weight of eviscerated carcass, thighs and de-boned breast. In contrast, drums weight was unaffected by environmental temperature: the negative effect of high temperature was not sufficiently great to result in significant difference between the low and high temperature groups. The relative weight of carcass, drums and thighs meat as a percentage of live body weight were significantly greater in high environmental temperature treatments while the opposite was true for the de-boned breast meat. The results indicate that the growth of breast was suppressed more severely relative to live body weight, and the development of carcass, drums and thighs were suppressed to a lesser extent than live body weight. Therefore, in agreement with the findings of Gu et al. (Citation2008) and Yalcin et al. (Citation1997, Citation2001), the results of the present study suggest that the growth of breast is more sensitive to high environmental temperature. Baziz et al. (Citation1996) suggested that high temperature environment would facilitate the development of abdominal fat. In our work, the relative (as a percentage of live weight) and absolute weight of abdominal fat increased with the increased temperature. Effects of dietary treatments were similar on live body weight at slaughtering and on weight gain. The live body weights of groups with supplemental methionine (in feed or water) were significantly higher than of those without added methionine. Among the dosage levels, significant differences were observed only between the lowest (0.025%) level of methionine supplementation in feed and the other dosage levels. With respect to the absolute weight of carcass parts, the effects of the dietary treatments on eviscerated carcass, drums, thighs, and de-boned breast was similar to that observed for weight gain. There were improvements in all carcass parts for all increments in methionine supplemented to basal diet in feed or water. It was more obvious with the highest supplementation (0.050%) in water and where the highest methionine consumption was observed. In contrast, the effects of dietary treatment were not significant on absolute weight of abdominal fat. In addition, comparisons made as percentages of live body weight revealed a tendency for the carcass and yield of major carcass parts to increase when the basal diet was fortified with methionine either in feed or water during the 21 days of the experimental period. In contrast, the opposite was true for the percentage of abdominal fat. However, the effect was not sufficiently great to result in a significant difference among any of the dietary treatments on all those parameters. In agreement with previous reports (Damron and Goodson-Williams, Citation1987; Damron and Flunker, Citation1992) our study demonstrated that methionine given to the drinking water of broilers rather than to their feed does not have adverse effects on the performance and carcass yields of birds. In addition, the present work showed that the interaction between methionine supplementation and temperature might positively affect feed efficiency.
Conclusions
Giving methionine to the drinking water of birds provides an easy way of methionine adjustment in accordance with needs without re-mixing the feed, thus saving the producer the cost of extra equipment, time and labour required when handling feed. An additional benefit of this method is the consistent supply of methionine to the birds and, consequently, improved feed efficiency. In addition, this practice would allow a better handling of stressful conditions (heat stress, crowdedness). Our data indicated that, under the conditions of this study, liquid DL-methionine can be effectively provided to growing birds through the drinking water, and that interaction between the ways and dosages of methionine supplementation and ambient temperature might positively affect feed efficiency. Substantial savings in the cost of production may be possible should delivering methionine via drinking water be proven feasible under commercial conditions. Therefore, further investigation is needed to verify the efficacy of delivering methionine in water compared to feed, with more dosage levels, with a particular emphasis on the impact of feed efficiency under the heat stress of broiler chickens between 21 to 42 days of age.
Acknowledgments
The authors wish to thank the University of Harran for financial support and facilities, and also thank Katalin Vajda for her general comments and advice during the project.
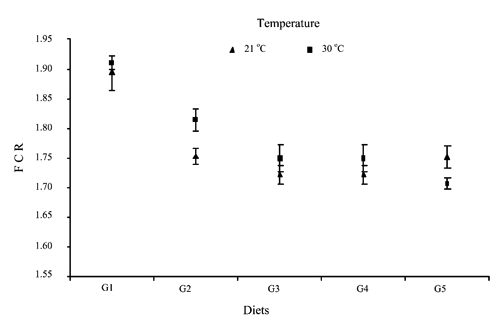
Table 1. Ingredient and nutrient composition of basal diet.
Table 2. Initial body weight, body weight gain, daily feed intake, water intake, methionine intake and feed conversion ratio of birds consuming methionine in either feed or water at 21 and 30°C.
Table 3. Body weight, carcass, major carcass parts yield and abdominal fat of birds consuming methionine in either feed or water at 21 and 30°C.
References
- Adams R.L. Andrews F.N. Gardiner E.E. Fontaine W.E. CarrickC., 1962. The effects of environmental temperature on the growth and nutritional requirements of the chick. Poultry Sci. 41 :588-594.
- AOAC,1980. Official Methods of Analysis, 13th ed. Association of Official Analytical Chemists, Washington DC, USA.
- Austic R.E., 1985. Feeding poultry in hot and cold climates. In: Yousef M.K. (ed.) Stress physiology in livestock. CRC Press, Boca Raton, FL, USA, pp 123-136.
- BalnaveD., 2004. Challences of accurately defining the nutrient requirements of heat-stressed poultry. Poultry Sci. 83 :5-14.
- BalnaveD. OlivaA., 1990. Responses of finishing broilers at high temperatures to dietary methionine source and supplementation levels. Aust. J. Agric. Res. 41 :557-564.
- Baziz H.A. Geraert P.A. Padilha J.C.F. GuillauminS., 1996. Chronic heat exposure enhances fat deposition and modifies muscle and fat partition in broiler carcasses. Poultry Sci. 75 :505-513.
- Borges S.A. Fischer da Silva A.V. MaiorkaA. Hooge D.M. Cummings K.R., 2004. Effects of diet and cyclic daily heat stress on electrolyte, nitrogen and water intake, excretion and retention by colostomised male broiler chickens. Int. J. Poultry Sci. 3 :313-321.
- CadirciS., 2001. Feeding methionine to laying hens in drinking water. Degree Diss., University of Glasgow, UK.
- CadirciS., 2012. Possible effects of delivering methionine to laying hens in drinking water. Ital. J. Anim. Sci. 11 :e46.
- CadirciS. Smith W.K. Mc Devitt R.M., 2009. Determination of the appetite of laying hens for methionine in drinking water by using colour cue. Arch. Geflügelk. 73 :21-28.
- Cerniglia G.J. Hebert J.A. Watts A.B., 1983. The effect of constant ambient temperature and ration on the performance of sexed broilers. Poultry Sci. 62 :746-754.
- Cheng T.K. Hamre M.L. Coon C.N., 1997. Responses of broilers to dietary protein levels and amino acid supplementation to low protein diets at various environmental temperatures. J. Appl. Poultry Res. 6 :18-33.
- Cheng T.K. Hamre M.L. Coon C.N., 1999. Effect of constant and cyclic environmental temperatures, dietary protein, and amino acid levels on broiler performance. J. Appl. Poultry Res. 8 :426-439.
- Cowan P.J. MichieW., 1978. Environmental temperature and choice feeding of the broiler. Brit. J. Nutr. 40 :311-315.
- Damron B.L. Flunker L.K., 1992. 2-Hydroxy-4(Methylthio) butanoic acid as a drinking water supplement for broiler chicks. Poultry Sci. 71 :1695-1699.
- Damron B.L. Goodson-WilliamsR., 1987. Liquid methionine as a drinking water supplement for broiler chicks. Poultry Sci. 66 :1001-1006.
- Geraert P.A. Padilha J.C.F. GuillauminS., 1996. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: biological and endocrinological variables. Brit. J. Nutr. 75 :205-216.
- Griggs J.E. Harris G.C. Waldroup P.W., 1971. The use of nutrient solutions for young turkey poults. Poultry Sci. 50 :1581 (abstr.).
- Gu H.X. Li S.S. LinH., 2008. Effects of hot environment and dietary protein level on growth performance and meat quality of broiler chickens. Asian Austral. J. Anim. 21 :1616-1623.
- HanY. Baker D.H., 1993. Effects of sex, heat stress, body weight, and genetic strain on the dietary lysine requirement of broiler chicks. Poultry Sci. 72 :701-708.
- HoehlerD. LemmeA. Jensen S.K. Vieira S.L., 2005. Relative effectiveness of methionine sources in diets for broiler chickens. J. Appl. Poultry Res. 14 :679-693.
- Howlider M.A.R. Rose S.P., 1987. Temperature and the growth of broilers. World. Poultry Sci. J. 43 :228-237.
- Howlider M.A.R. Rose S.P., 1989. Rearing temperature and the meat yield of broilers. Brit. Poultry Sci. 30 :61-67.
- HurwitzS. WeiselbergM. EisnerU. BartovI. RiesenfeldG. SharvitM. NivA. BornsteinS., 1980. The energy requirements and performance of growing chickens and turkeys as affected by environmental temperature. Poultry Sci. 59 :2290-2299.
- LeenstraF. CahanerA., 1991. Genotype by environment interactions using fast-growing, lean or fat broiler chickens, originating from the Netherlands and Israel, raised at normal or low temperature. Poultry Sci. 70 :2028-2039.
- LemmeA. RavindranV. Bryden W.L., 2004. Ileal digestibility of amino acids in feed ingredients for broilers. World. Poultry Sci. J. 60 :423-438.
- Leong K.C. Mc Ginnis J., 1952. An estimate of the methionine requirement for egg production. Poultry Sci. 31 :692-695.
- Llames C.R. FontaineJ., 1994. Determination of amino acids in foods. Collaborative study. J. AOAC Int. 77 :1362-1402.
- National Research Council 1994. Nutrient Requirements of Poultry, 9th ed. National Academy Press, Washington, DC, USA.
- North M.O. Bell D.D., 1990. Broiler, roaster, and capon management. In: Commercial Chicken Production Manual, 4th ed. Chapman & Hall Publ., New York, NY, USA, pp 453-504.
- Payne R.L. LemmeA. SekoH. HashimotoY. FujisakiH. KoreleskiJ. SwiatkiewiczS. SzczurekW. RostagnoH., 2006. Bioavailability of methionine hydroxy analog-free acid relative to DL-methionine in broilers. Anim. Sci. 77 :427-439.
- SAS, 2001. User’s Guide: Statistics, ver. 8.01. SAS Inst. Inc., Cary NC, USA.
- SyafwanS. Kwakkel R.P. Verstegen M.W.A., 2011. Heat stress and feeding strategies in meat-type chickens. World. Poultry Sci. J. 67 :653-674.
- Weast R.C., 1975. Handbook of chemistry and physics. CRC Press, Cleveland, OH, USA.
- YalcinS. OzkanS. TurkmutL. Siegel P.B., 2001. Responses to heat stress in commercial and local broiler stocks. 1. Performance traits. Brit. Poultry Sci. 42 :149-152.
- YalcinS. SettarP. OzkanS. CahanerA., 1997. Comparative evaluation of three commercial broiler stocks in hot versus temperate climates. Poultry Sci. 76 :921-929.
