Abstract
White striping defect (appearance of white striations parallel to muscle fiber on surface of breast) is considered an emerging issue in chicken breast meat which is related to increasing growth rate of modern hybrid birds. This study was aimed at evaluating the effect of white striping on chemical composition and nutritional value of chicken breast meat. During three replications, a total of 108 Pectoralis major muscles representing three degrees of white striping (absence=normal; presence classified in 2 levels as moderate or severe) were selected to determine proximate composition (moisture, protein, lipid and collagen) as well as sarcoplasmic and myofibrillar protein profile by sodium dodecyl sulphate-polyacrylamide gel electrophoresis analysis. The results showed that both severe and moderate white-striped fillets had higher fat content (2.53 vs 1.46 vs 0.78%; P<0.001), lower protein level (20.9 vs 22.2 vs 22.9%; P<0.001), decreased quality of protein as proven by higher collagen content (1.30 vs 1.37 vs 1.43%; P<0.001), and different pattern on myofibrillar and sarcoplasmic fractions when compared to normal fillets. Moreover, severe white-striped fillets exhibited higher energy content (450.7 vs 421.1 kJ/100g; P<0.01) with respect to normal meat. In conclusion, there was a large worsening of nutritional value of chicken breast meat following occurrence of white striping and this might impair consumer attitude towards poultry meat.
Introduction
In recent decades, the global consumption of chicken and turkey meat has dramatically increased and it is expected that in the few coming years the poultry meat becomes the first type of meat produced in the world. Outlooks of the year 2020 suggest that the world production of poultry meat will approach 122.5 million metric tons (Best, Citation2011). The two main reasons that are driving the success of poultry meat in both developed and developing countries are mainly lower cost and the perceived healthy nutritional profile with respect to pork and beef meat. In addition, the absence of religious constraints and the relative ease of culinary preparation together with large availability of convenient processed products have also played a positive role in favouring its consumption expansion (Petracci et al., Citation2013a).
The persistent growing demand on poultry meats put more has pressure on producers to improve the production performance of live birds (i.e. growth rate and feed conversion). Today, chickens and turkeys are marketed in about half the time and at about twice of body weight (BW) compared to 50 years ago (Havenstein et al., Citation2003; Barbut et al., Citation2008). In Europe and North America, the demand is mainly concentrated on breast meat, sold both fresh and processed due to nutritional quality, high tenderness and easiness of culinary preparation (Barbut et al., Citation2008). As a consequence, modern hybrid birds showed dramatic development in breast yield which represents more than 20% of BW (Havenstein et al., Citation2003). Regarding the nutritional aspects, poultry meat and in particular breast meat fits the modern consumer demand for a low-fat meat with a high unsaturation degree of fatty acids and low sodium and cholesterol levels (Cavani et al., Citation2009). It is noteworthy that in avian species lipid accumulation occurs mostly under the skin and so it is easy to separate this fat, while the deposition of intramuscular fat (marbling) is more limited (Mourota and Hermierb, Citation2001). Poultry meat may also be considered as a functional food as it provides bioactive substances with favourable effects on human health, e.g. long-chain n-3 polyunsaturated fatty acids (PUFA), conjugated linoleic acid (CLA), bioactive peptides, vitamins and antioxidants (Cavani et al., Citation2009; Gibbs et al., Citation2010; Ryan et al., Citation2011)
Recently, it has been observed that chicken meat does not still keep the same nutritional features as in the past. Some studies showed that today’s poultry meat contains higher lipid content compared with that produced some years ago (Wang et al., Citation2009; Crawford et al., Citation2010; Kuttappan et al., Citation2012a). On the other hand, several studies showed that modern hybrid birds selected for high growth rate have fostered the appearance of different abnormal conditions in breast muscles. Pale, soft and exudative (PSE)-like, deep pectoral disease and white striping (WS) are the most important current concerns in poultry meat quality (Petracci and Cavani, Citation2012). White striping is a new quality issue described by the appearance of white striation parallel to muscle fiber on the surface of Pectoralis major muscles (Petracci and Cavani, Citation2012). A recent survey has estimated that incidence of white-striped breast fillets was around 12% (Petracci et al., Citation2013b). The causes of WS are still unknown and no sign of systemic infections was found, but the histological evaluations showed that WS usually coupled with muscle degeneration and myopathic changes beneath the striation area (Kuttappan et al., Citation2013a; Petracci et al., Citation2013c; Sihvo et al., Citation2013). Occurrence of WS on the surface of chicken breasts impaired visual appearance and reduced the consumer willingness to buy this type of meat (Kuttappan et al., Citation2012b). For this reason, fillets showing severe WS may be downgraded in commercial plants and used for manufacturing further processed products (e.g. sausages, nuggets), while moderate WS fillets are not usually downgraded and marketed for fresh retailing. However, it has been found that the effect of WS was not confined on impairing visual appearance only, but it also involved chemical composition (Kuttappan et al., Citation2012a) and reduced technological properties of the meat (Petracci et al., Citation2013b).
Chicken breast meat is actually considered as a high quality meat as for the above mentioned nutritional aspects (i.e. low energy level, cholesterol content, and high polyunsaturated fatty acid content), but changing in the chemical composition due to occurrence of WS could have consequences on nutritional traits of chicken breast. The aim of this study was to evaluate the proximate chemical composition and nutritional value of breast meat produced from modern broiler hybrids which show increasing rates of WS defect.
Materials and methods
Sample selection and preparation
Three individual replicates were conducted using a total of 108 breast fillets (Pectoralis major muscles) from 7-week-old straight-run Ross 708 broilers (2.8 kg live weight) collected from the deboning area of a commercial processing plant at 24 h post-mortem. Ross 708 is one of the main commercial fast-growing and high-breast yield hybrids used in United States and Europe for chicken meat production. Breast fillets were classified into three degrees of WS depending on the intensity and thickness of white striations: normal fillet when there is no white striations; moderate when the fillets showed small thin lines (<1 mm); whereas those having thick (>1 mm) white striations covering most of the surface area were graded as severe (according to criteria suggested by Kuttapan et al., Citation2012b; ). In each replicate, breast fillets were bagged by group (12 fillets/group), packed on ice, and transported to the laboratory. The cranial part from each fillet was selected for determination of chemical composition (moisture, protein, lipid and collagen contents) and electrophoresis analysis.
Chemical composition
Proximate composition (moisture, protein and lipid contents) of breast meat was assessed using official methods of AOAC (Citation1990). The moisture content was determined on a ground sample of about 5 g which was dried in a conventional oven at 100 to 102°C for 16 h, crude protein content was measured by Kjeldahl method, while intramuscular fat content was estimated by petroleum ether extraction using soxhlet method. Hydroxyl proline was determined as a measure of collagen content using a colorimetric method (Kolar, Citation1990). About 4 g of finely minced meat was hydrolysed with sulphuric acid in air oven at 105°C for 16 h. Hydrolysed hydroxyl proline was diluted to proper concentration. Reddish purple complex was formed by oxidising with chloramine-T followed with 4-dimethylaminobenzaldehyde. The absorbance was measured by UV-Visible Spectrophotometer (UV-1601; Shimadzu, Tokyo, Japan) at 558 nm. Hydroxyl proline content was calculated as follows:
where h is hydroxyl proline in μg/mL in the final filtrate, read from standard curve; m is the weight of sample in grams; and v is the volume in mL that used to make 100 mL diluted solution. The percentage of collagen content was calculated by multiplying the percentage of hydroxyl proline by 8.
Electrophoresis analysis
Six samples from both normal and severe degrees of white-striped fillets were selected to separate the extracted proteins according to their molecular weights by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Minced meat sample (2 g) was added to 20 mL of rigor-buffer (RB) containing 75 mM KCl, 10 mM KH2PO4, 2 mM MgCl2, 2 mM EGTA (pH 7.0) and homogenised with high speed blender (ultra-turrax®, T 25 basic) on the lowest speed (11,000 rpm/min). The homogenate was centrifuged at 10,000 rpm keeping the temperature at 4°C for 10 min and the supernatant (S1) decanted and saved. Fresh RB 20 mL was added to the sediment and the homogenisation repeated. A sample (0.5 mL) of this homogenate (P1) was saved and the centrifugation repeated. This process was repeated to obtain S1 up to S4 and P1 up to P4 (Fritz et al., Citation1989). A composite sample from S1 to S4 was used for sarcoplasmic fractions, whereas P4 was used for myofibrils. Samples were mixed 1:1 with standard sample buffer which contained 8 M urea, 2 M thiourea, 3% (w/v) SDS, 75 mM Dl-dithothreitol, and 25 mM Tris HCl at pH 6.8 (Fritz et al., Citation1989), heated at 100°C for 5 min in a water bath, cooled, and applied to the gel. Fifteen μL of myofibrillar protein extract were loaded on 12% Mini-PROTEAN® TGX Stain-Free™ Gel (BioRad, Hercules, CA, USA) and the same amount of sarcoplasmic extract was loaded on Mini-PROTEAN® TGX any kD Stain-Free™ (BioRad). The separated protein bands were identified by comparing their mobilities against those of molecular weight markers (Precision Plus Standard protein, all blue prestained; BioRad) having several purified proteins with 10 different molecular weights (10, 15, 20, 25, 37, 50, 75, 100, 150, 250 kD). The reservoir buffer used in the Mini-protean® II cell small electrophoresis unit (BioRad) contained 50 mM Tris, 0.384 M glycine, and 0.1% (w/v) SDS. Small gels were run at a constant voltage of 120 V for stacking gel and 200 V for running gel.
Nutritional index calculation
The Atwater general factor system was used to estimate the total caloric content. This system is based on the total energy of combustion for protein, fat, and carbohydrates after they are corrected by losses in digestion, absorption and urinary excretion of urea. Irrespective of the food, it utilises a single factor for each of the energy-yielding substrates (protein, fat, carbohydrate) where the energy values are 17 kJ/g (4.0 kcal/g) for protein, 37 kJ/g (9.0 kcal/g) for fat, and 17 kJ/g (4.0 kcal/g) for carbohydrates. Moreover, collagen/total proteins ratio was calculated as follows (European Commission, Citation2005):
Finally, fat/protein ratio was determined as the following:
Statistical analysis
Data were subjected to ANOVA by testing the main effects for WS degree and replication, as well as the interaction term using the general linear model (GLM) (SAS, Citation1988). Means were separated using Tukey’s honestly significant difference multiple range test with P≤0.05 considered as significant.
Results and discussion
The results of chemical composition for different degrees of white-striped chicken breast meat are presented in . The chemical composition of normal fillets agreed with those reported in main food composition database (INFOODS, Citation2013; USDA, Citation2013). On the other hand, it was found that there were significant differences (P<0.001) in protein and lipid percentages among normal, moderate and severe groups. Protein content decreased as the degree of WS increased from normal to severe groups, respectively (22.9 vs 22.2 vs 20.9%; P<0.001), while the opposite trend was observed for lipid (0.78 vs 1.46 vs 2.53%; P<0.001) and collagen (1.30 vs 1.37 vs 1.43%; P<0.001) contents which exhibited the highest values in severe white-striped fillets. Finally, white striping defects did not show any effect on moisture content of breast meat. These results are consistent with Kuttappan et al. (Citation2012a) who found that severe white striped fillets had higher fat and lower protein content when compared with normal fillets, but there were no differences between moderate and normal fillets.
The changes in chemical composition of white striped fillets can be due to the occurrence of a degeneration process for muscle fibers. Some studies have pointed out the presence of different histological changes in the breast of modern chicken hybrids like increased eosinophilia, floccular/vacuolar degeneration and lysis of fibers, mild mineralisation, occasional regeneration (nuclear rowing and multinucleated cells), mononuclear cell infiltration, lipidosis and interstitial inflammation and fibrosis (Kuttappan et al., Citation2013a; Petracci et al., Citation2013c; Sihvo et al., Citation2013). Hence, these modifications explain the increase of intramuscular lipids (e.g. lipidosis) as well as the higher content of collagen (e.g. fibrosis) in fillets affected by WS, while lower protein level may be an indirect effect of increased accumulation of intramuscular lipid. The results of SDS-PAGE for meat proteins also evidenced great differences in the pattern of sarcoplasmic () and myofibrillar () fractions. Severe WS resulted in a reduction of total amount of sarcoplasmic and myofibrillar proteins. Certain protein bands of sarcoplasmic proteins showed low concentration while the other ranges of molecular weights did not exhibit any change (). Severe white-striped meat showed lower myofibrillar protein concentration at almost all molecular weights (). Degeneration of myofibrils (Kuttappan et al., Citation2013a; Petracci et al., Citation2013c; Sihvo et al., Citation2013), which coincided with an increase in the levels of some serum enzymes like creatine kinase and alanine transaminase (Kuttappan et al., Citation2013b), can explain the disparity in the pattern of sarcoplasmic and myofibrillar proteins as well as the differences in the concentration for both types of proteins between normal and severe white-striped fillets.
Based on the data obtained on proximate composition, some nutritional indexes were estimated (). The total energy content of normal chicken breast meat was in agreement with those reported in main food composition database (INFOODS, Citation2013; USDA, Citation2013). However, severe-white striped fillets had significantly higher total energy content in comparison with normal fillets (450.6 vs 421.1 kJ/100g; P<0.01), while moderate white-striped samples did not differ from one another. The effect of WS was not confined in increasing the total energy content, but there was a change in energy contribution from fat and protein. Energy contribution from protein, with respect to total energy, decreased from 93.0 to 78.8%, while energy contribution from fat increased from 7.0 to 21.2% when normal fillets were compared to severe ones (). Energy from fat in severe and moderate fillets were significantly higher (95.5 vs 54.9 vs 29.5 kJ/100g; P<0.001) than normal fillets. Fat/protein ratio was also significantly increased in moderate and severe white-striped fillets (0.067 and 0.118 vs 0.027; P<0.05) in comparison to normal.
Many factors may be involved in favouring this trend. However, it is very likely that intensive genetic selection towards increasing growth rate and breast yield, which have been achieved in modern chicken hybrids, have fostered some modifications in muscular anatomy and metabolism. White striping and its consequent strong effect on nutritional value of breast meat are an example (Petracci and Cavani, Citation2012).
Certainly, the use of high-energy diets in conjunction with farming systems, allowing a low mobility of the animals and increasing slaughter ages and weights, which have been employed by processors during the last years in order to optimise the production performance of meat to meet the persistent demand of processed products, may be other important factors involved in this problem (Wang et al., Citation2009; Crawford et al., Citation2010; Kuttappan et al., Citation2012a, Petracci et al., Citation2013c).
The ratio between collagen and total proteins was significantly increased () in severe and moderate fillets in comparison to normal (6.73 vs 6.19 vs 5.72; P<0.05). This increase means that the nutritional quality of proteins in white striped fillets may be reduced due to low digestibility of collagen and the deficiency of some essential amino acids (e.g. tryp-tophan, sulfur amino acids, and lysine) in connective tissue with respect to myofibrillar and sarcoplasmic proteins (Young and Pellett, Citation1984; Boback et al., Citation2007). In addition, Kuttappan et al. (Citation2012a) found that there were also differences in the fatty acid composition between normal and severe degrees of white striping. Indeed, severe white striped fillets had a lower amount of saturated fatty acids, but associated with lower levels of essential long-chain n-3 PUFA such as eicosapentaenoic acid and docosahexaenoic acid.
The impact of WS defect should not be underestimated because a recent survey estimated that current incidence rate under commercial production was around 12.0% (8.9 and 3.1% in moderate and severe degree, respectively) (Petracci et al., Citation2013b). At present, while fillets showing severe WS are usually downgraded by processors and used for manufacturing further processed products (e.g. sausages, nuggets) where chemical composition can be modified during formulation, fillets with moderate WS are not downgraded and marketed for fresh retailing. This means that an increasing share of chicken breast meat currently marketed in form of cut-up (whole or sliced) can have rather different nutritional characteristics with respect to those reported on the label and to consumer expectations towards poultry meat (e.g. low calories and fat).
Conclusions
The results of this study revealed that occurrence of WS defect, which is likely related to the increasing growth rate and breast yield achieved in most modern chicken hybrids, lead to an increase of fat content and energy density as well as to a decrease of protein content and quality of chicken breast meat. The effect of WS on the nutritional value of chicken breast was not confined on severe cases which can be separated in the plant production line, but moderate white-striped breasts also exhibited reduced nutritional quality. All these aspects might impair current consumer’s attitude towards poultry meat in comparison to red meats and hamper the future development of poultry meat market. Therefore, it is essential, in the near future, to identify the causes of the WS defect and overall emphasise relevance of meat quality traits among the selection criteria of commercial broiler chicken hybrids.
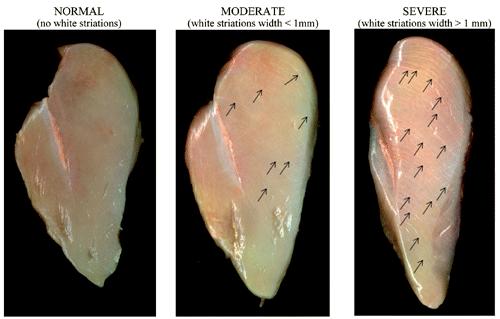
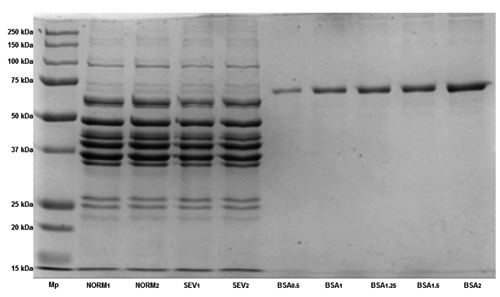
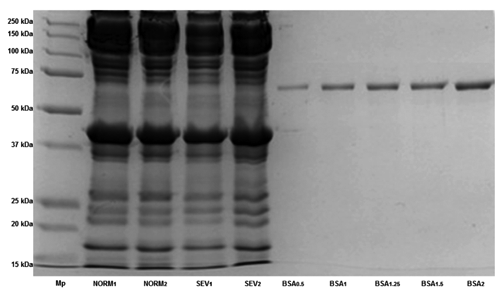
Table 1. Chemical composition of chicken breast meat affected by different degrees of white striping.
Table 2. Total energy content, energy distribution, and collagen:protein and fat:protein ratios of chicken breast meat affected by different degrees of white striping.
References
- AOAC, 1990. Official methods of analysis. 15th ed., Association of Official Analytical Chemists, Washington, DC, USA.
- BarbutB. SosnickiA.A. LonerganS.M. KnappT. CiobanuD.C. GatcliffeL.J. Huff-LonerganE. WilsonE.W., 2008. Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Sci. 79:46-63.
- BestP., 2011. Worldwide poultry meat production, consumption forecasts. Watt Executives ed., Rockford, IL, USA.
- BobackM.S. CoxL.C. OttD.B. CarmodyR. WranghamW.R. SecorM.S., 2007. Cooking and grinding reduces the cost of meat digestion. Comp. Biochem. Physiol. 148:651-656.
- CavaniC. PetracciM. TrocinoA. XiccatoG., 2009. Advances in research on poultry and rabbit meat quality. pp 741-750 in Proc. 18th ASPA Congr., Caserta, Italy. Ital. J. Anim. Sci. 8(Suppl.2):741-750.
- CrawfordM.A. WangY. LehaneC. GhebremeskelK., 2010. Fatty acid ratios in free-living and domestic animals. In: DeMeesterF. ZibadiS. WatsonR.R. ( eds.) Modern dietary fat intakes in disease promotion. Humana Press Inc., New York, USA, pp 95-108.
- European Commission, 2005. Council Regulation of 5 December 2005 laying down transitional arrangements for the implementation of Regulations (EC) No 853/2004, (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council and amending Regulations (EC) No 853/2004 and (EC) No 854/2004, 834/2007/EC. In: Official Journal, L 189, 20/07/2007, pp 1-23.
- FritzJ.D. SwartzD.R. GreaserM.L., 1989. Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Anal. Biochem. 180:205-210.
- GibbsR.A. RymerC. GivensD.I., 2010. Long-chain n-3 PUFA: intakes in the UK and the potential of a chicken meat prototype to increase them. Proc. Nutr. Soc. Aust. 69:144-155.
- HavensteinG.B. FerketP.R. QureshiM.A., 2003. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poultry Sci. 82:1500-1508.
- INFOODS, 2013. International Network of Food Data Systems. Available from: http://www.fao.org/infoods/en/
- KolarK., 1990. Colorimetric determination of hydroxyproline as measure of collagen content in meat and meat products: NMKL collaborative study. J. Assoc. Off. Ana. Chem. 73:54-57.
- KuttappanV.A. BrewerV.B. AppleJ.K. WaldroupP.W. OwensC.M., 2012a. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poultry Sci. 91:2677-2685.
- KuttappanV.A. HuffG.R. HuffW.E. HargisB.M. AppleJ.K. CoonC. OwensC.M., 2013b. Comparison of hematologic and serologic profiles of broiler birds with normal and severe degrees of white striping in breast fillets. Poultry Sci. 92:339-345.
- KuttappanV.A. LeeY.S. ErfG.F. MeullenetJ.F.C. MckeeS.R. OwensC.M., 2012b. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poultry Sci. 91:1240-1247.
- KuttappanV.A. ShivaprasadH.L. ShawD.P. ValentineB.A. HargisB.M. ClarkF.D. McKeeS.R. OwensC.M., 2013a. Pathological changes associated with white striping in broiler breast muscles. Poultry Sci. 92:331-338.
- MourotaJ. HermierbD., 2001. Lipids in monogastric animal meat. Reprod. Nutr. Dev. 41:109-118.
- PetracciM. CavaniC., 2012. Muscle growth and poultry meat quality issues. Nutrients 4:1-12.
- PetracciM. BianchiM. MudalalS. CavaniC., 2013a. Functional ingredients for poultry meat products. Trends Food Sci. Tech. 33:27-39.
- PetracciM. MudalalS. BonfiglioA. CavaniC., 2013b. Occurrence of white striping and its impact on breast meat quality in broiler chickens. Poultry Sci. 92:1670-1675.
- PetracciM. SirriF. MazzoniM. MeluzziA., 2013c. Comparison of breast muscle traits and meat quality characteristics in 2 commercial chicken hybrids. Poultry Sci. 92: 2438-2447.
- RyanJ.T. RossR.P. BoltonD. FitzgeraldG.F. StantonC., 2011. Bioactive peptides from muscle sources: meat and fish. Nutrients 3:765-791.
- SAS, 1988. SAS/STAT Guide for personal computers. Version 6.03. SAS Inst. Inc., Cary, NC, USA.
- SihvoH.K. ImmonenK. PuolanneE., 2013. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. ( In press).
- USDA, 2013. National Nutrient Database for Standard Reference Release 26. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl
- WangY. LehaneC. GhebremeskiK. CrawfordM.A., 2009. Modern organic and broiler chickens sold for human consumption provide more energy from fat than protein. Public Health Nutr. 13:400-408.
- YoungV.R. PellettP.L., 1984. Amino acid composition in relation to protein nutritional quality of meat and poultry products. Am. J. Clin. Nutr. 40:737-742.
