Abstract
One of the future-generation biofuel options that has recently recieved increased attention is the production of biofuels from microalgae. Besides the use of algae oil for physicochemical biodiesel production, biochemical and thermochemical pathways are possible. Although there is still a need to research algae production systems, downstream processing (e.g., biofuel production) needs to be researched in parallel. As there are several methods to produce biofuel from algae, different possible production processes are reviewed. By investigating the different steps of each of the processes and highlighting the challenges and risks that can occur, it is possible to make a decision regarding which pathway might be feasible for algal resources in the future.
†Denotes algae fuels.
DME: Dimethyl ether; FAME: Fatty acid methyl ester; FT: Fischer–Tropsch; SNG: Synthetic natural gas
With the upcoming shortage of fossil fuels and the environmental threat of global climate change, mankind is forced to search for alternatives concerning its energy supply. Today, huge efforts are made to maximize the productivity of biomass and identify new species of plants and processes to fulfill the future demand of food, fodder, materials and energy; the utilization of algae is seen as one of these possible alternatives. Algae are a feedstock that have certain advantages when compared with land-based feedstocks. Under favorable conditions, the growth rate of algae is estimated to be 5–10-times higher compared with land-based crops, implying a higher production rate of theoretically convertible biomass. Additionally, certain species may have a high fraction of lipids or carbohydrates of up to 70–80 wt% Citation[1].
The (macromolecular) composition of algal cells gives several options to utilize them. It is possible to convert the triglycerides of the lipid fraction with commonly known processes to biodiesel. Other alternatives include the conversion of algal biomass to fuel by biochemical or thermochemical processes. Depending on the process, proteins may be obtained as a byproduct for animal-feed production. As it is possible to produce microalgae on sites where no agriculture is possible and to use sea or brackish water, the competition to food production can be avoided or at least diminished.
Although research on microalgae production has been conducted for approximately 70 years, there is still a need for additional research in various aspects, such as optimal conditions for algae growth and type of reactor.
Besides research on the production of algae, which already has a long history Citation[2], there is a need for researching the postprocessing of algae. Microalgae are very diverse and offer many possibilities; however, their properties are different from land-based biomass. Therefore, applying known processes on microalgae without special adaptation is not suitable.
Although this publication is intended to review possible biofuel processes applicable for algae, first it is important to examine the important properties of algae. Therefore, the present state of development of the pre-processes algae production and harvesting, and their effect on the biofuel production, will be summarized. Following this, different options for providing liquid and gaseous biofuels are presented, with a focus on suitability for algae. Finally, economic and environmental challenges are briefly addressed and a comparative discussion of the advantages and disadvantages is presented.
Algae production & supply
Although there are already several algae production sites in Asia, the technique is still not in its full state of development; especially in regards to controlled and high-yield production of certain species for high-value application, there is still a lack of knowledge Citation[3]. Therefore, it is important to take a close look at all parts of this complex process. The term ‘algae production’ has to be specified further in terms of intention of usage or given requirements. Only then is it possible to provide a realistic estimation on possible utilization and, therefore, biofuel pathways.
Algae species
There are several different biological definitions of aquatic biomass summarized under the term ‘alga’. Firstly, algae can be divided into micro- and macro-algae. From an evolutionary perspective, macroalgae were land-based plants that returned to a wet environment. They consist of branches, leaves and may be enrooted, and can be divided into brown, red and green types, which are different kinds of multicellular eukaryotes with independent evolution pathways Citation[4]. By contrast, microalgae are unicellular and nano- to milli-meter sized. The phycologists definition of microalgae is an organism with chlorophyll-a and a body (thallus) not differentiated into roots, stem and leaves (thallophyte) Citation[5]. This includes both prokaryotes and eukaryotes. Although today blue–green algae are called cyanobacteria because of their bacterial characteristics (i.e., cell-wall constituents, ribosome structure and nucleic-acid structure) Citation[6], they are often mentioned in the context of ‘algae’ utilization pathways. Cyanobacteria are biomass and may also have the same properties that are important with regards to biofuel processes as other algae species; therefore, they are also regarded as algae in this article.
According to the literature, biomass production yield for algae, in general, is specified as 5–10-times higher than for land-based plants, giving a good chance of enhanced biomass production Citation[7]. There are several reasons for the higher production rate. One is the higher photosynthetic efficiency. The intrinsic solar-energy conversion efficiency (measured as energy released by complete combustion of biomass divided by the solar energy absorbed) can be up to 9% for microalgae, the theoretical maximum for land-based C3 crops (e.g., switchgrass) is 2.4% and for C4 crops (e.g., corn) is 3.7% Citation[8]. A reason for this is the unicellular morphology and, therefore, much shorter reproduction cycles – hours to several days.
Today, most of the research considering biofuels from algae is focused on microalgae due to their higher efficiency and better usability in comparison with macroalgae. Therefore, in this context, only microalgae will be regarded.
In general, approximately 100,000 different species of microalgae are estimated to exist worldwide. These species are adapted to their environment and all have specific characteristics. This has to be considered when designing the production and harvesting process.
Production processes
The production of algae in high quantities has been subject to investigation for many decades. In the past, the focus on using the algae was in regards to valuable products such as lipids Citation[9] or proteins Citation[10] for food and forage. Produced quantities per area were comparable to current values. Today, food and forage are still in focus Citation[11]; however, in addition, algae production for biofuel production has become an important issue, at least since the start of the Aquatic Species Program (ASP) Citation[12].
Two different concepts for algae production are favored and discussed in research today; open-pond reactors (OPR) and closed photobioreactors (PBR).
OPRs are open to the environment. The basins are often formed simliar to raceways, up to 300 m long and 15 m wide. In most reactors, a paddle-wheel is used to provide the flow of the medium. Advantages of this reactor are the relatively low investment costs and easy handling. The downside is the environmental influence (e.g., vaporization and contamination with other algae species or predators) that presents a serious disadvantage. The production rate is approximately 10–25 g dry matter (DM) of algae biomass per day per m2Citation[13]. The disadvantages and unstable production in OPRs led many research institutes to switch to closed PBRs.
In common PBRs, the algae medium is pumped through a closed system. In order to provide solar insolation to the algae, several designs are available such as vertical-ordered horizontal-coursed acrylic glass tubes, plastic bags or flat-plate reactors Citation[14]. Except for the solar radiation, the algae are sealed from any environmental influence and, therefore, a better control of the algae production is provided. The reported production rate of closed PBRs is usually between 20 and 100 g DM algae biomass per day per m2Citation[15]. However, the capital investment is approximately ten-times higher than for high-rate OPRs Citation[16]. Therefore, the main difference between the two reactor concepts lies in the quality and price of the biomass produced.
Harvesting processes
When algae grow, depending on the type of pond, the algae density from which growth is inhibited is between 0.5 g/l in OPRs Citation[12] to 20 g/l for closed PBR Citation[17]. As the diameter of algae is between 1 and 50 µm the separation process has to be adjusted to these parameters. For mass production, the separation process has to be energy and area efficient. Additionally, certain characteristics of the different algae species have to be considered when choosing the right harvesting process. These are:
▪ Morphology; | |||||
▪ Specific size, shape and appendages; | |||||
▪ Motility; | |||||
▪ ζ potential; | |||||
▪ Extracellular organic matter composition. | |||||
The characteristics influence the opportunity of harvesting the algae biomass with known processes such as sedimentation, filtration, centrifugation, flocculation or air flotation Citation[18].
Except for the biophotolysis, where algae are not the feedstock but the producing organism, next to consider before any other biofuel process is possible, is the extraction. In this context, extraction only refers to removing the substance out of the algae, in the quality and state needed for the biofuel process. As every biofuel process uses different parts of the biomass (e.g., oil and starch) it is necessary to pretreat the algae suitable for the different kinds of processes. The focus is on the degree of decomposition of the cell, moisture content or concomitant substances in the aqueous solution (e.g., flocculation substances). More general parameters that have to be considered, especially for mass production, are the detention time and the energy input. It can be concluded that there is no process to harvest and extract for all algae species and utilizations. Therefore, it is crucial for future algae biofuel production ventures to develop the whole process chain and to first consider all constraints between the different processing parts.
Biofuel options
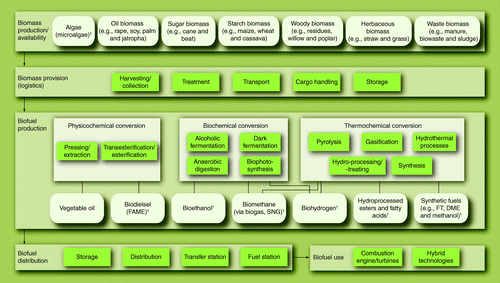
Over the last few decades, several processes to produce biofuels have been developed and partly commercialized. Until now, the feedstock for these processes has always been land-based biomass. Similar to land-based biomass, algae contain carbohydrates that might be converted with similar processes as already developed for land-based biomass. In regards to biomass, not every process is suitable for application in an efficient and economic manner. Therefore, well-known processes have to be examined with respect to their ability to process algal biomass. Additional to this, microalgae are offering novel pathways of producing biofuels, which have to be taken into account. provides an overview of general biofuel production options based on microalgae.
Biodiesel
The most common and frequently discussed biofuel from microalgae is biodiesel. The ASP focused on algae species with high lipid content and physicochemical transesterification to biodiesel as the biofuel production process Citation[12]. The impact of the ASPs goals and visions is still great.
▪ Pretreatment of raw material
Raw materials for the biodiesel process are triglycerides, which are one type of storage lipids. Together with wax esters, hydrocarbons, free fatty acids (FFAs) and sterols, they are classified as neutral lipids. Beside these, polar lipids such as phospho- and glyco-lipids also exist. They are part of the cell walls of microalgae and act like structural components Citation[19]. Similar to animal and microbial cells, 60–85% of the lipids in natural-growing algae are polar Citation[20], a fact already known for more than a century (“The greatest part of the fat is inside the chlorophyll band”, year 1887) Citation[21], but is sometimes forgotten. When looking at lipid analysis of algae produced, without focusing on lipid production, it has to be considered that only approximately 15–40% of all lipids are triglycerides and usable in the transesterification process . Polar lipids are unwanted in the biodiesel process as they strongly influence the processing and the quality of the product.
▪ Enhancement of lipid production
Besides screening for algae with the highest lipid content, there are several techniques to enhance the triglyceride content of algae, as described below.
Nitrogen deprivation stress is a well known and studied effect, which occurs when nitrogen concentration in the algal medium is depleted. This inhibits the reproduction/cell division process, as the nitrogen is needed for protein production; therefore, general biomass growth is inhibited. As for the production of storage lipids, no nitrogen is needed – the lipid production can still proceed and may be enhanced. Although the algal mass has a higher lipid content, the overall productivity of the cells is slowing down; thus, the photosynthetic efficiency decreases Citation[12]. Nonetheless, in other studies nitrogen stress leads to an overall positive effect for algae production Citation[22,23]. In addition to nitrogen, the depletion of phosphorus or the cultivation temperature has an influence on lipid yield and composition Citation[24].
Heterotrophic or mixotrophic growth of algae provides opportunities to increase the triglyceride content inside the cells. When producing algae via heterotrophic growth, the carbon source is organic, such as glucose or starch, and not CO2. In comparison with autotrophic growth Citation[25], the lipid content was increased from 15 to 55 wt% for Chlorella protothecoides. As formerly described in previous work Citation[26], heterotrophic or mixotrophic growth is a reasonable way to increase the yield of algal biomass. However, taking the energy balance into account, it is always a net loss, as an already high value and energy matter is converted into another (e.g., glucose into lipids). By using organic waste streams and running the production mixotrophically, it is possible to improve the energy balance.
The first genetic modification or metabolic engineering attempts to increase the lipid content of algae were already completed in the latter stages of the ASP Citation[12]. Although no ‘high-lipid strain’ was developed, significant progress was made in the understanding of environmental and genetic factors that affect lipid accumulation in microalgae and in the ability to manipulate these factors to produce strains with desired traits. Current research has gone further; however, still only one strain (Chlamydomonas reinhardtii) is thoroughly able to be metabolically engineered Citation[27]. Many of the genes involved in lipid metabolism in terrestrial plants have homologs in the sequenced microalgal genomes. Therefore, research is trying to apply the transgenic strategies that have been used to modify the lipid content of higher plants to microalgae Citation[28].
▪ Extraction of lipids
As only lipids, or more accurately the neutral triglycerides, are required for biodiesel production, the algae must be extracted; the method has to have a high selectivity towards triglycerides. Several extraction methods for lipids are known from research and practice; they can be divided into physical and chemical methods and their combinations. Usually, the physical methods destruct the algal cells. Physical destruction and extraction methods are sonication, homogenization, French pressing, expelling and bead milling. For the chemical solvent extraction, several extractors and mixtures are known; for example, hexane, chloroform, methanol, isopropanol and acetone, and their mixtures in various concentrations. For an industrial operation, the extraction solvents should be inexpensive, volatile (for easy removal afterwards), free from toxic or reactive impurities (to avoid reaction with the lipids), able to form a two-phase system with water (to remove nonlipids) and be poor extractors of unwanted components. The yields of the different extraction methods and combinations strongly depend on the process, its parameters Citation[29] and the algal strain extracted Citation[30,31].
In recent years, supercritical fluid extraction got into focus of industry for the extraction of high-value products from microalgae. This process can have a high selectivity towards specific extracts and may have higher yields than solvent extraction. The extraction medium is in many cases CO2. Under normal operating conditions, the supercritical solvent is gaseous, easy to remove, with no toxic organics used Citation[32].
The critical temperature of CO2 is 31.1°C at 73.8 bar. In the supercritical state it behaves as a lipophillic solvent and it is able to extract most nonpolar solutes. Unlike liquid solvents, the solving power of supercritical CO2 can be easily adjusted by slight changes in the temperature and pressure; this enables the specific extraction of compounds of interest. For further enhancement, modifiers such as ethanol as a cosolvent can be added Citation[33]. This process is well understood and used as a large-scale application in the industry, such as for decaffeination Citation[34]. The major costs and drawbacks concerning the mass utilization of supercritical fluid extraction for biofuel production are the energy costs, as high pressures have to be conducted to bring the solvent into its supercritical state Citation[35].
One process becoming increasingly focused in research is the in situ extraction (or so-called ‘milking’) of microalgae. In this case algae are not harvested and, therefore, do not need to be dewatered or dried. The lipids are extracted from living cells Citation[36]. In practice, this can be done by piping the algae medium through a solvent phase. The solvent should be immiscible with water to have a phase boundary for easy separation of the solvent/lipid mixture. By connecting the extraction vessel with the solvent to the PBR, a continuous operation is possible and the concentration of algae can be held at an optimum Citation[37]. Although this technique is not utilized in the industry yet, it opens several new options of algae processing.
Other extraction methods that are undergoing development (but are still in early stages) are ultrasonic-assisted extraction Citation[38] and pulsed electric field technology Citation[39,201]. For more information on oil extraction from microalgae a comprehensive review has been carried out by Mercer and Armenta Citation[33].
(Trans)esterification
Biodiesel production via (trans)esterification has been utilized in the industry for many years. It is defined as a physicochemical process that reorders the molecular structure of oils stirred at moderate temperatures with a homogeneous catalyst. For commercial application, several raw materials are used (e.g., rapeseed oil, soy oil, palm oil, waste grease and animal fat). For the transesterification process, triglycerides are needed as charge. Triglycerides are trivalent long-chained alcohols; fatty acids (FAs) of different chain length connected via a glycerin molecule. These connections are broken and the FAs are again esterified with methanol to a monovalent FA methyl ester, glycerin is a byproduct of this process Citation[40]. Several variations of the transesterification processes are known in science and industry, the most common and mature process being a one-step process with potassium- or natrium-hydroxide as the base catalyst. As the process parameters are comparably easy to achieve (mixing at approximately 50–70°C) the process can be established from a very small to large scale. However, this process only works well with oils and lipids with a low FFA content, as the FFAs are converted to soap, reduce the yield and may hinder the process. For raw materials with a high FFA content (e.g., animal fats or grease) other technologies are needed, such as a two-step process with an acid esterification of the FFA previous to the transesterification Citation[41]. This process is also common in industry, but increases the reaction time and effort and, therefore, costs Citation[42].
An advantage of making biodiesel from algal lipids is the wide variety of already utilized materials. Several multifeedstock plants exist, which are also able to handle more heterogeneous oils Citation[43] and may be appropriate for algal lipids and oils without great capital investments.
Another possibility to produce biodiesel from microalgae is in situ transesterification; comparable to the in situ extraction, the algae medium is directly mixed with the solvent, catalyst and alcohol Citation[44]. Ehimen et al. compare the method of in situ transesterification to a two-step extraction-transesterification method with two heterotrophic algal strains Citation[45]. The extraction efficiency of the methods (in situ and two-step) varied between 15 and 20% for one strain to 150% for the other strain. This study did not focus on the transesterification to produce biodiesel but showed that the amount of extracted lipids strongly depends on the extraction method (e.g., combined with a cell-wall destructing in situ transesterification). In a study with the green autotroph alga, Chlorella pyrenoidosa, FA content was low with all extraction methods and the two-step method resulted in a higher ester yield Citation[46]. It is further concluded that the recovery of excess alcohol and catalyst might reduce the achievable cost savings compared with the two-step process. The in situ transesterification method, in general, is a very complex process and is strongly influenced by several parameters such as algal species, time, temperature, moisture Citation[45], reaction mixture and the order in which the different chemicals were added to the reaction Citation[47]. Therefore, the best solvent system differs for different algal species and always has to be screened against a large solvent pool to achieve the appropriate reaction mixture Citation[48].
In summary, the biodiesel options, it can be concluded that the process of transesterification itself can be a very simple process. However, concerning algae, several constraints have to be overcome. Physical extraction methods, such as mechanical pressing of microalgae, are nearly impossible, as the small size plugging of the filter and press cake immediately occurs; other extraction methods have to be developed. Lipids in microalgae are not only storage lipids, hence not only triglycerides, but also to a great part membrane components (e.g. glyco- and phospho-lipids). These are not processable in the biodiesel process. To solve this, the extraction process needs a high selectivity towards triglycerides and the algae-production process has to be optimized to produce a high amount of storage lipids. Unfortunately, increasing the lipid content comes at the cost of lowering growth rate or manipulating the biological process, which leads to a higher effort.
Taking only the technical suitability and maturity of the biodiesel production process into account, the commercialization of algal biodiesel should be possible in the short to medium term. Nonetheless, these processes only use a specific part of the algae and, therefore, its efficiency strongly depends on the lipid yield and not only biomass productivity.
Hydroprocessed esters & FAs
In order to produce a biofuel with more favorable properties than biodiesel based on transesterification, hydroprocessing is possible, in order to produce fuels from algal lipids called hydroprocessed esters and FAs (HEFA). In this case, glyco- and phospho-lipids can also be utilized. Hydroprocessing is well known from crude oil refining and usually forms part of conventional oil refineries Citation[49,50]. The hydrotreating process typically takes place at temperatures of approximately 350–450°C and an elevated partial hydrogen pressure. Standard catalysts such as CoMo and NiMo are applied Citation[51]. During hydrotreating, oxygen is removed as water and CO2 by adding hydrogen. Triglycerides are converted into three separate branched chain paraffins. As cracking of the carbon chains also occurs (e.g., at double bonds), side products of the process are fuel gas, which can be used for energy recovery in the process, and small amounts of biogasoline. The main product, HEFA, has similar physical and chemical properties as synthetic biomass-to-liquid fuels (e.g., Fischer–Tropsch diesel). In comparison with biodiesel, HEFA fuels have the same components as fossil fuels and, therefore, inter alia better filter plugging and cold-flow properties Citation[52]. This makes HEFA fuels interesting for applications where biodiesel is not adequate; for example, as jet fuels. A number of aviation companies are contributing to research of this pathway to produce biofuel from algae Citation[53]; several flights have already been conducted partly Citation[54] or completely on algal-HEFA fuel Citation[55]. This process has many advantages, such as giving comparable fuel quality as well as possible integration in already-existing processes. On the other hand, HEFA fuels have the same constraints as biodiesel regarding the input raw material. Only parts of the algae are used for the process and, therefore, the production has to be optimized towards a high-lipid yield, with the same drawbacks that have been described earlier. Interestingly, in contrast to transesterification, phospholipids can be utilized by the hydroprocessing pathway Citation[56]. This increases the possibly usable fraction of the algae and, therefore, its conversion efficiency.
Biomethane
Although mainly liquid biofuels are used for transportation, gaseous fuels such as natural gas are implemented as fuel options for vehicles Citation[57]. With the infrastructure of additional natural gas filling stations, besides the usual petrol, it is also possible to utilize biogenic sources for natural gas as fuel. Several biochemical pathways for biofuels from algae are possible. Biomethane is produced either through biochemical conversion of biomass with a subsequent gas upgrading or thermochemical conversion of solid biomass via gasification with subsequent gas cleaning, methanation as synthesis and product gas upgrading (cf. synthetic fuels).
The biochemical conversion pathway is an anaerobic digestion of usually liquid or paste-like substrates by bacteria that produce a gas containing approximately two-thirds CH4, one-third CO2, some impurities and water vapor Citation[58]. This process is commercially available and well established in Europe. Recent developments have gone towards the upgrading of biogas to biomethane by cleaning and conditioning (e.g., CO2 and impurities removal and adjustment to gas pipeline specifications) Citation[59]. Regarding the utilization of algae, it is possible to use it as a substrate, since one general advantage of digestion is the possibility of feeding wet biomass. Thus, algal biomass does not need to be dried, only concentrated, as in wet-fermentation systems concentrations should not exceed 5 wt% DM biomass Citation[40]. In an early work by Golueke et al. the digestion of domestic wastewater sludge and green microalgal biomass (Scenedesmus and Chlorella) harvested from wastewater ponds was studied Citation[60]. In comparison with the digestion of raw sewage sludge, the yield was approximately 15–32% lower. The relatively low digestibility and, thus, the biogas yield of algae were suggested to be the result of cell walls resisting bacterial degradation. Recent studies confirmed that digestion of algae, in general, is suitable and that the biogas yield is dependent on the utilized algae strain and the pretreatment of the cells Citation[61]. By codigesting algae together with other substrates, the often seasonal production can be compensated and the biogas yield may be enhanced Citation[62]. This is due to the high protein content of algae, which contributes to high ammonium concentrations in the biogas sludge and may lead to toxic and inhibiting reactions Citation[63]. By adding organic substrates with low ammonium content this can be diminished. For an added value it is also possible to use the algal growth medium (from the PBR) as a ‘scrubber’ for the CO2 and, therewith, have an in situ conditioning of biogas to biomethane Citation[64]. The drawbacks of this combined application are the daily and seasonal fluctuations of the (phototrophic) microalgae production yield, which is difficult to adapt to the more steady biogas production Citation[65]. The advantage of the biogas process in general is the utilization of the whole algal cell and the possibility to use low-quality algae sources; for example, from wastewater treatment or blooms.
Bioethanol
Microalgae can be used for biochemical ethanol production via alcoholic fermentation. This process is well known and commercially established for sugar and starch crops (e.g., corn, wheat and sugarcane) Citation[66]. Alcoholic fermentation is realized at 35°C, which is the optimum temperature for the metabolism of the fermenting yeast. Within the fermentation step, monosaccharide is metabolized by the yeast to form ethanol, CO2 and biomass. Research is currently looking for additional raw materials for ethanol production. Beside lipids, many algae, especially green algae (Chlorophyceae), utilize starch as a storage compound Citation[12] and may also have different types of polysaccharides such as cellulose, xylose, galactose and arabinose entrapped in the cell walls Citation[67]. In most studies, starch is extracted by mechanic and enzymatic Citation[68,69] or only enzymatic Citation[70] pretreatment.
Afterwards it can be extracted and used for fermentation. As the common enzymes used are not capable of salt water, research on finding marine amylase has been conducted Citation[71]. Choi et al. found an ethanol yield of 235 mg ethanol per g algal biomass (C. reinhardtii) and suggest that algal biomass could be a potential feedstock for ethanol production on a large scale Citation[70].
Generally, microalgae seem to be suitable for ethanol fermentation. Comparable with biogas from microalgae, the ethanol yield depends on the pretreatment and chosen algal strain Citation[72].
Synthetic biofuels or hydrogen
In general, synthetic biofuels (i.e., ‘design fuels’ with clearly defined properties) or hydrogen are produced via thermochemical conversion. In principal, thermochemical conversion processes have the advantage to utilize the complete enclosed carbon of the biomass. As the cell wall is usually destroyed by the high reaction temperature, a destructive pretreatment is not necessary. In addition, the residues from an upstream high-value utilization of the algae can be charged.
The production of synthetic fuels is characterized by four main steps after appropriate biomass pretreatment:
▪ Gasification of biomass to a raw gas; | |||||
▪ Cleaning and conditioning of raw gas to synthesis gas with defined CO-to-H2 ratio or directly to hydrogen; | |||||
▪ Catalytic synthesis of gas to liquid or gaseous biofuels (e.g., by Fischer–Tropsch Citation[73], methanol Citation[74], dimethylether synthesis Citation[75] or methanation to biomethane [bio-synthetic natural gas] Citation[76]); | |||||
▪ Final product treatment. | |||||
Concerning the first step (gasification of biomass to a raw gas), different variations are possible, such as direct gasification of the biomass Citation[77] or with an intermediate step where the biomass is first liquefied or thermochemically densified and then gasified Citation[78].
Regardless, a long history of the development of a broad variety of system elements, as well as system layouts for the provision of liquid and/or gaseous fuels via biomass gasification, no market breakthrough has been realized so far. This is due to difficulties in combining system elements and the upscaling to achieve an economic and efficient production system. Additionally, some system elements are still under development.
▪ Dry & ambient pressure methods
Concerning direct gasification technologies, several alternatives are known. A well-known process from other biomass input materials is the gasification of dry solid particles in fluidized bed reactors; reaction temperature varies between 700 and 900°C Citation[40]. Also, gasification of wet algae slurry is possible; Hirano et al. gasified a Spirulina slurry (21 wt% DM) at ambient pressure and yielded gases with a high hydrogen content (48 vol% H2 at 1000°C) Citation[79]. A mixture with other materials was also tested. Gasification of coal–algae slurries resulted in good results but was mainly intended for the disposal of algal blooms Citation[80].
In general, direct gasification of microalgae is possible but not yet utilized. The main technical reason is the high moisture content of algae (which has to be dried out) or, alternatively, an adapted transportation system as well as major modifications of the gasification reactor have to be developed.
Besides direct gasification, another dry method for algae processing can be found in the literature; the fast or flash pyrolysis process as part of the synthetic biofuel production is an intermediate step of liquefaction and densification of biomass. It is defined as a thermochemical process where the input material is rapidly (seconds to minutes) heated to approximately 400–500°C in the absence of oxygen. As a result, the input material decomposes to generate mostly vapors, aerosols and charcoal. After rapidly cooling and condensing, a dark brown liquid is formed Citation[81]. Algae as an input for flash pyrolysis have been processed in several studies Citation[82–86]. The results from these studies were always comparable to other biomass and the yield of oil could be enhanced by using catalysts Citation[82]. Nonetheless, the oil yield of the pyrolysis of microalgae strongly depends on the algae, as, for example, heterotrophically grown algae do have a higher hydrocarbon content and, therefore, give a higher yield of a decomposed condensed pyrolysis product Citation[83]. Because of the chemical composition of pyrolysis oils, which is different to fossil oil, the direct use as a fuel is hard to achieve without further treatment or upgrading Citation[87] and also for pyrolysis oil from algae Citation[86]. On the other hand, by pyrolyzing microalgae, the heating value slightly increases and the biomass is liquefied. Therefore, it can be used in thermochemical processes as an intermediate.
One drawback concerning the pyrolyzation of algal biomass is the need to process dry biomass. All studies cited in this publication concerning pyrolyzation were at the laboratory scale and included prior oven drying of the algae. As the attained pyrolysis oil is hydrophilic, it contains 10–15% water produced within the process, plus the water contained in the load. The water content lowers the lower heating value and should, therefore, be avoided Citation[88]. This issue makes the processing of algae by pyrolyzation in an energy efficient way difficult, as drying to a maximum of 10% water content has to be applied.
▪ Wet & pressurized methods
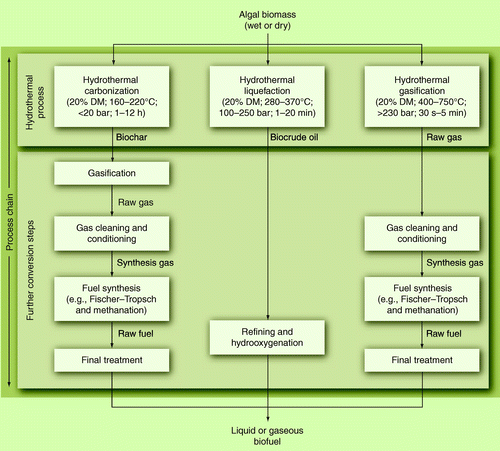
Another thermochemical process group that has become the focus of research in recent years is the hydrothermal treatment of biomass. A great difference to the aforementioned process is the utilization of water as reaction medium; the physical and chemical properties of water change when heated near or over the critical point (374°C, 221 bar). The solubility of nonpolar hydrocarbons rise and, therefore, the decomposition of the biomass is enhanced Citation[89]. Depending on temperature, pressure, catalyst and other parameters, it is possible to influence the process in the direction of solid carbon, liquid oil or synthesis gas. shows the possible hydrothermal pathways from microalgae to fuel.
Hydrothermal carbonization (HTC) was first investigated for the production of carbon nanomaterials from biomass Citation[90] but also gained interest as a process for the energetic use of biomass and carbon sequestration Citation[91]. The process itself is employed at relatively moderate conditions of less than 220°C, 20 bar and reaction times of up to several hours. The product has a coal-like consistency and might be used as an input for a gasification process. Several projects and companies are in the process of building demonstration plants for the utilization of wet-waste biomass, especially in Germany Citation[92]. The carbonization of microalgae has only been achieved a few times, thus far Citation[93]. In comparison to lignocellulosic-HTC chars, the algal chars of C. reinhardtii and Dunaleilla salina were more bituminous, had a higher hydrogen content, which is favorable for fuel syntheses, and had a high nitrogen content. Besides the production of biochar for gasification, this process is investigated towards the production of FAs by hydrolyzation and extraction from the char Citation[94,95]. Nonetheless, HTC of algae for biofuels is in its infancy and several fundamental questions concerning the composition of char and wastewater, besides others, have to be investigated.
From hydrothermal carbonization it is only a short transition to the process of hydrothermal liquefaction. Usually the liquefaction is conducted at 280–370°C and between 100 and 250 bar Citation[96]. By hydrothermally liquefying microalgae a high percentage of the chemical energy present in the educt can be recovered as so-called biocrude Citation[97]. Liquefaction of Chlorella and Spirulina was studied with different alkali and acidic catalysts Citation[98]. The liquid products had a higher heating value of 33.3–39.9 MJ/kg and, thus, are higher than for pyrolysis oils. Although the heating value is higher, with it the H/C ratio increases and oxygen content is lower, and the hydrothermal liquid (biocrude) is not comparable with fossil products such as diesel. It consists of a great variety of phenols and its alkylated derivatives, heterocyclic N-containing compounds, long-chain FAs, alkanes and alkenes, and derivatives of phytol and cholesterol Citation[97].
Since all hydrocarbons can be converted to liquid products, microalgae, with a low lipid content, can also be utilized for hydrothermal liquefaction. Yu et al achieved an oil (biocrude) yield of 39.4 wt% DM with C. pyrenoidosa, which had a lipid content of 0.1 wt% Citation[99].
The gasification in hydrothermal medium has been conducted in many studies. It can be divided into three different types (adapted from Citation[100]):
▪ Aqueous-phase reforming: biomass compounds such as glucose, sorbitol, glycerol, methanol and glycol are gasified at approximately 215–265°C to mainly hydrogen and CO2 in the presence of a heterogeneous catalyst (e.g., Pt, Ni, Ru, Rh, Pd and Ir). As only molecular compounds are processable, this type is not suitable for algae processing; | |||||
▪ Catalyzed near-critical gasification at around 350°C (liquid phase) or 400°C (supercritical state): biomass or organic compounds are gasified to mainly CH4 and CO2 in the presence of an added heterogeneous catalyst; | |||||
▪ Supercritical water gasification: biomass or organic compounds are gasified to mainly hydrogen and CO2 with or without the addition of a solid catalyst or in the presence of carbon catalysts. | |||||
In comparison with supercritical processes, subcritical or near-critical processes in the presence of a catalyst have a clear advantage of a lower temperature. This means lower capital investment, because reactor material, which is suitable for supercritical gasification, at around 600–700°C, is very costly. On the other hand, catalyst stability in the sub- and near-critical hydrothermal gasification processes is a challenge Citation[100]. A detailed review on possible catalysts can be found in Citation[101].
For the catalytic near-critical gasification, the applicability of microalgae was identified – Minowa and Sawayama demonstrated a positive net energy production (7.29 MJ/kg dry-cell) and that hydrogen and CO4 yield can be influenced by the addition of a nickel catalyst Citation[102]. Elliot et al. concluded that algae, in comparison with lignin feedstocks, were much more reliably processed Citation[103]. High conversions were obtained even with high slurry concentrations. Consistent catalyst operation in these short-term tests suggested good stability and minimal poisoning effects.
Supercritical conditions (400–700°C) were applied on Chlorella vulgaris with heterogeneous catalysts Ru/TiO2 and Inconel powder Citation[104]. The catalysts both increased the conversion rate and product gas, which was rich in hydrogen. Moving the reaction equilibrium towards a product-gas rich in methane is also possible by varying process conditions Citation[105].
Concerning the generally high protein content of microalgae, it was found that biogenic components with high nitrogen content (e.g., proteins) reduce the gas yield Citation[106,107]. However, on the other hand, catalytic gasification offers the possibility of recycling the nitrogen, as the proteins may be dissolved in the reaction water (as ammonia) and are, therefore, biologically available as fertilizer Citation[102].
As already noted, the benefit of the hydrothermal processes regarding algae biofuels is the utilization of wet biomass Citation[108]. The charge only needs prior concentration to approximately 20 wt%. Without heat cycling and management, this process may also get inefficient and uneconomic Citation[109]. Gasification in supercritical water is overall an endothermic reaction. Since supercritical water gasification is operated at very high water content, and given the considerably high specific heat of water, it is of great interest to reduce the reaction temperature as much as possible. It is crucial to recover the thermal energy of the reactor’s effluent to heat up the feed Citation[101], as this can increase the overall energy yield strongly Citation[110].
In comparison with thermochemical dry methods, wet hydrothermal methods do have the advantage of not needing dry algae as a prerequisite; nonetheless, it must be emphasized that a heat-recycling system has to be integrated, otherwise energy cost would be comparable.
Further alternatives
There are several other approaches to use microalgae as fuel. Three of them are briefly discussed below.
▪ Dark fermentation to ethanol
Beside the external fermentation of algal starch, microalgae may ferment intracellularly by dark fermentation Citation[68,111]. In this case, the microalgae grow up to the linear growth phase where they accumulate starch, after which light is excluded. Under dark aerobic conditions, microalgae keep themselves alive by consuming starch or glycogen stored in the cells and decomposing these molecules oxidatively to CO2. If anaerobic conditions are established, the oxidative reaction of starch becomes incomplete and, depending on the type of the microalga, ethanol and other short-chained hydrocarbon products are produced in varying proportions Citation[112]. As both starch accumulation and fermentation proceed in the same cell, this process variation seems to be a promising way to simplify the ethanol production from microalgae.
▪ Biophotosynthesis to ethanol or hydrogen
The understanding of the metabolic pathways on intracellular fermentation of algae leads to another biochemical production pathway; the biophotosynthesis pathway Citation[113]. The algal biomass is not used as a feed for a process, but the alga is the producer of the fuel itself. Certain algae species (green algae but also cyanobacteria) do produce small amounts of ethanol Citation[114] or hydrogen Citation[115] under special stress conditions, such as sulfur deprivation Citation[116]. In the case of green algae, enzymes called hydrogenases catalyze either the production or oxidation of molecular hydrogen Citation[117]. While hydrogen production in cyanobacteria is mostly coupled to nitrogen fixation, unicellular green algae utilize photosynthetically generated electrons for H+ reduction Citation[118].
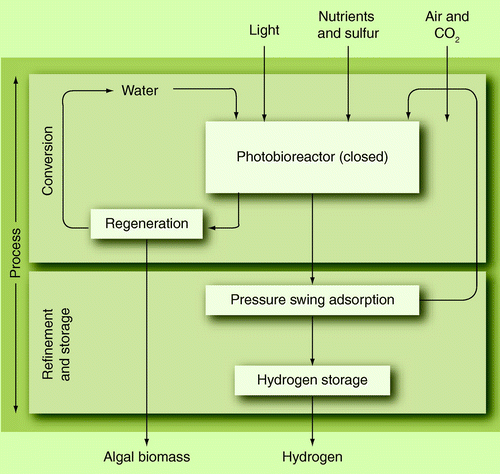
Because of the low conversion efficiency Citation[119] and hydrogen yields, improvement of these parameters has been attempted by screening of mutants and metabolic engineering. With metabolic engineering, the photosynthetic steps inside the cell are altered towards an elevated hydrogen production. For this kind of production it is import that the closed PBR has to be designed to fulfill the specific needs on nutrition and gas transport. shows a possible example process for the hydrogen production from microalgae. Research is still continuing to improve the production rate of single cells. Today the rate is approximately 2–7 ml(H2)/(l h) Citation[120,121]. Economic calculations showed that 100-fold of today’s production rate is the least necessary to achieve economic feasibility Citation[120].
The creation of a pathway for ethanol biosynthesis has been demonstrated with cyanobacteria, by insertion of pyruvate decarboxylase and alcohol dehydrogenase from the ethanologenic bacterium, Zymomonas mobilisCitation[122,123]. This pathway produces ethanol during photoautotrophic growth and could be incorporated into algae. However, these enzymes are not optimized for performance in toxic conditions and may need to be configured for eukaryotic systems Citation[28].
In general, the biophotosynthetic pathway is a direct method to produce ‘ready made’ fuels. Theoretically it can be an efficient process, since energy is not put into the production of biomass, which, afterwards, has to be converted to fuel but goes directly into the production of hydrogen or ethanol. Nonetheless, development is in the early stages and, therefore, it is not ready for efficient utilization at industrial scale until further research has been conducted.
▪ Direct application of microalgae as fuel
A physical and very straightforward possibility to use microalgae as a fuel is the direct use of the total cell in a diesel engine. As diesel engines may also combust solid particles of as little as a few micrometers in diameter, tests were conducted with milled wooden biomass emulsified with kerosene and proved the feasibility of this technology Citation[124]. The cell size of C. vulgaris is approximately 5–10 µm; therefore, Scragg et al. performed tests where the alga was emulsified with biodiesel (20 wt% algal slurry + 80 wt% biodiesel) and run in a single cylinder motor Citation[125]. As the lower heating value of the emulsion decreased, fuel consumption for the test runs increased; emissions of CO were slightly greater, but nitrogen oxides were lower Citation[125]. The technology of diesel engines, especially in automobiles, has strongly evolved in recent years and cannot handle solid particles any more, especially due to the development of sophisticated high-pressure injection systems. Therefore the utilization of such slurry fuel will be restricted to low-level technologies such as direct fuel burners. No more recent publications on this topic are known to the authors.
▪ Economic & environmental challenges
Despite the technical opportunities for using microalgae as a resource for biofuels, there are constraints from an economic and environmental point of view.
In most cases, biofuel production costs simply depend on biomass-supply costs (i.e., for raw material and their provision). Production sites for algal biomass, which are intended for mass production and energetic utilization, have not yet been built. Due to this and the small amounts of biomass currently produced (5000 tons DM/year microalgae worldwide Citation[27]), which are produced for other purposes as food supplements, feed and pharmaceuticals, it is difficult to estimate the possible costs of algae for biofuel production.
Today’s price for 1 kg of algae can vary between €15 for OPR algae from Asia to €30 for closed PBR algae from Europe. Several cost estimations are available, which consider the scale-up from current plant size to plants with several hectares in size. Wijffels et al. concluded that on a 1 ha production site, 1 kg of algae would cost approximately €10, but the costs could be reduced to approximately €4 by scaling up to 100 ha with technologies that are available today Citation[126].
Concerning the biofuel production itself, a comparative cost analysis, which harmonized the input assumptions of four different cost estimations (National Renewable Energy Laboratory, Sandia National Laboratories, New Mexico State University and Seambiotic), calculated costs of US$3.05 ± 0.31/l for triglycerides in a ‘near-term’ scenario Citation[127]. Although these estimations indicate an enormous reduction of the costs, they are still too high to compete with present land-based biomass used for biofuel. The current average biomass price (e.g., for rapeseed) is in the range of €0.25–0.45/kg (approximated from Marché A Terme d’Instruments Financiers, Paris; France) Citation[202] and rape oil between $0.84–1.26/l (Dutch, fob ex-mill, ISTA Oil world, 2010–2011) Citation[128]. For lignocellulosic biomass (e.g., for gasification) it is in a range of approximately €0.05–0.15/kg Citation[58].
Moreover, from the environmental viewpoint, biofuels based on microalgae have to fulfill international sustainability regulations such as the EU Renewable Energy Directive (2009/28/EC), which includes specification on minimum GHG mitigation potentials compared with fossil fuels. It has to be assumed that, similar to crop-based biofuels, for algae-based biofuels the biomass production step will have a major impact on the overall GHG balance. In this context, it is important to make a decision on the production process and applied algae strain dependent on the nutrition medium, as this has a great influence on the GHG balance Citation[129].
Comparative discussion
The mass production of microalgae in quantities required for noteworthy biofuel production has not yet been established. There are only a few sites worldwide that currently produce mentionable amounts of algae. Therefore, the first step for biofuel production from algae is the upscaling of the production processes and cost reduction to a level of current biofuel feedstocks, as algal biofuels are not economically feasible at present.
Algal biomass, in general, may come from different sources; these sources can be sorted by their nutrient supply and by the purpose of production. The nutrient supply can be divided into sources of explicit production (commercially produced CO2, nitrogen and phosphorus fertilizer and minerals) and sources of byproducts, residues or wastes (e.g., diluted CO2 from fermentation processes or flue gas from coal or oil combustion, and nitrogen and phosphorus from wastewater streams). The purpose of production may be explicitly for nutrition, feed, or biofuel production, as a byproduct of processes (e.g., wastewater treatment and CO2 capture) or as waste from, for example, water maintenance. Depending on the quality of the alga produced, its composition differs in terms of lipid, starch and protein content but also possible contamination with toxic substances and is, therefore, not suitable for every purpose.
This shows that the achievable price of the algae sources differs and that not all algae can be utilized for every application. As biofuel production means high quantities produced at relatively low biomass costs, the properties and conditions of the input material in the process plays a major role. The more specific the input must be, the higher the effort to reach this quality is and, thereby, production costs increase.
Concerning the possible biofuel pathways, this means that, for example, algae from wastewater treatment is not produced towards a specific lipid content and may contain heavy metals. Consequently, the biodiesel pathway would not be appropriate, since the extraction effort may be unproportionally high and heavy metals may reside in the lipids.
Therefore, all biofuel pathways presented in this review do have their advantages and disadvantages. For instance, biodiesel and ethanol from fermentation are both mature processes but do have constraints towards algae quality as well as only utilizing specific fractions (e.g., neutral lipids and starch). This can lead to an inefficient or uneconomic process. The hydroprocessing of algal lipids diminishes the drawbacks of the biodiesel process as more fractions can be used and the quality of the product is better. Still, a certain quality of the algae has to be reached (high total lipid content) and a hydrogen supply is needed. The production of biogas is established in many regions and can, therefore, be applied to algae very fast. The utilization as a co-substrate is allowing the usage of algae for energetic purposes and it is possible to use waste-stream algae as an input. On the other hand, it is important to include a disintegration process, otherwise the digestion would be inefficient. Nutrient cycling is feasible in this process, which may lower costs.
A more advanced biophotosynthetic pathway may lead to much better conversion efficiency as the fuels are produced directly without an additional downstream process. Nevertheless, today’s implementations have yields that are much too low and further progress in research is needed before an industrial application will be possible. Concerning all the biochemical pathways, no industrial implementation is known, but the technological state of development of mature processes, such as biogas and ethanol, ease a utilization of algae and can lead to short-term commercialization.
Advantages of the thermochemical pathways in general are the utilization of the whole carbon accumulated by the algae and the possibility to process algae from low-quality sources, as toxic substances are destroyed or immobilized in the process. Thermochemical dry methods have the drawback of being limited to biomass with a low moisture content but, as several pilot and demonstration plants are built and operated today for wood and straw, an implementation for research in a scaled-up pyrolysis or gasification plant is possible. Hydrothermal processes, on the other hand, allow the usage of wet biomass, which complies much more with the condition of harvested algae. The different process variations (carbonization, liquefaction and gasification) may lead to comparable products, such as the dry methods, but, in contrast, give the chance to recycle nutrients. Alternatively to this, hydrothermal liquefaction and gasification processes for energetic application are not as far developed as for dry methods. Processes such as supercritical water gasification for toxic organic substance destruction or the hydrothermal carbonization of biogenic wastes may be a base to start the implementation of hydrothermal biofuels from algae.
Conclusion
Overall, it can be concluded that without adaptation of the process chain to the source of algae, it is not possible to bring biofuels from algae into the market. Thus, beside the development of algae production processes it is important to develop the appropriate downstream processes at the same time. Currently, not all has to be invented from scratch – when it comes to biofuels there are already numerous processes on the market or in development. Each of these processes has advantages and drawbacks, but by combining them to an appropriate biomass source, efficient algal-biofuel production routes could be implemented and established. Despite the technical issues, economic (e.g., algae costs) and environmental (e.g., GHG mitigation) constraints need to be overcome.
Future perspective
Over the next few years the attempts to produce biofuels from algae will originate from two different directions. One will start with the product, which is the biofuel. It will be driven by a specific production of algae with certain attributes mandatory for specific biofuel processes (e.g., HEFA fuels). In this case, the main objective is to produce a biofuel for a defined application (e.g., aviation fuels) and, therefore, the production costs are crucial. The second direction will originate from the source; that is, the biomass. Algae will be produced or harvested for the purpose of biomass production, which may be for several reasons (e.g., water management, CO2 sequestration and wastewater treatment), but the main objective is not to produce a specific kind of algae with very specialized attributes. Nonetheless, the biomass produced may be used for an energetic application. In this case, other biofuel processes are needed (because of the lower algae quality) and the biofuel will be a byproduct, which gives an added value to an already established application.
Table 1. Neutral and polar lipid content of two algae species.
Lipid is sometimes used as a synonym for fats; fats are a subgroup of lipids called triglycerides. Only triglycerides are suitable for the biodiesel process (transesterification).
A jet fuel specification of the American Society for Testing and Materials International Committee, technically hydroprocessed esters and fatty acids fuels are comparable to hydrotreated vegetable oil fuels.
(Several definitions are known) Reaction that occurs in the process of combustion. Pyrolysis as a process itself is conducted in an oxygen-free atmosphere; depending on the reaction time, the organic input will be carbonized or condensates to a liquid product.
By comparing the H/C ratio of the input and output of a biofuel production process it is possible to analyze the quality of the conversion. As well as the H/C ratio, the O/C ratio is also important.
Algae production & supply
▪ Microalgae are very diverse and offer many possibilities; however, simply applying known processes for microalgae production does not work – processes have to be adapted. | |||||
▪ For future algae biofuel production ventures it is crucial to develop the whole process chain and consider all constraints between the different processing parts first. | |||||
Biofuel options
▪ The commercialization of the algal biodiesel process itself, that is, transesterification, should be possible in the short to medium term. Nonetheless, this process only uses a specific part of the algae and, therefore, its efficiency strongly depends on the triglyceride yield. | |||||
▪ The hydroprocessed esters and fatty acids (HEFA) process has many advantages, such as giving a comparable fuel quality and the possible integration in already existing processes. On the other hand, the same constraints identified for biodiesel also apply for HEFA fuels, when it comes to the input raw material. | |||||
▪ The biogas process may utilize the whole algal cell and also has the possibility to use low-quality algae sources, such as from wastewater treatment or blooms. | |||||
▪ ‘Thermochemical dry’ methods, such as direct gasification or flash pyrolysis, are possible processes for algal-biofuel production, but do have the main drawback of only utilizing dry biomass. Hence, a lot of energy would be needed for prior drying. | |||||
▪ In comparison with thermochemical dry methods, ‘wet hydrothermal’ methods do have the advantage of not needing dry algae as a prerequisite; nonetheless, it has to be emphasized that a heat-recycling system has to be integrated, otherwise energy cost would be comparable. | |||||
▪ Other processes that utilize an in situ extraction or production promise higher efficiencies but are not in a mature state of development. | |||||
Economic challenges
▪ Although the estimation of US$3.05 for 1 l of algal oil for biodiesel or HEFA production would already mean an enormous reduction of the costs, they are still too high to compete with present land-based vegetable oils (e.g., rape oil at $0.84–1.26/l) used for biofuel. | |||||
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Chisti Y. Biodiesel from microalgae. Biotechnol. Adv.25(3),294–306 (2007).
- Burlew JS. Algal Culture: From Laboratory to Pilot Plant. Carnegie Institute, WA, USA (1953).
- Lehr F, Posten C. Closed photo-bioreactors as tools for biofuel production. Curr. Opin. Biotechnol.20(3),280–285 (2009).
- Cock JM. Introduction to Marine Genomics. Springer Publishing, NY, USA (2010).
- Lee RE. Phycology. Cambridge University Press, Cambridge, UK (1999).
- Becker EW. Microalgae: Biotechnology and Microbiology. Cambridge University Press, Cambridge, UK (1994).
- Hill A, Feinberg D. Fuel from Microalgae Lipid Products. Solar Energy Research Institute, Golden Colorado, CO, USA (1984).
- Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC. Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol.19(3),235–240 (2008).
- Harder R, von Witsch H. Bericht über versuche zur fettsynthese mittels autotropher mikroorganismen. Forschungsdienst Sonderheft16,270–275 (1942).
- Müntz K. Die massenkultur von kleinalgen, bisherige ergebnisse und probleme. Die Kulturpflanze15,311–350 (1967).
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng.101(2),87–96 (2006).
- Sheehan J, Dunahay T, Benemann R, Roessler G, Weissman C. A Look Back at the US Department of Energy’s Aquatic Species Program: Biodiesel from Algae. Knowledge Publications, London, UK (1998).
- Borowitzka MA. Algal biotechnology products and processes – matching science and economics. J. Appl. Phycol.4(3),267–279 (1992).
- Posten C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci.9(3),165–177 (2009).
- Mirón AS, Gómez AC, Camacho FG, Grima EM, Chisti Y. Comparative evaluation of compact photobioreactors for large-scale monoculture of microalgae. In: Marine Bioprocess Engineering, Proceedings of an International Symposium organized under Auspices of The Working Party on Applied Biocatalysis of the Eurpean Federation of Biotechnology and The European Society for Marine Biotechnology (Volume 35). Elsevier, Amsterdam, The Netherlands, 249–270 (1999).
- Carlsson AS, van Beilen JB, Möller R, Clayton D. Micro- and Macro-Algae: Utility for Industrial Applications: Outputs from the EPOBIO Project. CPL Press, Thatcham, UK (2007).
- Posten C, Schaub G. Microalgae and terrestrial biomass as source for fuels – process view. J. Biotechnol.142(1),64–69 (2009).
- Henderson R, Parsons SA, Jefferson B. The impact of algal properties and pre-oxidation on solid–liquid separation of algae. Water Res.42(8–9),1827–1845 (2008).
- Neenan B. Fuels from Microalgae Technology Status, Potential and Research Requirements. Solar Energy Research Institute, Golden, CO, USA (1986).
- Medina AR, Grima EM, Giménez AG, González MJI. Downstream processing of algal polyunsaturated fatty acids. Biotechnol. Adv.16(3),517–580 (1998).
- Loew O, Bokorny T. Chemisch–physiologische studien über algen. J. Praktische Chem.36(1),272–291 (1887).
- Li Y, Horsman M, Wang B, Wu N, Lan CQ. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol.81(4),629–636 (2008).
- Illman AM, Scragg AH, Shales SW. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol.27(8),631–635 (2000).
- Subramaniam R, Dufreche S, Zappi M, Bajpai R. Microbial lipids from renewable resources: production and characterization. J. Indust. Microbiol. Biotechnol.37(12),1271–1287 (2010).
- Xu H, Miao X, Wu Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol.126(4),499–507 (2006).
- Kröger M, Müller-Langer F. Impact of heterotrophic and mixotrophic growth of microalgae on the production of future biofuels. Biofuels2(2),145–151 (2011).
- Wijffels RH, Barbosa MJ. An outlook on microalgal biofuels. Science329(5993),796–799 (2010).
- Radakovits R, Jinkerson RE, Darzins A, Posewitz MC. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell9(4),486–501 (2010).
- Doucha J, Lívanský K. Influence of processing parameters on disintegration of Chlorella cells in various types of homogenizers. Appl. Microbiol. Biotechnol.81(3),431–440 (2008).
- Shen Y, Pei Z, Yuan W, Mao E. Effect of nitrogen and extraction method on algae lipid yield. Int. J. Agric. Biol. Eng.2(1),51–57 (2009).
- Lee SJ, Yoon BD, Oh HM. Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol. Tech.12(7),553–556 (1998).
- Herrero M, Cifuentes A, Ibañez E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: plants, food by-products, algae and microalgae: a review. Food Chem.98(1),136–148 (2006).
- Mercer P, Armenta RE. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol.113(5),539–547 (2011).
- King J. Supercritical Fluid Technology in Oil and Lipid Chemistry. AOCS Press, Champaign, IL, USA (1996).
- Sievers U. Energy optimization of supercritical fluid extraction processes with separation at supercritical pressure. Chem. Eng. Process.37(5),451–460 (1998).
- Frenz J, Largeau C, Casadevall E. Hydrocarbon recovery by extraction with a biocompatible solvent from free and immobilized cultures of Botryococcus braunii. Enz. Microb. Technol.11(11),717–724 (1989).
- Hejazi MA, Wijffels RH. Milking of microalgae. Trends Biotechnol.22(4),189–194 (2004).
- Wei F, Gao G-Z, Wang X-F et al. Quantitative determination of oil content in small quantity of oilseed rape by ultrasound-assisted extraction combined with gas chromatography. Ultrason. Sonochem.15(6),938–942 (2008).
- Guderjan M, Elez-Martínez P, Knorr D. Application of pulsed electric fields at oil yield and content of functional food ingredients at the production of rapeseed oil. Innov. Food Sci. Emerg. Technol.8(1),55–62 (2007).
- Kaltschmitt M. Energie aus Biomasse: Grundlagen, Techniken und Verfahren (2nd Edition). Springer Publishing, Amsterdam, The Netherlands (2009).
- Kinast J. Production of Biodiesels from Multiple Feedstocks and Properties of Biodiesels and Biodiesel Diesel Blends Final Report Report 1 in a Series of 6. US Department of Energy, Washington, DC, USA (2003).
- Lam MK, Lee KT, Mohamed AR. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol. Adv.28(4),500–518 (2010).
- Amigun B, Müller-Langer F, von Blottnitz H. Predicting the costs of biodiesel production in Africa: learning from Germany. Energy Sustain. Dev.12(1),5–21 (2008).
- Belarbi EH, Molina E, Chisti Y. A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzyme Microb. Technol.26(7),516–529 (2000).
- Ehimen EA, Sun ZF, Carrington CG. Variables affecting the in situ transesterification of microalgae lipids. Fuel89(3),677–684 (2010).
- D’Oca MGM, Viêgas CV, Lemoes JS et al. Production of FAMEs from several microalgal lipidic extracts and direct transesterification of the Chlorella pyrenoidosa. Biomass Bioenergy35(4),1533–1538 (2011).
- Lewis T, Nichols PD, McMeekin TA. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J. Microbiol. Methods43(2),107–116 (2000).
- Xu R, Mi Y. Simplifying the process of microalgal biodiesel production through in situ transesterification technology. J. Am. Oil Chem. Soc.88(1),91–99 (2010).
- Robinson PR. Petroleum processing overview. In: Practical Advances in Petroleum Processing. Hsu CS, Robinson PR (Eds). Springer, NY, USA, 1–78 (2006).
- Huber GW, O’Connor P, Corma A. Processing biomass in conventional oil refineries: production of high quality diesel by hydrotreating vegetable oils in heavy vacuum oil mixtures. Appl. Catal. A Gen.329,120–129 (2007).
- Huber GW, Corma A. Synergien zwischen bio- und ölraffinerien bei der herstellung von biomassetreibstoffen. Angew. Chem.119(38),7320–7338 (2007).
- Donnis B, Egeberg RG, Blom P, Knudsen KG. Hydroprocessing of bio-oils and oxygenates to hydrocarbons. Understanding the reaction routes. Topics Catal.52(3),229–240 (2009).
- Warwick G. Biofuels could be cleared for aircraft use. Int. News Fat Oils Relat. Mater.21(12),724–780 (2010).
- Unknown. Analysis: algae and jatropha pilot planes take off. Chem. Eng.812,6 (2009).
- Wagner S. EADS aircraft runs on algae biofuel. The Engineer, 21 July 2010.
- Benson TJ, Miquez MS, Holmes WE, French WT, Hernandez R. Development of an ideal hydrotreating catalyst for the conversion of phospholipids to biofuels. Presented at: 21st International Symposium on Chemical Reaction Engineering. Philadelphia, PA, USA, 13–16 June 2010.
- Müller-Langer F, Scholwin F, Kaltschmitt M. Biomethane for Transport – a Worldwide Overview. From Today’s to Tomorrow’s Biofuels – from the Biofuels Directive to Bio Based Transport Systems in 2020. Springer Publishing, Amsterdam, The Netherlands (2009).
- Müller-Langer F, Majer S, Perimenis A. Biofuels – a technical, economic and environmental comparison. In: Encyclopedia of Sustainability Science and Technology. Springer Publishing, Amsterdam, The Netherlands (2012).
- Ryckebosch E, Drouillon M, Vervaeren H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy35(5),1633–1645 (2011).
- Golueke CG, Oswald WJ, Gotaas HB. Anaerobic digestion of algae. Appl. Microbiol.5(1),47–55 (1957).
- Mussgnug JH, Klassen V, Schlüter A, Kruse O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol.150(1),51–56 (2010).
- Yen H-W, Brune DE. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol.98(1),130–134 (2007).
- Salerno M, Nurdogan Y, Lundquist T. Biogas production from algae biomass harvested at wastewater treatment ponds. Presented at: Proceedings of the 2009 Bioenergy Engineering Conference. Florida, USA, 11–14 October 2009.
- Mann G, Schlegel M, Schumann R, Sakalauskas A. Biogas-conditioning with microalgae. Agron. Res.7(1),33–38 (2009).
- Schmack Biogas AG. Effizienzsteigerung der Biogasnutzung durch Solarenergie: EBSIE Technikumphase, Abschlussbericht. Schmack Biogas AG, Schwandorf, Germany (2008).
- Organization for Economic Cooperation and Development-FAO. Agricultural Outlook 2011–2020. Organization for Economic Cooperation and Development, Rome, Italy (2011).
- Ettl H. Grundriss der Allgemeinen Algologie. Gustav Fischer Verlag, Stuttgart, Germany (1980).
- Hirano A, Ueda R, Hirayama S, Ogushi Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy22(2–3),137–142 (1997).
- Shirai F, Kunii K, Sato C et al. Cultivation of microalgae in the solution from the desalting process of soy sauce waste treatment and utilization of the algal biomass for ethanol fermentation. World J. Microbiol. Biotechnol.14(6),839–842 (1998).
- Choi SP, Nguyen MT, Sim SJ. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol.101(14),5330–5336 (2010).
- Matsumoto M, Yokouchi H, Suzuki N, Ohata H, Matsunaga T. Saccharification of marine microalgae using marine bacteria for ethanol production. Appl. Biochem. Biotechnol.105(108),247–254 (2003).
- Harun R, Danquah MK, Forde GM. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol.85(2),199–203 (2010).
- Tijmensen MJA, Faaij APC, Hamelinck CN, van Hardeveld MRM. Exploration of the possibilities for production of Fischer–Tropsch liquids and power via biomass gasification. Biomass Bioenergy23(2),129–152 (2002).
- Dolan GA. Methanol production and utilization. In: Biomass to Biofuels: Strategies for Global Industries. John Wiley & Sons, Hoboken, NJ, USA, 435–455 (2010).
- Brown DM, Bhatt BL, Hsiung TH, Lewnard JJ, Waller FJ. Novel technology for the synthesis of dimethyl ether from syngas. Catal. Today8(3),279–304 (1991).
- Kopyscinski J, Schildhauer TJ, Biollaz SMA. Production of synthetic natural gas (SNG) from coal and dry biomass - a technology review from 1950 to 2009. Fuel89(8),1763–1783 (2010).
- Kirubakaran V, Sivaramakrishnan V, Nalini R et al. A review on gasification of biomass. Renew. Sustain. Energy Rev.13(1),179–186 (2009).
- Dahmen N, Dinjus E, Henrich E. The Karlsruhe process Bioliq® – synthetic fuels from the biomass. In: Renewable Energy: Sustainable Energy Concepts for the Future. Wengenmayr R, Bührke T (Eds). Wiley Publishing, IN, USA, 61–65 (2008).
- Hirano A, Hon-Nami K, Kunito S, Hada M, Ogushi Y. Temperature effect on continuous gasification of microalgal biomass: theoretical yield of methanol production and its energy balance. Catal. Today45(1–4),399–404 (1998).
- Li W, Li W, Liu H. The resource utilization of algae – preparing coal slurry with algae. Fuel89(5),965–970 (2010).
- Bridgwater A. Fast Pyrolysis of Biomass: a Handbook. CPL Press, Thatcham, UK (1999).
- Pan P, Hu C, Yang W et al. The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils. Bioresour. Technol.101(12),4593–4599 (2010).
- Miao X, Wu Q. High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol.110(1),85–93 (2004).
- Babich IV, van der Hulst M, Lefferts L et al. Catalytic pyrolysis of microalgae to high-quality liquid bio-fuels. Biomass Bioenergy35(7),3199–3207 (2011).
- Peng W, Wu Q, Tu P. Effects of temperature and holding time on production of renewable fuels from pyrolysis of Chlorellaprotothecoides. J. Appl. Phycol.12(2),147–152 (2000).
- Miao X, Wu Q, Yang C. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrol.71(2),855–863 (2004).
- Czernik S, Bridgwater AV. Overview of applications of biomass fast pyrolysis oil. Energy Fuels18(2),590–598 (2004).
- Bridgwater AV, Meier D, Radlein D. An overview of fast pyrolysis of biomass. Org. Geochem.30(12),1479–1493 (1999).
- Kruse A, Dinjus E. Hot compressed water as reaction medium and reactant: properties and synthesis reactions. J. Supercrit. Fluids39(3),362–380 (2007).
- Yu SH, Cui XJ, Li LL et al. From starch to metal/carbon hybrid nanostructures: hydrothermal metal-catalyzed carbonization. Adv. Mater.16(18),1636–1640 (2004).
- Titirici M-M, Thomas A, Antonietti M. Back in the black: hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem.31(6),787–789 (2007).
- Glasner C, Deerberg G, Lyko H. Hydrothermale carbonisierung: ein überblick. Chem. Ingen. Tech.83(11),1932–1943 (2011).
- Heilmann SM, Davis HT, Jader LR et al. Hydrothermal carbonization of microalgae. Biomass Bioenergy34(6),875–882 (2010).
- Heilmann SM, Jader LR, Harned LA et al. Hydrothermal carbonization of microalgae II. Fatty acid, char, and algal nutrient products. Appl. Energy88(10),3286–3290 (2011).
- Levine RB, Pinnarat T, Savage PE. Biodiesel production from wet algal biomass through in situ lipid hydrolysis and supercritical transesterification. Energy Fuels24(9),5235–5243 (2010).
- Toor SS, Rosendahl L, Rudolf A. Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy36(5),2328–2342 (2011).
- Brown TM, Duan P, Savage PE. hydrothermal liquefaction and gasification of Nannochloropsis spp. Energy Fuels24(6),3639–3646 (2011).
- Ross AB, Biller P, Kubacki ML et al. Hydrothermal processing of microalgae using alkali and organic acids. Fuel89(9),2234–2243 (2010).
- Yu G, Zhang Y, Schideman L, Funk TL, Wang Z. Hydrothermal liquefaction of low lipid content microalgae into bio-crude oil. Trans. ASABE54(1),239–246 (2011).
- Kruse A. Hydrothermal biomass gasification. J. Supercrit. Fluids47(3),391–399 (2009).
- Azadi P, Farnood R. Review of heterogeneous catalysts for sub- and supercritical water gasification of biomass and wastes. Int. J. Hydro. Energy36(16),9529–9541 (2011).
- Minowa T, Sawayama S. A novel microalgal system for energy production with nitrogen cycling. Fuel78(10),1213–1215 (1999).
- Pacific Northwest National Laboratory. Catalytic Hydrothermal Gasification of Lignin-Rich Biorefinery Residues and Algae Final Report. US Department of Energy, Washington, DC, USA (2009).
- Chakinala AG, Brilman DWF, Van Swaaij WPM, Kersten SRA. Catalytic and non-catalytic supercritical water gasification of microalgae and glycerol. Indust. Eng. Chem. Res.49(3),1113–1122 (2010).
- Stucki S, Vogel F, Ludwig C, Haiduc AG, Brandenberger M. Catalytic gasification of algae in supercritical water for biofuel production and carbon capture. Energy Environ. Sci.2(5),535 (2009).
- Kruse A, Maniam P, Spieler F. Influence of proteins on the hydrothermal gasification and liquefaction of biomass. 2. Model compounds. Ind. Eng. Chem. Res.46(1),87–96 (2011).
- Kruse A, Krupka A, Schwarzkopf V, Gamard C, Henningsen T. Influence of proteins on the hydrothermal gasification and liquefaction of biomass. 1. Comparison of different feedstocks. Ind. Eng. Chem. Res.44(9),3013–3020 (2011).
- Minowa T, Yokoyama S-Y, Kishimoto M, Okakura T. Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel74(12),1735–1738 (1995).
- Griffith JW, Raymond DH. The first commercial supercritical water oxidation sludge processing plant. Waste Manag.22(4),453–459 (2002).
- Calzavara Y, Joussot-Dubien C, Boissonnet G, Sarrade S. Evaluation of biomass gasification in supercritical water process for hydrogen production. Energy Conv. Manage.46(4),615–631 (2005).
- Ueno Y, Kurano N, Miyachi S. Ethanol production by dark fermentation in the marine green alga, Chlorococcum littorale. J. Ferment. Bioeng.86(1),38–43 (1998).
- John RP, Anisha GS, Nampoothiri KM, Pandey A. Micro and macroalgal biomass: a renewable source for bioethanol. Biores.Technol.102(1),186–193 (2011).
- Gaffron H. Reduction of carbon dioxide with molecular hydrogen in green algae. Nature27(5),273–283 (1940).
- Deng M-D, Coleman JR. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol.65(2),523–528 (1999).
- Zhang L, Melis A. Probing green algal hydrogen production. Philos. Trans. R. Soc. Lond. B Biol. Sci.357(1426),1499–1511 (2002).
- Tsygankov A, Kosourov S, Seibert M, Ghirardi ML. Hydrogen photoproduction under continuous illumination by sulfur-deprived, synchronous Chlamydomonas reinhardtii cultures. Int. J. Hydro. Energy27(11–12),1239–1244 (2002).
- Vignais PM, Billoud B, Meyer J. Classification and phylogeny of hydrogenases. FEMS Microbiol. Rev.25(4),455–501 (2001).
- Hemschemeier A, Melis A, Happe T. Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth. Res.102(2–3),523–540 (2009).
- Akkerman I, Janssen M, Rocha J, Wijffels RH. Photobiological hydrogen production: photochemical efficiency and bioreactor design. Int. J. Hydro. Energy27(11–12),1195–1208 (2002).
- Trudewind CA, Wagner H-J. Planung einer “großtechnischen” anlage zur photobiologischen wasserstofferzeugung aus mikroalgen. In: Der 4. Deutsche Wasserstoff Congress 2008 – Tagungsband. Forschungszentrum, Jülich, Germany, 247–257 (2008).
- Scoma A, Krawietz D, Faraloni C et al. Sustained H2 production in a Chlamydomonas reinhardtii D1 protein mutant. J. Biotechnol.157(4),613–619 (2012).
- Deng M-D, Coleman JR Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol.65(2),523–528 (1999).
- Dexter J, Fu P. Metabolic engineering of cyanobacteria for ethanol production. Energy Environ. Sci.2,857–864 (2009).
- Benter MM, Gilmour IA, Arnoux L. Biomass-oil slurry fuels: an investigation into their preparation and formulation. Biomass Bioenergy12(4),253–261 (1997).
- Scragg AH, Morrison J, Shales SW. The use of a fuel containing Chlorella vulgaris in a diesel engine. Enzyme Microb. Technol.33(7),884–889 (2003).
- Wijffels RH, Eppink M, Barbosa MJ. Biorefinery of microalgae. Presented at: 8th European Workshop Biotechnology of Microalgae. Potsdam, Germany, 7 June 2010.
- Sun A, Davis R, Starbuck M et al. Comparative cost analysis of algal oil production for biofuels. Energy36(8),5169–5179 (2011).
- ISTA. Oil World Statistics. ISTA/Mielke GmbH, Hamburg, Germany (2011).
- Kröger M, Majer S, Kaltschmitt M. Balancing of algae production and its impact on climate factors. Presented at: International Algae Congress. Hamburg, Germany, 1 December 2009.
▪ Websites
- Töpfl S. Dissertation. Pulsed electric fields (PEF) for permeabilization of cell membranes in food- and bioprocessing applications, Process and equipment design and cost analysis (2006). http://opus.kobv.de/tuberlin/volltexte/2006/1397
- Marché A Terme d’Instruments Financiers, Paris; Frankreich through LEL Schwäbisch Gmünd, Infodienst Landwirtschaft – Ernährung – Ländlicher Raum. www.landwirtschaft-bw.info/servlet/PB/menu/1042031_l1/index1221750829191.html