Abstract
The bioanalysis group at the Saclay site of the Commissariat à l‘énergie atomique et aux énergies alternatives focuses on MS for the discovery and quantification of biomarkers and drugs as well as metabolites. Key developments and achievements include intracellular pharmacokinetics of anti-HIV drugs, metabolomic studies, pioneering work in the bioanalysis of recombinant proteins, antibodies, probes for in vitro blood–brain barrier models and bioanalytical approaches to the quantification of biomarkers relevant to the threat of bioterrorism. Our activities are based on industrial collaboration and are pursued within the framework of national and international collaborations and strong partnerships with the pharmaceutical industry and clinicians.

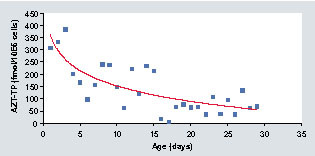
AZT-TP: Zidovudine triphosphate.
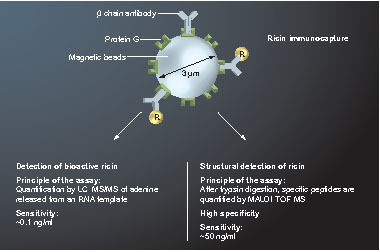
The N-glycosidase activity of ricin was first exploited by quantifying the adenine released from an RNA template by LC–MS/MS and isotope dilution. A complementary approach involving MALDI-TOF-MS has also been developed to quantitatively and specifically measure tryptic fragments from ricin. In both cases, ricin was first isolated by immunoaffinity to provide greater specificity and facilitate detection in complex matrices.
The Commissariat à l‘énergie atomique et aux énergies alternatives (Alternative Energies and Atomic Energy Commission [CEA]) is a research body with a technological focus on defense and national security, energy and healthcare technology. The CEA’s commitment to advanced technology enables its Life Sciences Division to combine high-level scientific progress with applied cross-disciplinary projects that favour innovation in healthcare and biotechnology, with a strong opening in diagnostics and therapeutics. The aim is to supply novel concepts and tools that are needed to improve our understanding of complex living systems, such as functional and structural genomics, protein engineering, diagnostics and imaging methods. A large part of the Life Sciences Division is located at the Saclay site , where our group belongs to the Laboratory for Drug Metabolism (Laboratoire d’Etude du Métabolisme des Médicaments [LEMM]), which develops analytical and cellular tools to support drug development and biomarker discovery. The LEMM is accredited by the French Authorities (Afssaps) for good laboratory practices since our activities are performed with strong partnerships with pharmaceutical companies. Our field of excellence is MS and we have 12 mass spectrometers (triple quadrupole, Q-TOF, MALDI-TOF, Orbitrap™ and Fourier-transform ion cyclotron resonance) that are used for the elucidation of drug metabolism, intracellular and tissue pharmacokinetics, characterization and bioanalysis of drug proteins and detection of biological warfare. In parallel, untargeted approaches for biomarker discovery are developed using metabolomic profiles in various cellular and biological fluids.
Bioanalysis & pharmacology
Our activities are mainly focused on the development of pharmacological models and tests for new strategies of treatment optimization and individualization and the development of state-of-the-art bioanalytical methods for studies of drug pharmacokinetics and metabolism. Our experience relies on LC coupled with radioactivity or MS (LC–MS/MS). With more than 20-years background in MS and biological sample preparation, we have developed many assay methods for various kinds of drugs in several biological fluids, with a special emphasis on quantification of drugs inside circulating cells or in tissues, such as nucleotides and anti-HIV or immunosuppressive drugs. For instance, we have shown that efflux proteins greatly affect the uptake of antiretroviral drugs by cells and hamper their access to the HIV type 1 replication site. We first focused our work on the factors that may lead to drug–drug interactions between emtricitabine, tenofovir and efavirenz, including the modulation of efflux transporter expression and function. Our work revealed for the first time a benefit from the use of combination therapy, like that achieved with Atripla® (emtricitabine, tenofovir and efavirenz) in terms of intracellular concentrations of emtricitabine and tenofovir phosphate metabolites, partly due to a decrease in ABCC functionality and to the direct interaction of the drugs with members of the ABCC transporter family. We have also reported that intracellular metabolism of zidovudine (the first nucleoside analogue, reverse transcriptase inhibitor approved against AIDS) in newborns of HIV-infected mothers leads to higher levels of the active triphosphorylated metabolites during the first days of life .
MS-based metabolomics
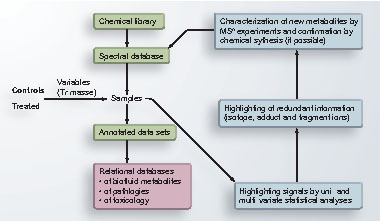
The metabolome is the set of all small organic molecules present in biological media. Metabolomics complements tools already available to biologists for characterization of gene functions, (i.e., transcriptomics and proteomics). It also opens new perspectives in the fields of nutrition, biomarker discovery in pathology and toxicity, or in the development of systems biology. Basic techniques required to mine metabolomes have existed for years. Initially, NMR spectroscopy was shown to provide reproducible and valuable information on metabolites in biofluids. However, MS provides higher sensitivity and complementary structural information. It is also a versatile technology since many ionization sources and mass analyzers are commercially available. The metabolic fingerprints obtained from MS or NMR analyses of biofluids provide information about the metabolic status of individuals. They contain a few hundred to thousands of signals which are related to both genetic and environmental contributions (i.e., diet, lifestyle, gut microbial activity, drug intake and exposure to pesticides, plasticizers or food preservatives, for example). In other words, metabolomics provides a unique opportunity to investigate relationships and interactions between individuals and their environment.
Our team is involved in several projects dealing with the effect of heavy toxicity on various organisms such as plants, yeast and animals (grants received from the CEA Nuclear and Environmental Toxicology program), the characterization of biomarkers for lysosomal storage diseases, detection of early metabolic disorders, neurological disorders and cancers (national and European grants received and as a collaboration with a pharmaceutical firm). Our methodological developments are based on ultra-high-resolution MS (LTQ-Orbitrap mass spectrometers) with a special focus on automatic data analyses , building of databases of small organic molecules present in various biofluids and biological media and, finally, on the application of our tools to functional genomic and biomarker discovery studies.
Biomarkers of recombinant protein exposure
In recent years, biotechnologically derived drugs have been a major focus of research and development in the pharmaceutical industry. Their pharmacokinetics and pharmacokinetic/pharmacodynamic relationships impact every stage of the development process and require their assessment in circulation in preclinical species and in humans. Validation of peptide and protein biomarkers is also a major area of clinical investigation. To this end, immunoassays are the most commonly used technique. However, standardization issues and long development times required to generate high-quality antibodies, as well as the limited possibility of multiplex assays are important restrictions. As an alternative, we have developed analytical strategies involving LC–MS. LC–MS is of proven sensitivity and specificity, especially for low-molecular weight drugs where it is considered the reference technique. However, in the case of peptides and proteins, sensitivity and robustness are mainly hampered by the complexity of biological samples, which contain milligram/milliliter concentrations of various proteins, that is, 106-times more than the targeted protein concentration. Our strategy is based on targeted approaches using the specific multiple reaction monitoring mode and isotope dilution (addition of internal standard peptides labeled with 13C or 15N). As an example, we have developed selective extraction by immunoaffinity for quantification of a small recombinant protein and for production of a therapeutic monoclonal antibody used for the treatment of colorectal cancer. In the field of biotechnology-derived drugs, immunogenicity is of growing interest, since their administration can lead to an unwanted immune response resulting in the generation of antidrug antibodies. Such antibodies are currently determined by immunological approaches, but these are subject to interference by high circulating concentrations of the therapeutic protein. Our previous work on antibody quantification is now being extended to this topic. MS can also play a crucial role in the structural elucidation of protein drugs and we have recently conducted indepth investigations of the structural variability of grass species allergens for therapeutic purposes.
Biomarkers of bioterrorism agents
We are involved in a program developed by the French Ministry of Defense, which is dealing with bioterrorism issues with the aim of developing and validating MS-based analytical methods to accurately identify and quantify biological agents involved in biodefense issues. This includes the fingerprints of both toxins and micro-organisms in various contaminated matrices (e.g., biofluids, food or environmental samples). Regarding toxins, we have used MS to explore their specific enzymatic activity. Such analytical methods were first developed for ricin (combined with a proteomic approach, ), botulinum neurotoxins and anthrax edema and lethal factors. The combination of such complementary activity- and structure-based approaches leads to the unambiguous identification of a given toxin and we are involved in a European program for testing these dual approaches. Regarding micro-organisms, we are now focusing our work on new MS techniques for fast bacterial identification.
Future perspective
Since the beginning of this century, there has been a considerable interest in the discovery and quantification of biomarkers in the exploration of pathological states and as an accompaniment to pharmacological interventions. This requires multiplexed techniques and bioinformatics tools to analyze the considerable amount of data generated by developments in MS over the last 10 years, such as soft ionization techniques and high-resolution analyzers. At the dawn of the 2010s, metabolomics and qualitative proteomics and peptidomics appear as promising approaches. In the coming years, we hope to capitalize on our expertise in biomarker discovery (the metabolome platform) and quantification of drug and protein/peptide biomarkers for applications in pharmaceutical developments and diagnosis. These activities will be conducted with the aim of proposing new developments in the context of a highly competitive and promising international context. For instance, metabolome applications have undergone huge development in the last 2 years (half of the 3000 publications have appeared during this time). However, a key point in the future will be the ability to rapidly analyze and identify putative metabolite biomarkers, which so far represents a bottleneck, and we aim to develop new tools and databases to accelerate metabolite identification. Using these tools, we are currently intensifying partnerships with clinicians in various fields (neurological and genetic diseases, and cancer) to expedite the identification of new biomarkers for diagnosis and therapeutics through the development of proprietary and software tools. This will be supported by the generation of the creation of start-up companies and the national recognition of our platform, which will promote partnerships with clinicians and industry.
The Commissariat à l‘énergie atomique et aux énergies alternatives is a research body with a strong focus on healthcare technology.
Targeted approaches such as those we developed for anti-HIV drugs and probes for in vitro blood–brain barrier drug-passage assessment should optimize drug intervention.
Drug metabolism studies should be analytically supported by a combination of in vitro metabolite profiling and high-resolution MS elucidation of metabolites.
Metabolomics is a novel platform that allows functional genomic and biomarker discovery studies.
High-resolution MS (LTQ-Orbitrap™) analyses enable building of databases of small organic molecules present in various biofluids and biological media.
Quantification of drug and biomarker proteins by MS offers greater precision than traditional immunological methods.
Bioterrorism issues necessitate the development of MS-based analytical methods to accurately identify and quantify biological agents.
Academic laboratories with strong expertise in MS for drug and biomarker quantification along with accreditations from national laboratories should provide valuable support to clinicians and pharmaceutical companies.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Representative publications
Bioanalysis & pharmacology
- Pruvost A , NegredoE, TheodoroFet al. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob. Agents Chemother. 53, 1937–1943 (2009).
- Bousquet L , PruvostA, GuyotACet al. Combination of tenofovir and emtricitabine plus efavirenz: in vitro modulation of ABC transporter and intracellular drug accumulation. Antimicrob. Agents Chemother. 53, 896–902 (2009).
- Pruvost A , TheodoroF, AgrofoglioLet al. Specificity enhancement with LC-positive ESI-MS/MS for the measurement of nucleotides: application to the quantitative determination of carbovir triphosphate, lamivudine triphosphate and tenofovir diphosphate in human peripheral blood mononuclear cells. J. Mass Spectrom. 43, 224–233 (2008).
- Durand-Gasselin L , PruvostA, DeheeAet al. High levels of zidovudine (AZT) and its intracellular phosphate metabolites in AZT- and AZT-lamivudine-treated newborns of human immunodeficiency virus-infected mothers. Antimicrob. Agents Chemother. 52, 2555–2563 (2008).
MS-based metabolomics
- Kaur H , KumarC, JunotCet al. Dug1p is a Cys-Gly peptidase of the g-glutamyl cycle of Saccharomyces cerevisiae and represents a novel family of Cys-Gly peptidases. J. Biol. Chem. 284, 14493–14502 (2009).
- Werner E , HeilierJF, DucruixCet al. Mass spectrometry for the identification of the discriminating signals from metabolomics: current status and future trends. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci871, 143–163 (2008).
- Madalinski G , GodatE, AlvesSet al. Direct introduction of biological samples into a LTQ-Orbitrap hybrid mass spectrometer as a tool for fast metabolome analysis. Anal. Chem. 80, 3291–3303 (2008).
- Werner E , CroixmarieV, UmbdenstockTet al. Mass spectrometry-based metabolomics: accelerating the characterization of discriminating signals by combining statistical correlations and ultrahigh resolution. Anal. Chem. 80, 4918–4932 (2008).
Biomarkers of recombinant protein exposure
- Ezan E , DuboisM, BecherFet al. Bioanalysis of recombinant proteins and antibodies by mass spectrometry. Analyst134, 825–834 (2009).
- Fenaille F , NonyE, ChabreHet al. Mass spectrometric investigation of molecular variability of grass pollen group 1 allergens. J. Proteome Res. 8, 4014–4027 (2009).
- Dubois M , FenailleF, ClementGet al. Immunopurification and mass spectrometric quantification of the active form of a chimeric therapeutic antibody in human serum. Anal. Chem. 80, 1737–1745 (2008).
- Becher F , PruvostA, ClementGet al. Quantification of small therapeutic proteins in plasma by liquid chromatography-tandem mass spectrometry: application to an elastase inhibitor EPI-hNE4. Anal. Chem. 78, 2306–2313 (2006).
Biomarkers of bioterrorism agents
- Manduzio H , MarteletA, EzanEet al. Comparison of approaches for purifying and desalting polymerase chain reaction products prior to electrospray ionization mass spectrometry. Anal. Biochem. 398(2), 272–274 (2010).
- Duriez E , GoossensPL, BecherFet al. Femtomolar detection of the anthrax edema factor in human and animal plasma. Anal. Chem. 81, 5935–5941 (2009).
- Duriez E , FenailleF, TabetJCet al. Detection of ricin in complex samples by immunocapture and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Proteome Res. 7, 4154–4163 (2008)
- Becher F , DuriezE, VollandHet al. Detection of functional ricin by immunoaffinity and liquid chromatography-tandem mass spectrometry. Anal. Chem. 79, 659–665 (2007).