Abstract
Biochar is not a structured homogeneous material; rather it possesses a range of chemical structures and a heterogeneous elemental composition. This variability is based on the conditions of pyrolysis and the biomass parent material, with biochar spanning the range of various forms of black carbon. Thereby, this variability induces a broad spectrum in the observed rates of reactivity and, correspondingly, the overall chemical and microbial stability. From evaluating the current biochar and black carbon degradation studies, there is the suggestion of an overall relationship in biochar stability as a function of the molar ratio of oxygen to carbon (O:C) in the resulting black carbon. In general, a molar ratio of O:C lower than 0.2 appears to provide, at minimum, a 1000-year biochar half-life. The O:C ratio is a function of production temperature, but also accounts for other impacts (e.g., parent material and post-production conditioning/oxidation) that are not captured solely with production temperature. Therefore, the O:C ratio could provide a more robust indicator of biochar stability than production parameters (e.g., pyrolysis temperature and biomass type) or volatile matter determinations.
References for data sources are rice husks Citation[126], pine 1 Citation[86], grass Citation[86], pine needles Citation[110], rapeseed straw Citation[15], almond shell Citation[127], pine 2 Citation[107], tall fescue grass Citation[107] and mixed wood Citation[128].
![Figure 1. Impact of pyrolysis temperature on the yield of biochar (dry weight basis of original biomass) from various biomass materials.References for data sources are rice husks Citation[126], pine 1 Citation[86], grass Citation[86], pine needles Citation[110], rapeseed straw Citation[15], almond shell Citation[127], pine 2 Citation[107], tall fescue grass Citation[107] and mixed wood Citation[128].](/cms/asset/77e72951-b906-40f5-b5a3-1432e8dfee98/tcmt_a_10816161_f0001.gif)
The term biochar spreads across all the divisions in the black carbon continuum describing multiple different black carbon forms.
Adapted from Citation[36,37,39] and also data shown in [Supplementary Table 1].
![Figure 2. The spectrum of the combustion product continuum as a result of the chemical–thermal conversion of biomass.The term biochar spreads across all the divisions in the black carbon continuum describing multiple different black carbon forms.Adapted from Citation[36,37,39] and also data shown in [Supplementary Table 1].](/cms/asset/a7a6af9c-75e2-4d23-9e43-77313f620a45/tcmt_a_10816161_f0002.gif)
Data from & [Supplementary Table 1].
![Figure 3. Comparison of the volatile matter versus the oxygen to carbon (O:C) molar ratio for the natural and synthetic biochars.Data from Tables 2 & [Supplementary Table 1].](/cms/asset/9702569f-35f2-43f0-aab6-63a919287104/tcmt_a_10816161_f0003.gif)
Dry heat sterilization (200°C for 72 h) was used.
Data from Citation[74].
![Figure 4. Averages (two replicates) of the counts per minute of 14C observed for non-sterile and sterile soils on the rate of degradation, originating from a 14C labeled char residue.Dry heat sterilization (200°C for 72 h) was used.Data from Citation[74].](/cms/asset/e0ebe4a9-f87a-4f0e-8600-315ec2053aee/tcmt_a_10816161_f0004.gif)
The sole exception to these divisions were biochars from Hamer et al.; shown in the rectangle Citation[94].
![Figure 5. Correlation of the oxygen to carbon (O:C) molar ratio and predicted half-life of synthetic biochar in various laboratory incubations from the literature studies presented in Table 2 (n = 35).The sole exception to these divisions were biochars from Hamer et al.; shown in the rectangle Citation[94].](/cms/asset/f0a4e5a3-de67-472b-9d4e-6ded99df5187/tcmt_a_10816161_f0005.gif)
![Figure 6. Overall relationship between the pyrolysis production temperature and the oxygen to carbon (O:C) molar ratio for the synthetic biochars listed in [Supplementary Table 1].](/cms/asset/2e00eab8-53dc-43bf-b85e-065ebc2fee2e/tcmt_a_10816161_f0006.gif)
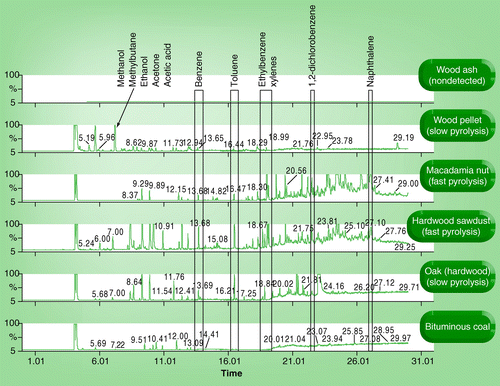
All chromatographic axes are scaled equally; therefore differing peak heights are directly related to differences in concentrations. Some volatiles are identified on the figure in relation to the elution time.
Traditional charcoal production, in a restricted oxygenated environment, has a long history in human civilization dating back to the 15th Century, and the use of charcoal in cave drawings dates back over 35,000 years Citation[201,202]. Despite this long history, there is a lack of uniformity in the scientific literature when it comes to the nomenclature for the products of biomass combustion. Char, charcoal, soot, graphitic carbon, ash, coal and black carbon (black C) have all been used to describe the solid residual products (e.g., see Citation[1]). Recently, biochar has been added to the list as the name applied to the carbonized product of pyrolysis that is returned to soil for its benefits for carbon sequestration, which consequentially, has also been linked to beneficial soil quality improvements Citation[2–6,]. However, the main purpose for the creation of biochar is its carbon sequestration potential Citation[7]. From this standpoint, biochar is new in terms of its overall application and purpose (carbon sequestration), but not in terms of its production. Biochar’s carbon sequestration benefit results from the fact that carbon from the atmosphere–biosphere pool is transferred to a slower cycling form (i.e., black C) that has the potential to exist for hundreds to thousands of years Citation[8,9] or possibly longer Citation[10,11].
Thermal conversion of biomass in the absence of oxygen (i.e., pyrolysis) yields three phases of products: liquid (bio-oil), solid and gas Citation[12–14]. From the standpoint of reducing the sources of global warming and sustainable resource management, biomass is also attracting attention as a renewable energy resource to replace current fossil fuel resources. Pyrolysis has been cited as one of the renewable processes that is most capable of competing with non-renewable fossil fuel resources Citation[15]. Typical pyrolysis conditions to optimize the production of solid residuals are the absence of oxygen (i.e., anaerobic conditions) and temperatures ranging from 350–800°C Citation[16]. As production temperature increases, there is a corresponding decrease in the yield of the solid residuals . An overall range of 15–60% of the dry-weight biomass is present as the solid residual as a function of the feedstock and pyrolysis conditions . Slow pyrolysis offers the technique that currently produces the highest yield of the solid residuals (i.e., biochar) Citation[11]. In addition to its new potential role in carbon sequestration, this solid residue has traditionally been used as a fuel source Citation[17], briquetted, mixed with water/oil to form slurries or subjected to further chemical processing to give more useful and/or valuable chemical products Citation[18,19]. One such example is the long standing use of activated charcoals, which takes charred biomass and chemically or physically activates the surface of the charcoal to create additional chemical reactive groups, particularly targeting sorption capacities Citation[20,21]. However, these energy and noncarbon-sequestration focused uses are not within purview of the definition of biochar Citation[6].
This article will summarize and evaluate the current observations on biochar stability in soil. This information on biochar’s carbon stability is relevant to improve current estimates of the residency time for biochar and its suitability as a vehicle for carbon sequestration. Furthermore, the hypothesis of utilizing the oxygen:carbon (O:C) molar ratio of biochar is presented as a means to predict the eventual stability of biochar in the soil system.
Black carbon
▪ Differences in natural versus synthetic black carbons
In this article, synthetic black C will refer to man-made black C materials created through the pyrolysis of biomass, which would include biochar. Conversely, natural black C is produced during natural events (e.g., lava flows, prairie and forest fires and geologic digenesis) and is present in sediments and the geologic strata Citation[9]. Despite differences in the type of production (natural or man-made), there are other differences between synthetic and natural black C in terms of feedstock purity, temperature control and post-production conditions. These factors could lead to significant differences in both the chemical composition and percentages of the produced products (i.e., solids, liquids and gases) Citation[14].
First, a significant factor for synthetic black C production is parent material purity, since synthetic black C is often produced from a homogenous feedstock stream and offers limited mixing with other materials during pyrolysis Citation[22]. Conversely, natural black C produced from wildfires (i.e., forest and prairie) and lava flows are often heterogeneous and the biomass is intermixed with soil and other impurities (including oxygen presence during production Citation[23]). Thereby, the carbon atoms can react with these other components, some of which can function as catalysts, during thermochemical conversion (pyrolysis) Citation[24,25] or substitutions in the graphitic (carbon) sheets Citation[24]. Inorganic salts also alter the final product distribution, with reduced liquid products and increased char-forming reactions during pyrolysis yielding higher proportions of char Citation[23]. Owing to the potential catalytic effect of these impurities, particularly metals and clay minerals, natural black C can possess differing crystalline structure and chemical stability for equivalent production temperatures Citation[25–28]. The presence of impurities also has other impacts. The presence of alkali earth metals with black C has also been observed to increase the oxidation of black C by up to a factor of 100,000 Citation[29].
Another difference between natural and synthetic black C is that production temperatures of natural black C are highly variable. Temperatures in a natural thermal event (fire or lava flow) can range from 80°C (smoldering) to over 1000°C Citation[30,31], which results in a spectrum of black C as a function of creation temperatures Citation[32]. Black C that has been intensively charred at high temperature has been observed to be entirely aromatic, whereas alkyl-carbon (i.e., non-aromatic) components are present in black C produced at lower temperatures Citation[33]. Furthermore, this natural black C is typically cooled under aerobic conditions as well as exposed to environmental weathering (e.g., temperature cycling, precipitation and/or UV exposure). These factors can lead to potential oxidation of the char Citation[25]. Therefore, one would expect a spectrum of stability and properties in natural black C as a result of the range of temperatures that could even exist within a single thermal event. This temperature variability imparts a fluctuating chemical composition as a function of the range in production temperatures. In addition, the cooling of freshly created black C with water or precipitation (as is done in some open pit synthetic production methods), creates the opportunity of activating the hot char with water or steam, altering the surface chemistry groups of the black C post-production Citation[25].
Natural formation of black C has been hypothesized to be connected to the missing carbon in the global carbon cycle (1.4–1.6 Gton year-1) Citation[34]. There are examples of where black C has been located in sediments as well as anoxic marine environments, with carbon-dating placing this material at hundreds to thousands of years old Citation[2,8,9]. However, keeping in mind the variable temperatures of natural fire events and different geologic burial histories, one can see how black C with different stabilities could be produced in the same natural event. Furthermore, it has been suggested that the anaerobic geologic burial protects black C, and without this protection it would have been degraded Citation[35].
Therefore, in nature we are dealing with variable production conditions of the black C that is not a homogenous single feedstock, created over a range of temperatures and with various post-production cooling and storage conditions. These factors complicate applying kinetic degradation models to natural black C since the material is not homogeneous and possesses different geologic burial histories. Owing to these differences, natural black C might not be a suitable direct chemical analogue for synthetic black C, particularly when it comes to overall thermochemical stability Citation[36].
▪ Black carbon continuum
Hedges et al. presented quantifiable divisions in this combustion black C continuum based on the molar ratio of oxygen to carbon (O:C) as a means to classify carbon combustion residues Citation[37]. The most stable carbon form in this spectrum is graphite, typically formed under extreme conditions (high temperature and pressure); and often progressively formed through numerous intermediate phases as a consequence of geologic organic matter burial and digenesis Citation[26]. The main distinguishing chemical property of graphite is the lack of oxygen (typically non-detected or <0.5% by weight). This indicates nearly defect-free carbon lattice structures. Graphite and soot are in the groupings of condensate particulates, since these products contain no relic structures of the original biomass material and both have low O:C ratios . Conversely, charcoal and char do possess relic structures from the original biomass, and therefore are classified as combustion residues. The main boundary between these two groupings of thermal–chemical conversion products is an O:C ratio of 0.2 and then the dividing line between conversion products and biomass is 0.6 Citation[37]. Incidentally, cellulose has a O:C molar ratio of 0.8, glucose 1.0, and lignin approximately 0.3.
This concept of an infinite continuum is common in mineral geology. The black C continuum is analogous to the solid chemical solution series of the calcium-sodium plagioclase minerals (e.g., transition from high temperature, calcium-rich plagioclase to low temperature sodium-rich plagioclase) Citation[38]. If we were dealing with isolated closed systems, logically there would be more uniformity. However, the pyrolysis process is a partially open system and the chemical transformations are further complicated, since an equilibrium state in the reaction chamber is not reached with reactions being quenched (e.g., removal of heat or material) before equilibrium or reaction completion. Therefore, biochar is not a single chemical entity and should instead be thought of as part of the black C continuum in both variable structure and chemical composition. This biochar variability has complicated efforts to standardize the assessment of the stability of biochar in the soil system, as well as general black C properties Citation[30,39–41].
▪ Chemical composition variability
There is a large range of chemical composition variability among biochars as analyzed through proximate and ultimate analyses [Supplementary Table 1]. The ranges of chemical composition are the result of often uncontrolled and incomplete chemical and physical transformations that occur as a function of pyrolysis temperature, feedstock material, cooling and post-production conditions Citation[25,32]. Typically, synthetic biochar yields (expressed as a dry weight percentage of the starting material) decreases with increasing pyrolysis temperatures , with a preference towards gaseous and liquid products at higher temperatures Citation[49]. However, higher production temperature increases fixed carbon amounts in the biochar and typically reduces oxygen content in the biochar [Supplementary Table 1]. Similar results would be expected in the processes of natural black C formation Citation[33,50]. Owing to this dependency, pyrolysis temperature is often cited as a deterministic property for biochar stability Citation[51].
Biochar is not a description of a material with one distinct structure or chemical composition. Rather, biochar has a range of potential chemical compositions and physical structures. All biochars have differing degrees of aromatic linked sheet development (graphitic sheets) and irregularities in the rhombohedral crystalline network comprised of multiple defects including layer, carbon isomerism, substitutions and edge defects Citation[24]. Substitutions of H-, N-, and O- containing functional groups also increase black C reactivity, and, correspondingly, decrease stability Citation[52]. These mineralogical defects result in wider atomic sheet carbon-spacing, which further increases the reactivity of the resulting chemical structure. The presence of polyaromatic ring structures can develop during the initial phases of carbonization Citation[53,54]. However, total graphitization typically can only be completed under extreme temperatures (>1100°C) and pressures Citation[55]. Lower temperatures and pressures would require excessively long thermal treatment times to achieve graphitization (>10,000 years Citation[26]). Therefore, it has been proposed that a better conceptual model for synthetic black C is a heterogeneous mixture of heat-altered biopolymers of relatively small cluster size, which possess significant substitution of N-, O- and S- functional groups Citation[52,56], versus pure graphitic sheets.
The range of O:C molar ratios (0–0.6; [Supplementary data]) places biochar across the entire spectrum of black C . This is important to remember, since thermal and chemical stability is governed by the position on the black C continuum Citation[36,37], with lower O:C ratios resulting in a more stable carbon product. Since biochar spans the entire spectrum of black C , the name biochar does not convey any information on the position of the solid residual in the black C spectrum. Rather, the nomenclature of the black C continuum should be used since this will provide a more meaningful basis of comparison for the solid residuals, particularly across multiple studies. This also emphasizes the need for adequate biochar characterization to be conducted as part of any study.
illustrates the overall relationship between O:C and volatile matter for both the synthetic black C [Supplementary Table 1] and the natural black C as well as corresponding biomass analogues . A similar trend exists with good agreement between the synthetic and natural black carbons. Oxygen content also plays a major role in determining the overall biochar surface chemistry behavior, particularly for surface pH Citation[25], which can be an important driver for chemical reactions and thus degradation potential.
In summary, biochar covers the range of black C forms, from slightly charred (more easily degradable) to highly condensed refractory black carbon soot, with a corresponding range of chemistries. The main purpose for the creation of biochar is for carbon sequestration. Biochar does not refer to a single characteristic solid residual. These residuals are more properly identified by the divisions of the black C continuum.
Biochar stability in soil
Despite differences in analytical methodologies, which could limit comparisons Citation[57], current estimates of the amount of natural black C present in prairie and agricultural soils range from 10–45% Citation[58,59], 10–60% in forest soils Citation[60] and 3–15% in ocean sediments Citation[61]. The general consensus is that natural black C is relatively inert and thus contributes to refractory soil organic matter Citation[3,30] and, recently, black C sources have also been linked as precursors of soil humic acids Citation[62]. The natural black C particles observed today in the soil profile could be the more recalcitrant fraction of the black C produced, thereby potentially biasing the results towards the more recalcitrant fraction of the black C. Owing to various protection mechanisms (e.g., anaerobic burial and aggregate protection), exceptions exist where studies have also shown that the oldest black C in the soil profile is not the most stable Citation[63].
▪ Microbial degradation of biochar
It has been suggested that biochar (black C) itself cannot be used as a sole fungal food source, and is therefore resistant to microbial degradation Citation[64]. However, this is contrary to the overwhelming evidence that black C can be degraded in soils. The spectrum of biochar characteristics will directly impact the observed degradation potential and thereby influence the residence time of biochar in the soil system. Potential hypotheses explaining these effects have focused mainly on abiotic interactions (e.g., pH changes, bulk density decreases, alterations in nutrient availability, water retention increases, biochar structure, and soil structure alterations), providing additional microbial habitat, as well as promotion of colonization by arbuscular mycorrhizal fungi Citation[65,66]. Recent research has also indicated the potential role of volatiles (sorbed or produced; Box 1) in the suppression of microbial nitrification rates and other microbial processes Citation[67–72], which could also influence biotic biochar degradation rates. This is an important facet since most of the estimates on biochar stability have resulted from short-term laboratory incubations.
Microbial degradation of charcoal has been noted for some time Citation[73,74] along with recent observations of wood-decaying fungi capable of degrading (solubilizing) low-grade coals (e.g., lignite) Citation[75–77]. These observations would suggest the role of fungi in the biological degradation of biochars, with the primary pathway through extracellular enzymes. Fungal actions on other materials would suggest that the degradation could also be due to physical breakdown of the biochar structure, since this has been observed in brick and concrete deterioration, which can be initiated by fungal hyphae entering microscopic cracks and crevices, escalating mechanical cracking Citation[78–81]. In addition, freezing cycles have also been shown to disintegrate black C particles Citation[82]. This physical breakdown of biochar would be vital in the ability of extracellular enzymes attacking the surface area of the biochar Citation[83], since the molecules of the biochar are too large to pass through microbial membranes Citation[37].
Some of the initial work on the biotic degradation potential of black C was performed in the early 1900s. Potter observed that the liberation of CO2 from charcoal (biochar) was increased in the presence of a soil inoculums and did not occur at temperatures and conditions where microbial life would not be viable (e.g., 100°C and various antimicrobial inhibitor chemicals) Citation[73]. Therefore, the degradation potential of biochar by soil microorganisms has been established for over 100 years. Further work was performed by Shneour who used a 14C labeled char mixed with various soils (forest soil exposed to fire, volcanic soil from lava flow edge and an urban soil) Citation[74]. The results, as shown in , illustrate the differences in the observed degradation (accumulated 14C counts min-1 trapped in a sodium hydroxide trap for the produced CO2), which would be directly linked to the 14CO2 production from the charcoal degradation. After 96 days, there was an observed maximum degradation of 2% of the 14C labeled char, with significant differences observed for the different soils . From this data, the rates are clearly a function of the ecosystem and the composition of the soil microbial consortia. This has also been shown more recently, with a broad range of pyrolysis biochars, further illustrating the different dynamics and impacts of biochar additions on various ecosystems Citation[84].
There is wide variability in the estimation of the decay rates of biochar from various laboratory studies . However, not all biochars are the same as a result of differences in both parent material and conditions of the pyrolysis (e.g., oxygen concentration, temperature and/or duration) Citation[32]. There are a variety of laboratory methods used in these studies, with varying analytical quantifications (e.g., direct gas chromatography or alkaline traps) and conditions of incubations (e.g., incubation time, temperature and soil moisture content). However, the majority of the studies examined the production of CO2 as a surrogate to document degradation of the biochar occurring in the incubation. Some of the incubations used soil samples Citation[74,85], while others used microbial inoculums Citation[86] for the biotic component of the laboratory degradation. This CO2 production data is then typically evaluated using first-order degradation models to estimate the half-life of the biochar. There were limited studies that have utilized carbon isotopes Citation[40,87]. Provided there is a significant difference between the soil organic pool and the biochar, isotopic analyses allow us to track the source of the evolved CO2. However, we do not know if there is a preferential location of the carbon-label in the charred material (e.g., higher percentage in aromatic ring structures), which could impact these results for partially carbon-labeled biochar.
Extrapolating greater than decade-length half-lives from relatively short term incubations (<2 years) is unreliable. This has been shown in the literature on the microbial degradation of agrochemicals, which is an equivalent laboratory experimental protocol used for biochar degradation experiments. Anderson observed that the rate of degradation of various herbicides decreased drastically with time, and this decrease was directly correlated to the observed decrease in microbial biomass Citation[88]. This is analogous to the results observed in biochar degradation studies Citation[40,86]. In other words, the longer the incubation time, the lower the observed rate of mineralization. Microbial biomass typically decreases in the soil sample from the time of collection owing to the interaction of the environment (e.g., soil temperature and moisture and storage conditions) as well as the availability of nutrients Citation[89,90]. Not surprisingly, the rate of microbial degradation of agrochemicals is directly correlated to the microbial abundance Citation[89]. Therefore, the rates of soil microbial reactions extrapolated from long-term laboratory incubations are particularly prone to uncertainties owing to the fact that the population of the active microbial community decreases with time during laboratory incubations and consequentially reduces the rate of degradation, which is important to keep in mind for laboratory biochar incubations.
Laboratory incubations are also idealized conditions, where the other natural processes (e.g., climate variability, infiltration, ozone, UV exposure, freeze/thaw cycling or run-off events) are often not considered. Incubations are typically run at conditions that optimize microbial activity Citation[86]. Abiotic processes, such as chemisorption of oxygen by the biochar Citation[91–93], can also liberate and produce CO2. This is an important factor that needs to be accounted for when evaluating biochar degradation by quantifying CO2 production from biotic and abiotic sources Citation[85,86]. In addition, soil moisture content also influences biochar degradation rates Citation[51,94–97]. Depending on the pH of the black C, sorption of CO2 into the black C can occur, thereby reducing the amount of CO2 accumulation in laboratory incubations Citation[98]. Microbial communities, particularly fungi, are believed to be the dominate drivers in the mineralization of black C. These microbial communities will therefore be related to specific soil conditions since these microclimate conditions influence the activity and diversity of the microbial communities Citation[74,99]. However, these microbial consortia vary widely between ecosystems, resulting in different observed responses following biochar amendments, particularly for green house gas production potentials Citation[84].
For biochar, it appears that minimum residency times on decade time periods are achieved, with some results indicating longer residency times . However, the length of this residency time does vary considerably. We need to find an adequate characteristic property for black C (biochar) that explains this variability in stability and at the same time is straightforward to determine.
▪ Oxygen to carbon molar ratio
When we examine the O:C ratio in the biochar , a general trend is observed suggesting that lower O:C molar ratios result in a longer predicted biochar half-life. This is in agreement with recent observations that an increased O:C ratio is an indication of weathering (oxidation) of the biochar Citation[100,101] as well as other black C literature that correlate O:C to black C properties and stability Citation[37]. The O:C ratio is related to the number and composition of the substituted functional groups, since graphitic sheets would have a O:C of 0 . In particular, the recent biochar studies has also linked the O:C ratio to cation exchange capacity and presence of oxygenated groups on the biochar Citation[102]. O:C ratios are typically higher near the surface than the interior of the black C particles, resulting in the use of the O:C ratio as an indicator of black C oxidation Citation[103]. These findings are in agreement with the hypothesis that black C can react with oxygen Citation[92] and some have observed that these reactions occur can occur immediately after production Citation[104]. This illustrates the ability of the O:C molar ratio in capturing this post-production black C oxidation.
From , there is the suggestion that lower O:C ratios result in more stable biochar material. When the molar O:C ratio is greater than 0.6 then biochar will probably possess a half-life on the order of less than 100 years, with some studies observing decadal time periods. The next grouping is for a half-life of between 100–1000 years, corresponding to the biochar O:C ratio between 0.2 and 0.6. Finally, if the O:C ratio is under 0.2, the resulting biochar will possess a half-life of greater than 1000 years. The sole exception to this was the synthetic biochars from the Hamer et al. study (shown in the rectangle in )Citation[94]. The exact cause is unknown. These divisions and half-life predictions make logical sense when they are compared with the black C continuum since these are the divisions between biomass, combustion residues and combustion condensates Citation[36,37]. In particular, this screening criteria explains the lower residency times observed from the biochars with high O:C ratios (near 0.6), which are close to biomass in the black C continuum. These groupings also explain the high degree of variability observed in biochar stability results in light of the divisions within the black C continuum, with some studies observing very short residence times for high O:C ratio biochar and other studies observing longer stability estimates for lower O:C ratio biochar.
Pyrolysis temperature has been suggested as a surrogate for biochar stability Citation[51]. The O:C molar ratio of the various biochars listed in is a function of the production temperature (R2 = 0.595) . However, the overall correlation is weak. As can be seen in , there are some biochars that meet the highest stability criteria (O:C <0.2) across a range of production temperatures (300–1000°C). This variability indicates that production temperature is not the sole determining factor in the stability of biochar. In addition to production temperature, the O:C molar ratio would also account for alterations that occur as a function of the cooling method used (i.e., anaerobic, aerobic and water), different heating rates and non-uniform heating, residency times, and storage time of the biochar owing to abiotic degradation Citation[86,92,104]. These factors explain the poor relationship with production temperature and suggest that production temperature is not the sole determining factor of biochar stability. Furthermore, these and other factors would be encapsulated by the O:C ratio, thereby suggesting that the O:C ratio would be a superior classification scheme than production temperature for estimation of the stability of biochars.
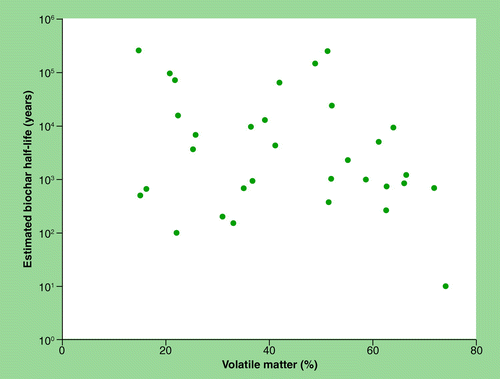
Volatile matter has also been suggested as a predictor for biochar stability Citation[86]. In this study, the relationship between volatile matter and biochar stability was statistically significant (R2 = 0.35–0.44 Citation[86]) for the chars produced, which were all from the same pyrolysis unit. When we compare the volatile matter to the O:C ratio across multiple biochar sources, we again observe variability in the predictability; as was seen for the production temperature across various O:C ratios . This variability increases with volatile content. When we compare the volatile matter to biochar stability , we do not observe any clear divisions as was observed for the O:C ratio . In addition, there is no literature reference for the divisions in the volatile matter content, as there are for the O:C ratio Citation[37]. However, the relationship between the O:C ratio and the volatile matter (n = 50; R2 = 0.76; ) is slightly stronger than the O:C and production temperature (R2 = 0.60; ). The reason for this improved agreement could be related to the fact that both of these quantities (volatile matter and O:C) are actual measurements of the black C material. However, volatiles can be desorbed from the black C at anytime and would be dependent on the pyrolysis conditions and time since production. Therefore, the timing of the volatile laboratory analysis and conditions of storage could be important factors in this variability. Furthermore, there are analytical methodology differences (e.g., in temperatures and duration) in the determination of volatile content among various biochar and black C studies, which hampers comparisons. Volatile matter is not a stationary property and does not necessarily correlate to the chemical composition or structure of the biochar material itself. However, volatile matter is a vital property for laboratory incubations, since these volatiles can have an impact on short-term microbial activity Citation[71,72], and is a good indicator for biochars produced from the same pyrolysis unit or process Citation[86]. The O:C ratio would account for volatiles as well as the non-volatile material, which could provide an improved basis for comparison, particularly across multiple pyrolysis units and conditions of production and storage.
A linked two-pool kinetic model for the liable and recalcitrant fractions of biochar has also been proposed Citation[105]. This concept fits some, if not the majority, of the experimental data. However, in reality we have an infinite number of pools, since the biochar is a spectrum of chemical structures and arrangements Citation[106,107]. Therefore, a model accounting for the liable and then the more stable fraction is a drastic simplification of the continuum of fragment stabilities within the heterogeneous biochar. Furthermore, there is no direct assessment of these kinetic parameters, except by fitting experimental data. The O:C ratio provides a direct comparison of a relatively simple and direct analysis, which permits black C to be compared from different studies.
There are upcoming innovations in examining the structure of black C (biochar) Citation[103,108–111]. These methods do provide additional insights into the atomic structure and arrangement of black C, which elucidate a detailed assessment of structural black C properties. These analytical techniques will improve our understanding and the implications of chemical structure on black C stability. However, there are limited studies to date that have utilized these more sophisticated structural analysis tools to draw any universal relationships.
Future perspective
This article highlights the lack of suitable terminology and standards in biochar stability research, particularly in relationship to microbial stability. Owing to the fact that microbial/fungal diversity differ across geographical locations, it will be necessary to develop a protocol for finding a proxy for microbial degradation for biochar stability in soils. One such possibility is a chemical–thermal stability evaluation that could be a proxy for determining both biotic and abiotic degradation potential Citation[36]. Otherwise, a standard mixture of microbial enzymes or cultures would be needed to ensure equal comparisons across laboratories Citation[112]. Furthermore, the particle size of the biochar needs to be included in the assessment of stability Citation[113]. Since the biologic degradation of biochar relies on the activity of extracellular enzymes Citation[37], smaller particle size could lead to higher activity of these enzymes. Furthermore, the effect of temperature fluctuations is another area that will need to be examined, particularly as a result of the impacts on fungal establishment and growth Citation[63]. As already mentioned in some studies Citation[85,114], the impact of abiotic degradation will need to be accounted for. In this fashion, consistent results for the predicted stability of the biochar will be achieved. The use of the O:C molar ratio is a step in this direction. However, further refinement and enhancement is needed in order to establish common analytical procedures to ensure reproducible results across laboratories in determining the microbial stability of biochar.
Conclusions
In conclusion, the degradation of biochar in the natural system is currently lacking fundamental scientific data to assign exact turnover rates. Our ability to predict the effects of long-term carbon sequestration from biochar additions to soil is currently limited by our lack of understanding in how biochar affects the soil microbial community mediating many of these processes. Compounding this issue is the variability of biomass combustion products as a function of the production conditions and parent material. Abiotic processes (e.g., chemisorption, transport and leaching) also cannot be neglected Citation[115].
This article has shown that a more reliable predictor of overall stability of biochar in soils might be the O:C molar ratio. This ratio is the net result of all of the multiple parameters during the production, cooling and storage of the biochar. Furthermore, the O:C ratio has explained the variability observed in the majority of the data to date that had sufficient characterization data on the evaluated biochar. Since all studies did not analyze the elemental composition, the universality of this conclusion still needs to be verified. Based on the literature studies examined in this article, biochar with an O:C molar ratio of less than 0.2 are typically the most stable, possessing an estimated half-life of more than 1000 years; biochar with an O:C ratio of 0.2–0.6 have intermediate half-lives (100–1000 years); and, finally biochar with an O:C ratio of greater than 0.6 possess a half-life in the order of over 100 years.
Table 1. Chemical composition of biomass and natural black carbon products.
Table 2. Summary of the residency times of biochar in various studies along with corresponding oxygen to carbon (O:C) ratio of the biochar.
▪ The role of soil volatile organic compounds (‘soil volatilomics’) is an active research area Citation[129]. These organic compounds can have stimulatory, inhibitory or no impact on microbial growth Citation[70,129]. As seen in , some biochars have high volatile matter contents. The source of these volatiles is not entirely known, but could be the result of sorbing the volatile organic compounds and other non- to semi-volatile organic compounds that are produced during pyrolysis Citation[130] or from direct contact with the bio-oil (liquid) Citation[131] and gas phases Citation[132]. Guillén and Manzanos have identified over 215 volatile species present in aqueous hardwood smoke Citation[68]. The water soluble portion of wood smoke has been shown to stimulate CO2 respiration and the associated increase in oxygen depletion in laboratory incubations, which have been linked to increases in microbial activity Citation[67,83]. This indicates that some portion of the volatile organics present in the wood smoke can be utilized by the soil microbial community as a food source. | |||||
▪ During the pyrolysis of cellulose, furans, pyranones, anhydrosugars and 5-hydroxymethylfurfural were the main components of black C produced at low-temperature pyrolysis (<300°C) Citation[133]. The role of these sorbed volatile organics on biochar has just recently been cited as a mechanistic factor in the response of soil microbes Citation[71,72] and plant responses Citation[134]. presents thermal desorption data from four different biochars, natural coal and a wood ash (aerobic combustion) analyzed by headspace thermal desorption mass-spectrometry, which is an analytical technique for the analysis of sorbed organic compounds to charcoal Citation[135], and when coupled to a mass-spectrometer allows identification of the volatile compounds Citation[136]. This technique was used here to visualize the types of organic compounds released when the material (e.g., biochar, coal or wood ash) is heated to 150°C (Perkin Elmer Gas Chromatograph [Clarus 600]; Agilent headspace sampler [7694E]; RTX-624 column [Restek ; 0.32 mm x 50 m] column with mass spectrometer [Perkin Elmer 600T] detection). As seen in , there are multiple types and classes of compounds observed on the biochars. Each peak on the chromatogram represents a unique compound, with smaller more volatile species eluting earlier and heavier molecular weight compounds eluting at later times. From this very limited data, there is the suggestion that fast pyrolysis produces more aromatic and higher molecular weight species (elution time >20 min) sorbed to the biochar, as can be seen by the presence of ethyl benzenes (and other aromatic species peaks) in the fast pyrolysis biochars. The main conclusion is that biochar also contains varying amounts and types of volatile organic compounds as a function of feedstock and pyrolysis conditions, which requires further investigation. | |||||
The name given to charred carbon, which comprises a continuum of various thermal–chemical conversion (pyrolysis) products, ranging from graphite, soot, char and charcoal . Incidentally, this term does include parent material from all biomass and fossil fuel sources, since we do get black carbon formation from the combustion of biomass as well as fossil fuels (e.g., coal, diesel and gasoline).
Forms of black carbon that contain no relic structures of the parent biomass material. Typically, these forms of black carbon have oxygen to carbon ratios (O:C) of less than 0.2, comprised of soot and graphite.
Forms of black carbon that contain relic structures of the parent biomass. Typical oxygen to carbon ratios (O:C) are between 0.2 and 0.6, which are called char or charcoal, depending on the position in the black carbon spectrum.
The name applied to the creation of black carbon (solid residual from the pyrolysis of biomass) for the purpose of carbon sequestration. Furthermore, the source of the original biomass for the pyrolysis is solely from renewable sources and not fossil fuel resources.
▪ Biochar is the solid residual product (black carbon [black C]) from pyrolysis that is created as a means of carbon sequestration. | |||||
Differences in natural and synthetic black C
▪ Significant differences between the purity of feedstock, temperature of production, and conditions of cooling can result in drastically different phase distributions and quality between natural and synthetic black C production. | |||||
Black C continuum
▪ The black C continuum describes various products of the thermochemical conversion of biomass. Biochar spans this entire spectrum of black C products. | |||||
▪ The oxygen to carbon (O:C) molar ratio is a characteristic property of black C that can be used to classify black C material and, correspondingly, the stability of the carbonized material. | |||||
Chemical composition variability
▪ Biochars possess a diverse range of chemical structure and compositions. | |||||
Biochar stability in soil
▪ There has been a wide range of predicted stabilities of biochar in the soil system ranging from decades to millennia, with limited attempts to unify these results. | |||||
Microbial degradation of black C
▪ There has been over a 100-year history of examining the stability of black C in soils, with limited success in developing an accurate predictor of the stability of black C in the soil environment. | |||||
▪ Biochar with an O:C molar ratio of less than 0.2 are typically the most stable black C forms possessing an estimated half-life of more than 1000 years; biochar with an O:C ratio of 0.2–0.6 have intermediate half-lives (100–1000 years); and, finally, biochar with an O:C ratio of greater than 0.6 possess a half-life on the order of less than 100 years. | |||||
tcmt_a_10816161_sm0001.doc
Download MS Word (185 KB)Financial & competing interests’ disclosure
This article was prepared by a US Department of Agriculture –Agricultural Research Service (USDA-ARS) employee as part of his official duties and is part of the USDA-ARS Biochar and Pyrolysis Initiative. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- Clark JS, North Atlantic Treaty Organization. Scientific Affairs Division. Sediment Records of Biomass Burning and Global Change. Springer, Berlin, New York (1997).
- Fowles M. Black carbon sequestration as an alternative to bioenergy. Biomass and Bioenergy31(6),426–432 (2007).
- Goldberg ED. Black Carbon in the Environment: Properties and Distribution (1985).
- Glaser B, Lehmann J, Zech W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal – a review. Biol. Fertil. Soils35(4),219–230 (2002).
- Laird DA. The charcoal vision: a win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J.100(1),178–181 (2008).
- Lehmann J. Bio-energy in the black. Front Ecol. Environ.5(7),381–387 (2007).
- Kuhlbusch TAJ, Crutzen PJ. Toward a global estimate of black carbon in residues of vegetation fires representing a sink of atmospheric CO2 and a source of O2. Global Biogeochem. Cycles9(4),491–501 (1995).
- Haberstroh PR, Brandes JA, Gélinas Y, Dickens AF, Wirick S, Cody G. Chemical composition of the graphitic black carbon fraction in riverine and marine sediments at sub-micron scales using carbon x-ray spectromicroscopy. Geochimica et Cosmochimica Acta70(6),1483–1494 (2006).
- Kuhlbusch TAJ, Zepp RG, Miller WL, Burke RA. Carbon monoxide fluxes of different soil layers in upland Canadian boreal forests. Tellus Series B-Chemical and Physical Meteorology50(4),353–365 (1998).
- Masiello CA, Druffel ER, nbsp, M. Black carbon in deep-sea sediments. Science280(5371),1911–1913 (1998).
- Masiello CA, Druffel ERM, Currie LA. Radiocarbon measurements of black carbon in aerosols and ocean sediments. Geochim. Cosmochim. Acta66(6),1025–1036 (2002).
- Bridgwater AV. Catalysis in thermal biomass conversion. Appl. Catal. A Gen.116(1–2),5–47 (1994).
- Bridgwater AV. Production of high grade fuels and chemicals from catalytic pyrolysis of biomass. Catal. Today29(1–4),285–295 (1996).
- Bridgwater AV, Peacocke GVC. Fast pyrolysis processes for biomass. Renew. Sust. Energy Rev.4(1),1–73 (2000).
- Ouml;zçimen D, Karaosmanoglu F. Production and characterization of bio-oil and biochar from rapeseed cake. Renew. Energy29(5),779–787 (2004).
- Bridgwater AV. Renewable fuels and chemicals by thermal processing of biomass. Chem. Engin. J.91(2–3),87–102 (2003).
- Abdullah H, Wu HW. Biochar as a fuel: 1. properties and grindability of biochars produced from the pyrolysis of mallee wood under slow-heating conditions. Energy Fuels23(8),4174–4181 (2009).
- Küçük MM, Demirbas A. Biomass conversion processes. Energ. Convers. Manag.38(2),151–165 (1997).
- Inyang M, Gao B, Pullammanappallil P, Ding WC, Zimmerman AR. Biochar from anaerobically digested sugarcane bagasse. Bioresour. Technol.101(22),8868–8872 (2010).
- Cao N, Darmstadt H, Soutric F, Roy C. Thermogravimetric study on the steam activation of charcoals obtained by vacuum and atmospheric pyrolysis of softwood bark residues. Carbon40(4),471–479 (2002).
- KütahyalI C, Eral M. Selective adsorption of uranium from aqueous solutions using activated carbon prepared from charcoal by chemical activation. Separ. Purif. Tech.40(2),109–114 (2004).
- Shafizadeh F. Pyrolysis and combustion of cellulosic materials. In: Advances in Carbohydrate Chemistry. Wolfrom ML (Ed.). Academic Press Inc., New York, USA, 419–474 (1968).
- Alexis MA, Rumpel C, Knicker H et al. Thermal alteration of organic matter during a shrubland fire: a field study. Org. Geochem.41(7),690–697 (2010).
- Ubbelohde AR, Lewis FA. Graphite and its Crystal Compounds. Clarendon Press, Oxford, UK (1960).
- Boehm HP. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon32(5),759–769 (1994).
- Bonijoly M, Oberlin M, Oberlin A. A possible mechanism for natural graphite formation. Int. J. Coal Geol.1(4),283–312 (1982).
- Baker RTK, Harris PS, Terry S. Unique form of filamentous carbon. Nature253(5486),37–39 (1975).
- Raveendran K, Ganesh A, Khilar KC. Pyrolysis characteristics of biomass and biomass components. Fuel75(8),987–998 (1996).
- Neeft JPA, Makkee M, Moulijn JA. Catalytic oxidation of carbon black – I. Activity of catalysts and classification of oxidation profiles. Fuel77(3),111–119 (1998).
- Schmidt MWI, Noack AG. Black carbon in soils and sediments: analysis, distribution, implications, and current challenges. Global Biogeochem. Cy.14(3),777–793 (2000).
- Pieri DC, Baloga SM. Eruption rate, area, and length relationships for some Hawaiian lava flows. J. Volcanol. Geoth. Res.30(1–2),29–45 (1986).
- Antal MJ, Grønli M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res.42(8),1619–1640 (2003).
- Knicker H, Totsche KU, Almendros G, Gonzalez-Vila FJ. Condensation degree of burnt peat and plant residues and the reliability of solid-state VACP MAS C-13 NMR spectra obtained from pyrogenic humic material. Org. Geochem.36(10),1359–1377 (2005).
- Shrestha G, Traina S, Swanston C. Black carbon’s properties and role in the environment: a comprehensive review. Sustainability2(1),294–320 (2010).
- Gonzalez-Perez JA, Gonzalez-Vila FJ, Almendros G, Knicker H. The effect of fire on soil organic matter – a review. Environment International30(6),855–870 (2004).
- Elmquist M, Cornelissen G, Kukulska Z, Gustafsson Ö. Distinct oxidative stabilities of char versus soot black carbon: implications for quantification and environmental recalcitrance. Global Biogeochem. Cy.20(2),GB2009 (2006).
- Hedges JI, Eglinton G, Hatcher PG et al. The molecularly-uncharacterized component of nonliving organic matter in natural environments. Org. Geochem.31(10),945–958 (2000).
- Ghent ED. Plagioclase-garnet-Al 2 SiO 5 -quartz; a potential geobarometer-geothermometer. Am. Mineral.61(7–8),710–714 (1976).
- Masiello CA. New directions in black carbon organic geochemistry. Mar. Chem.92(1–4),201–213 (2004).
- Bruun S, Jensen ES, Jensen LS. Microbial mineralization and assimilation of black carbon: Dependency on degree of thermal alteration. Org. Geochem.39(7),839–845 (2008).
- Schmidt MWI, Skjemstad JO, Czimczik CI et al. Comparative analysis of black carbon in soils. Global Biogeochem. Cy.15(1),163–167 (2001).
- Rocca PAD, Cerrella EG, Bonelli PR, Cukierman AL. Pyrolysis of hardwoods residues: on kinetics and chars characterization. Biomass Bioenergy16(1),79–88 (1999).
- Chen B, Zhou D, Zhu L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol.42(14),5137–5143 (2008).
- Karaosmanoglu F, Isigigur-Ergundenler A, Sever A. Biochar from the straw-stalk of rapeseed plant. Energy Fuels14(2),336–339 (2000).
- Ozcimen D, Karaosmanoglu F. Production and characterization of bio-oil and biochar from rapeseed cake. Renew. Energ.29(5),779–787 (2004).
- Encinar JM, Beltrán FJ, Bernalte A, Ramiro A, González JF. Pyrolysis of two agricultural residues: olive and grape bagasse. Influence of particle size and temperature. Biomass Bioenergy11(5),397–409 (1996).
- Kawamoto K, Ishimaru K, Imamura Y. Reactivity of wood charcoal with ozone. Japan Wood Research Society51,66–72 (2005).
- Shindo H. Elemental composition, humus composition, and decomposition in soils of charred grassland plants. Soil Sci. Plant Nutr.37,651–657 (1991).
- Bridgwater AV, Meier D, Radlein D. An overview of fast pyrolysis of biomass. Org. Geochem.30(12),1479–1493 (1999).
- Knicker H, Gonzalez-Vila FJ, Polvillo O, Gonzalez JA, Almendros G. Fire-induced transformation of C- and N-forms in different organic soil fractions from a Dystric Cambisol under a Mediterranean pine forest (Pinus pinaster). Soil Biol. Biochem.37(4),701–718 (2005).
- Baldock JA, Smernik RJ. Chemical composition and bioavailability of thermally, altered Pinus resinosa (Red Pine) wood. Org. Geochem.33(9),1093–1109 (2002).
- Knicker H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochem.85(1),91–118 (2007).
- Villey M, Oberlin A, Combaz A. Influence of elemental composition on carbonization pyrolysis of sporopollenin and lignite as models of kerogens. Carbon17(1),77–86 (1979).
- Oberlin A, Villey M, Combaz A. Influence of elemental composition on carbonization: pyrolysis of kerosene shale and kuckersite. Carbon18(5),347–353 (1980).
- Robertson SD. Graphite formation from low temperature pyrolysis of methane over some transition metal surfaces. Nature221(5185),1044–1046 (1969).
- Knicker H, Hilscher A, Gonzalez-Vila FJ, Almendros G. A new conceptual model for the structural properties of char produced during vegetation fires. Org. Geochem.39(8),935–939 (2008).
- Hammes K, Schmidt MWI, Smernik RJ et al. Comparison of quantification methods to measure fire-derived (black/elemental) carbon in soils and sediments using reference materials from soil, water, sediment and the atmosphere. Global Biogeochem. Cy.21(3) (2007).
- Schmidt MWI, Skjemstad JO, Gehrt E, Kogel-Knabner I. Charred organic carbon in German chernozemic soils. Eur. J. Soil Sci.50(2),351–365 (1999).
- Skjemstad JO, Reicosky DC, Wilts AR, McGowan JA. Charcoal Carbon in U.S. agricultural soils. Soil Sci. Soc. Am. J.66(4),1249–1255 (2002).
- Ponomarenko EV, Anderson DW. Importance of charred organic matter in Black Chernozem soils of Saskatchewan. Can. J. Soil Sci.81(3),285–297 (2001).
- Gustafsson Ö, Gschwend PM. the flux of black carbon to surface sediments on the new england continental shelf. Geochim. Cosmochim. Acta62(3),465–472 (1998).
- Haumaier L, Zech W. Black carbon – possible source of highly aromatic components of soil humic acids. Org. Geochem.23(3),191–196 (1995).
- Krull ES, Swanston CW, Skjemstad JO, McGowan JA. Importance of charcoal in determining the age and chemistry of organic carbon in surface soils. J. Geophys. Res. Biogeosci.111(G4) (2006).
- Ascough PL, Sturrock CJ, Bird MI. Investigation of growth responses in saprophytic fungi to charred biomass. Isot. Environ. Health Stud.46(1),64–77 (2010).
- Warnock DD, Lehmann J, Kuyper TW, Rillig MC. Mycorrhizal responses to biochar in soil – concepts and mechanisms. Plant Soil300,9–20 (2007).
- Zackrisson O, Nilsson M-C, Wardle DA. Key ecological function of charcoal from wildfire in the boreal forest. Oikos77(1),10–19 (1996).
- Focht U. The effect of smoke from charcoal kilns on soil respiration. Environ. Monit. and Assess.59(1),73–80 (1999).
- Guillén MD, Manzanos MJ. Study of the volatile composition of an aqueous oak smoke preparation. Food Chem.79(3),283–292 (2002).
- Asita AO, Matsui M, Nohmi T et al. Mutagenicity of wood smoke condensates in the Salmonella/microsome assay. Mutat. Res. Lett.264(1),7–14 (1991).
- Asita AO, Campbell IA. Anti-microbial activity of smoke from different woods. Lett. Appl. Microbiol.10(2),93–95 (1990).
- Spokas KA, Baker JM, Reicosky DC. Ethylene: potential key for biochar amendment impacts. Plant Soil333(1–2),443–452 (2010).
- Clough TJ, Bertram JE, Ray JL et al. Unweathered wood biochar impact on nitrous oxide emissions from a bovine-urine-amended pasture soil. Soil Sci. Soc. Am. J.74(3),852–860 (2010).
- Potter MC. Bacteria as agents in the oxidation of amorphous carbon. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character80(539),239–259 (1908).
- Shneour EA. Oxidation of graphitic carbon in certain soils. Science151(3713),991–992 (1966).
- Fakoussa RM, Hofrichter M. Biotechnology and microbiology of coal degradation. Appl. Microbiol. Biotechnol.52(1),25–40 (1999).
- Hölker U, Fakoussa RM, Höfer M. Growth substrates control the ability of Fusarium oxysporum to solubilize low-rank coal. Appl.Microbiol. Biotechnol.44(3),351–355 (1995).
- Hofrichter M, Fritsche W. Depolymerization of low-rank coal by extracellular fungal enzyme systems. Appl. Microbiol. Biotechnol.46(3),220–225 (1996).
- Hofrichter M, Bublitz F, Fritsche W. Fungal attack on coal: I. Modification of hard coal by fungi. Fuel Process. Tech.52(1–3),43–53 (1997).
- Burford EP, Fomina M, Gadd GM. Fungal involvement in bioweathering and biotransformation of rocks and minerals. Mineral. Mag.67(6),1127–1155 (2003).
- Fomina M, Podgorsky VS, Olishevska SV et al. Fungal deterioration of barrier concrete used in nuclear waste disposal. Geomicrobiol. J.24(7),643 (2007).
- Sand W. Microbial mechanisms of deterioration of inorganic substrates – a general mechanistic overview. Int. Biodeterior. Biodegradation40(2–4),183–190 (1997).
- Carcaillet C. Are Holocene wood-charcoal fragments stratified in alpine and subalpine soils? Evidence from the Alps based on AMS 14C dates. Holocene11(2),231–242 (2001).
- Willmann G, Fakoussa R. Extracellular oxidative enzymes of coal-attacking fungi. Fuel Process. Tech.52(1–3),27–41 (1997).
- Spokas K, Reicosky D. Impacts of sixteen different biochars on soil greenhouse gas production. Ann. Environ. Sci.3,15 (2009).
- Spokas KA, Koskinen WC, Baker JM, Reicosky DC. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere77(4),574–581 (2009).
- Zimmerman AR. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci. Tech.44(4),1295–1301 (2010).
- Steinbeiss S, Gleixner G, Antonietti M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem.41(6),1301–1310 (2009).
- Anderson JPE. Soil moisture and the rates of biodegradation of diallate and triallate. Soil Biol. Biochem.13(2),155–161 (1981).
- Anderson JPE. Herbicide degradation in soil: influence of microbial biomass. Soil Biol. Biochem.16(5),483–489 (1984).
- Wallenius K, Rita H, Simpanen S, Mikkonen A, Niemi RM. Sample storage for soil enzyme activity and bacterial community profiles. J. Microbiol. Methods81(1),48–55 (2010).
- Allardice DJ. The processes involved in the adsorption of oxygen on brown coal char. Carbon3(2),215–218 (1965).
- Allardice DJ. The adsorption of oxygen on brown coal char. Carbon4(2),255–262 (1966).
- Fujitsu H, Mochida I, Verheyen TV, Perry GJ, Allardice DJ. The influence of modifications to the surface groups of brown coal chars on their flue gas cleaning ability. Fuel72(1),109–113 (1993).
- Hamer U, Marschner B, Brodowski S, Amelung W. Interactive priming of black carbon and glucose mineralisation. Org. Geochem.35(7),823–830 (2004).
- Nguyen BT, Lehmann J, Kinyangi J, Smernik R, Riha SJ, Engelhard MH. Long-term black carbon dynamics in cultivated soil. Biogeochem.89(3),295–308 (2008).
- Middelburg JJ, Nieuwenhuize J, van Breugel P. Black carbon in marine sediments. Marine Chem.65(3–4),245–252 (1999).
- Neff JC, Harden JW, Gleixner G. Fire effects on soil organic matter content, composition, and nutrients in boreal interior Alaska. Can. J. Forest Res.35(9),2178–2187 (2005).
- Arenillas A, Smith KM, Drage TC, Snape CE. CO2 capture using some fly ash-derived carbon materials. Fuel84(17),2204–2210 (2005).
- Harden JW, Neff JC, Sandberg DV et al. Chemistry of burning the forest floor during the FROSTFIRE experimental burn, interior Alaska, 1999. Global Biogeochem. Cy.18(3) (2004).
- Bird MI, Moyo C, Veenendaal EM, Lloyd J, Frost P. Stability of elemental carbon in a savanna soil. Global Biogeochem. Cy.13(4),923–932 (1999).
- Cheng CH, Lehmann J, Engelhard MH. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta72,1598–1610 (2008).
- Lee JW, Kidder M, Evans BR et al. Characterization of Biochars Produced from Cornstovers for Soil Amendment. Environ. Sci. Tech.44(20),7970–7974 (2010).
- Brodowski S, Rodionov A, Haumaier L, Glaser B, Amelung W. Revised black carbon assessment using benzene polycarboxylic acids. Org. Geochem.36(9),1299–1310 (2005).
- Cheng CH, Lehmann J, Thies JE, Burton SD, Engelhard MH. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem.37(11),1477–1488 (2006).
- Liang B, Lehmann J, Solomon D et al. Stability of biomass-derived black carbon in soils. Geochim. Cosmochim. Acta72(24),6069–6078 (2008).
- Brewer CE, Schmidt-Rohr K, Satrio JA, Brown RC. Characterization of Biochar from Fast Pyrolysis and Gasification Systems. Environ. Progress Sustain. Energy28(3),386–396 (2009).
- Keiluweit M, Nico PS, Johnson MG, Kleber M. Dynamic molecular structure of plant biomass-derived black carbon (Biochar). Environ. Sci. Tech.44(4),1247–1253 (2010).
- Knicker H, Almendros G, Gonzalez-Vila FJ, Gonzalez-Perez JA, Polvillo O. Characteristic alterations of quantity and quality of soil organic matter caused by forest fires in continental Mediterranean ecosystems: a solid-state C-13 NMR study. Eur. J. Soil Sci.57(4),558–569 (2006).
- Ascough PL, Bird MI, Scott AC et al. Charcoal reflectance measurements: implications for structural characterization and assessment of diagenetic alteration. J. Archaeol. Sci.37(7),1590–1599 (2010).
- Fang XW, Chua T, Schmidt-Rohr K, Thompson ML. Quantitative C-13 NMR of whole and fractionated Iowa Mollisols for assessment of organic matter composition. Geochim. Cosmochim. Acta74(2),584–598 (2010).
- Kaal J, Rumpel C. Can pyrolysis-GC/MS be used to estimate the degree of thermal alteration of black carbon?. Org. Geochem.40(12),1179–1187 (2009).
- Hammes K, Torn MS, Lapenas AG, Schmidt MWI. Centennial black carbon turnover observed in a Russian steppe soil. Biogeosci.5(5),1339–1350 (2008).
- Nocentini C, Certini G, Knicker H, Francioso O, Rumpel C. Nature and reactivity of charcoal produced and added to soil during wildfire are particle-size dependent. Organic Geochem.41(7),682–689 (2010).
- Novak JM, Busscher WJ, Watts DW, Laird DA, Ahmedna MA, Niandou MAS. Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma154(3–4),281–288 (2010).
- Major J, Lehmann J, Rondon M, Goodale C. Fate of soil-applied black carbon: downward migration, leaching and soil respiration. Global Chang. Biol.16(4),1366–1379 (2010).
- Ghetti P. DTG combustion behaviour of coal: Correlations with proximate and ultimate analysis data. Fuel65(5),636–639 (1986).
- Merrick D. Mathematical models of the thermal decomposition of coal: 1. The evolution of volatile matter. Fuel62(5),534–539 (1983).
- Gaur S, Reed TB. Thermal Data for Natural and Synthetic Fuels. Marcel Dekker, NY, USA (1998).
- Nguyen BT, Lehmann J. Black carbon decomposition under varying water regimes. Org. Geochem.40(8),846–853 (2009).
- Steinbeiss S, Gleixner G, Antonietti M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem.41(6),1301–1310 (2009).
- Harden JW, Trumbore SE, Stocks BJ et al. The role of fire in the boreal carbon budget. Global Chang. Biol.6,174–184 (2000).
- Swift RS. Sequestration of carbon by soils. Soil Sci.166,858–871 (2001).
- Forbes MS, Raison RJ, Skjemstad JO. Formation, transformation and transport of black carbon (charcoal) in terrestrial and aquatic ecosystems. Sci. Total Environ.370(1),190–206 (2006).
- Kuzyakov Y, Subbotina I, Chen HQ, Bogomolova I, Xu XL. Black carbon decomposition and incorporation into soil microbial biomass estimated by C-14 labeling. Soil Biol. Biochem.41(2),210–219 (2009).
- Hilscher A, Heister K, Siewert C, Knicker H. Mineralisation and structural changes during the initial phase of microbial degradation of pyrogenic plant residues in soil. Org. Geochem.40(3),332–342 (2009).
- Williams PT, Nugranad N. Comparison of products from the pyrolysis and catalytic pyrolysis of rice husks. Energy25(6),493–513 (2000).
- González JF, Ramiro A, González-García CM et al. Pyrolysis of Almond Shells. Energy Applications of Fractions. Ind. Eng. Chem. Res.44(9),3003–3012 (2005).
- Williams PT, Besler S. The influence of temperature and heating rate on the slow pyrolysis of biomass. Renew. Energ.7(3),233–250 (1996).
- Insam H, Seewald M. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils46(3),199–213 (2010).
- Mara dos Santos Barbosa J, Ré-Poppi N, Santiago-Silva M. Polycyclic aromatic hydrocarbons from wood pyrolyis in charcoal production furnaces. Environ. Res.101(3),304–311 (2006).
- Sipilä K, Kuoppala E, Fagernäs L, Oasmaa A. Characterization of biomass-based flash pyrolysis oils. Biomass Bioenergy14(2),103–113 (1998).
- Zeng L, Qin C, Wang L, Li W. Volatile compounds formed from the pyrolysis of chitosan. Carbohydrate Polymers DOI:10.1016/j.carbpol.2010.10.007 (2010) (Epub ahead of print).
- McGrath TE, Chan WG, Hajaligol MR. Low temperature mechanism for the formation of polycyclic aromatic hydrocarbons from the pyrolysis of cellulose. J. Anal. Appl. Pyrol.66(1–2),51–70 (2003).
- Deenik JL, McClellan T, Uehara G, Antal MJ, Campbell S. Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J.74(4),1259–1270 (2010).
- Gan J, Yates SR, Spencer WF, Yates MV. Automated headspace analysis of fumigants 1,3-dichloropropene and methyl isothiocyanate on charcoal sampling tubes. J. Chromatog. A684(1),121–131 (1994).
- Kusch P, Knupp G. Headspace-SPME–GC–MS identification of volatile organic compounds released from expanded polystyrene. J. Polymers Environ.12(2),83–87 (2004).
▪ Websites
- The history of charcoal production. Grower Charcoal and Wood Company. www.gowercharcoal.co.uk/pages/charcoalproduction.php
- How Products Are Made – Volume 4: Charcoal Briquette. www.madehow.com/Volume-4/Charcoal-Briquette.html