Abstract
Purification of solvents is required to maintain quality and absorption capacity for better performance and economics for CO2 capture plants. An effective technique is needed to separate degradation products from their parent amines to prevent operational problems such as corrosion, foaming, fouling and change of solvent physicochemical properties. To overcome these problems, an amine reclamation process is used to clean up the solvent. Over 30 years ago, only five amine clean-up methods – solvent changeover, solvent purging/feeding, mechanical filtration, activated carbon filtration and neutralization of organic/inorganic acids – existed, which are reviewed in this article. More specific and advanced approaches developed later to achieve more effective removal of impurities and degradation products from the solvents are also summarized. This article also gives future trends for reclamation techniques in amine-based CO2 capture processes including hybrid processes or improvement of the current technologies such as extraction, ion exchange, thermal distillation and electrodialysis.
Reproduced with permission from Citation[55].
![Figure 1. Typical post-combustion CO2 capture process.Reproduced with permission from Citation[55].](/cms/asset/a50150b1-276c-4446-a775-1da2c8cdf779/tcmt_a_10816304_f0001.gif)
Reproduced with permission from Citation[204].
![Figure 2. Process flow diagram of AmiPur® amine reclaimer.Reproduced with permission from Citation[204].](/cms/asset/8c053ec0-9c1b-4deb-b13f-79565746733d/tcmt_a_10816304_f0002.gif)
A: Anode; C: Cathode.
Reproduced with permission from Citation[32] ElectroSep™ and Gas Processors Association.
![Figure 3. Electrodialysis Dow process: UCARSEP™.A: Anode; C: Cathode.Reproduced with permission from Citation[32] ElectroSep™ and Gas Processors Association.](/cms/asset/a85ccde9-d0af-416a-8f6c-23044ac8dbac/tcmt_a_10816304_f0003.gif)
A: Anode; C: Cathode.
Reproduced with permission from Citation[32] ElectroSep™ and Gas Processors Association.
![Figure 4. The ElectroSep™ electrodialysis process.A: Anode; C: Cathode.Reproduced with permission from Citation[32] ElectroSep™ and Gas Processors Association.](/cms/asset/9a91abaa-6752-4e69-95d0-19c1a665ab4c/tcmt_a_10816304_f0004.gif)
(A) Thermal stability test for monoethanolamine. (B) Thermal stability test for diethanolamine.
DEA: Diethanolamine; MEA: Monoethanolamine.
Reproduced with permission from Citation[14] Dow Chemical Company.
![Figure 5. Dow thermal stability tests for monoethanolamine and diethanolamine. (A) Thermal stability test for monoethanolamine. (B) Thermal stability test for diethanolamine.DEA: Diethanolamine; MEA: Monoethanolamine.Reproduced with permission from Citation[14] Dow Chemical Company.](/cms/asset/4618c4b7-ac07-4375-a460-b775715ce466/tcmt_a_10816304_f0005.gif)
Reproduced with permission from Citation[55].
![Figure 6. Traditional kettle amine reclaimer within amine plant.Reproduced with permission from Citation[55].](/cms/asset/8115e815-bcdb-432b-aee9-466c172e99b9/tcmt_a_10816304_f0006.gif)
Reproduced with permission from Citation[14] Dow Chemical Company.
![Figure 7. Liquid–gas equilibrium curve of monoethanolamine–water system.Reproduced with permission from Citation[14] Dow Chemical Company.](/cms/asset/ac2c6ca5-f78c-4780-a345-89a628a5f3ee/tcmt_a_10816304_f0007.gif)
HSAS: Heat-stable amine salt; HSS: Heat-stable salt.
Amines are extensively used in natural gas conditioning and oil refineries for acid gas removal (e.g., H2S and CO2). The same amines are also used for post-combustion CO2 capture processes. A typical post-combustion process for CO2 capture is given in . To make the process economically feasible and cost effective, the amine must be carefully selected, since it will determine the overall plant energy consumption. Another key factor is solvent loss, which could take three forms: carry over and entrainments; evaporation due to volatility; and degradation. Entrainment of liquid depends on the hardware and physical design of the absorber and stripper columns. However, these losses could be overcome by using a water washing section at the top of the absorber and stripper.
Solvent degradation is an important criterion in selection of the appropriate amine and whether the amine can be reclaimed easily Citation[1]. All amine solvents, including singles and blends, are subject to degradation with the impurities either in the feed gas or those introduced to the amine system from other sources (e.g., make-up water or leaks). Some flue gas impurities (e.g., O2 and SOx) can degrade amine to form heat-stable salts (HSSs) Citation[2]. Solvent resistance to O2 degradation varies largely on the capture conditions and type of solvent. A study ranked diisopropanolamine (DIPA) as the most sensitive amine to oxidation, followed by diethanolamine (DEA), monoethanolamine (MEA), diglycolamine (DGA) and methyldiethanolamine (MDEA) Citation[3]. Other factors that affect solvent degradation are temperature, residence time, and solvent pH and concentration Citation[4].
There are two types of degradation products that could be formed: HSSs and amine degradation products. Definitions of HSSs vary in the literature but we consider the following definition for HSSs: salts formed by reaction of amine and its acidic degradation products and impurities (e.g., organic acids and HCl), which cannot be regenerated under normal stripper conditions Citation[201]. explains the differences between heat-unstable salts and HSSs. The portion of HSSs that can form from the reaction with an amine is called heat-stable amine salt. Therefore, if there are no strong cations in the amine solution, heat-stable amine salts are the same as HSSs. HSS anions formed could include acetate, formate, thiosulfate, sulfate, thiocyanate, oxalate, butyrate and propionates. Other HSS anions introduced to the amine process from water make-up or flue gas are chloride, phosphates, cyanides and nitrates Citation[4]. The recommended HSS level in amine is less than 10% of amine concentration (e.g., 30 wt% MEA should contain a maximum of 3% HSSs) but 3% should be controlled to avoid operational problems Citation[3]. Further details of HSSs and their formation paths are extensively reported in the literature Citation[5].Operational problems triggered by the presence of HSSs and other amine degradation products include: reduction in solvent capacity and plant throughput; change of water balance and physical/chemical properties of solvent; high viscosity causing reduced mass transfer and increased pumping power; increase of foaming tendency and decrease of contact surface between gas and liquid; increase of solvent corrosivity affecting process equipment; fouling and plugging in the heat exchangers and mass transfer equipment; increase of energy/heating steam consumption; and increase of cooling duty (i.e., cooling water) Citation[6–8].
To operate amine processes more effectively, these problems should be addressed and solvent degradation should be controlled. Many temporary and long-termed solutions can be used but they are generally divided into two method categories: solvent cleaning and degradation prevention, and recycling and reclaiming .
Solvent cleaning & degradation prevention methods
▪ Change-over solvent inventory
Change-over solvent inventory is the oldest and the least effective method to overcome solvent degradation product build-up. The Standard Oil Development Company (USA) used online neutralization with caustic soda to control HSSs Citation[101]. However, sodium salts produced during neutralization tended to accumulate in the amine solvent, which led to increased viscosity and reduction of the solvent capture capacity. If the viscosity exceeds 8.75 cP at 37.7°C, the whole solvent inventory should be changed with fresh solvent Citation[101]. A complete replacement of solvent could still have up to 80% of usable solvent. Therefore, this option is no longer attractive due to concerns over the operating and disposal costs and environmental regulations Citation[9].
▪ Solvent purging/feeding
The purge/feed method was seemingly the first method used to overcome the build-up of contamination in amine solvent Citation[7,8,10,11]. This approach consists of the removal of a portion of contaminated solvent and replacement with fresh and clean solvent to reduce the contamination concentration. However, this approach is not only impractical but is unpleasant due to loss of useful solvent, increased disposal cost and environmental concerns.
▪ Mechanical filtration
Mechanical filters are usually used to remove suspended fine solid particles and corrosion products from amine solution Citation[3,10]. In the early stages of gas processing treatment using amines, it was thought that mechanical filtration could help maintain solvent quality Citation[7]. Mechanical filtration can remove neither HSSs nor degradation products; therefore, it will not be discussed in detail in this article. There are various types of filter materials that could be used in amine systems, which are summarized elsewhere Citation[10].
The normal design for a filter is based on the amine slipstream varying from 5 to 10% of total lean amine circulation Citation[12]. Slipstream of up to 15 or 20%, or sometimes a full filtration stream, is also used Citation[10,13]. Typical filters are selected for capturing 10 µm-sized particles. In reality, there is no standard design that can be followed; thus, selection still depends on rule of thumb in many cases. Some successful filtration systems are reported in the literature. MPR Services, Inc. has introduced a new technology, known as SSX™, which has a high efficiency in removing fine solid particles Citation[7].
▪ Activated carbon filtration
Activated carbon filtration was used as a method to purify and clean amine solvent Citation[7,14]. The activated carbon was traditionally considered for the removal of foaming formation sources such as surface active organic compounds, high molecular compounds (e.g., polymeric degradation products), dissolved hydrocarbons, lubricants and very small, fine solid particles Citation[10,15,16]. The carbon could also remove some degradation products at different rates; however, its adsorption capacity for those degradation products is very small, thus, not recommended for a continuous operation Citation[8,101]. MPR Services, Inc. has introduced a new technology, HCX®, which has a better performance in removing hydrocarbons compared with activated carbon Citation[7].
▪ Online neutralization of HSSs
Examples are given in showing neutralization processes of formic acid in a tertiary amine solution using sodium hydroxide (NaOH) and sodium carbonate (Na2CO3) Citation[7]. As shown in , 1 mol of NaOH could liberate 1 mol of amine. In comparison, 1 mol of Na2CO3 could liberate 2 mol of amine and generate 1 mol CO2, as given in . Although Na2CO3 may be preferred, its cost is higher than NaOH.
Equation 1
Equation 2
Equation 3
Online neutralization with Na2CO3 or NaOH was reported with respect to its use in many DEA systems when HSS levels reached 0.5 wt% or more Citation[15]. The addition of the strong base was usually more than the required quantity by 50%, and DEA solution can tolerate up to 20 wt% sodium salts before any precipitation would occur. Potassium compounds are more soluble by 25% than sodium compounds and, therefore, can be used; however, they are more expensive and also more corrosive than NaOH Citation[17].
The Standard Oil Development Co. utilized the online neutralization of HSSs with caustic soda at 5–30 wt% concentrations in order to control HSS levels and associated operational problems Citation[101]. However, sodium salts produced from neutralization would accumulate and increase the solvent viscosity, which reduced the solvent capture capacity. If the viscosity becomes very high, the whole solvent inventory requires a change-out Citation[101]. Tidewater Oil Company’s patent for the DEA reclaimer demonstrates the online neutralization technique for DEA amine plants Citation[102].
Dow Chemical Company suggested a method to reduce solid formation during neutralization by mixing the alkali metal-based solution with relevant amine prior to neutralization. However, the impact was essentially more of a dilution effect of the amine on the overall system rather than any other mechanism Citation[103]. In one of Dow’s publications, they claimed that the online neutralization could reduce corrosion; however, their investigation was only based on laboratory tests without confirmation from actual plant observations Citation[18]. Apparently, the expected reason for corrosion reduction is the increase of pH with addition of a strong base and the liberation of bound amine with HSSs that contributes to increase in the solvent pH Citation[19]. More advantages of online neutralization use are reported, including decreased fouling, increased efficiency of heat exchangers and absence of foaming Citation[19].
In some plants without on-site reclaimers, the online neutralization was utilized as a temporary solution to recover the amine until the solvent could be reclaimed off-site or with a mobile reclaimer Citation[20]. Online neutralization could give a false sense of stability when appear to be HSSs are under control, which is usually indicated by more stable operation with reduction of corrosion and foaming tendency, and restoration of solvent absorption capacity by freeing bound amine Citation[7,21]. In fact, the HSS organic/inorganic acid anions do not actually disappear from the solution. Rather, they form neutralized salts, which can subsequently lead to a number of problems such as increased corrosion, salt precipitation, high viscosity and density, reduction in surface tension and increased foaming formation tendency, formation of false high loading, and less solvent for scrubbing the acid gases Citation[7,22]. To overcome such problems, it is proposed to integrate online neutralization with an electrodialysis (ED) reclaimer, which will remove both acid anions and sodium cations Citation[20].
▪ Solvent degradation inhibition
Increase of solvent degradation resistance has been one of the most active R&D areas since the 1940s Citation[23–25]. This is achieved by selecting amine solvents that have high resistance for oxidative degradation. Also, chemical additives in small concentrations can be added either to inhibit the solvent degradation or eliminate/reduce the availability of degradation inducing impurities such as O2 and SO2. The US Naval Research Laboratory reported a comprehensive study done by the Girdler Corporation to evaluate potential amine solvents for CO2 removal and their resistance to oxidative degradation Citation[23,24]. The evaluation was done for 39 single and four mixed amines. The outcome from the Girdler Corporation evaluation showed that 12 single and three mixed amines were promising, showing high resistance to oxidation degradation. They concluded that the tertiary amines had the highest oxidation resistance followed by secondary and, finally, primary amines; however, MEA was the only one selected and used. The rest of the amines proposed by Girdler Corporation were not considered for real applications at the time, which was possibly due to lack of fundamental information Citation[25].
The US Naval Research Laboratory also issued a report on inhibition of MEA degradation Citation[25]. It indicated that N,N-diethanolglycine and ethylenediaminetetraacetate acid or their blends were found to stabilize MEA solvent by destroying the amine degradation products. However, these additives were not effective in the presence of metals (i.e., copper, iron, nickel and chromium), which acted as catalysts in the solvent degradation process Citation[25].
MEA could be more stable by adding a 0.5–5 wt% tertiary amine (i.e., triethanolamine [TEA], triisopropanolamine and triisobutanolamine) as shown in one of Union Carbide’s (now part of Dow Chemical Company, USA) patents Citation[104]. Based on laboratory tests, these inhibitors reduced the MEA degradation by over 98% in the presence of acid gases (i.e., CO2 and H2S); however, no evidence was found to show that this method was also tested in a real plant. Ten years later, Universal Oil Products (now part of Honeywell UOP, USA) found MDEA in concentrations of 4–25 wt% more suitable to inhibit MEA degradation, and also for DEA, than other trialkanolamines Citation[105]. UOP claimed that trialkanolamines could not be recovered during the regeneration (reclamation) step due to their high boiling points and molecular weights.
Another inhibition method for amine degradation is given by Cata Chem Inc., in which the formation of HSSs can be reduced by addition the of N,N-diethylhydroxyamine catalyzed by hydroquinone at a ratio of 0.5–6 ppm for every ppm of hydorquinone added Citation[106]. On the other hand, N,N-diethylhydroxyamine used alone has no impact on the inhibition. Recently, researchers from the University of Regina (Canada) have proposed a method to inhibit O2 and SO2 induced degradation of MEA solvent by addition of sodium sulfite (Na2SO3), potassium sodium tartrate tetrahydrate (KNaC4H4.4H2O), ethylenediaminetetraacetic acid, hydroxylamine (NH2OH) and their mixtures Citation[107].
Recycling & reclaiming methods
▪ Ion-exchange reclaimer
The ion-exchange action is similar to online neutralization with strong bases, but there is little difference in mechanisms of neutralization. Ion exchange occurs between liquid (i.e., amine solution) and solid (i.e., strong base resin containing hydroxide resin), whereas online neutralization occurs between liquid (amine solution) and liquid (strong base solution) Citation[7]. The ion-exchange technology has been adopted based on its success in other industrial applications such as treatment of boiler water and recovery of acids and metals. The two major ion-exchange commercial providers are Eco-Tec and MPR Services, Inc. based in Pickering (Ontario, Canada) and Dickinson (TX, USA), respectively. The ion-exchange-based reclaimer technologies commercialized respectively by Eco-Tec and MPR Services, Inc. are AmiPur® Plus and HSSX®. Keller et al. have shown illustrations of the typical location of the ion-exchange reclaimer in the amine plant Citation[26].
These technologies have been claimed to provide the optimum removal of major solvent contaminants (HSSs) with less chemical, energy and water consumption. Also, the waste (i.e., brine) produced is in small quantity and contains only traces of amine solvent or none at all. Previous ion-exchange reclaimers suffered from many drawbacks, as mentioned by MPR Services, Inc. or Eco-Tec. For instance, MPR has pointed out the drawbacks in the previous ion-exchange processes used in amine systems as being ineffective and producing large quantities of waste, which sometimes contained significant amounts of amine solvent, making it untreatable in waste water plants in some cases Citation[7].
The ion-exchange reclaimer was originally developed to treat contaminated amine solvents used in natural gas sweetening by removing the organic acid anions. In the process, the contaminated amine solvent was passed through a packed bed resin pretreated with a strong base such as sodium hydroxide or potassium hydroxide. The resin’s hydroxide ions (OH-) were exchanged with organic acid anions, thereby freeing amine to acid gas absorption cycle. Based on this, it can be seen that the effect of ion exchange is similar to acid–base neutralization, with the exception that the product of neutralization remains on the resin until the resin is regenerated Citation[7,22]; meanwhile, the cleaned solvent is left for the amine process. The typical ion-exchange process involves two steps, as shown in : the loading step (to remove contaminants from solution) and the regeneration step (treating the resin with strong base solution to remove contaminants from resin by replacing acid anions with hydroxide to restore the adsorption capacity of the resin and form base salts that are sent to a waste treatment facility).
Equation 4
Equation 5
The ion exchange usually operates in a continuous or semi-continuous mode to treat a small slipstream of cold and contaminated lean amine, but it can function as a batch operation in some cases. Sizes of reclaimer and slipstream depend on the severity of the contamination. Continuous operation has proven to be more efficient than batch treatment, as contaminants of acid anions are treated as they are formed, thereby instantly preventing operational problems. The process operates with a number of cycles in a specific sequence: loading (removing contaminants), regeneration (removing neutralized salts) and washing (water). After water washing, the bed is ready to accept a new batch of contaminated slipstream. The cycle is then repeated to maintain the target level of contamination in the lean amine solution. The waste is biologically degradable and, therefore, is sent to a wastewater plant Citation[7]. summarizes the ion-exchange reclaimer developers and providers.
It should be understood that the ion-exchange process might be acceptable in natural gas-treating services as the acid gas loading is preferred to be as small as possible (or even zero) to achieve pipeline specifications Citation[10]. Also, the acid gas in solvent or the bisulfate (HS-), carbonate (CO32-) and bicarbonate (HCO3-) would not interfere or compete with HSS adsorption in the ion-exchange reactions. In CO2 capture, particularly from flue gases, the lean amine usually contains a higher acid gas loading (0.1–0.2 mol CO2/mol amine, or even higher) compared with natural gas sweetening Citation[10,27]. The high loading is desired, as it helps reduce the energy demand of the reboiler, improving the plant economics and reduce the solvent degradation by reducing the ability of the solvent to dissolve the impurities in the flue gas feed Citation[28]. The high acid loading or high level of CO32- and HCO2- in the amine solution could interfere and compete with HSS anions in the resin leading to reduction of the effectiveness of the ion-exchange process in removing HSSs.
Informative dealing with water treatment for manufacture of industrial products mentioned that Eco-Tec’s AmiPur ion-exchange technology was integrated to Fluor Corporation’s Econamine® amine process Citation[202]. Furthermore, Fluor Corporation has mentioned that the ion-exchange process was used and tested in its Econamine technology Citation[29]; however, very little information is available on its performance. Beyond this case, it is understood that the performance of ion-exchange reclaiming in CO2 capture applications has not been satisfactory thus far. MPR Services, Inc. proposed a new ion-exchange process, known as Carbon Capture Amine Reclamation, to treat contaminated amine solvents used in CO2 capture system as given on the MPR Service website. However, no information on its performance or testing in any CO2 capture plant has been reported.
Despite the fact that ion-exchange technology can be applied for removal of organic acids from amine solution, the process alone cannot be considered an optimum solution for CO2 capture applications. Contamination of amine solvent used in treating flue gases is completely different from that in natural gas sweetening processes. The differences between both applications are summarized in . In addition to HSSs, major troublesome contaminants in CO2 capture applications from flue gas are amine degradation products, which cannot be removed by ion exchange directly. However, their formation, particularly as amides, has been reported to decrease after organic acid anions are removed by ion exchange Citation[4].
▪ ED reclamation
A typical ED process involves the utilization of electrical current, potential voltage and ion-selective membranes to separate ionic degradation products and HSSs. The separation is done with a process set-up consisting of a stack of alternating cation-selective and anion-selective membranes located between two electrodes. The filtered degraded amine feed can be treated with/without NaOH/NaCO3 prior to being introduced to specific channels between stacks of the cells. It is recommended to use cold lean amine at 43°C or less to be treated in the ED reclaimer Citation[20]. The separation is carried out between cations and anions toward their opposite electrodes and through the selective-ion membrane, which only allows cations or anions to pass, and prevents the others from passing. Therefore, the ED process is only capable of removing HSSs, while other degradation products or contaminants would be left in the amine solvent. Due to temperature sensitivity and limitations of the materials used in manufacturing the ion-exchange membrane, the recommended amine feed should not be very warm. Thus, downstream of the lean amine cooler would be the best location to extract the slipstream, which would then be fed through 10-µm filters before being sent to the ED unit to prevent membrane fouling Citation[10,20]. A schematic diagram illustrating the concept of the ED process is shown in .
During the design of an ED process, there are some important factors that affect the process performance, which are summarized as follows Citation[20]: ion-exchange membrane type, surface area, type of solution to be treated and the applied current, which is the main effluence factor that affects the removal efficiency.
Equation 6
Where: r is the removal rate (g-eq/sec); I is the current (Amps); n is the number of membrane cell pairs; F is the Faradays contact (96,480 Coulombs/g-eq); and E is the efficiency factor.
The ED reclamation process was pioneered by Union Carbide Corporation (which was later acquired by Dow) and the Dow Chemical Company Citation[10]. The process was patented for treating contaminated amine solvent in 1989 and was successfully tested at laboratory scale to remove formic acid from degraded MDEA solvent Citation[108,109]. The waste mobile phase was 0.1 N NaCl in water to facilitate the separation of formate from MDEA. The process was further tested and developed to be integrated with an amine process for capture of acid gases Citation[19,110]. Addition of a strong base such as caustic soda to amine prior to feeding to the ED process was found to help free amine from HSSs, allowing them to be removed more easily from the amine stream Citation[111]. The removal efficiency of HSSs was reported from 60 to 90%. Dow’s UCARSEP™ ED process uses the recirculation of amine and brine to remove HSSs. The process has helped to reduce chemical consumption and improved reclamation performance, as shown in .
The UCARSEP process has proven to be the effective online reclamation that helps resolve operational problems caused by HSSs during amine plant operation as well as maintain low level of HSSs (i.e., <1 wt%). Dow reported that the UCARSEP performance was found satisfactory when tested in various amine plants operated in oil refineries Citation[20,30]. ED Dow process used in an MDEA plant was also compared economically to solvent change-out and purging/feeding, ion exchange and vacuum distillation options Citation[31]. Briefly, the ED process would be economically and technically favored to solvent change-out and purge/feeding options, which would cause more environmental and disposal problems. Whereas, ion exchange and vacuum distillation could be used but there were other concerns on the chemical/utility consumptions and large disposal of possible hazardous wastes.
Another ED process known as ElectroSep™ was also tested in some oil refineries Citation[32,33]. ElectroSep, developed by another ED reclaimer provider, was likely adopted and modified from the Dow’s ED technology to reduce the utility consumption and enhance separation Citation[32]. As shown in one of ElectroSep patents, the improvements include recirculation of the salt mobile phase for enhanced removal of HSSs and reduction of water and chemical consumptions Citation[112]. Also, the technique proposed different arrangements for the membrane stack, as shown in Citation[32].
As reported by Dow, the UCARSEP process has some limitations and drawbacks. For instance, it is possible to lose 1–2% of amine inventory during the reclamation process and membrane fouling would also develop with time resulting in reduction of permeability Citation[30]. On the other hand, ElectroSep has been claimed to recover more than 99% of amine Citation[32]. MPR Services, Inc., an ion-exchange reclaimer competitor, mentions some drawbacks of the ED process as follows Citation[7]:
▪ ED requires filtration pretreatment of amine feed down to 1 µm to avoid fouling; | |||||
▪ ED demands more energy than distillation and ion exchange; | |||||
▪ ED produces more waste than distillation; | |||||
▪ Waste might be considered hazardous and cannot be treated in wastewater plants due to high levels of salts and chemicals. | |||||
Another limitation of the ED process is the loss of water from the amine stream due to osmosis, which could affect water balance in the amine system. Also, amine carbamate (CO2–amine), carbonate and bicarbonate could be separated and lost in the waste stream; this could occur especially if the acid gas loading is high. Furthermore, sodium cations used in the regeneration step could leak to the amine system from the ion exchange Citation[20]. Literature is also not available to provide a complete picture of the ED performance in terms of utility/energy consumption, removal efficiency, plant lifetime, HSS removal capacity and solvent recovery. Although the ED process has been used successfully in removing HSSs at different concentrations, the technique is not the optimum choice for CO2 capture processes, since it cannot remove neutral amine degradation products usually present with HSSs.
▪ Thermal reclamation
Thermal reclaimers use heat energy to purify an amine solvent by the evaporation of amine from its degradation products. Typically, the degraded amine slipstream is mixed with a strong base such as caustic soda or soda ash to release the amine trapped by HSSs. Then the mixture is either allowed to settle for some time to remove the solids and precipitated matters or sent directly to the reclaimer where evaporation is carried out inside special distillation equipment. Usually the added amount of sodium carbonates/hydroxide is slightly more or less than the stoichiometric amount of HSSs present in the degraded amine stream. 1 mol of sodium carbonate could release 2 mol of amine, while hydroxide could release 1 mol of amine, as explained earlier in .
The companies that have developed/commercialized thermal reclamation processes include Dow Chemical Company, ExxonMobil Corporation (USA), Huntsman (USA), Praxair (headquarters in the USA), Shell (headquarters in The Netherlands), Canadian Chemical Reclaiming (CCR) Technologies (Canada) and other companies that do not exist anymore. All have developed fixed and mobile reclamation units, some of which have sophisticated reclamation facilities that will reclaim the amine off-site. Typical thermal reclaimer’s drawbacks and merits are summarized in Citation[7].
The operating conditions of thermal reclaimers depend on the type of solvents, which have certain toleration of temperature after which thermal degradation can occur. shows the thermal stability tests for MEA and DEA carried out by Dow, which claimed MEA and DEA stability at up to 148 and 176°C, respectively (but could only be stable for short periods of exposure) Citation[14]. Dow also mentions that diluting the amine solvent with water would enhance the thermal stability of amine. MDEA has been thermally degraded at 182°C Citation[34]; thus, this temperature should be avoided during reclaiming operations. High boiling point amines such as DEA and MDEA should be reclaimed under vacuum Citation[3]. Reclamation of secondary and tertiary amine solvents, used singly or in mixed formulation, have not been extensively reported in the literature. However, secondary and tertiary amines or their mixtures are reported to be reclaimed by thermal distillation under vacuum with temperatures not exceeding 204°C Citation[10]. Limited information is available as a guideline in reclaiming these amines Citation[10]. DEA or DIPA reclamation is carried out under deep vacuum (using a tray distillation process with direct steam, pressure of 6.66–13.33 kPa and maximum temperature of 148°C) Citation[10]. The reason for this limitation is that the reclamation process may be quite complex and not ready to be utilized for many single, mixed or formulated amine solvents.
Classical thermal reclaimer (atmospheric or above)
Dow published a book giving details of classical amine thermal reclaimers (atmospheric, above atmospheric or vacuum) Citation[14]. According to Dow, operating pressure will control the reclamation cycle; for example, high-pressure operations would indicate a short cycle and large dumping, while atmospheric reclamation exhibited the opposite trend Citation[35]. As reported by Dow, the atmospheric reclaimer is suitable for easy-to-recover amines such as MEA, in which case it could be designed to operate in either batch, semicontinuous or continuous mode Citation[14]. shows a traditional kettle reclaimer used in an amine plant. Easy reclamation means degradation products have very low vapor pressure compared with the amine solvent Citation[36]. Dow suggested that 1–2% of lean amine slipstream be fed into the reclaimer in continuous mode. The slip ratio can be varied based on the amount of contamination in the solvent and amount of solvent inventory Citation[37]. Dow suggested heat flux be limited to 15,775 kW/m2 in order to control thermal degradation due to excess heat. The water and amine vapor from the reclaimer can be injected to the stripper bottom or mixed with the vapor stream from the stripper reboiler. Neutralization with sodium hydroxide or carbonate can be used to liberate amine trapped in HSSs. The size of the traditional atmospheric reclaimer is usually designed based on the slipstream to the reclaimer and targeted residence time, as shown in Citation[35]:
Equation 7
The scale-up factor can be varied depending on amine plant size, economics and expected solvent degradation rate. For instance, this factor could be varied from 50 to 100 Citation[35], in which small values are used for a large reclaimer and vice versa for large numbers Citation[38]. For preliminary sizing calculation, a factor of 75 can be used.
The classical reclaimers are designed based on the lean amine solvent concentration, temperature and pressure, as reported by Dow Citation[14]. Such a design is made to ensure an effective amine recovery operation using suitable temperature/pressure away from the thermal degradation temperature of amine. In the reclaimer operation, the boiling liquid inside the reclaimer is brought into equilibrium with the amine vapor leaving the reclaimer. This ensures that the exiting vapor also contains the same concentration as the feed amine stream. This relationship can be explained by the vapor–liquid equilibrium graph for a MEA system as shown in .
As the boiling liquid becomes more concentrated with salts and degradation products, its boiling temperature steadily increases Citation[10,39]. The reclaimer operation is held steady when the temperature reaches the maximum allowable to avoid thermal degradation. The diluting water (usually from reflux water) or steam is added to recover as much amine as possible. Addition of water also helps reduce the amine partial pressure and control the temperature inside the reclaimer. The reclaimer cycle is terminated when the temperature approaches the maximum temperature or the amount of amine in the vapor stream falls sharply below the amine concentration in the feed (i.e., reduced to 1wt% or less) Citation[14]. This means the maximum economic amine recovery is already achieved and can no longer be controlled by addition of water. Once the reclamation cycle is terminated, the reclaimer is emptied, cleaned and prepared for the next cycle. The reclaimer waste is usually thick and viscous, and might contain some active amine trapped in it. Due to high levels of many degradation products, reclaimer waste could be considered hazardous and should be handled through professional waste management. summarizes some other classical thermal reclaimer developers.
The concern with classical reclaimers is waste that could still contain high amounts of useable solvent. In one case reported by the Union Carbide Chemical Company, the residue waste from a MEA reclaimer could contain at least 74.6% MEA Citation[37]. MPR Services, Inc. claimed that in the classical reclaimer for primary amines (i.e., MEA and DGA), the waste contains high levels of amine. To explain this, MPR Services, Inc. has introduced a factor to evaluate this loss, which is the ratio of amine to HSS. It is advisable to have in waste this ratio be less than the ratio in the lean amine stream ([amine/HSS]_waste << [amine/HSS]_lean amine). However, based on MPR Services, Inc.’s observations, this does not happen in many cases Citation[7]. The ratio is worst in MEA and DGA reclaimers, which could lead to thermal degradation. The general advantages and disadvantages of thermal reclamation are given in .
Modern thermal reclaimers (atmospheric or below)
Modern thermal reclaimers can be considered the second generation of thermal reclaimers. The process is designed to reclaim high boiling point secondary and tertiary amine solvents or their blends, which are sensitive to decomposition at high temperature. It is well known that many amine solvents start to thermally decompose (degrade) if the temperature exceeds 204°C.
The leading company in developing modern thermal reclamation is CCR Technologies. According to CCR, their tests and evaluation of all the potential options to purify contaminated solvent showed vacuum distillation as the most effective method for purification Citation[40]. CCR claimed that their processes could reclaim any single or blended amines. The CCR reclaimer first appeared in detail in a patent by its founders in 1992 Citation[113,114]. Their first reclaimer, called version 1, was to be distinguished from the other versions proposed later. The CCR reclaimer objectives are the removal of contamination, enhancement of solvent recovery (90–95%), and reduction of the potential thermal degradation and corrosion within the reclaimer Citation[113,114]. The contaminations targeted by the CCR reclaimer are thermal degradation products, HSSs, hydrocarbons and suspended solid matters, with removal efficiency set to exceed 99% Citation[40]. DEA solvent was used to demonstrate reclaimer performance and a recovery rate of 90–95% could be achieved. Amine content in waste could vary from 5 to 15% to ensure fluidity within the process and handling of the reclaimer waste. The reclaimer can operate in continuous or batch mode Citation[41].
The CCR reclaimer (version 1) has a unique design. The amine feed (at 5–24°C) is supplied to a feed tank, which helps maintain constant flow to the reclaimer. After mixing the amine with hot, recycled amine bottom stream from heater, it is preheated in a heat exchanger and then fed to an evaporator at vacuum pressure (55–85 kPa). Water and some amine evaporate, and partial condensation, occurs in the heat exchanger where the coming amine feed is also preheated. Then, the partially condensed stream is sent to a separator to separate liquid (condensate) from vapor. The condensate (i.e., recovered amine) is cooled and filtered before being sent back to the amine system. The separator top stream consisting of water, noncondensable gases, light hydrocarbons and traces of amine is sent to the condenser for water and amine recovery. Noncondensable gases and light hydrocarbon are fed to the heater/burner to destroy them before they can be released to the atmosphere. In the evaporator, the bottom stream is split into two streams: recycled (at a ratio of 40-times to the feed Citation[40]) and mixed with amine feed after heating it up in the heater, while portions of the bottom is taken as waste to control the salt and degradation product content in the recycled stream and avoid fouling. The heater is designed to have limited heat transfer at 15,775 kW/m2, 30–60 s of amine solvent residence time with velocity varying from 1.8 to 3.0 ft/s and 204°C maximum temperature.
The version 1 reclaimer could be used to reclaim amine solvent (for removal of acid gas), glycol solvent (for dehydration) and physical solvent. Successful reclamation by this reclaimer includes recovery of MDEA, ethylene glycol, Sulfinol-M solution Citation[40], DEA, Sulfinol-D solution and formulated solvent (MDEA-based solvent) Citation[41]. In 1995, CCR generated a patent for a new application of their version 1 reclaimer to manage chemical waste and recover water to reduce the amount of waste produced for easy handling and disposal Citation[115,116].
CCR and the University of British Columbia (Canada) modified the version 1 reclaimer to overcome co-evaporation of amine and its degradation products of similar boiling points. The modified process (version 2) used a new separator with two separation steps connected in series: inert liquid extraction (i.e., normal paraffinic oil) to remove degradation products from the amine; and distillation to recover amine and water at the top while the inert liquid, richer in light degradation products and nonvolatile materials, is separated at the bottom Citation[41]. The case study of the version 2 reclaimer demonstrated by CCR was to recover DEA from its light degradation product, bis-hydroxyethyl piperazine. This reclaimer, as claimed by CCR, had the following features: high solvent recovery, low solubility of inert liquid in amine and significant reduction of thermal degradation of amine in the heater. Waste from a gravity separator was also claimed to mainly consist of degradation products with a very small amount of inert liquid; however, this has not been tested and unlikely will be. One shortcoming of the proposed process is that contamination and accumulation of the extractive liquid in the amine system could occur and possibly induce foaming and plugging problems.
A third version reclaimer was invented by CCR at the end of the 1990s; the CCR process proposed consisted of two heating zones Citation[117]. The first zone is used to evaporate water and amine solvent, while purging of the evaporator bottom is done from time to time for controlling solid build up. In this zone, the amine feed is first mixed with the hot recycled bottom liquid stream from the reclaimer evaporator using an appropriate type of mixing equipment. The hot mixture is introduced to the evaporator vessel operated under deep vacuum pressure (i.e., 50 kPa) at which most of the amine and water are evaporated. The evaporator bottom, consisting of solids, degradation products, and some water and amine, is heated and recycled to enhance recovery of solvent as well as to help heat up the coming feed. Some of the bottom liquid is recycled before heating and sprayed in the evaporator to control foaming formation. The second heating zone is composed of a distillation unit used to separate water and amine, as well as the noncondensable gases, in the vapor leaving the first heating zone. Within CCR’s patent, two other modifications of the first heating zone are proposed: one without the foaming control stream of the bottom and the second with the use of any filtration equipment to separate solids from the foaming control stream of the bottom going to the evaporator Citation[117].
CCR’s version 4 reclaimer was modified from their version 3 reclaimer Citation[118,119]. The difference between the two versions is the recycling of some of recovered water and recovered amine, and mixing them with incoming feed. This is believed to enhance solvent recovery and minimize the energy consumption. According to CCR, the enhancement in the reclaimer performance will help to control foaming and temperature and maximize solvent quality. CCR also suggested another configuration change of reclaimer of version 4 to version 5; the version 5 reclaimer has a second distillation zone to handle recovery of mixed amines Citation[118]. However, CCR has not provided any performance data on these two proposed reclaimers.
Molecular distillation reclaimers (deep vacuum)
Molecular distillation is specifically operated so that evaporation and condensation can occur in a short period of time. Evaporated molecules only travel a short distance from the evaporating surface to where they get condensed; thus, resistance in vapor space experienced by the molecules is reduced. As a result, there is no condensation back to the boiling surface, which is usually found in normal distillation. Moreover, the molecular distillation equipment is usually run at very deep vacuum (i.e., << 1.3 kPa), and the system is degassed from noncondensable gases before proceeding, which could interfere in the distillation process. A number of apparatus for molecular distillation could be used, and each apparatus has a different approach and productivity load in achieving molecular distillation. The apparatus include pot still, rotary still, falling film still, centrifugal still, and wiped file still. Molecular distillation is used to recover temperature sensitive chemicals Citation[42]; thus, this type of distillation is used to purify vitamins, fish liver oil, vegetable oils, lubricants and heavy petroleum residues Citation[115]. It has been reported that tertiary amine has been separated from other amines by molecular distillation Citation[42,43]. Shell has a patent proposing the use of a falling film or wiped-film evaporator to recover DIPA in two evaporation steps, in which water is first recovered followed by the amine Citation[120]. ChemGroup, Inc. (USA) has reported that wiped-film distillation is used to reclaim different types of amines (i.e., primary, secondary and tertiary), glycols, heating oil and lubricants. On their website, ChemGroup, Inc. reported two case studies for reclamation of DGA and DEA Citation[203]. However, molecular distillation is unlikely used in normal amine plants due to cost and complications of operation and maintenance.
▪ Other reclamation methods
Extraction
The conventional reclaiming process often uses distillation to separate the amine from the HSS contaminants. Since distillation is achieved based on boiling point difference, the process is very energy demanding due to the high energy input required to evaporate large volumes of amine and water from the HSSs. In addition, co-evaporation of these salts and other products with similar ranges of boiling point to that of amine could also occur, which still results in contamination of amine solution after distillation. Solvent extraction has found many uses in industrial separation, purification and product recovery (e.g., organic acids from fermentation broth, food, pharmaceuticals, refining of uranium, plutonium and radioisotopes). Its application in organic acid removal from fermentation processes probably was first considered for carbon capture operations because the extraction could potentially be applied to separating out HSSs, as well as associated forms of organic acid, from amine used in CO2 capture applications. To our knowledge, solvent extraction has never been publically presented regarding its use as a reclaiming technique for HSS separation in CO2 absorption operations, so very limited information is available. Therefore, the reviewed information given in this section is mostly based on extraction techniques used to remove organic acids in other fields, but is also applicable to amine-based CO2 absorption processes.
Carboxylic acids normally produced from biomass fermentation processes must be isolated from their aqueous solutions. These acids, including formic acid, acetic acid, propionic acid and oxalic acid, occur frequently in diluted concentrations, which require an effective separation technique for further uses. Reactive extraction using amine-based extractants have been used effectively to remove such low concentration acids from aqueous media for decades. Early research used aliphatic amine-based extractants, namely Ala-336 and Aliquat 336, prepared to desired concentration with kerosene and 2-octanol diluents to separate acetic acid, propionic acid, butyric acid and lactic acid from diluted aqueous phase for fermentation and wastewater applications Citation[44]. The results showed that the extraction performance using both extractants was pH dependent. Undissociated and dissociated acids were both extractable by quaternary amine Aliquat 336, allowing its working pH to cover acidic and basic ranges. The working concentration of Aliquat 336 was also found to be limited to 25% in order to prevent formation of emulsion between the aqueous and organic phases. Tertiary amine-based Ala-336, on the other hand, was found to only extract undissociated acid, implying a working pH range below 4. Also, 2-octanol diluent was found to boost the extractability of only Ala-336, while kerosene provided only physical improvement (i.e., reduce viscosity) without additional effects on both amine extractants. Amberlite LA-2, a secondary amine extractant in 1-octanol, was also used to recover acetic acid from diluted aqueous solution in calcium magnesium acetate production Citation[45]. The extraction performance was also compared with its tertiary (e.g., Ala-336) and quaternary (e.g., Aliquat 336) amine counterparts and tri-n-octylphosphine oxide (TOPO). Amberlite LA-2 and Ala-336 were among the highest in extraction capacity under pH 6, after which the capacity sharply dropped off. TOPO worked best at pH below 5 with a much lower acetic acid recovery rate than that produced by Amberlite LA-2 and Ala-336. Aliquat 336 ranked between Amberlite LA-2 and TOPO but exhibited a wider working pH range. However, its extraction capacity was boosted to close to that of Amberlite LA-2 when Cl- in its structure was partially exchanged for OH- ions. Aliquat 336 in butyl acetate diluent was later compared with tri-n-octylamine (TOA) for the extraction of 7-aminocephalosporanic acid Citation[46]. TOA showed a better extractability at a lower pH; in comparison, Aliquat 336 worked best on the opposite side of the pH scale, in which it also provided a higher extraction capacity than that of TOA at a higher pH range. Synergistic effects were also observed when mixed TOA and Aliquat 336 were applied to separate lactic acid from fermentation broth Citation[47]. The mixture extracted the acid more effectively than the individual extractants; more recent studies are available in the literature. Effect of various diluents (i.e., kerosene, n-octanol, chloroform, butyl acetate, tetrachloromethane and benzene) on Aliquat 336 extractability was also investigated Citation[48]. Specifically, at high pH conditions, protic diluents such as n-octanol and chloroform provided a better ion-exchange mechanism and, thus, better extraction capacity than that obtained from nonpolar diluents such as benzene and tetrachloromethane. Aliquat 336 mixed with other diluents, namely n-heptane, petroleum ether, 1-decanol and 1-octanol, was also used to recover propionic acid from biological production Citation[49]. Alcohol-type diluents such as 1-decanol and 1-octanol gave better extraction power when used with Aliquat 336. These diluents also prevented third-phase formation, which occurred when Aliquat 336 was mixed with n-heptane and petroleum ether.
TOA has also been investigated quite extensively for the past 10 years. One example was use of 30% TOA in 20% decanol and 50% dodecane to remove lactic acid from fermentation broth Citation[50]. The study found that extraction efficiency was dependent on pH and initial lactic concentration in that it dropped sharply when pH decreased but increased with lactic concentration. A separate study found a similar result, in which extraction efficiency of acetic and succinic acids by TOA/1-octanol decreased with pH of the aqueous solution Citation[51]. The study was also able to selectively extract acetic acid from succinic acid using a similar TOA/1-octanol combination with a multistage extraction. The influences of extraction temperature and phase volume ratio of organic to aqueous phase were later explored using TOA in 1-octanol and kerosene to separate glycolic acid from an aqueous glycolonitrile hydrolysate solution Citation[52]. The results showed that a lower temperature was beneficial to the extraction, while optimum phase ratio showed maximum acid removal efficiency. On the other hand, a higher temperature and phase ratio were preferred for regeneration of loaded extractant. Various solvents were examined to search for the most efficient diluent to be mixed with TOA for recovery of propionic acid from aqueous solution Citation[53]. Chemical extraction (i.e., TOA plus diluents) was highlighted as a better system, providing higher extraction efficiency than that obtained from diluent-based physical processes. Among diluents used in this study (i.e., n-heptane, petroleum ether, ethyl acetate and oleyl alcohol), oleyl alcohol gave the highest propionic acid extraction efficiency when mixed with TOA. The effect of binary extractants using various combinations of TOA, TOPO, Aliquat 336 and tri-n-butylphosphate in 1-decanol mixed with various diluents (e.g., n-octanol, dodecanol, methyl ethyl ketone and hexane) were evaluated, also for recovery of propionic acid for waste water and fermentation application Citation[54]. Overall, results reported within this study confirmed that dual extractants with certain active diluents (e.g., 2-octanol and methyl ethyl ketone) improved the extraction efficiency of a single extractant system.
These studies demonstrate that these extraction techniques have potential to be used to reclaim amine solvent effectively from at least HSS impurities, due to the similarity between acids formed in fermentation applications and those generated in CO2 absorption amine solvents. Since extraction is a multiparameter sensitive process, careful selection of extractant formulation (e.g., type of extractants and diluents) and conditions, which include aqueous pH, extractant to aqueous phase ratio and temperature, are essential to obtaining maximum extractability.
Hybrid reclamation processes
Hybrid reclamation uses a combination of more than one process to remove HSSs and degradation products. Early in 1955, Standard Oil Development Co. proposed a DEA reclamation process that consisted of five stages to manage solvent degradation problems. The process replaced the ineffective online neutralization of HSSs, which was thought to induce additional problems. They reported that online neutralization increased viscosity and reduced CO2 capture efficiency of the solvent. Heavily degraded solvent having high viscosity should be discarded and replaced with fresh solvent. The DEA recovery rate was reported to be as high as 90%; however, it is highly suspected this process will lead to solvent contamination over time, which was not reported in the patent Citation[101]. The five steps proposed in their patent starts with neutralization of HSSs with 5–30 wt% followed by dehydration of amine solvent by thermal distillation, extraction distillation of DEA with isopropyl alcohol, filtration to remove sodium salts and distillation to segregate alcohol (recycled) from amine (sent to amine system), respectively.
Mobil Oil Corporation’s first thermal reclaimer consists of reactive and separation zones Citation[121]. Their reclaimer was designed to initially convert amine degradation products back to their parent amines (i.e., DEA). Following this, a suitable separation method such as distillation or ion exchange was used to purify the reactor products from HSSs and unconverted degradation products. Conversion of the degradation products is carried out using metallic catalysts on alumina, silica or zeolite supports in a reactor operated at 243°C and mild pressure. Although the process provides high solvent recovery as claimed by Mobil, thermal degradation could occur in the reactor due to the sensitivity of amine solvent to high temperature (DEA is degraded once temperature exceeds 204°C).
Mobil Oil Corporation developed their second thermal reclaimer based on reactive distillation of amine solvents (i.e., DEA) Citation[122]. The process works by supplying the degraded amine loaded with acid gas to a distillation zone operated at 160°C and atmospheric or vacuum pressure (i.e., 2.8–20 kPa). Stripping steam is directly supplied to the distillation zone for heating, control of distillation temperature and hydration. The process involves conversion of amine degradation products back to the amine in the presence of steam. The residence time is a crucial factor in the conversion process and it should be adjusted to achieve the optimum conditions recommended within a range of 0.5–2 h. The recovered amine is taken from the top of the distillation zone while the waste, rich in degradation products and salts, is taken from the bottom. The experimental results show that few degradation products and HSSs will contaminate the distillate, but the overall conversion of amine is greater than the feed.
Conversion of degradation products to amines
The conversion of degradation products back to useful amines is one of the attractive reclamation methods. In 1972, a company called Union Chimique Belge (Belgium) patented a process to convert oxazolidone, thiocarbamate DIPA/COS and formamide to DIPA Citation[123]. Shell Oil Company also proposed a similar process to convert oxazolidones back to DIPA Citation[124].
MPR Services, Inc. has recently proposed a process to convert DIPA oxazolidones back to DIPA Citation[125]. In general, the MPR process has the same concept as those of UCB and Shell, but they further treated the recovered amine with an ion-exchange process to remove contaminated cations before returning the solvent to the amine system. Amine recovery rates as high as 99% were claimed by MPR Services, Inc.
Removal of undesired primary & secondary amines in tertiary amines
It is very important to remove DEA, which could form from oxidative degradation of TEA. Build-up of DEA concentration can reduce TEA solvent quality. Societe Francaise Hoechst suggested a process to remove undesired DEA in TEA solvent. The process uses glyoxal (dialdehyde) to initially convert DEA to N,N-bis(2-hydroxyethyl) glycine, which can be easily separated later from TEA by distillation Citation[126]. BASF, a world-leading chemical company, has also proposed a process to overcome contamination of tertiary amines with primary or secondary amines by adding carboxylate at ratios of 1:1 to 5:1 to undesired primary or secondary amines Citation[127]. The primary and secondary amines would form alkylamides, which could then be separated from the tertiary amine by distillation.
According to Rooney, DEA can be formed from MDEA oxidation and thermal degradation, which degrades further to other degradation products Citation[128]. Although DEA can be removed by vacuum distillation, the solvent loss rate could be high due to close boiling points between the degradation products and the amine. Activated carbon could be used, but it has a very limited adsorption capacity. Therefore, they proposed a method to convert primary/secondary amines (i.e., DEA) to tertiary amine (i.e., MDEA) using monoaldehyde or dialdehyde. The addition of these chemicals to tertiary amine solvent initially turns primary/secondary amines chemically to other forms before converting them further to tertiary amines by hydrogenation Citation[128].
Future perspective
The future trends for amine reclamation will likely take three paths: improving current technologies, process integration between the current technologies and introducing new methods for reclamation. Regardless of method types and operations, the techniques used must be able to handle newly developed and complicated formulated solvents. Also, the techniques should be readily available to help improve solvent quality and recovery, and process heat integration. In addition, they should be simple and should reduce the solvent loss in waste, chemical consumption, and capital and operational costs. Furthermore, overall wastewater quantity must be reduced, as these could also contain traces of amine. High levels of amine in wastewater could overload the wastewater treatment plant by killing the microbes Citation[4].
▪ Improving current technologies
The reclamation methods will be continuously improved to achieve the highest possible recovery rate and quality of amine solvents. Solvent degradation inhibition is expected to expand further to enhance solvent stability and resistivity to degradation. Solvent stability is also a key in improving the efficiency of any reclaiming technique. As opposed to unstable amines, their hard to degrade counterparts generate fewer types of degradation products and, thus, less contaminated solvent. As a result, the reclaiming process becomes an easier to handle and more effective operation. In addition, less frequent use of reclaimer processes should occur, as the unwanted product build-up rate is also smaller. Due to these reasons, the International Test Centre for CO2 Capture at the University of Regina has also developed inhibitors to prolong amine stability during CO2 absorption processes from flue gas emitted from fossil-fuel power plants. Our patented inhibitors include four individual compounds, and their combinations are specifically developed to reduce degradation effects of amines due to O2 and SO2. The optimum concentrations of these inhibitors that best prevent degradation if used alone were identified, as well as effective blend ratios of various blended formulations. The percent inhibitions of these inhibitors were found to be as high as 98%, depending on inhibitor formulation and CO2 capture conditions (e.g., flue gas concentration of O2 and SO2, CO2 loading and temperature). Based on these inhibitor data, as little as 2% of total amine can be exposed to degradation, thereby helping to improve reclaiming operations as discussed earlier or even eliminate the need for a reclaimer. Details of these inhibitors can be found in the literature Citation[129]. To further help identify a proper reclaiming process, inhibitors specifically tailored to prevent formation of certain types of degradation products can also be developed. For example, inhibitors made purposefully to limit formation of HSSs, leaving mostly neutral products in the amines, should help eliminate the need for an ion-exchange-based reclaimer. Conversely, a more effective technique, such as one based on solubility difference of neutral degradation products in an extraction solvent and amine can be chosen to achieve a better reclaiming efficiency.
In our view, solvent extraction stands out because it can clean up the HSS in the amine potentially down to less than 10 ppm level. In addition, the energy input is not as demanding as that of the distillation-based reclamation processes because extraction can be done at ambient temperatures. Therefore, the International Test Centre for CO2 Capture has also developed an extraction-based reclaiming technique to remove HSS and neutral degradation products from amine solution using reactive extracting solvents. Our work has proved that two well-selected organic extractants used in single, mixed and two-step extractions at optimum concentrations in a proper diluent, extractant and amine-phase ratio, temperature and CO2 loading can successfully remove HSSs, including formate, acetate, propionate, butyrate, oxalate, succinate, sulfate, sulfite and thiosulfate, and major degradation products such as imidazole, 2-oxazolidone and N-(2-hydroxyethyl) succinimide from MEA solution.
At optimum conditions, the extraction efficiency of most HSS and other degradation products was above 90%. Used extractants can be also regenerated for reuse. Most HSSs show regeneration efficiency over 50% with only a few below 50%. Details of our extraction technique can be found in the literature Citation[130]. The ion-exchange reclaimer will see more innovation in terms of design of the resin, enhancement of process capacity, shortened regeneration cycles, improvement of resin overall active life, resistance to solvent contamination such as hydrocarbons, and elimination/reduction of the loss of solvent carbamate, carbonate and bicarbonate in waste. Chemical and water consumption for regeneration will be also optimized.
ED reclaimers will need much further improvement than any other reclaiming techniques to resolve the design and operational challenges. The type of membrane would need more research to enhance its durability, resistance to fouling and ion selectivity. Reduction of power, chemical and water consumption, and solvent loss are also desired.
Classical and advanced thermal reclaimers would need much further improvement in terms of optimized energy for purification, enhanced solvent quality and recovery, and reduced solvent loss. Proper handling of difficult to reclaim amines such as those with antioxidant or corrosion inhibitors must be investigated. Also, use of different evaporation equipment with better heat transfer properties could be applied. For vacuum distillation, a steam ejector with a small vacuum pump versus a large vacuum pump alone could be tested to reduce operational costs.
▪ Need for hybrid processes
The integration of two or more reclaiming techniques, such as use of ion exchange or ED with thermal reclaimers to eliminate the HSS neutralization step, can be investigated. This might involve treatment of amine solvent with ion exchange/ED to remove HSSs. The HSS-free amine would be treated further in a thermal reclaimer to remove other degradation products and recover amine. Also, the sequence applied can be reversed. Process integration between the amine process and thermal reclaimer could be applied to utilize the energy exchange so that the external utility consumption and operating costs can be reduced.
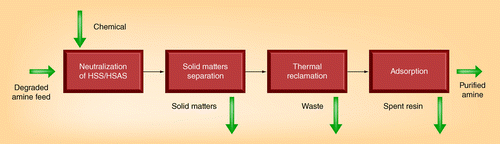
Combinations of different methods for controlling the degradation products would be feasible by using amine reclamation and solvent degradation inhibition. A new integrated technology for amine reclamation, as shown in , was tested at the University of Regina. Integration between neutralization treatments for HSSs, physical separation of solids and thermal reclamation, followed by adsorption treatment, is used.
Table 1. Comparison of heat-unstable and -stable salts.
Table 2. Ability comparison of different contamination mitigation methods.
Table 3. Development of ion-exchange reclaimer.
Table 4. Difference between natural gas and CO2 capture processes.
Table 5. Merits and limitations of thermal reclamation.
Table 6. Development of classical thermal reclaimer.
Table 7. Advantages and disadvantages of solvent thermal reclamation.
Group of salts that are formed from reactions of amine with its acidic degradation products. These salts are not regenerated under stripper conditions, leading to gradual loss of solvent capacity and introduction of operational problems in the amine system.
Undesired side reactions of active amine with flue gas impurities (e.g., CO2, O2, SOx and NOx) causing molecular deterioration after repeated use and long exposure during the capture process.
Purification method that uses current and ion-selective membranes to separate the ionic degradation species, such as heat-stable salts, from the degraded amine solvent.
Adsorption purification method that uses special types of resins to adsorb the heat-stable salt anions, ionic degradation products, from the degraded amine solvent.
Distillation purification method to separate the amine solvent from its degradation products by means of heating/steam under atmospheric or vacuum pressures.
Solvent degradation was managed in the early years by change-over and purge/feed of solvent, which have been proven to be unattractive options from technical and economic points of view. | |||||
▪ For some time, mechanical filtration and activated carbon were thought to reduce the effects of degradation on amine plant operation; however, this is inaccurate. | |||||
▪ Online neutralization to avoid the presence of heat-stable salts (HSSs) was used for some time, but it has introduced many operational problems and, therefore, has been discontinued. | |||||
▪ Solvent degradation inhibition seems to be one method of protecting solvent and will see more applications in the future with development of new inhibitors. | |||||
▪ Ion exchange and electrodialysis purification could be utilized to reduce HSSs and some degradation products; however, these technologies are not suitable for removal of the other degradation products, and this would make it challenging for these processes to be integrated into a CO2 capture plant. | |||||
▪ At present, thermal reclamation is the only technology that can remove all degradation products, HSSs and nonvolatile impurities (i.e., fine solid particles, corrosion products and salts). Therefore, thermal reclamation is likely the most attractive purification option for CO2 capture plants. | |||||
▪ Primary single amines can be thermally reclaimed at atmospheric pressure, but vacuum reclamation must be used for secondary and tertiary amines to avoid thermal degradation. In cases of blended solvents (i.e., primary/secondary or tertiary amines), solvent reclamation becomes a difficult task for purging methods. However, solvent degradation products can be handled by some current thermal processes. | |||||
▪ Extraction can be applied to effectively separate HSSs and neutral degradation products from amine using proper extractant and diluent combinations with extracting conditions as demonstrated in International Test Centre for CO2 Capture’s patented work. | |||||
▪ Effective degradation inhibitors such as those developed by the International Test Centre for CO2 Capture can protect up to 98% of amine from being degraded, thereby reducing reclaimer load in CO2 capture operations. | |||||
▪ Combined reclamation processes have seen steady growth; however, they are only applicable in certain cases. | |||||
▪ Many solvent reclamation technologies are under improvement/development with the aim of enhancing the process technology’s key performance parameters such as solvent recovery and utility consumption reduction, and efforts are also being made to develop simple and easy to operate processes. | |||||
▪ Many methods of amine reclamation have been used, and each one has a different capacity for removing contaminants, as illustrated in . It is clear that a single process cannot remove all degradation products. Some processes are not attractive due to their limited ability to remove the contaminants. | |||||
Acknowledgements
The authors would like to acknowledge the research support over the past many years to the International Test Centre for CO2 Capture by the following organizations: Natural Sciences and Engineering Research Council of Canada, Canada Foundation for Innovation, Saskatchewan Ministry of Energy & Resources (Canada), Western Economic Diversification (Canada), EnCana Energy Inc. (Canada), E.ON Energy (UK), RWE Corp. (UK), Saudi Aramco (Saudi Arabia), Saskatchewan Power Corporation (Canada), HTC Purenergy (Canada), StatOil Hydro (Norway), SaskFerco Inc. (Canada), Sulzer Chemtech (Switzerland), Fluor Corporation (USA), the Canada Centre for Mineral and Energy Technology, Alberta Energy Research Institute (Canada), and the Research Institute of Innovative Technology for the Earth (Japan). W ElMoudir would also like to acknowledge the financial assistance received from HTC Purenergy and the Government of Libya for his PhD program.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Astarita G. Chapter 14: process selection. In: Gas Treating with Chemical Solvents. John Wiley & Sons, Inc., Hoboken, NJ, USA, 356–388 (1983).
- Wilson M, Tontiwachwuthikul P, Chakma A et al. Test results from a CO2 extraction pilot plant at boundary dam coal-fired power station. Energy29(9–10),1259–1267 (2004).
- Bacon TR. Amine solution quality control through design, operation and correction. Presented at: Laurance Reid Gas Conditioning. Norman, OK, USA, 2 March 1987.
- Cummings AL, Mecum SM. Increasing profitability and improving environmental performance by maintaining amine solvent purity. Presented at: 50th Annual Laurance Reid Gas Conditioning Conference. Norman, OK, USA, 27 February–1 March 2000.
- Supap T, Saiwan C, Idem R, Tontiwachwuthikul P. Part 2: Solvent management: solvent stability and amine degradation in CO2 capture processes. Carbon Management2(5),551–566 (2011).
- Ciferno JP, Fout TE, Jones AP, Murphy JT. Capturing carbon from existing coal-fired power plants. Chem. Eng. Prog. April, 33–34 (2009).
- Cummings AL, Smith GD, Nelsen DK. Advances in amine reclaiming – why there’s no excuse to operate a dirty amine system. Proc. Laurance Reid Gas Conditioning Conference227–244 (2007).
- Jouravleva D, Davy P. Impact of continuous removal of heat stable salts on amine plant operation. 50th Annual Laurance Reid Gas Conditioning Conference. Norman, OK, USA, 27 February–1 March 2000.
- Abdi MA, Golker MM, Meisen A. Improve contaminant control in amine systems. Hydrocarb. Process.102 (2001).
- Kohl AL, Nielsen RB. Gas Purification (5th Edition). Gulf Publishing Company, Houston, TX, USA (1997).
- Shao J, Lu G, Ye M. Removal of heat stable salts – a solution to amine plant operational problems. Chem. Eng. Petroleum Natural Gas J. December (2002).
- Harston JD, Ropital F. Amine Unit Corrosion in Refineries (EFC 46). Woodhead Publishing, Cambridge, UK (2007).
- Ballard D. How to operate an amine plant. Hydrocarb. Process. April, 137–144 (1966).
- Dow. Gas Conditioning Fact Book. Dow Chemical Company, Midland, MI, USA (1962).
- Scheirman WL. Filter DEA treating solution, Hydrocarb. Process. August, 65–66 (1973).
- Perry D. What you should know about filters. Hydrocarb. Process. April, 145–148 (1966).
- Berlie EM, Estep JW, Ronicker FJ. Preventing MEA degradation. Chem. Eng. Prog.61(4),82–85 (1965).
- Asperger RG, Liu HJ, Dean JW. Accurate, reproducible measurment of reduced corrosion in gas treating amine systems after application of judicious neutralization techniques. Presented at: AICHE 1995 Spring National Meeting, Houston, TX, USA, 21–23 March 1995.
- Liu HJ, Dean JW, Bosen SF. Neutralization technology to reduce corrosion from heat stable amine salts. Presented at: NACE International, Corrosion 95. Orlando, FL, USA, March 1995.
- Burns D, Gregory RA. The UCARSEP process for on-line removal of non-regenerable salts from amine units. Presented at: Laurance Reid Gas Conditioning Conference. Norman, OK, USA, March 1995.
- Cummings AL, Mecum SM. Remove heat stable salts for better amine plant performance. Hydrocarb. Process. August, 63–67 (1998).
- Verma N, Verma A. Amine system problems arising from heat stable salts and solutions to improve system performance. Fuel Process. Technol.90(4),483–489 (2009).
- Kindrick RC. A Prolonged Oxidation Test on Amine Solutions Resistant to Oxidation. The Girdler Corporation, Gas Processing Division, Process Development and Research Labratories, Louisville, KY, USA (1950).
- Kindrick RC, Atwood K, Arnold MR. The Relative Resistance to Oxidation of Commercially Avilable Amines. The Girdler Corporation, Gas Processing Division, Process Development and Research Labratories, Louisville, KY, USA (1950).
- Blachly CH, Ravner H. The Stabilization of Monoethanolamine Solutions for Submarine Carbon Dioxide Scrubbers. US Naval Research Labratory, Washington, DC, USA (1964).
- Keller AE, Kammiller RM, Veatch FC, Cummings AL, Thompsen JC, Mecum SM. Heat-stable salt removal from amines by the HSSX process using ion exchange. Presented at: Laurance Reid Gas Conditioning Conference. Norman, OK, USA, 2–4 March (1992).
- Hendriks C. Carbon Dioxide Removal from Coal-Fired Power Plants (1st Edition). Springer, NY, USA (1994).
- Uyanga IJ, Idem RO. Studies of SO2- and O2-induced degradation of aqueous MEA during CO2 capture from power plant flue gas streams. Ind. Eng. Chem. Res.46(8),2558–2566 (2007).
- Reddy S, Johnson D, Gilmartin J. Fluor’s Econamine FG PlusSM technology for CO2 capture at coal-fired power plants. Presented at: Power Plant Air Pollutant Control Mega Symposium. Baltimore, MD, USA, 25–28 August 2008.
- Burns D, Gregory A. Waste minimization and compliance benefits to be gained from a good amine management program. Presented at: 1995 NPRA Environmental Conference. San Francisco, CA, USA, 15–17 October 1995.
- Price J, Burns D. Clean amine solvents economically and online. Hydrocarb. Process.74(8),140–141 (1995).
- Parisi P, Bosen S. Electrodialysis – effective amine reclamation with minimal operational impact. Presented at: 85th GPA Annual Convention. Grapevine, TX, USA, 15–17 March 2006.
- Parisi P, Bosen S. Amine reclamation with minimal operational impact through electrodialysis. Presented at: Laurence Reid Gas Conditioning Conference. Norman, OK, USA, 27 February–1 March 2006.
- Bedell SA, Worley CM, Darst K, Simmons K. Thermal and oxidative disproportionation in amine degradation – O2 stoichiometry and mechanistic implications. Int. J. Greenhouse Gas Control5(3),401–404 (2011).
- Blake RJ, Rothert KC. Reclaiming monoethanolamine solutions. Presented at: Laurance Reid Gas Conditioning Conference. Norman, OK, USA, 1962.
- Butwell KF. How to maintain effective MEA solutions. Hydrocarb. Process.47(4),111–113 (1968).
- Wonder DK, Blake RJ, Frager JH, Tierney JV. An approach to monoethanolamine solution control: chemical analysis and its interpretation. Presented at: Laurance Reid Gas Conditioning Conference. Norman, OK, USA, 1959.
- Jefferson Chemicals. Monoethanolamine reclaiming part II design considerations. Hydrocarb. Process. Refinery42(10),225 (1963).
- Blake RJ. Why reclaim monoethanolamine solutions? Oil Gas J.9,130–134 (1963).
- Millard MG, Beasley T. Contamination consequences and purification of gas treating chemicals using vacuum distillation. Presented at: Laurance Reid Gas Conditioning Conference. Norman, OK, USA, 1–3 March 1993.
- Meisen A, Abedinzadegan M, Abry RGF, Millard MG. Degraded amine solutions: nature, problems and distillative reclamation. Presented at: Laurance Reid Gas Conditioning Conference. Norman, OK, USA, 3–6 March 1996.
- Lohwater RK. Advances in the technology of molecular distillation. Vacuum17(1),1–3 (1967).
- Hollo J, Kurucz E, Borodi A. The Application of Molecular Distillation. Akadémiai Kiadó, Budapest, Hungary (1971).
- Yang ST, White SA, Hsu ST. Extraction of carboxylic acids with tertiary and quaternary amines: effect of pH. Ind. Eng. Chem. Res.30(6),1335–1342 (1991).
- Reisinger H, King CJ. Extraction and sorption of acetic acid at pH above pKa to form calcium magnesium acetate. Ind. Eng. Chem. Res.34(3),845–852 (1995).
- Bora MM, Dutta NN, Bhattacharya KG. Extraction of 7-aminocephalosporanic acid with secondary, tertiary, and quaternary amines. J. Chem. Eng. Data43(3),318–324 (1998).
- Kyuchoukov G, Marinova M, Molinier J, Albet J, Malmary G. Extraction of lactic acid by means of a mixed extractant. Ind. Eng. Chem. Res.40(23),5635–5639 (2001).
- Li Z, Qin W, Dai Y. extraction behavior of amino sulfonic acid by tertiary and quaternary amines. Ind. Eng. Chem. Res.41(23),5812–5818 (2002).
- Keshav A, Chand S, Wasewar KL. Recovery of propionic acid from aqueous phase by reactive extraction using quarternary amine (Aliquat 336) in various diluents. Chem. Eng. J.152(1),95–102 (2009).
- Yankov D, Molinier J, Albet J, Malmary G, Kyuchoukov G. Lactic acid extraction from aqueous solutions with tri-n-octylamine dissolved in decanol and dodecane. Biochem. Eng. J.21(1),63–71 (2004).
- Hong YK, Hong WH. Removal of acetic acid from aqueous solutions containing succinic acid and acetic acid by tri-n-octylamine. Sep. Purif. Technol.42(2),151–157 (2005).
- Yunhai S, Houyong S, Deming L, Qinghua L, Dexing C, Yongchuan Z. Separation of glycolic acid from glycolonitrile hydrolysate by reactive extraction with tri-n-octylamine. Sep. Purif. Technol.49(1),20–26 (2006).
- Keshav A, Wasewar KL, Chand S. Extraction of propionic acid with tri-n-octyl amine in different diluents. Sep. Purif. Technol.63(1),179–183 (2008).
- Keshav A, Wasewar KL, Chand S, Uslu H. Effect of binary extractants and modifier–diluents systems on equilbria of propionic acid extraction. Fluid Phase Equilibria275(1),21–26 (2009).
- Idem R, Wilson M, Tontiwachwuthikul P et al. Pilot plant studies of the CO2 capture performance of aqueous MEA and mixed MEA–MDEA solvents at the University of Regina CO2 capture technology development plant and the Boundary Dam CO2 capture demonstration plant. Ind. Eng. Chem. Res.45,2414–2420 (2006).
- Cummings AL, Street D, Lawson G. Contaminants and their effects on operations – yes! You can have better operating amine and glycol systems! Presented at: The Brimstone Sulfur Conference. Banff, Alberta, Canada, May 2003.
- Cummings AL, Waite SW, Nelsen DK. Corrosion and corrosion enhancers in amine systems. Presented at: The Brimstone Sulfur Conference. Banff, Alberta, Canada, April 2005.
- Pearson H, Dandekar S, Shao J, Norton D. Case study of effects of bicine in CO2 only amine treater service. Presented at: Laurance Reid Gas Conditioning Conference. Norman, OK, USA, February 2005.
- Reddy S, Scherffius J, Freguia S, Roberts C. Fluor’s Econamine FG PlusSM technology an enhanced amine-based CO2 capture process. Presented at: The Second National Conference on Carbon Sequestration. Alexandria, VA, USA, 5–8 May 2003.
- Dandekar S, Shao J. Continuous removal of contaminants from amine solutions. Petroleum Technology Quarterly December, 81–87 (2011).
- Morgan C, Klare T. Chloride removal from DEA by ion exchange. Presented at: Laurance Reid Gas Conditioning Conference. Norman, OK, USA, March 1977.
- Jameh AA. Amine solution recovery package and controlling corrosion in regeneration tower. World Acad. Sci. Eng. Technol.69,107–110 (2010).
- Huntsman Performance Products. Diglycolamine® Agent Product Information. Huntsman Performance Products, The Woodlands, TX, USA, 1079–1399 (2005).
▪ Patents
- Paulsen HC, Holtzelaw JB, McNamara TP: US270175008 (1955).
- Anderson HM: US289277530 (1959).
- Rooney PC: US591238715 (1999).
- Singh KP: US353526020 (1970).
- Faucher JA: US484077720 (1989).
- Soria J: US576654816 (1998).
- Idem R, Tontiwachwuthikul P, Saiwan C, Supap T: WO065218A128 (2009).
- Bedell SA, Tsai SSK: US480828428 (1989).
- Bedell SA, Tsai SSK: US481405121 (1989).
- Roy V, Hakka LE, Sarlis JI: US529240708 (1994).
- Gregory RA, Cohen MF: US591061108 (1999).
- Byszewski CH: US0020625 A121 (2002).
- Beasley T, Merritt DA: US515288706 (1992).
- Beasley T, Merritt DA: US515864927 (1992).
- Beasley T, Merritt DA: US544160515 (1995).
- Beasley T, Merritt DA: US538920814 (1995).
- Abry RG, Beasley TS, Carlson SW, Kresoyak SG: US599360830 (1999).
- Razzaghi M, Kresnyak SG, Keast BA, Giles TW: WO0076624 A221 (2000).
- Razzaghi M, Kresnyak SG, Keast BA, Giles TW: US6508916 B121 (2003).
- Van Grinsven BFA, Van Heeringen GJ: US615299428 (2000).
- Yan TY: US513770211 (1992).
- Yan TY: US510855128 (1992).
- Pottiez F, Verbeest R: US366493023 (1972).
- Van Scoy RW: US365846225 (1972).
- Turoff ML, Cummings AL, Waite SW, Horan RL: US7323600 B129 (2008).
- Claud G, Blanc A: US529295808 (1994).
- Fuchs E, Witzel T, Stadler KP: US548103702 (1996).
- Rooney PC: US6353138 B105 (2002).
- Idem R, Tontiwachwuthikul P, Saiwan C, Supap T, Pitipuech P: US8105420B2 (2012).
- Saiwan C, Idem C, Tontiwachwuthikul P, Akkarachalanont P, Supap T: USPCT/CA2012/0000632012 (2012).
- Coberly SH, Laven TH, Cummings AI: US578886404 (1998).
- Brown CJ: US467350716 (1987).
- Keller AE: US504529103 (1991).
- Cummings AL, Veatch FC, Keller AE, Thompson JC, Severson RA: US516208410 (1992).
- George JT: US6245128 B112 (2001).
- Sarlis NJ: WO2007/104134 A220 (2007).
- Anderson HM, Draemel FC, Lyons DJ: US291446924 (1959).
- Sokolik JE, Kosseim AJM, Awtood GR: US438938321 (1983).
- Burgers KL, Chakravarti S, Williams WR: US0148068 A128 (2007).
▪ Websites
- MPR Services, Inc. Amine Headache # 1 Understanding Amine System Terminology. www.mprservices.com/PDFs/amine_headache1.pdf
- IDSWater. Ecotec AmiPur for CO2 Recovery, 2011. www.idswater.com/water/us/carbon_recovery/933/products.html
- ChemGroup, Inc. www.chem-group.com/services/amines.tpl
- Amine Purification Services. What is AmiPur®-PLUS? http://aminepurification.com/whatisamipur.html