Abstract
Cancer Therapeutics CRC Pty Ltd (CTx), established in 2007, is Australia’s first translational drug-discovery and development company. It was formed to bridge the gap between leading cancer research and the discovery and development of the next generation of cancer drugs, precision small-molecule drugs. These small-molecule drugs are expected to make up half of the global market in cancer drugs within 10 years. Funded under the Australian Government’s Cooperative Research Centres scheme, CTx has garnered approximately AU$ 148 million in cash and in-kind contributions from the federal government and its participating institutes to operate for 7 years. These funds are being used to expand and integrate previously separate drug-discovery and development centers into one large multidisciplinary organization with the requisite resources and capabilities to make a significant impact in the global cancer drug-discovery industry.
The WEHI Biotechnology Centre contains state-of-the-art medicinal chemistry research services and 14 custom-built fume hoods, as well as the high-throughput screening facilities.

(A) The installed and commissioned Zymark Mini Staccato system and (B) the Perkin Elmer Minitrak IX at the WEHI laboratories.

(A) The Bundoora labs: only a few years old and well equipped. NMR access is within metres of the main laboratory (shown). (B) Fumehoods are set up with in-house dry nitrogen, compressed air, recirculating chilled water, in-house vacuum and 12-turn needle valves for fine-controlled delivery of gases. As can be seen, this makes chemists happy! The laboratories are equipped with, for example: Bruker 300 MHz UltraShield Avance II NMR Spectrometer with Autosampler, Argonaut Flashmaster II, Waters Alliance Analytical HPLC with an additional ELS Detector, Finnigan LCQ Advantage Max LCMS, Mettler Toledo 12/24 MiniBlock for multiple parallel synthesis, CEM Microwave Reactor, Waters-Micromass ZQ 3100 Mass-directed Auto Purification platform with PDA and quadrupole mass analyser (ZQ detector) and autosampler and automated mass-directed fraction collector, Thales-Nano H-Cube continuous Flow Hydrogenator, Bruker Tensor Fourier Transform IR with ATR Bellingham and Stanley ADP220 Polarimeter, Isco Combi Flash Rf Purification System, CEM Microwave reactor LabMate and CEM Microwave reactor Explorer 24 autosampler and Braun Solvent Purification System.

For decades, Australia has been home to many world-leading cancer research biologists. However, for several reasons, this has not translated into a corresponding output in drug discovery. In the past few years, a number of organizations, mainly small biotechnology companies and departments within academic research institutes, have tried to begin the process of drug discovery using research from their respective institutes. These efforts have been difficult to fund and now tend to run largely as contract research organizations or fee-for-service operations, with little ownership in the outcome of the work.
Cancer Therapeutics CRC Pty Ltd (CTx) aims to dramatically change the paradigm in Australia by using significant long-term funding to gather the resources, capabilities and first-class expertise required to springboard an effective and sustainable drug-discovery operation Citation[101]. This effort will potentially see a number of cancer drug candidates reach clinical evaluation with one or two eventually ending up on the market, successfully treating cancer patients.
To achieve this, CTx’s focus is on the early stages of cancer drug discovery and development, with an eye to producing well-validated novel cancer drug candidates with evidence of safety and effectiveness in preclinical models of cancer. CTx will then license these drug candidates to biotechnology and pharmaceutical companies for further regulatory development followed by clinical trials. For more information, see the websites Citation[102,103].
Critical mass
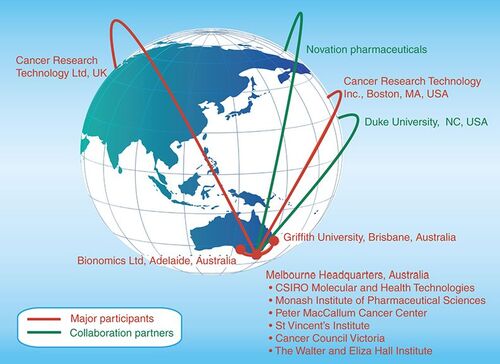
Many of Australia’s leading cancer research institutes have come together to contribute to CTx, both as investing shareholders in the company and to provide people, expertise and facilities to further CTx in its mission. These institutes are:
Walter and Eliza Hall Institute of Medical Research (WEHI)
St Vincent’s Institute of Medical Research
Monash University’s Institute of Pharmaceutical Sciences (MIPS)
Peter MacCallum Cancer Institute
Griffith University
Commonwealth Scientific and Industrial Research Organisation (CSIRO)
Bionomics Ltd
Cancer Research Technology Ltd (CRT)
Cancer Council of Victoria
Cancer Therapeutics CRC Pty Ltd also has a number of international collaboration partners. These global interactions are depicted in .
It is often the case that throwing together a bunch of institutes and hoping for a center of excellence is a concept that can receive funding but not necessarily work. A cynic might wonder why CTx is different. The answer here is that the process required to successfully enter the CRC scheme was extraordinarily rigorous and took place over a number of years: only participants who can work well together and have the same vision to succeed could survive this process.
Road from promising target to promising drug candidate
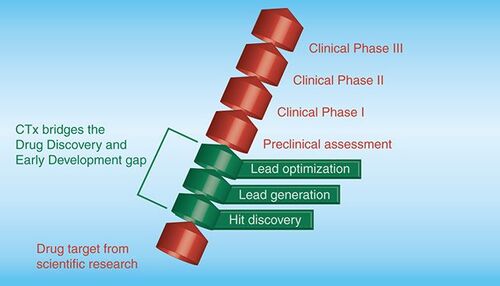
As shown in , the path from target to clinic is long and arduous. CTx hopes to increase the efficiency of this process by specializing in early drug discovery, producing optimized lead compounds engendered with favorable progression properties. CTx works closely with all prominent Australian cancer research institutes, and some overseas, to identify new cancer targets suitable for drug discovery. After an extensive review of the target that takes in the probability of a screen based on the target yielding suitable hits, the commercial potential of these hits and the intellectual property position, a committee of CTx and independent cancer drug-discovery experts decides whether to progress the target into an initial high-throughput screening phase. CTx has two screening centres, one at WEHI, Melbourne and the other at Griffith University, Brisbane. CTx funds have been used to expand, integrate and jointly manage CTx’s cancer projects in these two leading independently operating centres. WEHI and Griffith University staff run high-throughput screens, which search for hit compounds that interact with the selected target of choice. The compound library consists of 250,000 carefully chosen drug-like compounds that can be brought to the task Citation[1].
The infrastructure of the high-throughput chemical screening (HTCS) facilities have been designed to provide maximum flexibility with regard to the types of assays and assay technologies that can be employed while still providing high levels of throughput. They can accommodate assays based on both molecular targets (e.g., enzymes, receptors and protein–protein interactions) and mammalian cells.
The range of assay technologies supported for formatting molecular screens includes, AlphaScreen, time-resolved fluorescence (DELPHIA and LANCE), fluorescence polarization, steady-state fluorescence, photometry, chemiluminescence, ELISA, scintillation proximity and filtration-based separations. For cell-based screens, supported assays include reporter genes (e.g., luciferase, fluorescent proteins, alkaline phosphatase and β-galactosidase), BRET (e.g., protein–protein interactions) or assays measuring biochemical responses such as cell-surface antigen expression, cytokine expression, cell proliferation and cytotoxicity. The group has considerable expertise in developing and formatting high-capacity assays. In addition, for many projects it is planned that the staff from the HTCS facilities will work with the project originator to develop the high-capacity assays necessary for the screening campaign. The infrastructure of the HTCS facilities has been designed to deliver maximum throughput with assays formatted in 384-well microtiter plates. However, assays formatted in 96-well microtiter plates can also be accommodated. For assays formatted in 384-well microtiter plates, assay volumes as small as 5 µl can be used, reducing the consumption of precious and expensive reagents and thereby lowering the overall cost of the screening campaign. Downstream assessment of direct binding kinetics is undertaken using a Biacore S51, which is sensitive enough to be able to detect the binding of small molecules, while thermodynamic analysis uses isothermal calorimetry.
Assay automation systems
Cancer Therapeutics CRC Pty Ltd is well equipped to undertake high-throughput screening. For example, at the Bundoora site, the HTCS facility has two core automation systems, a Zymark Mini staccato system and a Perkin Elmer Minitrak IX . These automation systems were chosen to complement each other’s strengths and weaknesses, as well as to provide some redundancy in function. The Mini Staccato system provides the flexibility necessary to automate a wide range of assay technologies and to run cell-based assays under sterile conditions. The Mini Staccato system has been integrated with an Envision 2100 plate reader providing online measurement for TRF, FP, photometric, fluorescence and chemiluminescence-based assays.
The Mini Staccato system also provides a cherry-picking capability allowing fully automated access to each individual compound in the discovery library for confirmation and follow-up studies. The Minitrak IX provides the capability to run simple mix and read assays and routine plate-replicating tasks at high throughput; this system is also the most efficient way of automating ELISA and DELPHIA assays. The Minitrak IX is integrated with a Multiprobe II and a Fusion Alphaplate reader. The Multiprobe II provides a secondary cherry picking capability and a means of efficiently automating statistical assay optimization methods. The Fusion Alpha is used for on-line measurement of Alphascreen, steady-state fluorescence, photometric, luminescent and BRET assays.
Data management
ActivityBase, an Oracle-based data management system is used as the primary method of managing the screening work-flow, data processing and data-mining activities of high-throughput screening. ActivityBase is also used for managing the inventory of the lead-discovery library.
Medicinal chemistry optimization
The primary screening hits that result from an HTS campaign using the above equipment are generally triaged through a battery of control and secondary screens in order to remove most of the less interesting compounds. At this point, CTx’s medicinal chemists will typically analyse the screening library for development potential and for signs of early structure–activity relationships, and then source commercially available analogues. Several of the most potent compounds will be tested in cellular models of cancer, allied to measurement of appropriate biomarkers, in order to determine whether the compounds do inhibit the selected target and actually produce the desired cellular response. At this early stage cell-based activity is not expected but some indication of in vitro biomarker response is highly desirable. Much of this work is undertaken at the Peter MacCallum Cancer Centre.
After one or more interesting compound hit series are identified, the serious medicinal chemistry work begins as the project progresses into lead generation. CTx is fortunate to have approximately 25 expert chemists on task in three main centers: WEHI , Griffith University and MIPS.
This significant chemistry capability addresses an issue that arose early in the CRC’s lifetime – an initial lack of pharmaceutical industry-trained medicinal chemists in Australia available to CTx. Thus, CTx has set out to recruit a coterie of chemists, ironically at the same time as several large pharmaceutical companies in the UK were down sizing their internal medicinal chemistry teams. This enabled CTx to successfully recruit to its ranks; making essential additions to the already excellent teams at the three CTx centers. Coupled with well-credentialed medicinal chemists such as Simon Campbell and Bill Denny on the Scientific Advisory Board, CTx medicinal chemistry is well prepared for the rigors of drug discovery.
During the lead-generation process, compounds are subjected to increasingly stringent assessment as they progress into lead optimization. Full selectivity profiles, pharmacokinetics, metabolite profiling and early toxicology are all brought into play alongside assessment of anticancer efficacy in vivo in suitable animal models. MIPS and the Peter MacCallum Cancer Centre play crucial roles here. Early on during this phase, protein production experts at CSIRO are engaged so that protein suitable for crystallization trials can be provided to structure-based drug design experts at St Vincent’s Institute of Medical Research. Here, it is hoped that structure-based drug design can accelerate any given drug-discovery program through enabling rational design of next-generation synthetic targets.
The end result of a lot of medicinal chemistry effort is an optimized lead series of compounds with the right properties for the best exemplar to be considered as a clinical drug candidate.
Commercialization of CTx products
A major participant in CTx, CRT is the technology transfer and drug-discovery company wholly owned by the UK charity Cancer Research UK. CRT operates from London and Boston and has been in the business of technology transfer between academic cancer research centers and the biotechnology/pharmaceutical industry for more than 25 years as a means of successfully developing and partnering many cancer drug candidates into clinical trials and onto the market. An example of a current CRT commercialized drug is the brain cancer drug, temozolimide, sold by Merck. CTx’s association with CRT provides it with an excellent advisor and first-class route to market partner. As CTx’s major commercializing partner, CRT is responsible for finding late-stage development partners for CTx projects and negotiating suitable financial arrangements to reward CTx and its target providers for its drug-discovery efforts.
CTx projects
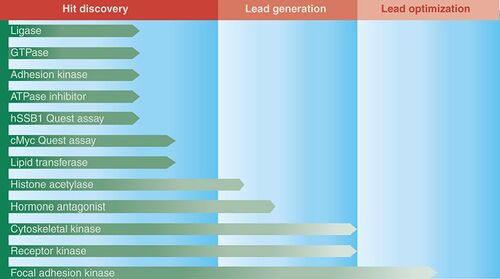
CTx has ten projects that are being actively pursued, while two have been discontinued . Seven are early hit-generation projects, mostly involving high-throughput screening and two are lead-generation projects where several interesting hit compounds are being tested further for the appropriate biological and chemical characteristics required. One project has entered the final lead-optimization phase where medicinal chemists are trying to engineer optimal drug characteristics into three lead series compounds.
After 2.5 years of operation, CTx is now running at full capacity, although inevitably some current projects will not progress to the next phase. However, we are aiming for one or two projects to be licensed out to partners in the next year. CTx is always on the lookout for new projects to keep its pipeline full, working closely with cancer research institutes to identify and generate potential exciting new targets for new drug-discovery projects.
Cancer Therapeutics CRC Pty Ltd also works with local biotechnology companies and has undertaken projects for Bionomics and Cytopia (recently merged with the Canadian company YM Biosciences). CTx has also worked with developers of drug-discovery technology, in particular another Canadian company, Novation, to find new ways of accessing previously undruggable targets. Recently, CTx has in-licensed a project from Duke University in the USA, which was discovered by CRT’s US operations in Boston.
Current position
CTx has been in operation now since 2008. In that time it has successfully expanded several drug-discovery and medicinal chemistry operations in Australia and brought them together in a coordinated and integrated fashion to produce a large, well-resourced organization with considerable expertise focused on early-stage drug discovery and development in the cancer field. CTx has hired a number of pharmaceutical-trained medicinal chemists to complement its existing drug-discovery base, as well as fund a number of additional biologists and other drug-discovery experts to produce a world-class organization capable of delivering on its promise of producing novel cancer drugs from Australia’s research base.
Future perspective
With this new expertise on board and funds to support significant improvements in facilities and equipment, CTx is working with many of Australia’s leading cancer research institutes and biotechnology companies on several promising new projects, one or two of which are already yielding exciting results.
The structure of pharmaceutical drug discovery in recent years has been turned on its ear, with an increasing emphasis of outsourcing the innovation deficitCitation[2]. We believe CTx is well placed to occupy an important position in the future drug discovery paradigm. It is a challenging, exciting and optimistic place to work and has the scientific and management team to drive new molecules to the oncology market place. We are interested in receiving project proposals, from target-based screening to lead optimization (contact Guy Heathers at CTx for further information).
Lead likeness
Concept that for a screening hit, you ideally want a small, simple, fairly polar molecule so that you can optimize potency and selectivity in parallel with pharmacokinetic properties. This notion arose from the relative failure of the combinatorial chemistry cameo in the 1990s that delivered nanomolar screening hits with drug-like complexity but not drug-like properties – and could not be efficiently developed into drugs
PAINS
These compounds are insidious, in that they may interfere with assay readouts via several different mechanisms and give rise to incorrect conclusions about biochemical pathways
Cooperative Research Centers scheme
Australian Government initiative. The CRC Program began in 1991 with the objective ‘to deliver significant economic, environmental and social benefits to Australia by supporting end-user driven research partnerships between publicly funded researchers and end users to address clearly articulated, major challenges that require medium to long term collaborative efforts‘
Innovation deficit
Term relates to the fact that despite unprecedented investment in pharmaceutical R&D, the number of new drugs approved by the US FDA remains low. A lack of innovation has been blamed. Recent analysis of the approximately1,200 new drugs that have been approved by the FDA since 1950 shows that the new-drug output from pharmaceutical companies in this period has been essentially constant and remains so despite the attempts to increase it. This suggests that, contrary to common perceptions, the new-drug output is not depressed, but may simply reflect the limitations of the current R&D model
PKPD and biomarker response
Pharmacokinetic (‘what the body does to the drug’) and pharmacodynamic (‘what the drug does to the body’) analysis is increasingly important earlier in drug discovery. The sooner one can see that meaningful serum levels of a lead compound after dosing correlate for example with lower levels of a mechanistically logical biomolecule (‘biomarker response’), the better
Financial & competing interests disclosure
Jonathan Baell is a Medicinal Chemistry group leader for Cancer Therapeutics CRC Pty Ltd. Guy Heathers is Chief Business Officer for Cancer Therapeutics CRC Pty Ltd and Cathy Sage is Education and Communications Manager for Cancer Therapeutics CRC Pty Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
Bibliography
- Baell JB , HollowayGA. New substructure filters for removal of pan assay interference compounds [PAINS] from screening libraries and for their exclusion in bioassays.J. Med. Chem.53, 2719–2740 (2010).
- Munoz B . Lessons from 60 years of pharmaceutical innovation.Nature Rev. Drug Discov.8, 959–968 (2009).
Websites
- Cancer Therapeutic CRC www.cancercrc.com
- High-throughput chemical screening – Walter and Eliza Hall Institute of Chemical Research www.wehi.edu.au/faculty/advanced_research_technologies/high_throughput_chemical_screening/
- The Medicinal Chemistry Group– Walter and Eliza Hall Institute of Chemical Research www.wehi.edu.au/faculty_members/research_projects/the_medicinal_chemistry_group