Zebrafish Model Provides Insights into Development of Parkinson’s Disease
Singaporean investigators have developed a zebrafish model that can be used for understanding the mechanism underlying the development of Parkinson’s disease (PD) and that could also serve as a suitable vertebrate model for large-scale drug screening.
The study, led by Liu Jianjun and fellow researchers at the Genome Institute of Singapore, describes the first zebrafish model for leucine-rich repeat kinase 2 (LRRK2) mutation-related PD. The team showed that their model is able to overcome some of the limitations of other animal models of LRRK2 and that zebrafish can be used to study the development of human diseases. LRRK2 is known to play an important role in PD, but its biological functions are largely unknown. The researchers performed the first in vivo loss-of-function study of LRRK2 in zebrafish. They cloned the zebrafish homolog of human LRRK2 and performed a series of molecular and genetic analyses to characterize its expression and biological functions, particularly the role of the WD40 domain, in embryonic and neuronal development. Blocking the normal function of LRRK2 resulted in the zebrafish displaying features of neurodegeneration and locomotion defects, similar to those seen in PD patients. In addition, they showed that the fish could be rescued by expressing the normal protein of LRRK2, while the locomotion defect could also be rescued by the administration of Levo-dopa (L-dopa), an agent widely used in the treatment of PD patients.
Liu Jianjun believes the model could hold future potential for use in large-scale drug screening. Speaking to Future Medicinal Chemistry, he said, “Our work demonstrated zebrafish as a suitable model for PD studies. Due to the numerous advantages of the zebrafish model – easy to maintain and breed, rapid development, transparent and development externally – our zebrafish PD model could be utilized for large-scale drug screening for PD in the future.”
“In our study, the truncation of LRRK2 protein and, thus, the neurodegenerative defects in zebrafish are generated by some transient knockdown techniques, which are not suitable for deep biological investigation and large-scale drug screening”.
Commenting on where the researchers intend to next focus their efforts, Liu Jianjun added, “We are therefore working to use zinc finger nuclease technology to generate stable mutant zebrafish lines of LRRK2 to pursue further studies.”
Source: Sheng D, Qu D, Kwok KHH, Ng SS, Lim AYM et al. Deletion of the WD40 domain of LRRK2 in zebrafish causes Parkinsonism-like loss of neurons and locomotive defect. PLoS Genet. DOI:10.1371/journal.pgen.1000914 (2010) (Epub ahead of print).
Computational Method May Shed Light on Gene Regulation
The model reconstructions are shown as a dashed line along with shaded posterior confidence intervals.
Reproduced with permission from Antti Honkela (Aalto University).
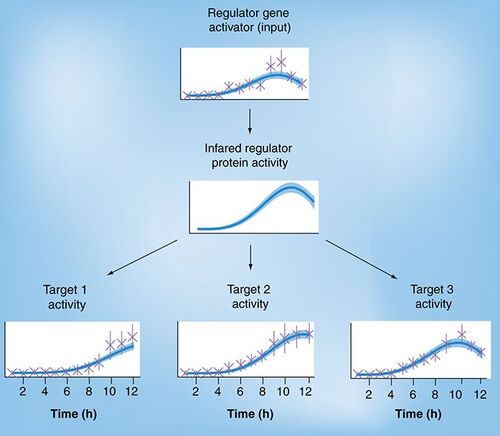
A new computational method has been developed for identifying potential targets of regulator genes using wild-type gene-expression time-series data.
The body’s cell make up depends on how instructions contained in the human genome are read. This process is controlled by gene-regulatory mechanisms, the better understanding of which is crucial for grasping how cells and tissues interpret the information contained in the genome. One important regulatory mechanism concerns genes that actively promote or repress the activity of other genes.
A new study, conducted by researchers at the University of Manchester (UK), Aalto University (Finland) and the European Molecular Biology Laboratory, Heidelberg (Germany), addresses the problem of identifying the targets these regulator genes affect.
The researchers based their method on careful modeling of time series measurements of gene activity. They combined a simple biochemical model of the cell with a probabilistic modeling technique to deal with incomplete and uncertain measurements.
Speaking to Future Medicinal Chemistry about the significance of their findings, Antti Honkela (Aalto University) said, “the paper presents a significantly more sensitive method for detecting active regulation by a transcription factor from time-series expression data. This is especially useful in situations where knockdown mutants are not available or uninformative”
The researchers hope that the work will help convince experimentalists that time-series expression assays can be very informative for inferring regulatory relationships.
The teams now plan to extend the method for combinatorial regulation by multiple transcription factors. “This is a much more difficult problem that usually requires some prior information to constrain the many alternative hypotheses to consider.”, said Honkela, “we recently received funding through the European Research Area Networks in systems biology initiative, along with other European partners in the SYNERGY consortium, to apply these techniques to increase our understanding of nuclear receptors, an important class of transcription factors affecting many physiological and pathological processes.”
Source: Honkela A, Girardot C, Gustafson EH, Liu YH, Furlong EEM, Lawrence ND, Rattray M. Model-based method for transcription factor target identification with limited data. Proc. Natl Acad. Sci. USA 107(17), 7793–7798 (2010)..
Humira® Will Be World’s Best-Selling Drug, Says Report
A recent report has claimed that Abbott Laboratories’ rheumatoid arthritis treatment, Humira®, will be the world’s best-selling drug by 2016.
The report, commissioned by EvaluatePharma, states that revenues from Humira (adalimumab) will rise 9% annually to US$10.1 billion in 2016. Roche’s cancer treatment Avastin® (bevacizumab) is also expected to perform well, despite recent clinical setbacks in the company’s efforts to expand the label. The report claims that 2016 sales will be in the region of US$8.9 billion.
Currently, the world bestseller is Pfizer’s cholesterol-lowering drug Lipitor® (atorvastatin), which will soon be hit by patent expiries. However, the report maintains that the company will still be the biggest in terms of sales in 2016, closely followed by Merck & Co, which completed its US$41 billion merger with Schering-Plough in 2009.
The other top 10 best-selling products include Amgen’s Enbrel® (etanercept) and Prolia® (denosumab), Roche/Biogen Idec’s Rituxan® (rituximab), Roche’s Herceptin® (bevacizumab), Johnson & Johnson/Merck & Co’s Remicade® (infliximab) and Sanofi-Aventis’ Lantus® (insulin glargine). Also making the list are the conventional small-molecule drugs Crestor® (AstraZeneca; rosuvastatin) and Advair/Seretide® (GlaxoSmithKline; salmeterol and fluticasone), which the report states is “proving incredibly resilient to generic challengers”. A surprise entrant to the top sales list is Teva Pharmaceutical Industries, which the report predicts, “will become an increasingly large pharma player as the industry’s patent cliff looms”. The company is anticipated to occupy tenth position over the next 7 years, ahead of the likes of Bristol-Myers Squibb, Eli Lilly and Amgen.
Commenting on the report’s findings, Jonathan de Pass, EvaluatePharma’s chief executive, said that the next 7 years will see “huge growth in sales of complex biologics, driven in part by the premium price they can command and the industry’s productivity in getting these compounds to market”.
Source: Press release: Pfizer reigns supreme after patent cliff as Humira kicks Avastin off top spot: www.evaluatepharma.com/PressRelease_Humira_kicks_Avastin_off_top_spot.aspx
Greece to Slash Medicine Prices
The Greek government has announced that it is to put in place measures aimed at bringing drug prices in line with those of its neighbours, thereby saving €1.96 billion as part of a package to tackle the country’s debt crisis. The measures will see the prices of over 12,500 medicines cut by 20–30%. The drug price commission is to take action to adjust price lists and ensure the reductions reach consumers.
Products whose wholesale price is no more than €1 will not be subject to price cuts, while those with a wholesale price of €1.01–5 will be cut only 3%. For products priced €5.01–100, the reductions will increase incrementally from 20% up to 27% for those over €100. The price cuts will not apply to orphan drugs and medicines produced from blood products will be exempted from price cuts.
The country’s pharmaceuticals market was previously one of the most dynamic in Central and Eastern Europe, with drug spending having increased 64% over the period 2005–2009 to reach €6.24 billion last year. A recent report from Companies and Markets has stated that market growth is expected to drop to 1.5% this year and to total 3.71% during the period to 2014, when the market will reach a value of €7.49 billion.
The report warns of the implications of the recent government action for drug producers. It also adds that the country’s financial crisis has highlighted the “deep culture” of corruption in the health care sector.
When addressing parliament about progress in the country’s applications for fiscal aid from the International Monetary Fund and the EU, the Greek Prime Minister George Papandreou said that his government “will mercilessly strike against waste, corruption and speculation in the health sector” but vowed to maintain healthcare spending.
Pharmaceutical industry leaders in the country have raised concerns that the regulations will result in domestic drug shortages because the marketing of certain products in the country will no longer be economically viable.
Source: Market research reports and company profiles: www.companiesandmarkets.com
Study Shows Pea-15 Protein Regulates T-Cell Proliferation
A key protein that stops T-cell proliferation by blocking the cell’s ability to reproduce has been identified by US researchers. The findings could have far-reaching implications in understanding how the immune response is controlled.
In examining the PEA-15 protein, which acts as a tumor suppressor in certain cancers, the researchers from the University of Hawaii Cancer Research Center (HI, USA), Rutgers University (NJ, USA) and Washington University in St. Louis (MO, USA) found that PEA-15 normally controls lymphocyte (white blood cell) proliferation. In an effort to determine the protein’s normal role, the team studied mice PEA-15 in which the PEA-15 coding region was deleted. They found that those without the protein exhibited spatial learning disabilities and a pronounced increase in lymphocyte proliferation.
Significantly, the researchers also reported that loss of PEA-15 particularly affected T-cells, a group of lymphocytes that are involved in killing invading pathogens and stimulating more long-term immunity.
The PEA-15 protein effectively functions to put a brake on a group of proteins that activate cell cycling and proliferation when they identify a signal from an invading organism. PEA-15-deficient lymphocytes are seen to continue proliferating beyond normal response levels as if they lack the ability to stop.
“Understanding how T-cell expansion is controlled at the molecular level should lead to new methods for controlling the immune response during infection, as well as perhaps helping the development of novel ways to utilize these cells to attack tumors,” said Joe Ramos, the study’s principal investigator. “Dysregulation of PEA-15 function might also play a role in the development or progression of lymphomas or leukemias,” he added, “finding ways to regain normal function of PEA-15 might contribute to identification of new approaches to treat these cancers.”
Source: Pastorino S, Renganathan H, Caliva MJ et al. The death effector domain protein PEA-15 negatively regulates T-cell receptor signalling. DOI: 10.1096/fj.09-144295 FASEB J. (2010) (Epub ahead of print).