Abstract
In the biofilm form, bacteria are more resistant to various antimicrobial treatments. Bacteria in a biofilm can also survive harsh conditions and withstand the host's immune system. Therefore, there is a need for new treatment options to treat biofilm-associated infections. Currently, research is focused on the development of antibiofilm agents that are nontoxic, as it is believed that such molecules will not lead to future drug resistance. In this review, we discuss recent discoveries of antibiofilm agents and different approaches to inhibit/disperse biofilms. These new antibiofilm agents, which contain moieties such as imidazole, phenols, indole, triazole, sulfide, furanone, bromopyrrole, peptides, etc. have the potential to disperse bacterial biofilms in vivo and could positively impact human medicine in the future.
The ability of bacteria to form biofilm has drawn considerable interest from researchers over the past decade [Citation1]. Biofilms are usually made up of diverse microorganisms, which are attached to a surface. These microorganisms are usually embedded in polymeric matrix. Bacteria foul medical devices and implants, such as catheters, components of cardiac pacemakers, artificial heart valves and joints. These implant-associated biofilms can be treated with antibiotics but in certain situations replacement surgeries, which of course come with risks, would be needed [Citation2,Citation3].
Data taken from [Citation88–90].
![Figure 12. Cembranoids library for biofilm inhibition.Data taken from [Citation88–90].](/cms/asset/ee8e9ccf-2f6e-4fd5-b4be-9b1fe5446e69/ifmc_a_12363421_f0004.jpg)
(A) Carolacton structure. (B) Analog 59 structure. (C) Confocal microscopy imaging of Streptococcus mutans UA159 biofilm cells treated with 250 μM: (i) DMSO, (ii) carolacton and (iii) 59.
Reproduced with permission from [Citation104] © American Chemical Society (2014).
![Figure 14. Biofilm inhibition by carolacton. (A) Carolacton structure. (B) Analog 59 structure. (C) Confocal microscopy imaging of Streptococcus mutans UA159 biofilm cells treated with 250 μM: (i) DMSO, (ii) carolacton and (iii) 59.Reproduced with permission from [Citation104] © American Chemical Society (2014).](/cms/asset/628b7475-42cc-49e9-8fca-7ac874aec223/ifmc_a_12363421_f0006.jpg)
A potential antibiofilm drug that can either facilitate the dispersion of preformed biofilms or inhibit the formation of new biofilms in vivo is needed. So far, a plethora of potential antibiofilm agents with unique structures, mainly inspired by natural products, have been developed and shown great promise in dispersing existing biofilms or preventing bacteria from forming biofilms in vitro. In contrast to conventional antibiotics, the majority of the recently developed antibiofilm molecules do not directly affect bacterial survival and thus the expectation is that resistance to these molecules will not readily occur. In the coming years it is hoped that some of these lead compounds would be translated into antibiofilm drugs. A complementary approach to prevent biofilm formation on medical is to passivate the surfaces of the implants with molecules that discourage biofilm formation. In this review, we summarize the current state-of-the-art compounds that are capable of inhibiting biofilm formation of a spectrum of clinically relevant Gram-positive and Gram-negative bacteria.
Biofilm inhibition by small molecules
Nature-inspired synthetic molecules
Nature continues to inspire the discovery of novel compounds with interesting structures and biological activity. These naturally derived compounds have served as scaffolds for the development of a plethora of synthetic therapeutic agents. Currently, many groups are focusing their research on the discovery of novel compounds capable of inhibiting biofilms [Citation4,Citation5]. To date, many antibiofilm compounds have been identified from diverse natural sources, for example, brominated furanones [Citation6], garlic [Citation7], ursine triterpenes [Citation8], corosolic acid and asiatic acid [Citation9], ginseng [Citation10] and 3-indolylacetonitrile [Citation11].
Imidazole derivatives
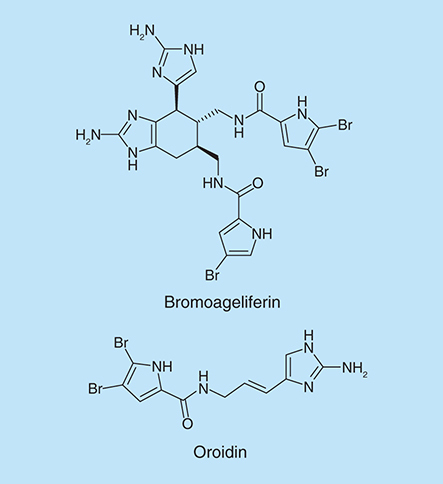
The 2-aminoimidazole functionality is essentially a guanidine mimetic and is found in numerous marine natural products. Prototypical examples in this class include natural products such as bromoageleferin and oroidin (), which were isolated from the sponge Agelas conifer.
The Melander group has developed an array of novel molecular scaffolds that both inhibit and disperse bacterial biofilms [Citation12–16]. The 2-aminoimidazole heterocycle has proven to be crucial for the observed biological activity of these compounds.
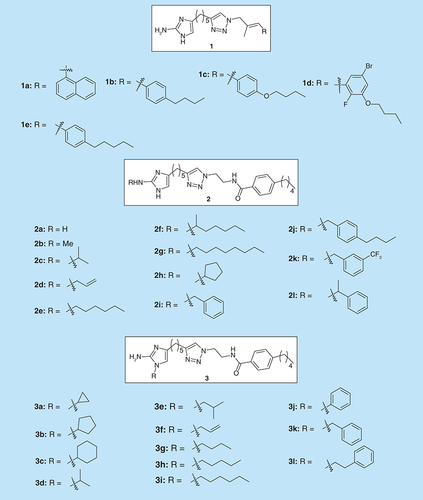
Reyes et al. have synthesized a library of 2-aminoimidazole triazoles (2-AITs) and checked their activity on Acinetobacter baumannii (ATCC 19606) and Methicillin-resistant Staphylococcus aureus (MRSA) [Citation17]. Compounds 1(a-e) () were found to inhibit biofilm formation upwards of 94% at the initial concentration of 100 µM. However, compounds 1(b-d) were also found to have potent antibacterial activities. Compounds 1(a-d) also possessed the highest biofilm dispersal activity at 200 µM, with compound 1c as the most effective at dispersing preformed A. baumannii biofilms with an EC50 of 44.70 µM. In addition these compounds were tested against MRSA (ATCC BAA-44) in biofilm inhibition assays. Compounds 1a, 1c and 1d showed IC50 values of 9.86, 8.55 and 4.50 µM, respectively and were nonmicrobicidal.
Melander et al. synthesized a series of analogs (2b-l, ) of compound 2a, a potent antibiofilm compound [Citation18], and tested them against three MRSA strains: BAA-1770, BAA-1765 and 43 300 (200 µM) [Citation19]. Analogs with alkyl chain substituents of less than four carbons (2b-d) possessed a similar inhibition activity to compound 2a. Analogs substituted with alkyl chains more than four carbons or benzyl substituent exhibited an increased ability to inhibit biofilm formation in comparison to compound 2a, especially analog 2j. The 2-amino substituted library was also screened against MRSA strain for oxacillin resistance suppression activity. Substitution with short aliphatic chains (2c and 2d) led to an increase in synergetic activity with oxacillin against MRSA.
Recently, Melander and colleagues discovered another series of 1,4-disubstituted-2-aminoimidazoles (3a-l, ) and explored their antibiofilm activity against multidrug resistant bacteria [Citation20]. These compounds were found to be active biofilm inhibitors of MRSA with the lead compound 3h exhibiting single digit IC50 value (IC50 = 4.14 ± 2.03 µM).
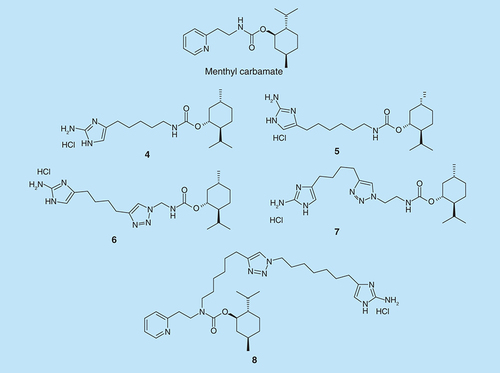
Melander et al. also found a class of molecules based on a menthyl carbamate scaffold that possesses potent nonmicrobicidal biofilm inhibition activity against various staphylococcal strains [Citation21]. A series of blended 2-aminoimidazole (2-AI) head group together with menthyl carbamate moiety were synthesized () and tested for antibiofilm activity. The antibiofilm activity and their ability to disperse mature preformed biofilms were tested against MRSA, Staphylococcus aureus (ATCC# 29213), Pseudomonas aeruginosa (PA14) and A. baumannii (ATCC# 19606) [Citation22]. Compounds 4–7 inhibited biofilm formation in P. aeruginosa (PA14) with IC50 of 18, 18, 58.7 and 40.3 µM, respectively, while 8 inhibited biofilm in A. baumannii with IC50 of 16.7 µM.
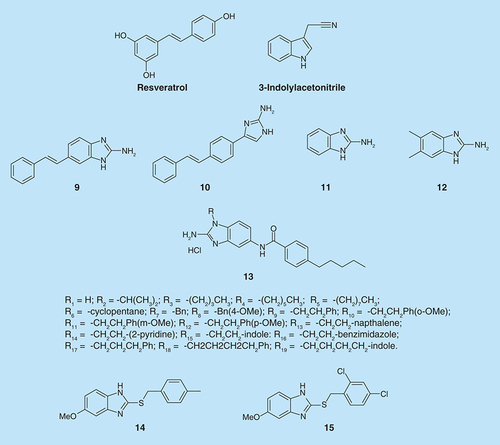
Frei et al. showed that 2-aminobenzimidazoles (2-ABIs) are biofilm inhibitors in P. aeruginosa [Citation23]. Based on the three known inhibitors: bromoageliferin (), 3-indolylacetonitrile and resveratrol (), they proposed a scaffold that combined all of the three compounds. They designed and synthesized a small set of stilbenes containing 2-aminoimidazole or indole moieties (). Compounds 9 and 10 (100 µM) were able to inhibit P. aeruginosa biofilm growth at 24 h by 56 and 48%, respectively.
In order to check which feature in those molecules is responsible for their antibiofilm activity, they synthesized each part separately and found that molecule 11 () exhibited greater activity than the lead compound 9 and almost completely inhibited biofilm formation at 24 h (94% inhibition, IC50= 47 µM). Then, the effect of different substitutions on the ring was investigated. Halide or methyl substitution on 2-ABI aryl ring exhibited greater activity. The most potent biofilm inhibitor identified was compound 12 (IC50= 4.0 µM). It is one of the most active P. aeruginosa biofilm inhibitors known to date. Moreover, it was found that the 2-ABIs, 10 and 11, were capable of strongly dispersing P. aeruginosa biofilms. The mechanism of these 2-ABIs is still not fully understood.
Another modification by the Melander group in the 2-aminobenzimidazole (2-ABI) class of compounds (compounds 13 (R1-R19), ) involved the preparation of N-1-substituted-2-aminobenzimidazole as zinc-dependent S. aureus biofilm inhibitors [Citation24]. The indole derivative (13, R15) was the most potent of these 2-ABIs with IC50 values of 3.7 and 4.4 μM for 43300 and BAA-44, respectively. However, none of these compounds were effective dispersal agents.
Sambanthamoorthy et al. investigated the activities of two structurally related benzimidazole compounds, 14 and 15 (), which possess antibiofilm activity in Vibrio cholerae [Citation25]. Both compounds inhibited biofilm formation in V. cholerae at concentration of 10 µM. Another six human pathogens were examined for the effect of compound 14: P. aeruginosa (CF-145), Klebsiella pneumoniae, Erwinia amylovora, Shigella boydii, MRSA strain USA300 and S. aureus strain Newman [Citation25]. Compound 14 was used at a concentration of 100 µM for the Gram-negative bacteria and 25 µM for S. aureus. Compound 14 inhibited biofilm formation in each of these bacteria in a static biofilm assay, while it did not have an effect of their growth. Biofilm formation by P. aeruginosa strain PA01 on the surface of a medical silicone catheter, under flow conditions, was inhibited by 100 µM of compound 14.
Indole derivatives
Indole, which is generated by the degradation of tryptophan by tryptophanase [Citation26], is an intercellular signal molecule that can affect multiple aspects of some bacterial species [Citation27]. Particularly, it has the potential to inhibit biofilm formation in several clinical relevant bacterial strains. Escherichia coli O157:H7 biofilm formation and bacterial motility was inhibited by indole at a concentration of 500 μM [Citation28,Citation29]. Gene expression in indole-treated biofilms was investigated and it was found that indole reduced expression of cold shock regulator genes (cspGH) by 2.5- to fourfold. Phosphate-related genes of E. coli O157:H7 were also affected by indole [Citation28].
There are many oxygenases in bacteria and indoles are found to be readily converted to hydroxylated indoles by these oxygenases. These hydroxylated products include 5-hydroxyindole and 7-hydroxyindole (see Supplementary Figure 1) [Citation30]. As indole can affect bacterial biofilm formation, the antibiofilm activities of oxidized indole derivatives were investigated. Indole was found to decrease E. coli O157:H7 biofilm formation by sixfold, while 7-hydroxyindole decreased biofilm formation by tenfold [Citation31]. On polystyrene, biofilm formation of E. coli O157:H7 and E. coli K12 could be diminished both by 7-hydroxyindole and 5-hydroxyindole [Citation31]. Specifically, 1000 µM concentration of 7-hydroxyindole caused 27-fold reduction on E. coli O157:H7 biofilm formation while 1000 µM 5-dydroxyindole caused 11-fold reduction. For E. coli K12, bacterial biofilm was inhibited eightfold by 1000 µM 7-hydroxyindole and sixfold by 1000 µM 5-hydroxyindole.
The plant pathogen Rhodococcus sp. BFI 332 produces significant amounts of indole-3-acetaldehyde and indole-3-acetic acid (see Supplementary Figure 1.). Indole-3-acetaldehyde was found to be better than indole at reducing E. coli O157:H7 biofilm formation. E. coli O157:H7 biofilm formation was diminished by tenfold using 100 µg/ml indole-3-acetaldehyde and only by fourfold using 100 µg/ml indole [Citation32].
The plant secondary metabolites, 3-indolylacetonitrile (IAN) and indole-3-carboxyaldehyde (I3CA) (see Supplementary Figure 1), were also proven to reduce E. coli O157:H7 biofilm formation [Citation11]. Indole, IAN and I3CA were compared under the same conditions for their ability to inhibit E. coli O157:H7 biofilm formation. IAN and I3CA were more effective than indole. E. coli O157:H7 biofilm formation was diminished by threefold in the presence of 100 µg/ml indole (see Supplementary Figure 2). Meanwhile, 100 µg/ml IAN and I3CA suppressed E. coli O157:H7 biofilm formation by 24-fold and 11-fold, respectively [Citation11]. In addition, biofilm formation by P. aeruginosa was also inhibited by IAN (2.3-fold) and I3CA (1.9-fold). On the other hand, biofilm formation by P. aeruginosa was induced by indole and 7-hydroxyindole.
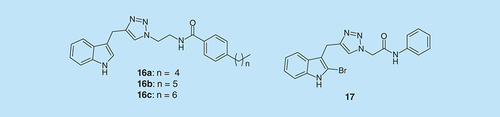
As some compounds with pyrroloindoline-triazole-amide scaffold have been identified to affect bacterial biofilm formation in E. coli, A. baumannii, S. aureus and MRSA [Citation33], indole–triazole-amide analogs were investigated () [Citation34]. Compounds 16(a-c) decreased 50% biofilm growth, relative to control, of S. aureus at the concentrations of 44.4, 173.6 and 174.8 μM, respectively [Citation34]. Also, compound 17 inhibited 12% biofilm formation of E. coli at concentration of 150 μM, about 50% biofilm growth of A. baumannii and MRSA at concentrations of 325.5 and 151.4 μM, respectively [Citation34]. Moreover, these analogs inhibited biofilm formation in a nontoxic way. They did not affect planktonic bacterial growth.
Plants-derived compounds
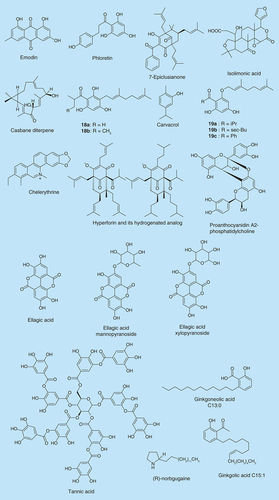
Emodin () is a naturally occurring anthraquinone found in the roots and barks of numerous plants, molds and lichens. Ding et al. showed that emodin significantly inhibits biofilm formation at 20 µM in P. aeruginosa and Stenotrophomonas maltophilia [Citation35]. Cells that were incubated with emodin were detached and dispersed from the surface. It is likely that emodin penetrated into the biofilm and interfered with the quorum sensing (QS) system in P. aeruginosa.
Flavonoids have been shown to exhibit antibiofilm activity. Phloretin (), a flavonoid found in apples was observed to control E. coli O157:H7 biofilm formation by inhibiting the production of fimbriae, which is required for biofilm formation [Citation36]. Lee et al. showed that 50 µg/ml phloretin achieved a 98% decrease in E. coli O157:H7 biofilm without affecting the growth of planktonic cells. Most importantly phloretin also exhibited no inhibitory activity against the commensal E. coli K-12 biofilm [Citation36]. This is particularly advantageous since a potential antibiofilm agent should selectively inhibit pathogenic strains without wiping out the commensal flora [Citation37,Citation38].
Extracts of ginger were shown to reduce biofilm formation in P. aeruginosa strain PA14 by 39–56% without affecting the growth of the cells [Citation39]. Further analysis revealed that the extracts caused a reduction in the extracellular polymeric substance, an increase in swarming motility and reduced levels of cellular c-di-GMP. In that same study, ginger extract showed broad spectrum antibiofilm activity in the Gram positive bacteria S. aureus and Bacillus megaterium and in the Gram negative bacteria E. coli.
The therapeutic effects of the extracts of Hypericum perforatum (also known as St. John's Wort) are well known. Hyperforin is a major constituent of H. perforatum and because it is unstable, it was probably believed that it did not contribute to the therapeutic effects of H. perforatum. Contrary to this notion, Schiavone et al. showed that a stable form of hyperforin, hydrogenated hyperforin (), inhibited the growth and biofilm formation of S. aureus ATCC 29213, MRSA, Enterococcus faecalis ATCC 29212 and S. aureus Ig5 [Citation40]. Treating S. aureus ATCC 29213 and S. aureus Ig5 with 150 and 37.5 µg/ml of the hydrogenated hyperforin, respectively, caused over 50% biofilm reduction. Also, at a concentration of 37.5 µg/ml, hydrogenated hyperforin reduced the biofilms of S. aureus ATCC 43300 and Enterococcus faecalis ATCC 29212 by 47 and 45%, respectively.
Sarkisian et al. have also tested other secondary metabolites from Hypericum spp. for biofilm inhibition [Citation41]. They found that five of these compounds (18a-b and 19a-c, ) inhibited biofilm formation by Staphylococcus epidermidis and S. aureus. The minimum biofilm inhibitory concentrations of these five compounds were lower than 8 μg/ml.
7-Epiclusianone () is a natural compound purified from Rheedia brasiliensis. Murata et al. reported that 250 μg/ml 7-epiclusianone with or without 125 ppm fluoride (F) inhibited Streptococcus mutans biofilm development [Citation42]. 7-Epiclusianone could also reduce the formation of dental caries in vivo.
Isolimonic acid (), a triterpenoid secondary metabolite from citrus species has previously been shown to interfere with QS in Vibrio harveyi and also exhibit a dose-dependent inhibition of biofilm formation with an IC50 value of 94.18 µM [Citation43]. Isolimonic acid inhibited E. coli O157:H7 biofilm formation at IC50 of 19.7 µM. It also demonstrated an ability to modulate the type III secretion system and adhesion of E. coli O157:H7 [Citation44].
Chelerythrine () was isolated from the plant Chelidonium majus and it showed inhibitory effects on biofilm formation of S. aureus ATCC 6538P and S. epidermidis ATCC 35984 in a dose-dependent manner with IC50 values of 15.2 µM and 8.6 µM respectively [Citation45]. Another plant derived compound, proanthocyanidin A2-phosphatidylcholine () was isolated from the medicinal plant Krameria lappacea and was also shown to inhibit biofilm formation in the Staphylococcus strains with EC50 values of 6.9 and 7.6 µM, respectively. Both molecules exhibited no inhibitory activity on the growth of the Staphylococcus strains, as there was no significant difference between growth curves in the presence and absence of sub-minimum inhibitory concentrations (MIC) of the compounds. They also lack the ability to disrupt mature biofilm.
Different adherent S. aureus biofilm forming cells were observed to form scattered clumps when treated with fractionated root extracts of Rubus ulmifolius Schott (Rosaceae-Elmleaf blackberry), a wild shrub native to the Mediterranean. A limited number of published studies have explored the antibacterial properties of R. ulmifolius [Citation46,Citation47]. Quave et al. observed an inhibition of S. aureus biofilm formation by R. ulmifolius extracts with concentrations in the range of 50–200 µg/ml [Citation48]. The extract had no effect on mature biofilms, but when dosed together with selected antibiotics its efficacy against mature biofilms was significantly enhanced. LC-UV/MS/MS analysis of the extract revealed that the antibiofilm activity of the extract was probably due to its constituent ellagic acid and its derivatives (ellagic acid mannopyranoside and ellagic acid xylopyranoside; ).
The polyphenolic compound tannic acid () also inhibits S. aureus biofilm formation without inhibiting cell growth (20 µM) [Citation49]. Tannic acid is found in teas and other plant-derived foods. Black tea, a source of tannic acid, also causes inhibition of biofilm formation in S. aureus. When cells were grown in the presence of tannic acid, an increased level of the protein IsaA was found. IsaA is a putative lytic transglycosylase that has previously been implicated in cleaving peptidoglycan.
Ginkgolic acid C15:1 was found among several plant-derived compounds to inhibit biofilm formation of E. coli O157:H7 at 5 µg/ml without affecting the growth of the cells (see Supplementary Figure 3) [Citation50]. The inhibition was observed to be as a result of the suppression of curli genes and consequently the production of fimbriae. When tested against the commensal E. coli K-12 strain, ginkgolic acid C15:1 had no inhibitory effect but interestingly it boosted biofilm formation. At 5 µg/ml, ginkgolic acid C15:1 inhibited biofilm formation by MRSA and other S. aureus strains but had no inhibitory effect on cell growth.
One of the major components of ginkgolic acid, ginkgoneolic acid (C13:0) has been demonstrated to possess antibacterial activities [Citation51]. He et al. observed that at 2 μg/ml, ginkgoneolic acid inhibited the adherence of S. mutans, a dental pathogen, on saliva-treated hydroxyapatite (S-HA) beads [Citation52]. Ginkgoneolic acid at 32 μg/ml caused 50% or more inhibition of S. mutans biofilm formation. It also caused a change of the biofilm morphology. The study by He et al. therefore demonstrates that ginkgoneolic acid has potential to be used to treat dental caries.
Carvacrol (), a component of oregano essential oil, reduces bacterial motility and virulence at sublethal concentrations [Citation53]. Nostro et al. reported that vapor forms of oregano oil, carvacrol and thymol were effective at inhibiting biofilm formation as well as eradicating the preformed biofilms of S. aureus and S. epidermidis [Citation54–56]. Carvacrol reduces biofilm formation by Chromobacterium violaceum (0.1–0.3 mM), Salmonella typhimurium (0.75–1.25 mM) and S. aureus (0.5–1 mM) [Citation57]. At these concentrations there was no effect on bacterial growth. Preformed biofilms were not affected by the addition of carvacrol. Carvacrol affects genes coding for QS and inhibits the production of acylhomoserine lactones (AHLs), chitinase and violacein in C. violaceum. Thyme oil, oregano oil and carvacrol also reduced the amount of biofilm produced at 0.012% by the S. typhimurium strains [Citation55].
Bgugaine is a pyrrolidine alkaloid extracted from the tubers of Arisarum vulgare [Citation58]. Recently Majik et al. described the synthesis of the N-demethylated form of the natural pyrrolidine alkaloid and explored its QS inhibition in P. aeruginosa [Citation59]. P. aeruginosa showed a decrease in biofilm density, with increasing concentration of (R)-norbgugaine (0–4 mM). Eighty three percent inhibition was observed at a concentration of 1.8 mM and above. P. aeruginosa growth was not affected by norbgugaine. Besides biofilm inhibition, norbgugaine was also found to inhibit motility, pyocyanin, LasA protease and rhamnolipid productions.
Another natural product, casbane diterpene (), has been identified to have antibiofilm activity [Citation60]. Casbane diterpene is an extract of the plant Croton nepetaefolius and was screened against both Gram-positive and Gram-negative bacteria. Casbane diterpene was able to inhibit the biofilm formation of both Gram-positive and Gram-negative bacteria. 125 and 250 µg/ml casbane diterpene significantly reduced biofilm formations by S. aureus and S. epidermidis CECT 4183, respectively. 15.6 and 250 µg/ml of casbane diterpene reduced biofilm formation of K. pneumoniae ATCC 11296 (45%) and P. aeruginosa ATCC 10145 (80%), respectively. Also, biofilm formations of P. aeruginosa CGCT 111, E. coli K12 strains and Pseudomonas fluorescens were reduced by high concentrations of casbane diterpene.
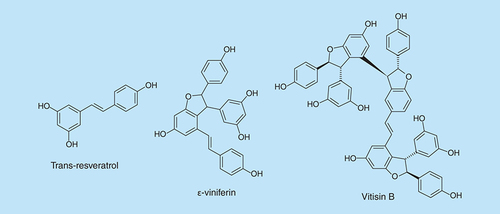
A screen of close to 500 plant extracts revealed that, trans-resveratrol (), a major component of the extracts of Carex dimorpholepsis, demonstrated antibiofilm formation activity against E. coli O157:H7 [Citation61]. Interestingly, no inhibitory effect was observed against commensal E. coli strains. Resveratrol is found in red grapes, peanuts and some woody plants and has been reported to have other antibacterial and antioxidant properties in humans [Citation62,Citation63].
Resveratrol oligomers are also found to have nutritional benefits [Citation64,Citation65]. ε-Viniferin, a dimer of resveratrol (), is found in grape vines and carex plants and has been reported to have fungicidal and antioxidant activities [Citation66]. Biofilm inhibition experiments showed that trans-resveratrol and ε-viniferin inhibited the biofilm formation of two P. aeruginosa strains, PAO1 and PA14, in a dose-dependent fashion [Citation67]. Trans-resveratrol and ε-viniferin (50 μg/ml) inhibited biofilm formation by P. aeruginosa PAO1 and P. aeruginosa PA14 by 92 and 82%, respectively. Also ε-viniferin inhibited E. coli O157:H7 biofilm formation at 10 μg/ml by 98%, without affecting planktonic growth.
Lee et al. [Citation68] tested trimers and tetramers of resveratrol for biofilm inhibition; they found vitisin B () to inhibit biofilm formation of E. coli O157:H7 and P. aeruginosa PA14. In E. coli, 5 μg/ml vitisin B inhibited biofilm formation by more than 90%. In addition, at concentrations lower than 50 μg/ml, visitin B did not inhibit E. coli O157:H7 growth. The mechanism of the inhibition of E. coli O157:H7 biofilm formation was found to involve inhibition of fimbriae production.
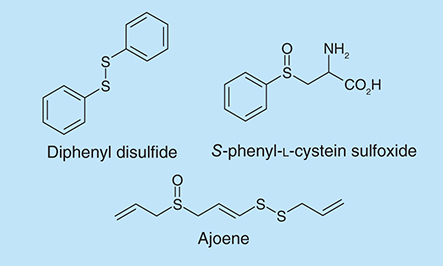
Garlic-derived natural products have been reported to inhibit QS systems in Pseudomonas and Vibrio species [Citation7,Citation69]. Bioassays showed that the main compounds in the garlic extracts responsible for QS inhibition are ajoene, sulfides, polysulfides and vinyl dithiins [Citation7,Citation70]. Ajoene (), a component extracted from garlic, has been identified to be able to decrease the production of QS signal molecules of P. aeruginosa and can synergize biofilm inhibition by tobramycin [Citation70]. Ajoene (80 µg/ml) inhibited the production of N-butyryl-l-homoserine lactone in P. aeruginosa by almost threefold. Tobramycin (as high as 340 µg/ml) was ineffective at killing bacteria in a P. aeruginosa biofilm but in the presence of ajoene, up to 90% of biofilm-forming P. aeruginosa were killed by 10 µg/ml of tobramycin.
Extracts from the Amazonian medicinal plant, Petiveria alliacea L. (Phytolaccaceae), contain sulfur derivatives such as S-phenyl-l-cystein sulfoxide and diphenyl disulfide [Citation71]. The effects of these compounds on P. aeruginosa biofilms were investigated and it was found that at 1 mM concentration, both S-phenyl-L-cystein sulfoxide and diphenyl disulfide () did not inhibit planktonic cell growth but lowered cell viability within a biofilm by 41 and 45%, respectively (determined by the MTT assay) [Citation71].
Marine-derived compounds
Peach et al. reported the discovery of a new structural class of biofilm inhibitors by applying an image-based, high-throughput-screening platform to a marine natural products library [Citation72]. Auromomycin (see Supplementary Figure 4) was found to have biofilm inhibition activity against V. cholerae with IC50 of 60.1 µM. Moreover, addition of subinhibitory concentrations of antibiotics (tetracycline, ceftazidime and ciprofloxacin) enhanced the biofilm inhibitory activity of auromomycin [Citation72].
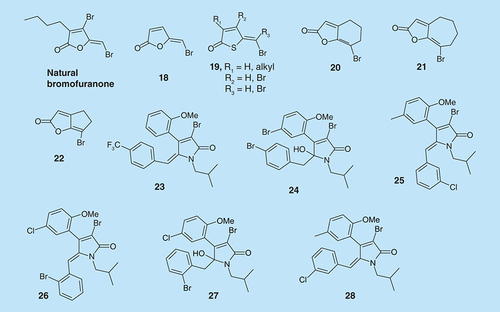
Natural halogenated furanones, isolated from the marine red algae Delisea pulchra, have also shown antibiofilm properties. Both natural and synthetic brominated furanones have been identified as the effective QS inhibitors (QSIs) in both Gram-positive and Gram-negative bacteria [Citation6,Citation73–76]. Some synthetic furanones show an ability to penetrate P. aeruginosa biofilm and cause changes in biofilm maturation (compound 18, ) [Citation6]. Both nitrogen and sulfur analogs of brominated furanones have also been synthesized and tested (see ) [Citation77,Citation78].
Compound 19 (R1/R2/R3= H) had an inhibitory effect on biofilm formation (IC50= 20 µM) with minimum effect on planktonic cell growth. However, the use of halogenated furanones is limited due to their toxicity in mammalian cells. Analogs 20–22, () on the other hand, reduced biofilm formation and thickness in E. coli and P. aeruginosa (see Supplementary Figure 5) and inhibited elastase B production by P. aeruginosa at nonmicrobicidal concentrations and are less toxic to human neuroblastoma SK-N-SH cells than natural bromofuranones [Citation79]. However, these analogs are not as active as the more toxic halogenated furanones and require concentrations that are far greater than 100 µM to achieve significant antibiofilm effects.
Pereira et al. evaluated the antibiofilm activity of brominated alkylidene lactams (see , compounds 23–28), on S. aureus, P. aeruginosa, S. epidermidis and S. mutans [Citation80]. Among all of the compounds that were evaluated, the most active against S. epidermidis were (E)-γ-alkylidene-γ-lactam 23 with IC50 = 12.2 µg/ml (25.4 µM) and the γ-hydroxy-γ-lactam 24 with IC50 = 13.3 µg/ml (22.6 µM). Against P. aeruginosa, the most active compounds were (Z)-γ-alkylidene-γ-lactam 25 and 26 with IC50 values of 0.6 and 0.7 µ/ml (1.3 µM), respectively. The most active compound against S. aureus biofilm was γ-hydroxy-γ-lactam 27 and 44 µg/ml (80.9 µM) inhibited 53.1% of biofilm formation. Compounds 23, 25 and 28 () inhibited S. mutans biofilm formation, with 23 being the most active.
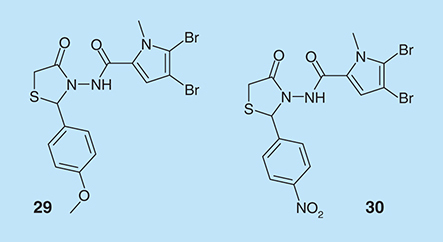
Marine sponges produce an array of secondary metabolites, which they use to ward off fish that prey on them. One of such classes of compounds is the bromopyrrole alkaloids, which have been shown to exhibit a range of biological activities, including antibiofilm activities. 4-Thiazolidinones derivatives of pyrroles, in addition to antibiofilm properties, also have anti-HIV-1 [Citation81] and anti-inflammatory [Citation82,Citation83] activities. 4-Thiazolidinone bromopyrrole derivatives (compounds 29 and 30, ) are against S. aureus biofilms [Citation84]. Interestingly, the MIC of 29 and 30 against S. aureus biofilm is 0.78 µg/ml, which is three-times less than the MIC value for vancomycin (3.125 µg/ml).
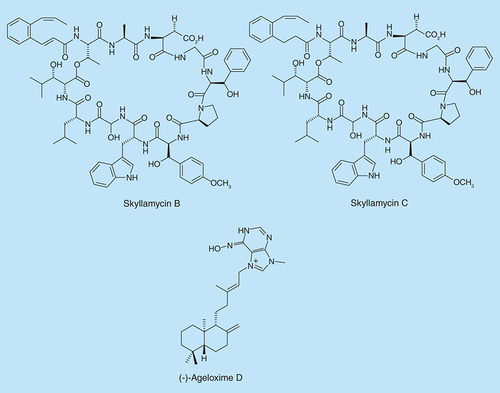
Streptomyces spp. has served as a source of a plethora of structurally unique molecules with various biological activities [Citation85]. In a screening of marine-derived microbial prefractions for P. aeruginosa biofilm inhibitors, a class of structurally unique molecules, skyllamycins, were purified from cultures of Streptomyces spp [Citation86]. The cyclic depsipeptide skyllamycins B and C () were found to inhibit biofilm formation with respective EC50 values of 30 and 60 µM. Skyllamycin B demonstrated the ability to clear mature biofilms of P. aeruginosa strain PAO1 ΔwspF (designed to overexpress c-di-GMP and hence is expected to form strong biofilms) in a nontoxic manner.
(-)-Ageloxime D () is a diterpene alkaloid, which was isolated from the Indonesian marine sponge Agelas nakamurai. Hertiani et al. found that (-)-ageloxime D inhibits biofilm formation in S. epidermidis, but does not affect bacterial growth [Citation87].
Tello et al. have reported the antibiofilm activities of natural (obtained from Colombian Caribbean octocoral Pseudoplexaura flagellosa and Eunicea knighti) and synthetic cembranoid compounds () [Citation88–90]. At 100 ppm, cembranoid epimers at C8 inhibited P. aeruginosa biofilm maturation [Citation88]. In V. harveyi, compounds 31 and 33 (10 ppm) inhibited V. harveyi biofilm maturation by 75%. Compounds 35 and 36 (100 ppm) inhibited by V. harveyi biofilm maturation by 40 and 95%, respectively. Compounds 32, 33 and 34 (10 ppm) inhibited S. aureus biofilm formation by 70%. These cembranoid epimers did not inhibit bacterial growth at 100 ppm.
Compound 38 was found to be a QSI in C. violaceum. Against P. aeruginosa, compounds 37–43 were found to inhibit biofilm formation with IC50 values less than 50 μM. Compounds 38 and 39 were particularly effective against S. aureus biofilm formation with IC50 values of 0.16 and 0.08 μM, respectively. Amongst the cembranoids that were tested, biofilm formation by V. harveyi was inhibited only by compounds 41–43, with IC50 values of 5.20, 30.80 and 9.91 μM, respectively. These compounds did not affect bacterial growth.
AHLs-based inhibitors
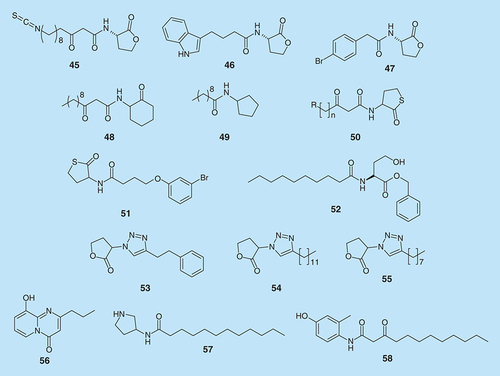
N-acyl homoserine lactones mediate QS in Gram-negative bacteria. There are several types depending on the length of the acyl side chain. A considerable amount of modified AHL derivatives have been developed and tested for quorum sensing inhibition and sometimes for biofilm inhibition () [Citation89–95].
Lactone based analogs of AHL are susceptible to hydrolysis but a few hydrolytically more stable AHL analogs have also been developed and have the potential to be used in practical applications. For example, thiolactanone 51, which is hydrolytically more stable than the lactone analog, inhibits biofilm formation by P. aeruginosa [Citation96]. N-decanoyl-l-homoserine benzyl ester (52, ), a nonlactone analog but which could also undergo ester hydrolysis nonetheless could repress virulence factors production, swarming and the production of rhamnolipids by P. aeruginosa without affecting growth [Citation97]. Compound 52 affects virulence by repressing las and rhl systems. In a recent study by Weng et al., it was found that compound 52 (200 µM) inhibited biofilm formation by PAO1 and reduced biofilm tolerance to tobramycin [Citation98].
The QS system in the bacterium Burkholderia cenocepacia is controlled by two AHLs, N-octanoyl-l-homoserine lactone and N-hexanoyl-l-homoserine lactone. Brackman et al. reported the synthesis of a series of AHL analogs, triazolyldihydrofuranones (Compounds 53–55, ), in which the amide function was replaced by a triazole group and they evaluated their effect on QS and biofilm formation in B. cenocepacia and P. aeruginosa [Citation99]. In P. aeruginosa PAO1, the most effective were compounds 53 and 54, whereas in B. cenocepacia LMG16656, 54 and 55 were active.
Porphyromonas gingivalis is a major pathogen of periodontal disease. The QS mechanism in this bacterium is still not clear, although AI-2 signaling has been implicated. Planktonic P. gingivalis cells respond to N-acyl HSL molecules and AHL analogs (56–58, ) have been shown to reduce P. gingivalis biofilm thickness [Citation100]. Some of these analogs could also enhance the potency of antibiotics (cefuroxine, minocycline and ofloxacin) against P. gingivalis biofilms (see Supplementary Figure 6) [Citation101].
Other small molecules
Metabolites isolated from the myxobacterium Sorangium cellulosum strain So ce960 have been demonstrated to have antibiotic properties. One of such metabolites is carolacton ( A), a macrolide ketocarbonic acid [Citation102]. The antibiofilm activity of carolacton against caries and endocarditis-associated bacterium S. mutans has been investigated. It was observed that 5 µg/ml of carolacton reduced S. mutans biofilms by 35%, with very little effect on planktonic cells growth [Citation103]. Hallside et al. reported the effect of carolacton and its analog 59 ( B) on biofilm morphology [Citation104]. When carolacton (>500 nM) and compound 59 (>62.5 µM) were incubated with S. mutans in the presence of biofilm-inducing media, dramatic changes in the integrity and morphology of the biofilm matrix were observed ( C).
Peptides & biofilms
Microbial amyloids
Many neurodegenerative diseases such as Alzheimer's and Parkinson's are probably caused by the aggregation of proteins into amyloid fibers. Amyloid formation was originally viewed as a consequence of protein misfolding and aggregation. Ongoing studies show that functional amyloids have a ubiquitous role in living systems, especially in bacteria. Proteins that are folded into amyloid state are found in many microorganisms and they have a role in cell-cell communication and biofilm formation [Citation105,Citation106]. Once formed, amyloid fibers are resistant to disassembly by enzymatic and chemical digestion [Citation107].
S. aureus biofilm matrix contains polysaccharides and DNA that interact with structural and enzymatic proteins. At certain conditions, S. aureus biofilms are composed of small peptides, amyloid-like fibers that are called phenol soluble modulins (PSMs) [Citation108]. PSMs control biofilm integrity. Mutants incapable of producing PSMs formed biofilms that were degraded by enzymes and mechanical stress. PSMs can modulate biofilm disassembly using amyloid-like aggregation as a control point for their activity. It is known that PSMs are regulated by the agr QS network [Citation109,Citation110]. Schwartz et al. have found that an agr deficient strain did not produce fibers [Citation108]. PSMs may be stored as inert fibrils in a sessile biofilm until conditions are suitable for their dissociation and promoting biofilm disassembly.
These microbial amyloid fibers are structurally similar to the pathogenic variants found in humans; therefore, they are good targets in screening for molecules with antibiofilm and antiamyloids activities.
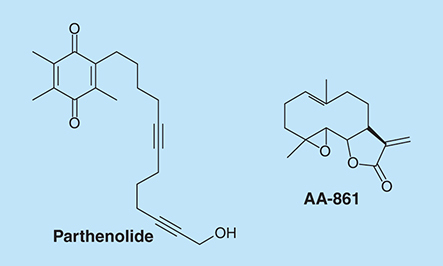
Bacillus subtilis produces a spore-coat protein called TasA. TasA forms amyloid structures in these bacteria's biofilms. AA-861 () is a benzoquinone derivative with anti-inflammatory activity. Parthenolide () is a sesquiterpene lactone with anti-inflammation and anticancer activities [Citation111]. Romero et al. showed that AA-861 and parthenolide inhibit biofilm in B. subtilis by interfering with the formation of amyloid-like fibers [Citation112]. Parthenolide was also shown to interfere with pre-established biofilms. These compounds also inhibit biofilm formation by Bacillus cereus and E. coli, which have amyloid proteins as major matrix components.
Cationic peptides
Antimicrobial peptides in plants and animals are usually cationic molecules (contain an excess of lysine and arginine residues) and are composed of 12–50 amino acids residues [Citation113]. Cationic peptides are able to pass through the cell membrane, bind to the DNA and induce gene expression. They play an important role in the innate defenses of all species of life [Citation114].
The human cathelicidin peptide LL-37 is able to block biofilm formation by P. aeruginosa [Citation115]. LL-37 contains 37 residues and is a α-helical peptide that is produced at mucosal surfaces by epithelial cells (). The attachment of P. aeruginosa cells to the surface was significantly reduced in the presence of LL-37.
Looking for shorter peptides for biofilm inhibition, de la Fuente-Núñez et al. found four peptides with antibiofilm activity (). One of them, a 9-amino acid peptide 1037, has a weak antimicrobial activity MIC (304 µg/ml) but demonstrated significant decrease in biofilm mass (78%) at half the MIC value [Citation114]. When they compared the sequences of the peptides they found a conserved part (RIRVR). In B. cenocepacia and Listeria monocytogenes, treatment with 5 µg/ml 1037 resulted in a substantial reduction in biofilm growth in both organisms [Citation114].
LL-37 also has antimicrobial activity toward group A streptococcus (GAS) and it can cause cell lysis by disturbing cell membranes [Citation116]. hCAP18, which LL-37 is originated from, is widespread in human cells and tissues, including neutrophils, monocytes, NK cells and mast cells. Also, LL-37 can attract neutrophils and CD4 T cells to infection sites.
LL-37 is also active against S. epidermidis [Citation117]. In this bacterium, exopolysaccharide intercellular adhesion mediates immune evasion. LL-37 has higher antibacterial activities toward ica-mutant strain, which lacks exopolysaccharide intercellular adhesion, than the wild type strain. Interaction between S. epidermidis and LL37 was also investigated by Hell et al. [Citation118] They showed that at low concentration (1 mg/l), LL37 inhibits bacterial attachment and biofilm formation significantly. LL37 up to 16 mg/l did not inhibit bacterial growth.
d-amino acids
Cells in B. subtilis biofilm are held together by exopolysaccharides and amyloid-like fibers. The dispersal of biofilm involves the release of planktonic cells from the exopolysaccharides and protein components of the matrix. The d-amino acids, d-Tyr, d-Leu, d-Trp and d-Met, which are incorporated into the peptidoglycan, can trigger the release of the TasA fibers [Citation119,Citation120]. d-amino acids, such as d-tyrosine, d-methionine, d-tryptophan or d-leucine, are also known to inhibit biofilm formation in B. subtilis, P. aeruginosa and S. aureus [Citation119]. The biofilm inhibitory effects of d-amino acids could be reversed by their cognate l-amino acids.
B. subtilis biofilm has been documented to be dependent on the availability of the polyamine spermidine. Kolodkin-Gal et al. found that a shorter structural analog, norspermidine, is another biofilm-disassembly factor, present in conditioned medium from aging B. subtilis biofilms and it directly interacts with the exopolysaccharide [Citation121]. Exogenous norspermidine (25 µM) added to the growth medium prior to inoculation, fully inhibited biofilm formation without inhibiting planktonic growth. d-amino acids and norspermidine acted together in breaking down existing, mature pellicles. Norspermidine was also found to inhibit biofilm formation by S. aureus and E. coli. These findings have however been refuted by Hobley et al., who observed that exogenous norspermidine inhibited B. subtilis planktonic growth and pellicle development in an exopolysaccharide-independent manner and was not required in the formation of robust biofilms [Citation122]. They also found that norspermidine is not synthesized by B. subtilis and is not naturally present in biofilms formed by B. subtilis. They reconfirmed the requirement of spermidine for the formation of robust B. subtilis biofilms.
Polysaccharides & biofilms
Kingella kingae is a Gram negative bacterium that can cause infections such as endocarditis, osteomyelitis and arthritis. Its ability to cause infections is mainly due to its ability to form biofilms. Bendaoud et al. showed that Kingella kingae produces extracellular galactan, a polysaccharide that inhibits its own biofilm formation and also the biofilm formation by other bacteria such as, Aggregatibacter actinomycetemcomitans, K. pneumoniae, S. aureus, S. epidermidis and Candida albicans [Citation123]. Galactan consists of a linear polymer of galactofuranose residues. The biological role of galactan is still unknown. One hypothesis is that it regulates the biofilm architecture. Another possibility is that it mediates the release of cells in the dispersal stage.
Galactose is a monosaccharide that is utilized by many organisms. Galactose metabolism plays a role in the biofilm formation in B. subtilis [Citation124]. The sugar-nucleotide UDP-galactose is toxic for planktonic cells. However, it is essential for exopolysaccharide biosynthesis during biofilm formation. The metabolism of galactose is generally catalyzed by the enzymes GalK, GalT and GalE, each of which catalyzes one of three steps in converting galactose to UDP-glucose in the Leloir pathway (see Supplementary Figure 7) [Citation125]. A deficiency of any one of these enzymes has been demonstrated to have deleterious effects on planktonic bacteria growth in the presence of galactose [Citation124]. In the third step, GalE catalyzes the reversible conversion of UDP-galactose to UDP-glucose. Chai et al. studied a B. subtilis galE deletion mutant and observed that addition of galactose caused planktonic cell lysis due to the accumulation of UDP-galactose [Citation124]. In that study, it was observed that biofilm-forming B. subtilis ΔgalE on the other hand survived in the presence of galactose by producing exopolysaccharide (EPS). Analysis of the EPS showed a large amount of galactose and so it was proposed that UDP-galactose was channeled into EPS production.
The antimicrobial properties of sugar esters have been previously demonstrated. One of such studies used enzymatically synthesized lauroyl glucose to probe its antimicrobial properties against selected bacterial and fungal test organisms [Citation126]. It was observed that lauroyl glucose after 48 h of incubation with P. aeruginosa and Pseudomonas aureofaciens resulted in 51 and 57% disruption of preformed biofilms, respectively. Against the fungal test organisms, lauroyl glucose dislodged 45% of C. albicans and 65% of Candida lipolytica biofilms.
In another study, the tropical marine bacteria, Serratia marcescens was isolated from the hard marine coral Symphyllia sp [Citation127]. The bacteria were found to produce a biosurfactant. A glycolipid made up of palmitic acid and glucose was subsequently purified from S. marcescens and was determined to possess antimicrobial, antiadhesive and antibiofilm activities. It was observed that 50 µg/ml of the purified glycolipid surfactant dislodged 70.6% of preformed P. aeruginosa PAO1 biofilms whilst at 100 µg/ml, 90.5% of the P. aeruginosa PAO1 biofilms were disrupted.
Fatty acids & biofilms
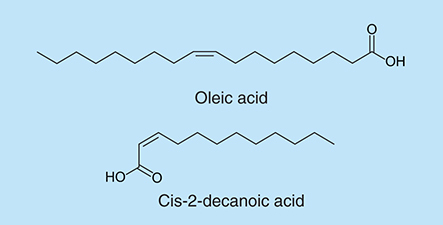
Free fatty acids (FFAs) derive from fatty acids that escape from lipids with the aid of enzymes [Citation128]. FFAs were observed to have antibacterial functions against both Gram-negative bacteria, such as Chlamydia trachomatis [Citation129], Neisseria gonorrhoeae [Citation130] and Gram-positive bacteria [Citation131], such as Bacillus larvae [Citation132]. FFAs exist in human skin [Citation133] and they were found to be functional in controlling skin infection against bacteria [Citation134]. The relationship between FFAs and bacterial biofilm formation has been studied extensively. FFAs have been proven to have the ability of inhibiting biofilm formation [Citation135,Citation136]. Oleic acid (), which was identified as one of the primary unsaturated fatty acids, is able to repress biofilm formation of S. aureus by blocking bacterial adhesion [Citation135]. Biofilm production of eight strains of S. aureus was reduced dramatically when oleic acid was added during the initial adhesion step. Another fatty acid, cis-2-decenoic acid (), which is produced by P. aeruginosa is able to induce the dispersion of established biofilms and inhibit biofilm development in P. aeruginosa, E. coli, K. pneumoniae, Proteus mirabilis, Streptococcus pyogenes, B. subtilis, S. aureus and the yeast C. albicans [Citation136]. Cis-2-decenoic acid at concentrations greater than or equal to 125 µg/ml inhibited biofilm formation in microtiter plates, but at concentrations at or above 500 µg/ml it also inhibited bacterial growth. Jenings et al. showed that the combination with antibiotics resulted in decreased biofilm formation, especially the combination with the antibiotic linezolid resulted in biofilm inhibition at concentrations 2–16-times lower than for either antimicrobial alone [Citation137]. The mechanism of fatty acids antibacterial activity remains unclear. FFAs are able to affect some fundamental processes of bacteria by affecting the cell membrane, especially the process of energy production [Citation128].
The combination of AI-2-based antibiofilm molecules with traditional antibiotics
Biofilm-associated infections are often very difficult to treat with conventional antibiotics. Little is known about the relationship between the antibiofilm effect of QSI and the susceptibility of biofilms to antibiotics in the presence of these molecules. AI-2 is an autoinducer found in both Gram-negative and Gram-positive bacteria. AI-2 signaling plays an important role in biofilm formation. AI-2 analogs, isobutyl-DPD and isopropyl-DPD, were found to be QSIs in E. coli, S. typhimurium and V. harveyi [Citation138,Citation139], and phenyl-DPD inhibits QS-related pyocyanin production in P. aeruginosa [Citation140]. AI-2 analogs, isobutyl-DPD and phenyl-DPD were also tested for their ability to alter biofilm formation, maturation and removal among E. coli and P. aeruginosa [Citation141]. Biofilms treated with AI-2 analogs were thinner and less ordered. When AI-2 analogs were added together with the antibiotic gentamicin, pre-existing E. coli biofilm could be dispersed (see Supplementary Figure 8).
Nitric oxide & biofilm dispersal
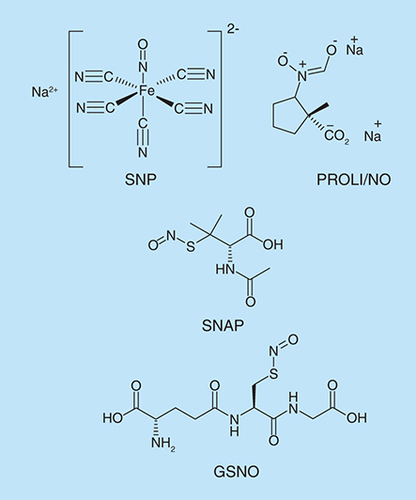
Strategies to induce biofilm dispersal are of interest because in most settings, therapeutic interventions would be applied after the biofilm has already formed. It has been shown that the important biological messenger, nitric oxide (NO) is a signal for biofilm dispersal, inducing the transition from the biofilm mode of growth to the free swimming planktonic state [Citation142,Citation143]. The delivery of exogenous NO to biofilms was achieved via NO donors (). At low concentrations of NO donors, Barraud et al. observed a decrease in biofilm biomass and an increase in planktonic biomass [Citation142]. NO donors also enhanced the efficacy of antibacterial compounds, such as tobramycin, in the dispersal of P. aeruginosa biofilms.
Proteins harboring heme nitric oxide/oxygen binding (HNOX) domains are known to play a role in NO sensing and modulation of intracellular c-di-GMP levels in bacteria, such as Shewanella woodyi and Shewanella oneidensis [Citation144]. The HNOX protein forms a regulatory complex with a DGC/PDE (diguanylate cyclase/phosphodiesterase) protein. NO binding to HNOX inhibits cyclic-di-GMP production and sometimes increases PDE activity, lowering cyclic-di-GMP concentrations, which causes biofilm dispersal [Citation145]. It is known that NO induces an increase in PDE activity and as a result induces the dispersion of P. aeruginosa biofilms [Citation146]. This is followed by a decrease in intracellular c-di-GMP levels [Citation142,Citation143]. In P. aeruginosa, the genome encodes two proteins containing an MHYT domain. MHYT domain acts as a sensor for diatomic gases such as O2, CO or NO.
In order to identify proteins controlling c-di-GMP turnover in response to NO in P. aeruginosa, Li et al. [Citation146] searched the Pseudomonas genome database for proteins containing c-di-GMP modulating activity, in particular the largely unexplored MHYT domain predicted to possess putative gas sensor function. The P. aeruginosa genome encodes two proteins containing MHYT domains: the membrane-anchored proteins MucR (PA1727) and NbdA (NO-induced biofilm dispersion locus A; PA3311). NbdA is responsible for the NO induced dispersion response. They concluded that MucR is an active PDE under planktonic growth conditions but act as a DGC in biofilms.
NO signaling is also found in Gram positive bacteria such as B. subtilis, where it provides protection against oxidative stress caused by H2O2. NO is proposed to activate catalase, the H2O2 degrading enzyme [Citation147]. In addition, NO suppresses the formation of DNA damaging OH· radicals from the oxidation of Fe2+ with H2O2 [Citation147]. Biofilm formation in B. subtilis is characterized by the formation of robust pellicles at the air-liquid interface and the formation of structurally complex spot colonies on agar surfaces. Schreiber et al. examined the effect of exogenously supplied NO on biofilm formation and dispersal in B. subtilis [Citation148]. They found that NO affects biofilm dispersal of B. subtilis.
Antibiofilm surfaces
Attachment of cells to surfaces is important for biofilm formation. This community of sedentary cells has become a problem in healthcare settings where biofilms are found to colonize and contaminate the surfaces of medical implants. It has therefore become necessary to develop surfaces that can inhibit the formation and growth of biofilms. Efforts have generally been targeted toward the use of materials that are mostly used for medical implants.
In 2007, Chung et al. studied the effect of engineered microtopographies on the biofilm formation ability of S. aureus. They used engineered microtopography Sharklet AFTM on poly (dimethyl siloxane) elastomer (PDMSe) with 2 µm feature width and spacing and 3 µm feature height [Citation149]. By comparing the smooth PDMSe to the topographically modified surface, it was observed that S. aureus biofilms were inhibited on the Sharklet AFTM, recording only a 7% growth as compared with the 54% growth observed on the smooth PDMSe. The cultures were allowed to grow for 21 days, enough for S. aureus to contaminate an implanted device in vivo. The disruption of S. aureus biofilms on this topographically modified surface did not involve the release of any bactericidal agents.
Monomeric trimethylsilane (TMS) was used to coat the surfaces of stainless steel and titanium alloy grade 5 and used to assess S. epidermidis biofilm formation [Citation150]. Ma et al. showed that TMS coated surfaces inhibited the formation of biofilms by preventing the attachment of cells. Biofilms of S. epidermidis were formed successfully on uncoated stainless steel and titanium alloy surfaces. It was also demonstrated that susceptibility of the biofilms to antibiotic treatment was significantly enhanced with TMS coating of the surfaces tested.
A different study used polystyrene coated with nonionic surfactants containing poly (ethylene oxide), a brush coating, to study its effect on biofilm formation by S. epidermidis. The study observed that Pluronic F127 was the most active surfactant, as it achieved 90% inhibition of S. epidermidis biofilm [Citation151]. Both studies however showed that the coated surfaces did not inhibit biofilms formed by P. aureginosa.
Ionic liquids
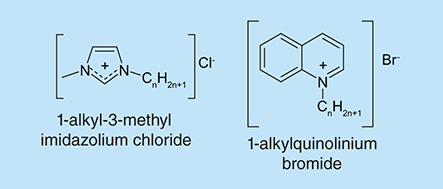
Ionic liquids are a class of liquid salts with discrete anions and cations that can independently be modified. They are widely used in both chemistry and biology fields. Due to their flexibility, it is thought that ionic liquids could be designed to exhibit antimicrobial and antibiofilm properties [Citation152,Citation153]. A typical example of such class is the 1-alkylquinolinium bromide ionic liquids (). These compounds have been shown to possess broad spectrum antimicrobial and antibiofilm activities. They significantly inhibited the planktonic cell growth and biofilm formation of a panel of Gram positive and Gram negative test organisms [Citation152]. A previous study also demonstrated the antibiofilm activity of a series of 1-alkyl-3-methylimidazolium chloride ionic liquids against a range of clinically relevant pathogens [Citation153]. Both studies observed a dependence of antibiofilm potency on alkyl chain length, generally peaking at an alkyl chain length of 14.
Conclusion
In the last decade, there has been an explosion in the discovery of small molecules that modulate bacterial biofilm formation. The majority of the antibiofilm molecules that have been developed have either been natural product-inspired or modifications of signaling molecules that regulate biofilm formation. For example the modification of several quorum sensing molecules, which regulate biofilm formation, has resulted in modulators of biofilm formation. The biofilm inhibitory concentrations of the majority of the antibiofilm molecules discovered or developed to date are in the micromolar range, and hence further optimizations of these molecules to improve potency are required if any of these molecules are to become clinical candidates.
Future perspective
Our current understanding of the molecular players of bacterial biofilm formation has increased [Citation154] but there is still a great challenge in the development of antibiofilm drugs and to date, no antibiofilm drug has been registered and is in clinical use. This has made the treatment of biofilm-related infections very problematic. Research toward identifying antibiofilm agents includes but not limited to the development of various technologies ranging from synthetic/extracted small molecules to modified surfaces. Approaches such as the modified surfaces, if successful, would be instrumental in combating medical and dental implant-associated biofilms. Nature continues to inspire researchers by providing unique chemical scaffolds such as the bromoageleferin and oroidin, which have been demonstrated to exhibit antibiofilm activities. Efforts could be targeted at fine-tuning such natural scaffolds to arrive at more potent antibiofilm drugs. Any potent antibiofilm agent that makes it to the drug development stage could be administered as a single drug compound to alleviate biofilm infections. Nonetheless antibiotics, which are otherwise ineffective in the treatment of bacterial infections, could be combined with potent antibiofilm agents to augment the activities of the antibiotics and hence afford some leverage in the treatment of biofilm-related infections. Antibiofilm agents that can both disperse and kill biofilm bacteria could have some useful applications but remain rare [Citation155,Citation156,Citation157].
Table 1. Peptides with antibiofilm activity.
IC50: Quantifies the ability of a compound to inhibit a specific biological or biochemical function. For this review, it refers to the concentration of a compound that inhibits biofilm formation by 50%.
Quorum sensing: Response to a signal in a population-dependent manner. In bacteria, this is used to coordinate gene expression.
C-di-GMP: Cyclic dinucleotide containing two guanine nucleobases with two 2’-5’-phosphodiester linkages and which regulates biofilm-related genes in many bacteria.
Minimum inhibitory concentration: Refers to the lowest concentration of a compound to inhibit bacterial growth after overnight incubation.
N-acyl homoserine lactones: Signaling molecules that are involved in quorum sensing. They are secreted by different bacteria and their structures differ by the length of the side chain and the substitution of a carbonyl at the third carbon.
Biofilm matrix: Network of macromolecules that form a protective scaffold around bacteria in a biofilm.
Amyloids: Misfolded polypeptides that aggregate to form a cross-β structure. They are associated with diseases such as Alzheimer and diabetes. They are also found in the matrices of some bacterial biofilms.
Planktonic bacteria: Bacteria living in a free state without being attached to a surface or physically connected to other bacterial cells and can freely drift.
Ionic liquids: Salts that are liquids under 100°C and could even be a liquid at room temperature. The anion is poorly coordinated and thus no stable crystal is formed.
Background
Biofilms are defined as aggregated microorganism communities attached to surfaces and embedded in a self-produced matrix.
In the biofilm structure the cells are tolerant to harsh environmental conditions, resistant to antibiotics and host immune systems.
Biofilm inhibition by small molecules
Natural compounds (isolated from plants, marine and bacteria) have served as biofilm inhibitors or as scaffolds for the development of synthetic antibiofilm compounds.
Peptides & biofilms
Amyloids exist in many microorganisms and they are involved in cell–cell communication and biofilm formation. Cationic peptides are able to alter gene expression directly. D-amino acids also demonstrated the ability to inhibit biofilm formation.
Polysaccharides & biofilms
Galactan can inhibit biofilm formation. Galactose metabolism plays a role in the biofilm formation in B. subtilis. Sugar esters and a biosurfactant, produced by S. marcescens, possess antibiofilm properties.
Fatty acids & biofilms
Free fatty acids can affect bacterial cell membrane, especially the process of energy production. Some free fatty acids, such as oleic acid and cis-2-decenoic acid, inhibit biofilm of some bacteria.
The combination of antibiofilm molecules & traditional antibiotics
The combination of QS molecule analogs (such as AI-2 analogs) together with the antibiotic gentamicin resulted in reduction in biofilm formation.
Nitric oxide & biofilm dispersal
Nitric oxide induces the transition from the biofilm mode to planktonic state.
Antibiofilm surfaces
Various methods have been used to develop surfaces that can inhibit the formation and growth of biofilms.
Ionic liquids
Ionic liquids are designed to be biofilm inhibitors, such as 1-alkylquinolinium bromide ionic liquids.
Supplemental Material 1
Download MS Word (2.5 MB)Supplemental Material
Financial & competing interests disclosure
The authors are grateful to NSF (CBET 1264509 and CHEM 1307218) and Camille Dreyfus Foundation (Teacher-Scholar fellowship to HOS) for funding. Yue Zheng is supported by Kraybill biochemistry fellowship. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Rasmussen TB , GivskovM. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Biol.296 (2–3), 149–161 (2006).
- Donlan RM , CostertonJW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev.15 (2), 167–193 (2002).
- Stewart PS , William CostertonJ. Antibiotic resistance of bacteria in biofilms. Lancet358 (9276), 135–138 (2001).
- Worthington RJ , RichardsJJ, MelanderC. Small molecule control of bacterial biofilms. Org. Biomol. Chem.10 (37), 7457–7474 (2012).
- Worthington RJ , RichardsJJ, MelanderC. Non-microbicidal control of bacterial biofilms with small molecules. Anti-Infective Agents12 (1), 120–138 (2014).
- Hentzer M , RiedelK, RasmussenTBet al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology148 (1), 87–102 (2002).
- Bjarnsholt T , JensenPØ, RasmussenTBet al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology151 (12), 3873–3880 (2005).
- Hu J-F , GaroE, GoeringMGet al. Bacterial biofilm inhibitors from Diospyros dendo. J. Nat. Prod.69 (1), 118–120 (2006).
- Garo E , EldridgeGR, GoeringMGet al. Asiatic acid and corosolic acid enhance the susceptibility of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob. Agents Chemother.51 (5), 1813–1817 (2007).
- Wu H , LeeB, YangLet al. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol. Med. Microbiol.62 (1), 49–56 (2011).
- Lee JH , ChoMH, LeeJ. 3-indolylacetonitrile decreases Escherichia coli O157:H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ. Microbiol.13 (1), 62–73 (2011).
- Huigens RW , RichardsJJ, PariseGet al. Inhibition of Pseudomonas aeruginosa biofilm formation with bromoageliferin analogues. J. Am. Chem. Soc.129 (22), 6966–6967 (2007).
- Richards JJ , BallardTE, MelanderC. Inhibition and dispersion of Pseudomonas aeruginosa biofilms with reverse amide 2-aminoimidazole oroidin analogues. Org. Biomol. Chem.6 (8), 1356–1363 (2008).
- Richards JJ , BallardTE, HuigensRW, MelanderC. Synthesis and screening of an oroidin library against Pseudomonas aeruginosa biofilms. ChemBioChem9 (8), 1267–1279 (2008).
- Rogers SA , MelanderC. Construction and screening of a 2-aminoimidazole library identifies a small molecule capable of inhibiting and dispersing bacterial biofilms across order, class, and phylum. Angew. Chem. Int. Ed. Engl.47 (28), 5229–5231 (2008).
- Ballard TE , RichardsJJ, AquinoA, ReedCS, MelanderC. Antibiofilm activity of a diverse oroidin library generated through reductive acylation. J. Org. Chem.74 (4), 1755–1758 (2009).
- Reyes S , HuigensIII RW, SuZ, SimonML, MelanderC. Synthesis and biological activity of 2-aminoimidazole triazoles accessed by Suzuki-Miyaura cross-coupling. Org. Biomol. Chem.9 (8), 3041–3049 (2011).
- Su Z , PengL, WorthingtonRJ, MelanderC. Evaluation of 4,5-disubstituted-2-aminoimidazole–triazole conjugates for antibiofilm/antibiotic resensitization activity against MRSA and Acinetobacter baumannii.ChemMedChem6 (12), 2243–2251 (2011).
- Yeagley AA , SuZ, McCulloughKD, WorthingtonRJ, MelanderC. N-substituted 2-aminoimidazole inhibitors of MRSA biofilm formation accessed through direct 1,3-bis(tert-butoxycarbonyl)guanidine cyclization. Org. Biomol. Chem.11 (1), 130–137 (2013).
- Furlani RE , YeagleyAA, MelanderC. A flexible approach to 1,4-di-substituted 2-aminoimidazoles that inhibit and disperse biofilms and potentiate the effects of β-lactams against multi-drug resistant bacteria. Eur. J. Med. Chem.62 (0), 59–70 (2013).
- Rogers SA , WhiteheadDC, MullikinT, MelanderC. Synthesis and bacterial biofilm inhibition studies of ethyl N-(2-phenethyl) carbamate derivatives. Org. Biomol. Chem.8 (17), 3857–3859 (2010).
- Rogers SA , LindseyEA, WhiteheadDC, MullikinT, MelanderC. Synthesis and biological evaluation of 2-aminoimidazole/carbamate hybrid anti-biofilm and anti-microbial agents. Bioorg. Med. Chem. Lett.21 (4), 1257–1260 (2011).
- Frei R , BreitbachAS, BlackwellHE. 2-aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa biofilms. Angew. Chem. Int. Ed. Engl.51 (21), 5226–5229 (2012).
- Lindsey EA , BrackettCM, MullikinT, AlcarazC, MelanderC. The discovery of N-1 substituted 2-aminobenzimidazoles as zinc-dependent S. aureus biofilm inhibitors. MedChemComm.3 (11), 1462–1465 (2012).
- Sambanthamoorthy K , GokhaleAA, LaoWet al. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother.55 (9), 4369–4378 (2011).
- Martino PD , FursyR, BretL, SundararajuB, PhillipsRS. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can. J. Microbiol.49 (7), 443–449 (2003).
- Lee JH , LeeJ. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev.34 (4), 426–444 (2010).
- Bansal T , EnglertD, LeeJ, HegdeM, WoodTK, JayaramanA. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun.75 (9), 4597–4607 (2007).
- Domka J , LeeJ, WoodTK. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl. Environ. Microbiol.72 (4), 2449–2459 (2006).
- Rui L , ReardonKF, WoodTK. Protein engineering of toluene ortho-monooxygenase of Burkholderia cepacia G4 for regiospecific hydroxylation of indole to form various indigoid compounds. Appl. Microbiol. Biotechnol.66 (4), 422–429 (2005).
- Lee J , BansalT, JayaramanA, BentleyWE, WoodTK. Enterohemorrhagic Escherichia coli biofilms are inhibited by 7-hydroxyindole and stimulated by isatin. Appl. Environ. Microbiol.73 (13), 4100–4109 (2007).
- Lee JH , KimYG, KimCJ, LeeJC, ChoMH, LeeJ. Indole-3-acetaldehyde from Rhodococcus sp. BFI 332 inhibits Escherichia coli O157:H7 biofilm formation. Appl. Microbiol. Biotechnol.96 (4), 1071–1078 (2012).
- Bunders C , CavanaghJ, MelanderC. Flustramine inspired synthesis and biological evaluation of pyrroloindoline triazole amides as novel inhibitors of bacterial biofilms. Org. Biomol. Chem.9 (15), 5476–5481 (2011).
- Minvielle MJ , BundersCA, MelanderC. Indole/triazole conjugates are selective inhibitors and inducers of bacterial biofilms. MedChemComm4 (6), 916–919 (2013).
- Ding X , YinB, QianLet al. Screening for novel quorum-sensing inhibitors to interfere with the formation of Pseudomonas aeruginosa biofilm. J. Med. Microbiol.60 (12), 1827–1834 (2011).
- Lee J-H , RegmiSC, KimJ-Aet al. Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun.79 (12), 4819–4827 (2011).
- Kolter R , GreenbergEP. Microbial sciences: the superficial life of microbes. Nature441 (7091), 300–302 (2006).
- Clatworthy AE , PiersonE, HungDT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol.3 (9), 541–548 (2007).
- Kim H-S , ParkH-D. Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS One8 (9), e76106 (2013).
- Schiavone BIP , RosatoA, MarilenaMet al. Biological evaluation of hyperforin and its hydrogenated analogue on bacterial growth and biofilm production. J. Nat. Prod.76 (9), 1819–1823 (2013).
- Sarkisian SA , JanssenMJ, MattaH, HenryGE, LaPlanteKL, RowleyDC. Inhibition of bacterial growth and biofilm production by constituents from Hypericum spp.Phytother. Res.26 (7), 1012–1016 (2012).
- Murata RM , Branco-de-AlmeidaLS, FrancoEMet al. Inhibition of Streptococcus mutans biofilm accumulation and development of dental caries in vivo by 7-epiclusianone and fluoride. Biofouling26 (7), 865–872 (2010).
- Vikram A , JesudhasanPR, JayaprakashaGK, PillaiSD, PatilBS. Citrus limonoids interfere with Vibrio harveyi cell-cell signalling and biofilm formation by modulating the response regulator LuxO. Microbiology157 (1), 99–110 (2011).
- Vikram A , JesudhasanP, PillaiS, PatilB. Isolimonic acid interferes with Escherichia coli O157:H7 biofilm and TTSS in QseBC and QseA dependent fashion. BMC Microbiol.12 (1), 261 (2012).
- Artini M , PapaR, BarbatoGet al. Bacterial biofilm formation inhibitory activity revealed for plant derived natural compounds. Biorg. Med. Chem.20 (2), 920–926 (2012).
- Flamini G , CatalanoS, CaponiC, PanizziL, MorelliI. Three anthrones from Rubus ulmifolius.Phytochemistry59 (8), 873–876 (2002).
- Panizzi L , CaponiC, CatalanoS, CioniPL, MorelliI. In vitro antimicrobial activity of extracts and isolated constituents of Rubus ulmifolius.J. Ethnopharmacol.79 (2), 165–168 (2002).
- Quave CL , Estévez-CarmonaM, CompadreCMet al. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS One7 (1), e28737 (2012).
- Payne DE , MartinNR, ParzychKR, RickardAH, UnderwoodA, BolesBR. Tannic acid inhibits Staphylococcus aureus surface colonization in an isaa-dependent manner. Infect. Immun.81 (2), 496–504 (2013).
- Lee J-H , KimY-G, RyuSY, ChoMH, LeeJ. Ginkgolic acids and Ginkgo biloba extract inhibit Escherichia coli O157:H7 and Staphylococcus aureus biofilm formation. Int. J. Food Microbiol.174 (0), 47–55 (2014).
- Wu X-Y , YangL-Q, ChenJ, YuanX-H, XiaG-H. Preparation of ginkgolic acid monomers and their antifungal activity. Chem. Industry Forest Products23 (4), 17–21 (2003).
- He J , WangS, WuT, CaoY, XuX, ZhouX. Effects of ginkgoneolic acid on the growth, acidogenicity, adherence, and biofilm of Streptococcus mutans in vitro. Folia Microbiol.58 (2), 147–153 (2013).
- Inamuco J , VeenendaalAKJ, BurtSAet al. Sub-lethal levels of carvacrol reduce Salmonella typhimurium motility and invasion of porcine epithelial cells. Vet. Microbiol.157 (1–2), 200–207 (2012).
- Nostro A , RoccaroAS, BisignanoGet al. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol.56 (4), 519–523 (2007).
- Soni KA , OladunjoyeA, NannapaneniRet al. Inhibition and inactivation of Salmonella typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J. Food Prot.76 (2), 205–212 (2013).
- Nostro A , MarinoA, BlancoARet al. In vitro activity of carvacrol against Staphylococcal preformed biofilm by liquid and vapour contact. J. Med. Microbiol.58 (6), 791–797 (2009).
- Burt SA , Ojo-FakunleVTA, WoertmanJ, VeldhuizenEJA. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS One9 (4), e93414 (2014).
- Benamar M , MelhaouiA, ZyadA, BouabdallahI, AzizM. Anti-cancer effect of two alkaloids: 2R and 2S-bgugaine on mastocytoma P815 and carcinoma Hep. Nat. Prod. Res.23 (7), 659–664 (2009).
- Majik MS , NaikD, BhatC, TilveS, TilviS, D'SouzaL. Synthesis of (R)-norbgugaine and its potential as quorum sensing inhibitor against Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett.23 (8), 2353–2356 (2013).
- Carneiro VA , SantosHS, ArrudaFVet al. Casbane diterpene as a promising natural antimicrobial agent against biofilm-associated infections. Molecules16 (1), 190–201 (2011).
- Lee J-H , ChoHS, JooSWet al. Diverse plant extracts and trans-resveratrol inhibit biofilm formation and swarming of Escherichia coli O157:H7. Biofouling29 (10), 1189–1203 (2013).
- Jang M , CaiL, UdeaniGOet al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science275 (5297), 218–220 (1997).
- Cottart C-H , Nivet-AntoineV, Laguillier-MorizotC, BeaudeuxJ-L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res.54 (1), 7–16 (2010).
- Li L , HenryGE, SeeramNP. Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J. Agric. Food. Chem.57 (16), 7282–7287 (2009).
- González-Sarrías A , GromekS, NiesenD, SeeramNP, HenryGE. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food. Chem.59 (16), 8632–8638 (2011).
- Piver B , BerthouF, DreanoY, LucasD. Differential inhibition of human cytochrome P450 enzymes by ∊-viniferin, the dimer of resveratrol: comparison with resveratrol and polyphenols from alcoholized beverages. Life Sci.73 (9), 1199–1213 (2003).
- Cho HS , LeeJ-H, RyuSY, JooSW, ChoMH, LeeJ. Inhibition of Pseudomonas aeruginosa and Escherichia coli O157:H7 biofilm formation by plant metabolite ∊-viniferin. J. Agric. Food. Chem.61 (29), 7120–7126 (2013).
- Lee J-H , KimY-G, RyuSY, ChoMH, LeeJ. Resveratrol oligomers inhibit biofilm formation of Escherichia coli O157:H7 and Pseudomonas aeruginosa. J. Nat. Prod.77 (1), 168–172 (2014).
- Persson T , HansenTH, RasmussenTB, SkindersoME, GivskovM, NielsenJ. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem.3 (2), 253–262 (2005).
- Jakobsen TH , van GennipM, PhippsRKet al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother.56 (5), 2314–2325 (2012).
- Cady NC , McKeanKA, BehnkeJet al. Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS One7 (6), e38492 (2012).
- Peach KC , ChengAT, OliverAG, YildizFH, LiningtonRG. Discovery and biological characterization of the auromomycin chromophore as an inhibitor of biofilm formation in Vibrio cholerae. ChemBioChem14 (16), 2209–2215 (2013).
- Ren D , BedzykLA, SetlowPet al. Differential gene expression to investigate the effect of (5Z)-4-bromo- 5-(bromomethylene)-3-butyl-2(5H)-furanone on Bacillus subtilis. Appl. Environ. Microbiol.70 (8), 4941–4949 (2004).
- Manefield M , de NysR, NareshKet al. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology145 (2), 283–291 (1999).
- Manefield M , RasmussenTB, HenzterMet al. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology148 (4), 1119–1127 (2002).
- Ren D , SimsJJ, WoodTK. Inhibition of biofilm formation and swarming of Bacillus subtilis by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Lett. Appl. Microbiol.34 (4), 293–299 (2002).
- Jayaraman A , WoodTK. Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease. Annu. Rev. Biomed. Eng.10 (1), 145–167 (2008).
- Benneche T , HerstadG, RosenbergM, AssevS, ScheieAA. Facile synthesis of 5-(alkylidene)thiophen-2(5H)-ones. A new class of antimicrobial agents. RSC Advances1 (2), 323–332 (2011).
- Yang S , Abdel-RazekOA, ChengFet al. Bicyclic brominated furanones: a new class of quorum sensing modulators that inhibit bacterial biofilm formation. Biorg. Med. Chem.22 (4), 1313–1317 (2014).
- Pereira UA , BarbosaLC, MalthaCR, DemunerAJ, MasoodMA, PimentaAL. γ-Alkylidene-γ-lactones and isobutylpyrrol-2(5H)-ones analogues to rubrolides as inhibitors of biofilm formation by Gram-positive and Gram-negative bacteria. Bioorg. Med. Chem. Lett.24 (4), 1052–1056 (2014).
- Barreca ML , ChimirriA, De LucaLet al. Discovery of 2,3-diaryl-1,3-thiazolidin-4-ones as potent anti-HIV-1 agents. Bioorg. Med. Chem. Lett.11 (13), 1793–1796 (2001).
- Goel B , RamT, TyagiRet al. 2-Substituted-3-(4-bromo-2-carboxyphenyl)-5-methyl-4-thiazolidinones as potential anti-inflammatory agents. Eur. J. Med. Chem.34 (3), 265–269 (1999).
- Allen S , NewhouseB, AndersonASet al. Discovery and SAR of trisubstituted thiazolidinones as CCR4 antagonists. Bioorg. Med. Chem. Lett.14 (7), 1619–1624 (2004).
- Rane RA , SahuNU, ShahCP. Synthesis and antibiofilm activity of marine natural product-based 4-thiazolidinones derivatives. Bioorg. Med. Chem. Lett.22 (23), 7131–7134 (2012).
- de Lima Procópio RE , SilvaIR, MartinsMK, AzevedoJL, AraújoJM. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis.16 (5), 466–471 (2012).
- Navarro G , ChengAT, PeachKCet al. Image-based 384-well high-throughput screening method for the discovery of skyllamycins a to c as biofilm inhibitors and inducers of biofilm detachment in Pseudomonas aeruginosa. Antimicrob. Agents Chemother.58 (2), 1092–1099 (2014).
- Hertiani T , Edrada-EbelR, OrtleppSet al. From anti-fouling to biofilm inhibition: new cytotoxic secondary metabolites from two Indonesian Agelas sponges. Biorg. Med. Chem.18 (3), 1297–1311 (2010).
- Tello E , CastellanosL, Arevalo-FerroC, RodríguezJ, JiménezC, DuqueC. Absolute stereochemistry of antifouling cembranoid epimers at C-8 from the Caribbean octocoral Pseudoplexaura flagellosa: revised structures of plexaurolones. Tetrahedron67 (47), 9112–9121 (2011).
- Amara N , MashiachR, AmarDet al. Covalent inhibition of bacterial quorum sensing. J. Am. Chem. Soc.131 (30), 10610–10619 (2009).
- Geske GD , WezemanRJ, SiegelAP, BlackwellHE. Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J. Am. Chem. Soc.127 (37), 12762–12763 (2005).
- Geske GD , O'NeillJC, MillerDM, MattmannME, BlackwellHE. Modulation of bacterial quorum sensing with synthetic ligands: systematic evaluation of n-acylated homoserine lactones in multiple species and new insights into their mechanisms of action. J. Am. Chem. Soc.129 (44), 13613–13625 (2007).
- Smith KM , BuY, SugaH. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol.10 (1), 81–89 (2003).
- Smith KM , BuY, SugaH. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol.10 (6), 563–571 (2003).
- Glansdorp FG , ThomasGL, LeeJKet al. Synthesis and stability of small molecule probes for Pseudomonas aeruginosa quorum sensing modulation. Org. Biomol. Chem.2 (22), 3329–3336 (2004).
- Ishida T , IkedaT, TakiguchiN, KurodaA, OhtakeH, KatoJ. Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl. Environ. Microbiol.73 (10), 3183–3188 (2007).
- O'Loughlin CT , MillerLC, SiryapornA, DrescherK, SemmelhackMF, BasslerBL. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl Acad. Sci. USA110 (44), 17981–17986 (2013).
- Yang Y-X , XuZ-H, ZhangY-Q, TianJ, WengL-X, WangL-H. A new quorum-sensing inhibitor attenuates virulence and decreases antibiotic resistance in Pseudomonas aeruginosa. J. Microbiol.50 (6), 987–993 (2012).
- Weng L-X , YangY-X, ZhangY-Q, WangL-H. A new synthetic ligand that activates QscR and blocks antibiotic-tolerant biofilm formation in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol.98 (6), 2565–2572 (2014).
- Brackman G , RisseeuwM, CelenSet al. Synthesis and evaluation of the quorum sensing inhibitory effect of substituted triazolyldihydrofuranones. Biorg. Med. Chem.20 (15), 4737–4743 (2012).
- Asahi Y , NoiriY, IgarashiJ, AsaiH, SugaH, EbisuS. Effects of N-acyl homoserine lactone analogues on Porphyromonas gingivalis biofilm formation. J. Periodontal Res.45 (2), 255–261 (2010).
- Asahi Y , NoiriY, IgarashiJ, SugaH, AzakamiH, EbisuS. Synergistic effects of antibiotics and an N-acyl homoserine lactone analog on Porphyromonas gingivalis biofilms. J. Appl. Microbiol.112 (2), 404–411 (2012).
- Kunze B , ReckM, DotschAet al. Damage of Streptococcus mutans biofilms by carolacton, a secondary metabolite from the myxobacterium Sorangium cellulosum. BMC Microbiol.10 (1), 199 (2010).
- Jansen R , IrschikH, HuchVet al. Carolacton: a macrolide ketocarbonic acid that reduces biofilm formation by the caries- and endocarditis-associated bacterium Streptococcus mutans. Eur. J. Org. Chem.2010 (7), 1284–1289 (2010).
- Hallside MS , BrzozowskiRS, WuestWM, PhillipsAJ. A concise synthesis of carolacton. Org. Lett.16 (4), 1148–1151 (2014).
- Blanco LP , EvansML, SmithDR, BadtkeMP, ChapmanMR. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol.20 (2), 66–73 (2012).
- Maury CP . The emerging concept of functional amyloid. J. Intern. Med.265 (3), 329–334 (2009).
- Schwartz K , BolesBR. Microbial amyloids: functions and interactions within the host. Curr. Opin. Microbiol.16 (1), 93–99 (2013).
- Schwartz K , SyedAK, StephensonRE, RickardAH, BolesBR. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus Biofilms. PLoS Pathog.8 (6), e1002744 (2012).
- Vuong C , SaenzHL, GötzF, OttoM. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis.182 (6), 1688–1693 (2000).
- Queck SY , Jameson-LeeM, VillaruzAEet al. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell.32 (1), 150–158 (2008).
- Mathema VB , KohYS, ThakuriBC, SillanpääM. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation35 (2), 560–565 (2012).
- Romero D , Sanabria-ValentinE, VlamakisH, KolterR. Biofilm inhibitors that target amyloid proteins. Chem. Biol.20 (1), 102–110 (2013).
- Izadpanah A , GalloRL. Antimicrobial peptides. J. Am. Acad. Dermatol.52 (3), 381–390 (2005).
- de la Fuente-Núňez C , KorolikV, BainsMet al. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother.56 (5), 2696–2704 (2012).
- Overhage J , CampisanoA, BainsM, TorfsEC, RehmBH, HancockRE. Human host defense peptide ll-37 prevents bacterial biofilm formation. Infect. Immun.76 (9), 4176–4182 (2008).
- Johansson L , ThulinP, SendiPet al. Cathelicidin LL-37 in severe Streptococcus pyogenes soft tissue infections in humans. Infect. Immun.76 (8), 3399–3404 (2008).
- Vuong C , VoyichJM, FischerERet al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol.6 (3), 269–275 (2004).
- Hell E , GiskeCG, NelsonA, RömlingU, MarchiniG. Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of Staphylococcus epidermidis. Lett. Appl. Microbiol.50 (2), 211–215 (2010).
- Kolodkin-Gal I , RomeroD, CaoS, ClardyJ, KolterR, LosickR. D-amino acids trigger biofilm disassembly. Science328 (5978), 627–629 (2010).
- Leiman SA , MayJM, LebarMD, KahneD, KolterR, LosickR. D-amino acids indirectly inhibit biofilm formation in Bacillus subtilis by interfering with protein synthesis. J. Bacteriol.195 (23), 5391–5395 (2013).
- Kolodkin-Gal I , CaoS, ChaiLet al. A self-produced trigger for biofilm disassembly that targets exopolysaccharide. Cell149 (3), 684–692 (2012).
- Hobley L , Kim SokH, MaezatoYet al. Norspermidine is not a self-produced trigger for biofilm disassembly. Cell156 (4), 844–854 (2014).
- Bendaoud M , VinogradovE, BalashovaNV, KadouriDE, KachlanySC, KaplanJB. Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide. J. Bacteriol.193 (15), 3879–3886 (2011).
- Chai Y , BeauregardPB, VlamakisH, LosickR, KolterR. Galactose metabolism plays a crucial role in biofilm formation by Bacillus subtilis. mBio3 (4), e00184–12 (2012).
- Holden HM , RaymentI, ThodenJB. Structure and function of enzymes of the leloir pathway for galactose metabolism. J. Biol. Chem.278 (45), 43885–43888 (2003).
- Dusane DH , RajputJK, KumarAR, NancharaiahYV, VenugopalanVP, ZinjardeSS. Disruption of fungal and bacterial biofilms by lauroyl glucose. Lett. Appl. Microbiol.47 (5), 374–379 (2008).
- Dusane DH , PawarVS, NancharaiahYV, VenugopalanVP, KumarAR, ZinjardeSS. Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling27 (6), 645–654 (2011).
- Desbois AP , SmithVJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol.85 (6), 1629–1642 (2010).
- Bergsson G , ArnfinnssonJ, KarlssonSM, SteingrímssonO, ThormarH. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob Agents Chemother.42 (9), 2290–2294 (1998).
- Bergsson G , SteingrímssonO, ThormarH. In vitro susceptibilities of Neisseria gonorrhoeae to fatty acids and monoglycerides. Antimicrob Agents Chemother.43 (11), 2790–2792 (1999).
- Kabara JJ , SwieczkowskiDM, ConleyAJ, TruantJP. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother.2 (1), 23–28 (1972).
- Feldlaufer M , KnoxD, LusbyW, ShimanukiH. Antimicrobial activity of fatty-acids against bacillus-larvae, the causative agent of American foulbrood disease. Apidologie24 (2), 95–99 (1993).
- Wille JJ , KydonieusA. Palmitoleic acid isomer (C16:1delta6) in human skin sebum is effective against Gram-positive bacteria. Skin Pharmacol. Appl. Skin Physiol.16 (3), 176–187 (2003).
- Georgel P , CrozatK, LauthXet al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with Gram-positive bacteria. Infect. Immun.73 (8), 4512–4521 (2005).
- Stenz L , FrançoisP, FischerAet al. Impact of oleic acid (cis-9-octadecenoic acid) on bacterial viability and biofilm production in Staphylococcus aureus. FEMS Microbiol. Lett.287 (2), 149–155 (2008).
- Davies DG , MarquesCN. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol.191 (5), 1393–1403 (2009).
- Jennings JA , CourtneyHS, HaggardWO. Cis-2-decenoic acid inhibits S. aureus growth and biofilm in vitro: a pilot study. Clin. Orthop. Relat. Res.470 (10), 2663–2670 (2012).
- Roy V , SmithJA, WangJ, StewartJE, BentleyWE, SintimHO. Synthetic analogs tailor native AI-2 signaling across bacterial species. J. Am. Chem. Soc.132 (32), 11141–11150 (2010).
- Smith JA , WangJ, Nguyen-MauSM, LeeV, SintimHO. Biological screening of a diverse set of AI-2 analogues in Vibrio harveyi suggests that receptors which are involved in synergistic agonism of AI-2 and analogues are promiscuous. Chem. Commun. (45), 7033–7035 (2009).
- Gamby S , RoyV, GuoMet al. Altering the communication networks of multispecies microbial systems using a diverse toolbox of ai-2 analogues. ACS Chem. Biol.7 (6), 1023–1030 (2012).
- Roy V , MeyerMT, SmithJAet al. AI-2 analogs and antibiotics: a synergistic approach to reduce bacterial biofilms. Appl. Microbiol. Biotechnol.97 (6), 2627–2638 (2013).
- Barraud N , HassettDJ, HwangSH, RiceSA, KjellebergS, WebbJS. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol.188 (21), 7344–7353 (2006).
- Barraud N , SchleheckD, KlebensbergerJet al. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-gmp levels, and enhanced dispersal. J. Bacteriol.191 (23), 7333–7342 (2009).
- Liu N , XuY, HossainSet al. Nitric oxide regulation of cyclic di-gmp synthesis and hydrolysis in Shewanella woodyi. Biochemistry51 (10), 2087–2099 (2012).
- Plate L , MarlettaMA. Nitric oxide-sensing H-NOX proteins govern bacterial communal behavior. Trends Biochem. Sci.38 (11), 566–575 (2013).
- Li Y , HeineS, EntianM, SauerK, Frankenberg-Dinkel N. NO-induced biofilm dispersion in Pseudomonas aeruginosa is mediated by an MHYT domain-coupled phosphodiesterase. J. Bacteriol.195 (16), 3531–3542 (2013).
- Gusarov I , NudlerE. NO-mediated cytoprotection: instant adaptation to oxidative stress in bacteria. Proc. Natl Acad. Sci. USA102 (39), 13855–13860 (2005).
- Schreiber F , BeutlerM, EnningDet al. The role of nitric-oxide-synthase-derived nitric oxide in multicellular traits of Bacillus subtilis 3610: biofilm formation, swarming, and dispersal. BMC Microbiol.11 (1), 1–12 (2011).
- Chung KK , SchumacherJF, SampsonEM, BurneRA, AntonelliPJ, BrennanAB. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases2 (2), 89–94 (2007).
- Ma Y , ChenM, JonesJE, RittsAC, YuQ, SunH. Inhibition of Staphylococcus epidermidis biofilm by trimethylsilane plasma coating. Antimicrob. Agents Chemother.56 (11), 5923–5937 (2012).
- Treter J , BonattoF, KrugC, SoaresGV, BaumvolIJR, MacedoAJ. Washing-resistant surfactant coated surface is able to inhibit pathogenic bacteria adhesion. Appl. Surf. Sci.303, 147–154 (2014).
- Busetti A , CrawfordDE, EarleMJet al. Antimicrobial and antibiofilm activities of 1-alkylquinolinium bromide ionic liquids. Green Chem.12 (3), 420–425 (2010).
- Carson L , ChauPKW, EarleMJet al. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem.11 (4), 492–497 (2009).
- Rabin N , ZhengY, Opoku-TemengC, DuY, BonsuE, SintimHO. Biofilm formation mechanisms and targets for developing anti-biofilm agents. Future Med. Chem.7 (3), 493–512 (2015).
- Abouelhassan Y , GarrisonAT, BurchGM, WongW, NorwoodVM4th, HuigensRW3rd. Discovery of quinoline small molecules with potent dispersal activity against methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis biofilms using a scaffold hopping strategy.. Bioorg. Med. Chem. Lett.24 (21), 5076–5080 (2014).
- Jennings MC , AtorLE, PaniakTJ, MinbioleKP, WuestWM. Biofilm-eradicating properties of quaternary ammonium amphiphiles: simple mimics of antimicrobial peptides.Chembiochem.5 (15), 2211–2215 (2014).
- Garrison AT , BaiF, AbouelhassanY, PaciaroniNG, JinS, HuigensIII RW. Bromophenazine derivatives with potent inhibition, dispersion and eradication activities against Staphylococcus aureus biofilms.RSC Adv.5, 1120–1124 (2015).