Abstract
Non-islet cell tumor hypoglycemia (NICTH) is a paraneoplastic syndrome characterized by persistent, severe hypoglycemia with a wide variety of solid tumors. It is considered to cause hypoglycemia by increasing the insulin-like bioactivity of the circulating insulin-like growth factor (IGF) system, however, the precise mechanism of hypoglycemia remains unclear. In this manuscript, we report on a patient suffering from NICTH caused by a small cell carcinoma of the colon. This is the first report focusing on the role of bioactive IGFs for this pathological condition. First, we demonstrated that the IGF signal pathway has been activated in this tumor in an autocrine and/or paracrine manner using immunohistochemical analysis. Second, we confirmed that bioactive IGFs in the patient's serum were increased using a modified kinase receptor activation assay, thus bioactive IGFs (mainly IGF-2) could be considered to play a major pathogenic role in enhanced hypoglycemic insulin-like activity. Third, increased IGF bioactivity in the patient's serum was completely inhibited by an anti-IGF neutralizing antibody in vitro. These results suggest that neutralization of bioactive IGFs might become a novel therapeutic strategy for NICTH to relieve the hypoglycemic symptoms together with the tumor suppressive effect.
Abbreviations
| NICTH | = | non-islet cell tumor hypoglycemia |
| IGF | = | insulin-like growth factor |
| IGF-1R | = | IGF type 1 receptor |
| IR | = | insulin receptor |
| IGFBP | = | IGF binding protein |
| HMW IGF-2 | = | high molecular weight IGF-2 |
| KIRA | = | the kinase receptor activation assay |
Introduction
Insulin-like growth factor (IGF)-1 and IGF-2 are ligands for IGF type 1 receptor (IGF-1R), which is a cell-surface tyrosine kinase signaling receptor, and for insulin receptor (IR). The physiological activities of IGFs are modulated by six IGF binding proteins (IGFBP-1 through -6) that may inhibit the signaling pathway by capturing free IGFs, thus blocking receptor binding.Citation1,2 Therefore, receptors are only activated by free/bioactive IGFs released from the IGF-IGFBP complex, mainly by proteolysis of IGFBPs.Citation1,2 Recently, it has been recognized that these receptors are widely expressed on neoplastic tissues, and overexpression of ligands, particularly IGF-2, is common in many malignancies.Citation1
Non-islet cell tumor hypoglycemia (NICTH) is a paraneoplastic syndrome characterized by persistent, severe hypoglycemia with a wide variety of tumor types that are either of mesenchymal or epithelial origin, and should be suspected in any patient with hypoglycemia without clear etiology.Citation3-6 It is associated with secretion of incompletely processed IGF-2 (high molecular weight (HMW) IGF-2) by the tumor into the circulation, and some reports attribute the etiology of NICTH to the increasing insulin-like activity of systemic IGF, mainly HMW IGF-2.Citation3-9 Thus, the mechanism of hypoglycemia has been partially elucidated by previous studies, but is still not sufficiently well understood.Citation3-9 Therapies that are directed at reduction of the tumor burden should theoretically improve NICTH.Citation3-6 However, because many NICTH causing tumors are found in the advanced stage, when surgical resection is no longer possible, we have no choice but to perform systemic chemotherapy, often with very limited success.Citation3-5
Here, we report a patient with NICTH caused by an advanced small cell carcinoma of the colon, focusing on the role of bioactive IGFs for this pathological condition and the possibility of bioactive IGFs being a novel therapeutic target for NICTH.
Case Report
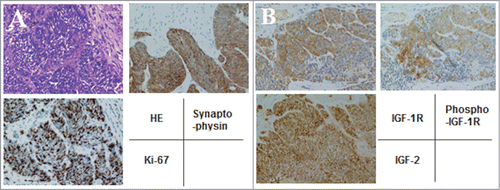
A 54-year-old male required emergency admission to the hospital due to a loss of consciousness. On admission, severe hypoglycemia was noted with a blood glucose level of 20 mg/dl. Computed tomography of the abdomen revealed multiple tumors in the liver () and thickening of the ascending colonic wall (, arrow). A total colonoscopy demonstrated a large ulcerated and circumferential tumor at the ascending colon (, inset). Biopsy specimens from the primary and liver tumors revealed that the tumor cells were composed of highly atypical small cells with hyperchromatic nuclei and scanty cytoplasm (). Immunohistochemistry was performed using anti-synaptophysin antibody (DAKO, Carpinteria, CA, USA), anti-Ki67 antibody (DAKO). The tumor cells were strongly positive for synaptophysin, and the Ki-67 labeling index was over 70% (). Based on these findings, the colonic tumor was diagnosed as a small cell carcinoma with multiple liver metastases (Neuroendocrine cell carcinoma, small-cell type, WHO classification 2010). Moreover, immunostaining was also performed using anti-IGF-2 antibody (ab9574, Abcam, Cambridge, UK), anti-IGF-1R antibody (Ab 1161, Applied Biological Materials, Richmond, BC, Canada), and anti-phospho-specific IGF-1R antibody (Phospho-Tyr 1161, Applied Biological Materials). The tumor cells were positive for IGF-2, IGF-1R, and phosphorylated IGF-1R, suggesting that the IGF signal in this tumor was activated in an autocrine and/or paracrine manner ().
Figure 1. Radiological findings of this case. (A) Abdominal computed tomography revealed multiple metastatic tumors in the liver and (B) thickening of the ascending colonic wall. (B, inset) Total colonoscopy demonstrated a large ulcerated and circumferential tumor at the same region.
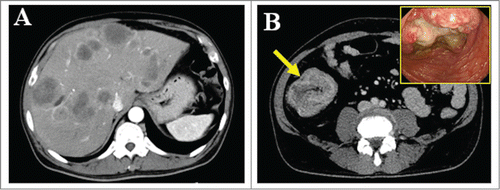
Figure 2. Immunohistochemical analysis of tumor cells. (A) The tumor cells were highly atypical small cells with hyperchromatic nuclei and scanty cytoplasm, and strongly positive for synaptophysin and Ki-67 (labeling index; 70%) (×400). (B) The tumor cells were positive for IGF-2, IGF-1R, and phosphorylated IGF-1R (×400).
The laboratory data on admission are summarized in . Decreased levels of insulin, C-peptide, IGF-1, and growth hormone, together with a normal level of total IGF-2, are typical laboratory data for NICTH.Citation3-5 HMW IGF-2 could only be detected in the patient's serum by western blot analysis (). With these laboratory data, the cause of the severe hypoglycemia was diagnosed as NICTH caused by an advanced neuroendocrine cell carcinoma of the colon.
Table 1. Laboratory data on admission
Figure 3. Western blot analysis and the kinase receptor activation assay of the patient's serum. (A) High molecular weight (HMW) and mature IGF-2 were detected in serum by western blot analysis using anti-IGF-2 antibody (ab9574). Each serum (40 μg of protein per lane) was separated by 15% SDS-PAGE. (B) IGF bioactivity in the patient's serum was greater than in a normal subject, and increased IGF bioactivity was inhibited to normal bioactivity level by the anti-IGF-2 specific antibody, ab9574, and increased IGF bioactivity was completely inhibited by the anti-IGF neutralizing antibody, KM1468. KRB; Krebs–Ringer bicarbonate buffer as a negative control, Ab; ab9574 (anti-IGF-2 specific antibody, final concentration 10 μg/ml), KM; KM1468 (anti-IGF neutralizing antibody, final concentration 10 μg/ml), hIGF-2; recombinant human IGF-2 (final concentration 10 ng/ml) as a positive control.
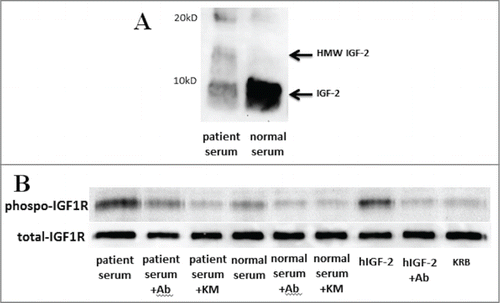
To evaluate bioactive IGFs in the circulation, we developed the novel bioassay directed against the human phosphorylated IGF-1R using protein gel blot analysis as a modification of the kinase receptor activation assay (KIRA) reported by Chen et al.Citation10 Briefly, subconfluent serum-free embryonic mouse hypothalamic (N7) cells overexpressing the human IGF-1R (N7-IGF-1R cells) were stimulated by a diluted blood sample (1mg protein) in Krebs-Ringer bicarbonate buffer (protein concentration was adjusted to 1 mg/mL) and incubated for 15 min. Each sample (10 μg of cell lysate) was fractionated by 7.5% SDS-PAGE under the reducing condition. Phosphorylated and total IGF-1R proteins were detected by western blot analysis using anti-phospho-specific IGF-1R antibody (Invitrogen, Camarillo, CA, USA) and anti-IGF-1R antibody (C-20, Santa Cruz Biotechnology, Santa Cruz, CA, USA), respectively. Serum IGF bioactivity can be evaluated by the phosphorylation levels of IGF-1R. As a result, IGF bioactivity in the patient's serum was greater than in a normal subject (, lanes 1 and 4). In addition, to clarify the ability of the anti-IGF neutralizing antibodies to inhibit the patient's IGF bioactivity in vitro, N7-IGF-1R cells was stimulated by the diluted serum sample (1mg protein) with anti-IGF-2 specific antibody (ab9574) and anti-IGF-1 and IGF-2 neutralizing antibody (KM1468; rat IgG2b), and bioactivity was evaluated by the same procedure of protein gel blot analysis as described above. The detailed characterization of KM1468 has been published elsewhere.Citation11 Increased IGF bioactivity in the patient's serum was inhibited by the anti-IGF-2 specific neutralizing antibody, ab9574 (, lane 2) to almost normal level of IGF bioactivity (, lane 4). Furthermore, Increased IGF bioactivity in the patient's serum was completely inhibited by KM1468 (, lane 3), whereas in a normal serum no difference of inhibitory effect was observed between the anti-IGF-2 specific antibody and KM1468 (, lanes 5 and 6). These results demonstrated that not only bioactive IGF-2 but also bioactive IGF-1 were increased in the NICTH patient's serum.
The mainstay of treatment is surgical resection,Citation3-5 but in the present case the primary and metastatic lesions included large masses with rapid progression. Thus, systemic chemotherapy with cisplatin and etoposide was initiated, and the patient achieved a stable condition after the first 5 cycles. Furthermore, to relieve hypoglycemia, therapies recommended by previous studies, such as glucagon, recombinant human growth hormone, or high-dose glucocorticoids, were not used because the patient's blood glucose levels were able to be maintained with the administration of glucose via a central venous line.Citation3-5 Despite systemic chemotherapy being repeated, the patient succumbed to cancer progression 8 months after diagnosis.
Discussion
NICTH can cause an emergent and life-threatening condition, and should be suspected in any patient suffering from various solid tumors with hypoglycemia without a clear etiology.Citation3-6 In addition, NICTH is considered to cause hypoglycemia by increasing the insulin-like bioactivity of the circulating IGF system.Citation3-9 In the present case, we confirmed for the first time that the levels of circulating bioactive IGFs, mainly bioactive IGF-2, were highly elevated in the NICTH patient serum by using the modified KIRA.
Highly decreased levels of total IGF-1, insulin, and C-peptide are typical laboratory data for NICTH.Citation3-6 Because it has been demonstrated in many reports that HMW IGF-2 is secreted by the tumor itself and disappeared with relief from the hypoglycemic symptoms after successful removal of the tumor, this characteristic peptide in the patient circulation has been thought to play an important role in activation of the IGF system in situations without elevation of total IGF-2.Citation3-9
HMW IGF-2 severely compromises IGFBP-3-based ternary complex formation, whereas all IGFBPs are able to form both mature and HMW IGF-2.Citation8,Citation12-14 Consequently, a shift to binary complexes is likely to occur.Citation8,Citation12-14 Binary complexes are thought to enable paracellular penetration of the vascular barriers to diffuse more effectively in the tissue microenvironment.Citation8,12,13 In addition, the short half-life and high turnover rate of binary complexes lead to increased delivery of bioactive IGFs to tissue receptors.Citation8,12,13,15 Moreover, it has been reported in recent studies that HMW IGF-2 exhibits a similar degree of bioactivity to both IGF-1R and IR as mature IGF-2.Citation16 Even in situations with an increase of HMW IGF-2 in the circulation, free IGFs in bioactive forms still need to be released from the IGF–IGFBP complex. In fact, Frystyk et al. demonstrated that the levels of free IGFs were highly elevated in the patient serum.Citation9 On the other hand, some reports are available about NICTH cases involving only a small increase of HMW IGF-2.Citation8,9 Although the mechanism responsible for the increase of free IGFs has not yet been fully elucidated, free IGFs have been strongly suggested as being of major importance in provoking hypoglycemia in NICTH patients.Citation3,4,6,9,14
IGF-2 binds to IR-A and -B with an affinity of 35–40% and about 5% that of insulin, respectively. However, the serum concentration of IGF-2 is about 100–1000 times higher than that of insulin.Citation4,17 In theory, in patients with NICTH, increased IGF bioactivity on the IR may fully induce hypoglycemia.Citation3,4,Citation7-9 The mechanism of hypoglycemia is attributed not only to enhanced glucose uptake by the tumor itself, but also to glucose consumption in the peripheral muscles and inhibition of glucose production in the liver through the IR (because no IGF-1R is expressed by the adult liver).Citation18
In the present case, western blot analysis showed that HMW IGF-2 was detected faintly and total IGF-2 was lower than in the control serum. However, the levels of bioactive IGFs (IGF-1 and IGF-2) were obviously elevated in the patient; thus, bioactive IGFs could be considered to play a major pathogenic role in enhanced hypoglycemic insulin-like activity. Bioactive IGF-2 was thought to be the main etiological peptide because increased IGF bioactivity in the patient serum was inhibited to almost normal IGF bioactivity level by the anti-IGF-2 specific neutralizing antibody in vitro. In addition, immunohistochemical analysis demonstrated that the IGF signal was activated in this tumor, suggesting that tumor growth, at least in part, is dependent on the IGF pathway in an autocrine and/or paracrine manner, in agreement with previous reports.Citation3,4
Furthermore, we have previously reported on the therapeutic efficacy of the anti-IGF-1 and IGF-2 neutralizing antibody, KM1468, in treating various tumors such as liver metastasis of colon cancers, bone metastasis of prostate cancer and breast cancer, and multiple myeloma in vivo.Citation11,Citation19-21 In the present study, KM1468 completely inhibited increased IGF bioactivity in vitro. Frystyk et al. demonstrated that not only the levels of free IGF-2 but also free IGF-1 were highly elevated in the patient serum, and levels of free IGF-1 were fourfold increased in patients compared to that in normal subjects.Citation9 Therefore, both free IGF-1 and IGF-2 may contribute to the progression of the hypoglycemia.
Our finding suggested that by direct neutralization of bioactive IGFs, a tumor growth signal and insulin-like signal mediated by both IGF-1R and IR can be blocked simultaneously. Therefore, neutralization of bioactive IGFs might become a novel effective therapeutic strategy for NICTH to relieve the hypoglycemic symptoms together with the tumor suppressive effect.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
Acknowledgments
We thank Dr Jan Frystyk for useful advice about IGF bioassays. We also thank Dr Axel Ullrich for generously providing the N7-IGF-1R cells.
Funding
Funding received from the Japan Society for the Promotion of Science (JSPS) nos. 23590939 and 24229005.
References
- Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: update. Nat Rev Cancer 2012; 12:159-69; PMID:22337149
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 2002; 23:824-54; PMID:12466191; http://dx.doi.org/10.1210/er.2001-0033
- de Groot JWB, Rikhof B, van Doorn J, Bilo HJG, Alleman MA, Honkoop AH, van der Graaf WTA. Non-islet cell tumour-induced hypoglycemia: a review of the literature including two new cases. Endocr Relat Cancer 2007; 14:979-993; PMID:18045950; http://dx.doi.org/10.1677/ERC-07-0161
- Dynkevich Y, Rother KI, Whitford I, Qureshi S, Galiveeti S, Szulc AL, Danoff A, Breen TL, Kaviani N, Shanik MH, et al. Tumors, IGF-2 and hypoglycemia: insight from the clinic, the laboratory, and the historical archive. Endocr Rev 2013; 34:798-826; PMID:23671155; http://dx.doi.org/10.1210/er.2012-1033
- Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab 2014; 99:713-722; PMID:24423303; http://dx.doi.org/10.1210/jc.2013-3525
- Fukuda I, Hizuka N, Ishikawa Y, Yasumoto K, Murakami Y, Sata A, Morita J, Kurimoto M, Okubo Y, Takano K. Clinical features of insulin-like growth factor-II producing non-islet-cell tumor hypoglycemia. Growth Horm IGF Res 2006; 16:211-6; PMID:16860583; http://dx.doi.org/10.1016/j.ghir.2006.05.003
- LeRoith D. Non-islet cell hypoglycemia. Ann Endocrinol (Paris) 2004; 65:99-103; PMID:15122103; http://dx.doi.org/10.1016/S0003-4266(04)95641-7
- Zapf J, Futo E, Peter M, Froesch ER. Can “big” insulin-like growth factor II in serum of tumor patients account for the development of extrapancreatic tumor hypoglycemia? J Clin Invest 1992; 90:2574-84; PMID:1281841; http://dx.doi.org/10.1172/JCI116152
- Frystyk J, Skjaerbaek C, Zapf J, Ørskov H. Increased levels of circulating free insulin-like growth factors in patients with non-islet cell tumour hypoglycaemia. Diabetologia 1998; 41:589-94; PMID:9628278; http://dx.doi.org/10.1007/s001250050951
- Chen JW, Ledet T, Ørskov H, Jessen N, Lund S, Whittaker J, De Meyts P, Larsen MB, Christiansen JS, Frystyk J. A highly sensitive and specific assay for determination of IGF-1 bioactivity in human serum. Am J Physiol Endocrinol Metab 2003; 284:1149-55
- Goya M, Miyamoto S, Nagai K, Ohki Y, Nakamura K, Shitara K, Maeda H, Sangai T, Kodama K, Endoh Y, et al. Growth inhibition of human prostate cancer cells in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice by a ligand-specific antibody to human insulin-like growth factors. Cancer Res 2004; 64:6252-8; PMID:15342412; http://dx.doi.org/10.1158/0008-5472.CAN-04-0919
- Zapf J, Schmid Ch, Guler HP, Waldvogel M, Hauri Ch, Futo E, Hossenlopp P, Binoux M, Froesch ER. Regulation of binding proteins for insulin-like growth factors (IGF) in humans. Increased expression of IGF binding protein 2 during IGF I treatment of healthy adults and in patients with extrapancreatic tumor hypoglycemia. J Clin Invest 1990; 86:952-61; PMID:1697608; http://dx.doi.org/10.1172/JCI114797
- Bond JJ, Meka S, Baxter RC. Binding characteristics of pro-insulin-like growth factor-II from cancer patients: binary and ternary complex formation with IGF binding proteins-1 to-6. J Endocrinol 2000; 165:253-60; PMID:10810289; http://dx.doi.org/10.1677/joe.0.1650253
- Greenall SA, Bentley JD, Pearce LA, Scoble JA, Sparrow LG, Bartone NA, Xiao X, Baxter RC, Cosgrove LJ, Adams TE. Biochemical characterization of individual human glycosylated pro-insulin-like growth factor (IGF)-II and big-IGF-II isoforms associated with cancer. J Biol Chem 2013; 288:59-68; PMID:23166326; http://dx.doi.org/10.1074/jbc.M112.432013
- Guler HP, Zapf J, Shmid C, Froesch ER. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh). 1989; 121:753-8; PMID:2558477
- Qui Q, Yan X, Bell M, Di J, Tsang BK, Gruslin A. Mature IGF-II prevents the formation of “big” IGF-II/IGFBP-2 complex in the human circulation. Growth Horm IGF Res 2010; 20:110-7; PMID:19962924; http://dx.doi.org/10.1016/j.ghir.2009.11.001
- Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor insoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 1999; 19:3278-88; PMID:10207053
- Eastman RC, Carson RE, Orloff DG, Cochran CS, Predue JF, Rechler MM, Lanau F, Roberts, Jr CT, Shapiro J, Roth J, et al. Glucose utilization in a patient with hepatoma and hypoglycemia. Assessment by a positron emission tomography. J Clin Invest 1992; 89:1958-63; PMID:1318326; http://dx.doi.org/10.1172/JCI115803
- Miyamoto S, Nakamura M, Shitara K, Nakamura K, Ohki Y, Ishii G, Goya M, Kodama K, Sangai T, Maeda H, et al. Blockade of paracrine supply of insulin-like growth factors using neutralizing antibodies suppresses the liver metastasis of human colorectal cancers. Clin Cancer Res 2005; 11:3494-502; PMID:15867252; http://dx.doi.org/10.1158/1078-0432.CCR-04-1701
- Araki K, Sangai T, Miyamoto S, Maeda H, Zhang SC, Nakamura M, Ishii G, Hasebe T, Kusaka H, Akiyama T, et al. Inhibition of bone-derived insulin-like growth factors by a ligand-specific antibody suppresses the growth of human multiple myeloma in the human adult bone explanted in NOD/SCID mouse. Int J Cancer 2006; 118:2602-8; PMID:16353147; http://dx.doi.org/10.1002/ijc.21653
- Sangai T, Fujimoto H, Miyamoto S, Maeda H, Nakamura M, Ishii G, Nagai K, Nagashima T, Miyazaki M, Ochiai A. Roles of osteoclasts and bone-derived IGFs in the survival and growth of human breast cancer cells in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice. Clin Exp Metastasis 2008; 25:401-410; PMID:18307047; http://dx.doi.org/10.1007/s10585-008-9144-8
