Abstract
This study compared the efficacy and toxicity of Gefitinib, Methotrexate and Methotrexate plus 5-Fluorouracil (5-FU) in patients of recurrent squamous cell carcinoma of head and neck (SCCHN) treated with palliative intent. Patients with recurrent SCCHN not amenable to curative treatment were randomly assigned to Gefitinib, Methotrexate or Methotrexate plus 5-FU arm. The primary end point was overall survival. Secondary end points of interest were objective response rate, toxicity and quality of life. Total 117 patients were analyzed. Median overall survival and objective response rates were 8.8 months, 7.8 months and 8.1 months and 7.7%, 5.0% and 7.9% in Gefitinib, Methotrexate and Methotrexate plus 5-FU arms respectively with no statistically significant difference between 3 arms. Gefitinib had different toxicity profile compared with other arms. Majority of toxicities were Grade 1 or Grade 2. Gefitinib had significant improvement in quality of life during initial months over Methotrexate. There was no suggestion that Gefitinib significantly prolonged overall survival compared with Methotrexate and Methotrexate plus 5-FU. However, improved Quality of Life with manageable toxicities was observed.
Abbreviations
| HNSCC | = | Head and neck squamous cell carcinoma |
| EGFR | = | Epidermal growth factor receptor |
| ECOG | = | Eastern Cooperative Oncology Group |
| QOL | = | Quality of life |
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a major form of cancer in India accounting for 23% of all cancer in males and 6% in females. Most cases (80%) present in late stages and survival is poor with all modalities of treatment.Citation1 Recurrence and metastasis is the usual disease course in more than 50% of these patients.Citation2 These patients have poor prognosis with median survival in range of 6–12 months.Citation3
Treatment options are limited for recurrent/metastatic disease, only few patients are suitable for surgery or re-radiation. Palliative chemotherapy is considered as standard of care in these patients. Platinum based regimen with 5-FU is often considered as standard in patients with good performance status.Citation4-6 The other drugs that have been used are Methotrexate, Bleomycin, Capacitabine and Ifosphamide.Citation7 These drugs produce response rate up to 30% while introduction of new chemotherapy agents such as taxane can increase response rate up to 43%.Citation8 Despite advancements in chemotherapy, none of these drugs as single agents or in combination, have been found to prolong survival.Citation9
As most of these patients present with poor general health or prior platinum based chemotherapy, methotrexate as a single agent remains a drug of choice in a significant proportion of patients.Citation7 Methotrexate and 5-FU are anti metabolites and many of the studies have suggested synergistic effect in combination therapy.Citation10,11
Various novel targeted therapies have been tested; of which epidermal growth factor receptor (EGFR) inhibitors are of particular interest. EGFR is over-expressed in 90% of HNSCC.Citation12 Dysfunction of this receptor and its associated pathways tend to have significant implication in susceptibility and prognosis of head and neck cancer.Citation13,14
Gefitinib, an EGFR tyrosine kinase inhibitor has shown promising results showing response rate and median survival of 10.6%–1.4% and 8.1 months-5.5 months at a dose of 500 mg/250 mg daily respectively.Citation15-17
As of yet although there have been studies wherein gefitinib at 500 mg/day and methotrexate at 40 mg/m2/week were found to be equivalent in survival in recurrent/metastatic SCCHN, this study was proposed in view of the possibility that there would be different outcomes in a study of patients from India, based upon genetic and disease presentation differences.
We therefore conducted a study to compare Gefitinib, Methotrexate and Methotrexate plus 5-FU in palliation of recurrent head and neck squamous cell carcinoma. The primary aim of this study was to analyze the efficacy and toxicity of Gefitinib versus Methotrexate and Methotrexate plus 5-FU in Asian patients.
Results
A total of 120 patients were enrolled and randomized in the study between July 2010 and September 2012. However, 3 patients were not analyzed for results. One patient died before commencing treatment and 2 patients developed second primary tumor/brain metastasis (these patients never received the drugs). Out of 117 analyzed cases, 39 patients were on Gefitinib arm, 40 patients were on Methotrexate arm and remaining 38 patients were on Methotrexate plus 5-FU arm.
Patient population
Patient's demographic and clinical characteristics are presented in . The treatment arms were well balanced with respect to age, gender, ECOG performance status, primary disease site, site of recurrence and prior chemotherapy. The number of male patients being higher than the female patients is in agreement with published Indian Literature. Majority of the patients had oral cavity followed by oropharynx as primary disease site. Approximately 60% of the patients in each arm had nodal recurrence. Most of the patients in the study received prior chemotherapy as a part of palliative or initial curative intent treatment. Half of the patients in each arm had received Platinum plus Taxane as prior chemotherapy.
Table 1. Patient's demographic and clinical characteristics of 3 groups
Treatment administration
The mean exposure duration was 8.89, 6.42 and 6.50 months for Gefitinib, Methotrexate and Methotrexate plus 5-FU respectively (P = 0.054). The most common reason for treatment discontinuation was progression of disease and poor general condition. Other reasons were toxicity, patient preference, logistic and unknown. Eight patients of Gefitinib arm subsequently received Methotrexate and 5 patients of Methotrexate plus 5-FU later received Methotrexate.
Clinical response
The response to therapy is summarized in . The term “disease control rate” is often used to describe the proportion of patients with response or stable disease. None of the patients in each arm achieved complete response (CR). In Gefitinib arm, 3 (7.7%) patients achieved partial response (PR) and 23 (59%) patients had stable disease as their best response, such that disease control rate was 66.7%. In Methotrexate, 2(5.0%) patients achieved PR and 21(52.5%) patients had SD, such that disease control rate was 57.5%. On the other hand in Methotrexate plus 5-FU arm, 3(7.9%) patients observed PR and 21(55.3%) had stable disease, such that disease control rate was 63.2%. However, response rate did not differ significantly (P > 0.05) among these groups. Also, there was no difference in tumor and node response rate in 3 groups.
Table 2. Treatments response of 3 groups
Toxicity
Toxicities observed during treatment are summarized in . Toxicities encountered mostly were in Grade 1 or 2 in severity on each arm. The most common toxicity reported with Gefitinib was rash, diarrhea and mucositis. Patients in Methotrexate and Methotrexate plus 5-FU arm, observed mucositis, nausea, vomiting, anemia and leucopenia. The Grade 3 toxicities were slightly higher in Methotrexate plus 5-FU arm. However, all these groups showed manageable and insignificant difference in toxicities.
Table 3. Treatments related adverse events of 3 groups
Overall survival
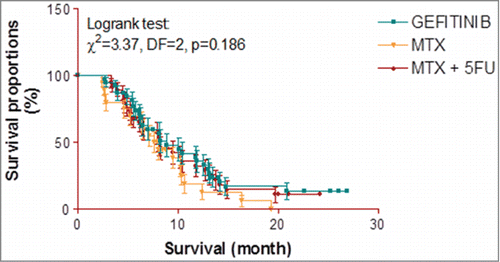
The recurrent patients of 3 groups were followed up to 27 months. After 27 months, there were comparable 9 survivals in GEFTINIB group (23.1%) followed by 8 in MTX + 5FU group (21.1%) and 4 the least in MTX group (10.0%) with also comparable overall median survival of 8.8 months, 8.1 months and 7.8 months respectively and comparing the overall median survivals of 3 groups, Logrank test revealed similar survivals among the 3 groups (χ2 = 3.37, P = 0.186) i.e. not differed statistically ().
Quality of life
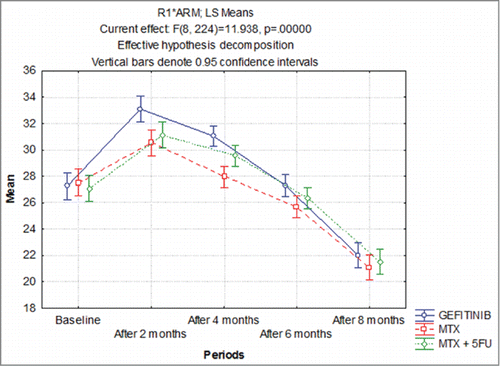
The pre (baseline) and post (after 2 month, after 4 month, after 6 month and after 8 month) QOL of 3 groups are summarized graphically in . After treatment, the QOL in all 3 groups improved at after 2 month and after 4 month and thereafter decrease gradually in all 3 groups. Comparing the effect of groups and periods together on QOL, ANOVA revealed significant effect of both groups (F = 3.63, P = 0.033) and periods (F = 1083.05, P < 0.001) on QOL. Further, the interaction effect of both (groups × periods) on QOL was also found significant (F = 11.94, P < 0.001). Further, for each group comparing the mean QOL within the groups (i.e., between periods) Tukey test revealed significantly (P < 0.001) different QOL between the periods in all 3 groups. Similarly, for each period, comparing the mean QOL between the groups, Tukey test revealed significantly (P < 0.05 or P < 0.01) different and higher QOL of GEFITINIB at after 2 month and after 4 month as compared to MTX. However, the mean QOL did not differ (P > 0.05) between GEFITINIB and MTX + 5 FU and MTX and MTX + 5FU, i.e., found to be statistically the same.
EGFR expression
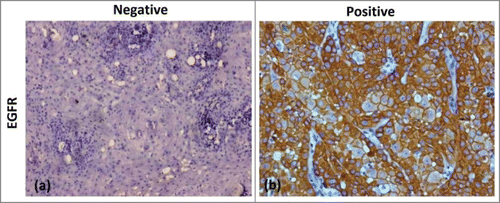
The immunohistochemical expression of molecular marker in tumor cells for EGFR expression were found to be positive in more than 90% patients so it was not correlated with response/survival.
Discussion
This study compared Gefitinib, Methotrexate and Methotrexate plus 5-FU as palliatively intended therapeutic option for recurrent SCCHN. The hypothesis of this study was that in recurrent SCCHN, 500 mg daily Gefitinib would have activity comparable or superior to Methotrexate or Methotrexate plus 5-FU. As of yet except for few there are no studies comparing standard palliative Methotrexate with newer targeted agents in recurrent/metastatic SCCHN.Citation18
Stewart et al. compared 2 doses of Gefitinib with Methotrexate in recurrent/metastatic SCCHN.Citation20 This study showed response rate of 7.7%, 5.0% and 7.9% respectively for Gefitinib, Methotrexate and Methotrexate plus 5-FU which was found insignificant among all the 3 groups. While the dose response of Gefitinib in this study was found to be similar as reported in previous studies, the response rate of Methotrexate and Methotrexate plus 5-FU was reported lower as compared to previous studies.Citation17,19
Some investigators have concluded from clinical studies that recurrent/metastatic SCCHN has poor prognosis and have reported response rate of 10% with Methotrexate and Methotrexate plus 5-FU whereas others believe treatment with Methotrexate and Methotrexate plus 5-FU can bring about higher response rate of 50%.Citation20-23
Gefitinib has a different toxicity profile compared to Methotrexate and Methotrexate plus 5-FU. The most common adverse events reported with Gefitinib were Cutaneous rash, diarrhea and oral mucositis as observed in previous studies.Citation15-17 In Methotrexate and Methotrexate plus 5-FU arm, oral mucositis, nausea, vomiting and hematological toxicity were common adverse events. Higher grade adverse reaction observed in more patients of Methotrexate plus 5-FU arm compared to Methotrexate. In our study, we observed longer mean exposure duration of Gefitinib (8.89 months) arm compared to Methotrexate (6.42 months) and Methotrexate plus 5-FU (6.50 months), reflecting more patients compliance with Gefitinib.
In the present study, the primary end point was overall survival. The median overall survival (8.8 months) observed with Gefitinib was in range of previously reported studies.Citation15-17 We observed comparable median survival in Gefitinib (8.8 months), Methotrexate (8.1 months) and Methotrexate plus 5-FU arm (7.8 months), as it was statistically insignificant, however Gefitinib appears to contribute to good compliance in our patients probably in relation to better tolerability during treatment period even if tumor response was not achieved. Hence there was no suggestion of prolongation of overall survival with either arm in our study. Similarly none of the trials previously performed have shown to prolong survival in recurrent/metastatic SCCHN since the introduction of cisplatin. A recently published phase III EXTREME study showed that adding Cetuximab (human chimeric monoclonal antibody against EGFR) to platinum and 5-FU significantly prolonged the median OS from 7.4 months to 10.1 months in recurrent and/or metastatic SCCHN.Citation24
Quality of life (QOL) measurement was done with a recently developed instrument, the Functional Assessment of Cancer Therapy- Head & Neck Symptom Index-10 (FHNSI-10). It is a 10 item symptom-focused index specially designed for advanced and/or recurrent/refractory head and neck cancer. This index was selected for current study due to its brevity and feasibility in patients with poor status and co-morbid condition. It was acceptable to patients with high rate of compliance. Our study showed improvement in QOL in initial 2nd and 4th month and then it declined from baseline. Although Gefitinib and Methotrexate plus 5-FU were not found to increase survival, significant improvement in QOL was observed in gefitinib arm. It was different as recorded in Stewart et al. and Cohen et al.Citation15,18 It could be because Cohen et al. study documented QOL measurement with patients receiving 250 mg of Gefitinib and in Stewart et al. study multiple QOL instrument were used which might have resulted in poor patients compliance rate.
In conclusion, there was no suggestion that incorporation of Gefitinib in the treatment algorithms significantly prolonged overall survival compared with Methotrexate and Methotrexate plus 5-FU. However reasonable improvement in Quality of Life with manageable toxicities was observed in Gefitinib arm. Therefore QOL does improve with second line therapy, regardless of agent. It does reinforce that second line therapy should be considered in some patients, despite the poor performance of these agents in response and durable survival. Hence learning how to incorporate these targeted agents in our current treatment strategies by using correlative science should be the focus of our future endeavors in recurrent SCCHN in efforts to improve quality of life and survival.
Patients and Methods
Eligibility
Patients with recurrent SCCHN who were ineligible for Salvage surgery or Radiotherapy were eligible for treatment. Also the patients, who had prior systemic chemotherapy (for recurrent disease) or had not, were eligible.
Patients were required to have Eastern Co-operative Oncology Group (ECOG) performance status of 2 or less, Hemoglobin ≥10 g/dl, leukocyte count ≥4000/μL, platelets count ≥100,000/μL, adequate renal function (creatinine level ≤1.5 mg/dL), total bilirubin within normal institutional limits and Plasma Hepatic trans-aminase ≤2.5 times the institutional upper limit of normal.
Treatment plan
Patients were randomized to receive Gefitinib, Methotrexate and Methotrexate plus 5-FU. Therapy was continued until disease progression, unacceptable toxicity or patient withdrawal.
In Gefitinib arm, patients were administered at a fixed dose of Gefitinib 500 mg/day. Patients, who were unable to swallow, were allowed to dissolve the tablets in water.
In Methotrexate (MTX) arm, the dose selected was 40 mg/m2 which were administered weekly as a slow intravenous push. In Methotrexate plus 5-FU arm, MTX 40 mg/m2 and 5-FU 600 mg/m2 was administered weekly. Each drug was given by slow intravenous push.
Study assessment
All patients were assessed clinically and radiologically before starting the treatment and were subsequently assessed clinically, with radiological assessment (where possible). Patients were assessed on a monthly basis by standard RECIST (Response Evaluation Criteria in Solid Tumors) as either a complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD).
Toxicity was graded according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 4.0.
Quality of life
Quality of Life (QOL) was measured using the Functional Assessment of Cancer Therapy- Head & Neck Symptom Index-10 (FHNSI-10) designed by David Cella et al.Citation25 Overall survival was defined as time from randomization to death from any cause or censored at last date the patient was known to be alive.
EGFR biomarker assessment
For immunohistochemical study, tumor samples were fixed in 10% buffered formalin and then embedded in paraffin. Further sections were processed for EGFR by immunohistochemistry using primary monoclonal antibodies and a polymer based secondary antibody detection kit from Dakopatts, Denmark Standard.
Immunohistochemistry protocol was used. Sections were deparaffinized in xylene and rehydrated through various grades of ethanol. Deparaffinized rehydrated sections were blocked for endogenous peroxidases in 0.3% hydrogen peroxide in methanol, followed by a rinse in distill water for 30 min. Antigen retrieval was achieved at 121°C in 10 mM citrate buffer (pH 6.0) for 10 min using Pascal retrieval system from Dakopatts, Denmark. Slides cooled to room temperature were washed thrice with Tris-buffered saline (TBS) and incubated overnight with Primary Antibody EGFR (BioGenex, USA) at 4°C. After washing with Tris-buffered saline; the sections were incubated for 30 min with secondary antibody. Biomarkers were visualized with Liquid Diaminobenzidine substrate chromogen and counterstained with diluted Mayer's haematoxylin. Sections mounted with DPX were inspected under a Zeiss metasystem and photographed at 40× magnification.
The immunohistochemical evaluation was carried out in tumor hotspots including the invasion front, which was regarded as most indicative of the biological activity of the tumor, in 10 high power fields. About 1500–2000 tumor cells were observed in all tumors at a magnification of 40× in 10 selected fields. For EGFR tumors were labeled as negative if <10% and positive if ≥10% tumor cells expressed the antigen.Citation26 ()
Statistical Consideration
Continuous data were summarized as Mean ± SD while discrete (categorical) in no. and%. Continuous groups were compared by one way analysis of variance (ANOVA) or repeated measures ANOVA using general linear models (GLM) and the significance of mean difference within and between the groups was done by Tukey post hoc test. Categorical groups were compared by chi-square (χ2) test. Survival between the groups was done by Kaplan-Meier method using Logrank test. A 2-sided P value less than 0.05 (P < 0 .05) was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Al-Sarraf M. Treatment of locally advanced head and neck cancer: historical and critical review. Cancer Control 2002; 9:387-99; PMID:12410178
- Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, Mittal BB, Pelzer H, Fung BB, Witt ME, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol 2004; 15:1179; PMID:15277256; http://dx.doi.org/10.1093/annonc/mdh308
- Brockstein BE. Management of recurrent head and neck cancer: recent progress and future directions. Drugs 2011; 71:1551-9; PMID:21861540; http://dx.doi.org/10.2165/11592540-000000000-00000
- Jacobs C, Lyman G, Velez-Garcia E, Sridhar KS, Knight W, Hochster H, Goodnough LT, Mortimer JE, Einhorn LH, Schacter L, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992; 10:257-63; PMID:1732427
- Forastiere AA, Mitch B, Schuller DE, Ensley JF, Hutchins LF, Triozzi P, Kish JA, McClure S, VonFeldt E, Williamson SK, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous cell carcinoma of the head and neck: A Southwest Oncology Group Study. J Clin Oncol. 1992; 10:1241-51; PMID:1634913
- Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005; 23:8646-54; PMID:16314626; http://dx.doi.org/10.1200/JCO.2005.02.4646
- Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006; 24:2644-52; PMID:16763278; http://dx.doi.org/10.1200/JCO.2005.05.3348
- Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010; 21:252-61; PMID:20943624; http://dx.doi.org/10.1093/annonc/mdq453
- Price KA, Cohen EE. Current Treatment Options for Metastatic Head and Neck Cancer. Curr Treat Options Oncol 2012; 13:35-46; PMID:22252884; http://dx.doi.org/10.1007/s11864-011-0176-y
- Asaumi J, Nishijima K. Low dose sequential methotrexate and 5-fluorouracil administration is effective and safe as neo-adjuvant chemotherapy in oral cancer. In Vivo 1996; 10:559-62; PMID:8986464
- Browman GP, Archibald SD, Young JE, Hryniuk WM, Russell R, Kiehl K, Levine MN. Prospective randomized trial of one-hour sequential versus simultaneous methotrexate plus 5-fluorouracil in advanced and recurrent squamous cell head and neck cancer. J Clin Oncol 1983; 1:787-92; PMID:6366132
- Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. U Clin Oncol 2006; 24:2666; PMID:16763281; http://dx.doi.org/10.1200/JCO.2005.04.8306
- Ang KK, Andratschke NH, Milas L. Epidermal growth factor receptor and response of head-and-neck carcinoma to therapy. Int J Radiat Oncol Biol Phys 2004; 58:959-65; PMID:14967456; http://dx.doi.org/10.1016/j.ijrobp.2003.07.010
- Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, Jarrett C, Carter J, Murphy BA, Netterville J, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. Clin Oncol 2006; 24:4170-6; PMID:16943533; http://dx.doi.org/10.1200/JCO.2006.07.2587
- Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, Vokes EE. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 2003; 21:1980-7; PMID:12743152; http://dx.doi.org/10.1200/JCO.2003.10.051
- Cohen EE, Kane MA, List MA, Brockstein BE, Mehrotra B, Huo D, Mauer AM, Pierce C, Dekker A, Vokes EE. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 2005; 11:8418-842; PMID:16322304; http://dx.doi.org/10.1158/1078-0432.CCR-05-1247
- Kirby AM, A'Hern RP, D'Ambrosio C, Tanay M, Syrigos KN, Rogers SJ, Box C, Eccles SA, Nutting CM, Harrington KJ. Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or metastatic head and neck cancer. Br J Cancer. 2006; 94:631-6; PMID:16495923
- Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D, Vodvarka P, Rischin D, Garin AM, Hirsch FR, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2009; 27:1864-71; PMID:19289630; http://dx.doi.org/10.1200/JCO.2008.17.0530
- Airoldi M, Pedani F, Brando V, Gabriele P, Orecchia R. Comparison of methotrexate and sequential methotrexate-5-fluorouracil for patients with recurrent squamous cell carcinoma of the oral cavity. Chemioterapia 1987; 6:390-2; PMID:3435920
- Tortochaux J, Tao Y, Tournay E, Lapeyre M, Lesaunier F, Bardet E, Janot F, Lusinchi A, Benhamou E, Bontemps P, et al. Randomized phase III trial (GORTEC 98-03) comparing re-irradiation plus chemotherapy versus methotrexate in patients with recurrent or a second primary head and neck squamous cell carcinoma, treated with a palliative intent. Radiother Oncol 2011; 100:70-5; PMID:21741720; http://dx.doi.org/10.1016/j.radonc.2011.06.025
- Schornagel JH, Verweij J, de Mulder PH, Lapeyre M, Lesaunier F, Bardet E, Janot F, Lusinchi A, Benhamou E, Bontemps P, et al. Randomized phase III trial of edatrexate versus methotrexate in patients with metastatic and/or recurrent squamous cell carcinoma of the head and neck: a European Organization for Research and Treatment of Cancer Head and Neck Cancer Cooperative Group study. J Clin Oncol 1995; 13:1649-55; PMID:7602354
- Pitman SW, Kowal CD, Bertino JR. Methotrexate and 5-fluorouracil in sequence in squamous head and neck cancer. Semin Oncol 1983; 10:15-9; PMID:6346497
- Ringborg U, Ewert G, Kinnman J, Lundqvist PG, Strander H. Sequential methotrexate-5-fluorouracil treatment of squamous cell carcinoma of the head and neck. Cancer 1983; 52:971-3; PMID:6603896; http://dx.doi.org/10.1002/1097-0142(19830915)52: 6<971::AID-CNCR2820520606>3.0.CO;2-B
- Rivera F, García-Castaño A, Vega N, Vega-Villegas ME, Gutiérrez-Sanz L. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther 2009; 9:1421-8; PMID:19828002; http://dx.doi.org/10.1586/era.09.113
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993; 11:570-9; PMID:8445433
- Shiraki M, Odajima T, Ikeda T, Sasaki A, Satoh M, Yamaguchi A, Noguchi M, Nagai I, Hiratsuka H. Combined expression of p53, cyclin D1 and epidermal growth factor receptor improves estimation of prognosis in curatively resected oral cancer. Mod Pathol 2005; 18:1482-9; PMID:16007067; http://dx.doi.org/10.1038/modpathol.3800455