Abstract
Protective immunity against Mycobacterium tuberculosis (Mtb) requires IFNG. Besides, IFNG-mediated induction of autophagy suppresses survival of virulent Mtb in macrophage cell lines. We investigated the contribution of autophagy to the defense against Mtb antigen (Mtb-Ag) in cells from tuberculosis patients and healthy donors (HD). Patients were classified as high responders (HR) if their T cells produced significant IFNG against Mtb-Ag; and low responders (LR) when patients showed weak or no T cell responses to Mtb-Ag. The highest autophagy levels were detected in HD cells whereas the lowest quantities were observed in LR patients. Interestingly, upon Mtb-Ag stimulation, we detected a positive correlation between IFNG and MAP1LC3B-II/LC3-II levels. Actually, blockage of Mtb-Ag-induced IFNG markedly reduced autophagy in HR patients whereas addition of limited amounts of IFNG significantly increased autophagy in LR patients. Therefore, autophagy collaborates with human immune responses against Mtb in close association with specific IFNG secreted against the pathogen.
Abbreviations:
- AG, antigen
- ATG, autophagy-related
- FBS, fetal bovine serum
- GAPDH, glyceraldehyde-3-phosphate dehydrogenase
- GTP, guanosine triphosphate
- HD, healthy donors
- HR TB, high-responder tuberculosis patient
- IFNG, interferon gamma
- IL, Interleukin
- LC3, microtubule-associated protein 1A/1B-light chain 3
- LR TB, low-responder tuberculosis patients
- mAb, monoclonal antibody
- Mtb-Ag, Mycobacterium tuberculosis antigen
- PBS, phosphate-buffered saline
- PBMC, peripheral blood mononuclear cells
- rIFNG, recombinant IFNG
- SLAM, signaling lymphocytic activation molecule
- TB, tuberculosis
- Th, T helper
Introduction
The immune response elicited after M. tuberculosis infection is critically dependent on CD4+ T cells. In particular, T helper (Th) 1 cells play an important role in granuloma formation and clearance of M. tuberculosis.Citation1-3 In fact, deficiencies in the IL12 (interleukin 12)-IFNG (interferon gamma)-STAT1 (signal transducer and activator of transcription 1, 91kDa) signaling pathway lead to the dissemination of mycobacterial infections.Citation4,5 The degree of reduction in IFNG production by peripheral blood mononuclear cells (PBMC) is a marker of disease severity in patients with tuberculosis;Citation6 therefore IFNG secretion is lower in patients with the most severe manifestation of tuberculosis.Citation6 Thus, elucidation of the cellular mechanisms affected by reduced IFNG production in individuals that develop the disease will enhance our knowledge of the pathogenesis of tuberculosis, contributing to a better understanding of the immune response to this intracellular pathogen.
IFNG, a cytokine produced primarily by T cells and natural killer cells, is an important mediator of macrophage activation in controlling several intracellular pathogens, including mycobacteria.Citation2 Injection of recombinant IFNG into lesions of lepromatous leprosy patients results in the migration of large numbers of Th cells and monocytes to the site of injectionCitation7,8 and a decrease in acid-fast bacilli load.Citation7 IFNG has many effects, including induction of autophagy.Citation9 In fact, IFNG increases proteolysis of long-lived proteins and processing of MAP1LC3/LC3 (microtubule-associated protein 1A/1B-light chain 3) (i.e., punctate distribution), both hallmarks of autophagy induction.Citation10,11 Upon induction of autophagy, LC3-I is converted to LC3-II through lipidation by a system involving ATG7 (autophagy-related 7) and ATG3, which then allows LC3 to associate with autophagic vesicles.Citation12 Thus, both LC3 presence in autophagosomes and its conversion to the lower migrating form, LC3-II, are commonly used indicators of autophagy.Citation13 Interestingly, a polymorphism on IRGM1 (immunity-related GTPase family M member 1), an autophagy-related gene downstream of the IfngCitation14 gene, has been associated with protection against M. tuberculosis.Citation15,16 In a more recent work, another gene family of the IFNG inducible GTPases has been described, which comprises the guanylate-binding proteins.Citation17 It has been shown that members of this family interact with autophagy-related (ATG) proteins, establishing a link between this family of GTPases and autophagy-mediated defense mechanisms against invading pathogens.
Previously it has been shown that induction of autophagy promotes maturation of Mycobacterium-containing phagosomes and concomitantly suppresses mycobacterial survival.Citation10 Besides, it has been demonstrated that activation of RAW 264.7 cells with IFNG induces autophagic activation.Citation10 Moreover, Harris et al. have demonstrated that Th2 cytokines (IL4 and IL13) abrogate autophagy and autophagy-mediated killing of intracellular mycobacteria in human U937 or THP-1 cells.Citation9 Thus, critical cytokines modulate both positively and negatively the autophagic response affecting mycobacterial survival.
Most of the studies about the role of autophagy as a defense mechanism against Mycobacterium have been performed using cell lines infected with the pathogen or primary culture cells. However, very limited information exists about the autophagic response in patients with active tuberculosis disease. Therefore, in this work we investigated the potential role of autophagy during the human immune response against M. tuberculosis antigens. To this end, we studied M. tuberculosis-induced autophagy in 2 populations of patients with active disease previously identified by our laboratory.Citation18 These 2 groups of individuals were classified based on their T-cell responses to the bacterium: High-responder (HR) tuberculosis patients displayed significant M. tuberculosis-dependent T- cell proliferation and IFNG production, while low-responder (LR) tuberculosis patients displayed weak or no T-cell responses to M. tuberculosis.Citation18 On the basis of the magnitude of the immune response of these 2 groups of patients against the pathogen, we hypothesized that IFNG produced by T cells might increase the autophagic response in human monocytes. In fact, we showed that sonicated M. tuberculosis (Mtb-Ag) induced the highest levels of autophagy in healthy donors and the lowest levels in LR tuberculosis patients, in direct association with the amounts of IFNG secreted by each individual. Interestingly, addition of IFNG augmented autophagy in patients with the weakest production of IFNG against the bacteria. Together, our present data indicate that autophagy is being modulated by the innate and adaptive immune response of the human host against M. tuberculosis. To our knowledge, this is the first study that establishes a link between autophagy and IFNG production in cells obtained from tuberculosis patients.
Results
M. tuberculosis antigen induces significantly lower autophagy levels in tuberculosis patients with the poorest immune response against the pathogen
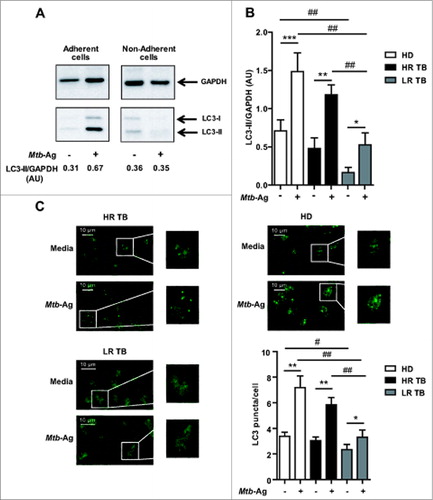
Studies in human cell lines and in mice have shown that autophagy is a defense mechanism that inhibits mycobacterial survival.Citation10,19 Furthermore, it has been recently demonstrated that the virulent M. tuberculosis strain H37Rv is able to inhibit basal autophagy in human macrophages and dendritic cells.Citation20 In order to analyze the role of autophagy in the defense of the human host against M. tuberculosis, we investigated this process in healthy donors and patients with active disease. To do this, peripheral blood mononuclear cells (PBMC) were cultured during 16 h without stimulus. The cells were then incubated with sonicated M. tuberculosis antigen (Mtb-Ag) for 24 h and the levels of the autophagic marker LC3, were assessed by western blotting. As shown in , endogenous levels of LC3-II accumulated upon Mtb-Ag stimulation of adherent cells from a high-responder tuberculosis patient. In contrast, no detectable levels of LC3-II were observed in the nonadherent fraction of the PBMC. These data suggested that Mtb-Ag-induced autophagic response was occurring in monocytic cells. Densitometry of the blots showed that the amount of LC3-II was significantly increased in Mtb-Ag-treated cells obtained from healthy donors (HD) and tuberculosis patients, as compared to nonstimulated cells (). However, both HD and HR patients showed significantly higher amounts of LC3-II as compared to LR patients (). Furthermore, we detected marked differences between the basal levels of LC3-II in cells from HD as compared to LR tuberculosis patients ().
Figure 1. Levels of autophagy induced in response to Mtb-Ag in tuberculosis patients and healthy donors. (A and B) PBMC from HD, HR TB and LR TB were cultured at 3 × 106 cells/ml in RPMI with 10% FBS. Cells were then cultivated for 16 h without stimulus to allow the adherence of monocytes. Afterwards, PBMC were stimulated with sonicated M. tuberculosis (Mtb-Ag) for 24 h, protein extracts were obtained and western blot was performed. Densitometry of the blots was performed and the means of the ratios of LC3-II to GAPDH were expressed as arbitrary units (AU). (A) A representative example of a HR TB patient is shown. (B) Bars represent the mean values ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, Wilcoxon matched-pairs signed rank test; #P < 0.05, ##P < 0.01 Mann-Whitney Test. (C) PBMC from HD and HR TB and LR TB patients were incubated at 2 × 106 cells/ml in RPMI with 10% FBS. PBMC were then stimulated with or without Mtb-Ag for 24 h and immunofluorescence for LC3 was performed. Samples were then analyzed in a confocal microscope. Representative images of a HD, a HR TB, and a LR TB patient are shown. Bars represent the mean values of the number of LC3 puncta/cell ± SEM. *P < 0.05, **P < 0.01, Wilcoxon matched-pairs signed rank test; ##P < 0.01 Mann-Whitney Test.
To assess the rate of autophagy we determined the autophagic flux in the absence or presence of bafilomycin A1 (BafA1), which inhibits vacuolar-type H+-ATPases and prevents fusion between autophagosomes and lysosomes, leading to inhibition of LC3-II degradation.Citation21 Thus, the LC3-II levels upon exposure of the cells to the Mtb-Ag preparation in the presence or absence of BafA1 were analyzed. We observed that, in adherent cells, Mtb-Ag increased LC3-II puncta levels significantly in the presence of BafA1, strongly suggesting that this antigen induced autophagosome formation and that the autophagic pathway was functional (Fig. S1). Similar results were observed by protein gel blot and flow cytometry (data not shown).
To further analyze the ability of human monocytes to undergo autophagy in response to Mtb-Ag, cells from tuberculosis patients and HD were cultured in the presence of the antigen for 24 h to induce the autophagic process. After fixation, cells were labeled with antibodies recognizing endogenous LC3 and the samples were analyzed by confocal microscopy. LC3 is a useful marker of autophagy by immunofluorescence, as it gets conjugated to autophagic membranes when autophagy is induced, thus forming puncta in the cytosol. As shown in , in LR tuberculosis patients, only a few LC3 puncta could be detected in Ag-stimulated cells (). In contrast, in HD and HR tuberculosis patients, LC3 puncta accumulated strongly when Mtb-Ag was added to the culture medium (). Moreover, quantification of the dots confirmed that Mtb-Ag induced significantly higher levels of autophagy in HD and HR patients as compared to LR individuals (). Taken together, our results indicate that LR TB patients showed diminished autophagy in response to Mtb-Ag as compared to HR patients and HD.
M. tuberculosis antigen induces autophagy in CD14+ cells from patients with active disease
As shown above, Mtb-Ag induced autophagy in the adherent cell fraction of PBMC (). Thus, in order to confirm that the autophagic response specifically occurred in monocytes (i.e., CD14+ cells) from tuberculosis patients and HD, we next measured intracellular saponin-resistant LC3-II by FACS, as previously described.Citation22 As shown in , few LC3-II-positive (LC3+) monocytes were detected among cells cultured with media (controls). However, incubation with Mtb-Ag significantly augmented the percentage of CD14+LC3+ cells in tuberculosis patients and HD (), in agreement with our findings shown by western blot (). Nevertheless, we detected significantly lower numbers of CD14+ LC3+ cells in LR tuberculosis patients as compared to HR patients and HD (). To further characterize the autophagic response to Mtb-Ag in PBMC obtained from tuberculosis patients, we analyzed intracellular LC3-II not only in CD14+ monocytes, but also in CD3+ lymphocytes. shows that the autophagic response to Mtb-Ag is not a general response in all cell types, since it clearly affects CD14+ monocyte cells but not CD3+ T lymphocytes. Together, these findings demonstrate that Mtb-Ag induced autophagy in monocytes from patients with active tuberculosis, but the process is markedly reduced in CD14+ cells from LR tuberculosis patients, individuals with weak immune responses against the pathogen.
Figure 2. Mtb-Ag induces autophagy in CD14+ cells from tuberculosis patients and healthy donors. PBMC from healthy donors (HD) and tuberculosis patients (TB) (High responders [HR TB] and low responders [LR TB]) were incubated at 3 × 106 cells/ml in RPMI with 10% FBS. PBMC were stimulated with or without sonicated M. tuberculosis antigen (Mtb-Ag) for 24 h and intracellular saponin-resistant LC3 determination was performed on CD14+ and/or CD3+ cells by flow cytometry. (A) Dot plots were obtained first gating on monocytes by light scatter, then on CD14+ cells and finally on LC3+ cells. Representative dot plots from a HD and a LR TB are shown. (B) Bars represent the mean values of the percentage of CD14+LC3+ cells ± SEM. *P < 0.05, Wilcoxon matched-pairs signed rank test; #P < 0.05, ##P < 0.01, Mann-Whitney Test. (C) Intracellular LC3-II was determined by flow cytometry first gating on lymphocytes by light scatter and then gating on CD3+ T cells (upper panel); or first gating on monocytes by light scatter and then on CD14+ cells (lower panel). A representative dot plot from a HR tuberculosis patient is shown.
![Figure 2. Mtb-Ag induces autophagy in CD14+ cells from tuberculosis patients and healthy donors. PBMC from healthy donors (HD) and tuberculosis patients (TB) (High responders [HR TB] and low responders [LR TB]) were incubated at 3 × 106 cells/ml in RPMI with 10% FBS. PBMC were stimulated with or without sonicated M. tuberculosis antigen (Mtb-Ag) for 24 h and intracellular saponin-resistant LC3 determination was performed on CD14+ and/or CD3+ cells by flow cytometry. (A) Dot plots were obtained first gating on monocytes by light scatter, then on CD14+ cells and finally on LC3+ cells. Representative dot plots from a HD and a LR TB are shown. (B) Bars represent the mean values of the percentage of CD14+LC3+ cells ± SEM. *P < 0.05, Wilcoxon matched-pairs signed rank test; #P < 0.05, ##P < 0.01, Mann-Whitney Test. (C) Intracellular LC3-II was determined by flow cytometry first gating on lymphocytes by light scatter and then gating on CD3+ T cells (upper panel); or first gating on monocytes by light scatter and then on CD14+ cells (lower panel). A representative dot plot from a HR tuberculosis patient is shown.](/cms/asset/f2165dbc-0520-4f3b-83ba-d7a519c52a38/kaup_a_981791_f0002_b.gif)
Autophagy levels correlate with endogenous Mtb-induced IFNG production
In view of our results (), we hypothesized that the immune response of the human host against M. tuberculosis might be related to the induction of autophagy. As mentioned before, HR and LR tuberculosis patients were classified according to their T cell responses against the antigen (Mtb-Ag). In particular, a pattern of considerable severity is detected in LR patients:Citation23 HR patients displayed significant IFNG production whereas LR tuberculosis patients exhibited low IFNG secretion. In fact, we have previously demonstrated that immunological features parallel common clinical parameters analyzed in patients with tuberculosis in Argentina: HR patients have significant higher percentages of total lymphocytes compared with LR patients; HR patients exhibit higher tuberculin skin test diameters than LR patients. In addition, LR individuals have severe pulmonary lesions, a striking loss of weight, and have been ill longer than HR individuals.Citation18 Recently, we have further expanded those studies by finding that the leukocyte count in tuberculosis patients correlates directly with the proportion of CD4+ IFNG+ lymphocytes and that elevated ratios of CD4+ IFNG+ are associated with shorter length of disease progression.Citation23 Therefore, we wondered whether the production of IFNG in response to Mtb-Ag might influence the antigen-induced autophagic response.Citation24-26 shows the secretion of IFNG upon 24 h of Mtb-Ag-stimulation of PBMC from HD and tuberculosis patients. As expected, HR patients produced higher levels of antigen-induced IFNG in comparison to LR patients (mean Mtb-Ag-induced IFNG ± SEM: 3,933 ± 905.3 pg/ml HR group; 1,346 ± 613.4 pg/ml LR group, P < 0.05, Mann Whitney Test) (). Moreover, HD secreted the highest levels of IFNG in response to the antigen (mean Mtb-induced IFNG ± SEM: 8,897 ± 1,465 pg/ml) (). In order to confirm that the levels of IFNG detected by ELISA () were secreted by T cells, we performed intracellular cytokine staining. Therefore, PBMC from HR and LR tuberculosis patients and HD were stimulated with Mtb-Ag and CD4+ IFNG+ lymphocytes were analyzed. As shown in , in LR tuberculosis patients, very low percentages of IFNG-secreting T lymphocytes were expanded by Mtb-Ag. In contrast, higher numbers of CD4+ IFNG+ T cells were observed in HR tuberculosis patients and HD as compared to LR patients (). Furthermore, the percentages of specific Mtb-Ag induced IFNG producing cells in HR patients and HD were higher at 48 h as compared to 24 h, whereas no modification in the number of CD4+ IFNG+ lymphocytes was detected in LR patients at 48 h (). We analyzed the percentage of CD4+ IFNG+ cells induced by Mtb-Ag at 24 h (as measured by ELISA, ) and at a later time (i.e., 48 h) to show the increase in IFNG producing lymphocytes over time. In fact, we previously demonstrated that in HR patients and HD, the highest numbers of CD4+ IFNG+ lymphocytes expanded by Mtb-Ag were detected after 4 d of Ag stimulation.Citation27,28 These findings demonstrate that at least one of the sources of IFNG generated in response to Mtb-Ag were CD4+ T cells.
Figure 3. The levels of autophagy are directly correlated with the amount of IFNG secreted in response to Mtb-Ag. PBMC from healthy donors (HD) and tuberculosis patients (TB) (High responders [HR TB] and low responders [LR TB]) were incubated at 3 × 106 cells/ml in RPMI with 10% FBS. Cells were cultured for 16 h without stimulus to allow the adherence of monocytes. Then, PBMC were stimulated with sonicated M. tuberculosis (Mtb-Ag) for 24 and/or 48 h. (A) IFNG levels in Mtb-Ag-stimulated cell culture supernatant fractions assayed by ELISA after 24 h of incubation with Mtb-Ag. Bars represent the mean values ± SEM. #P < 0.05, ##P < 0.01, Mann-Whitney Test. (B) PBMC from HR and LR TB patients and HD were stimulated with Mtb-Ag for 24 and 48 h, and then, IFNG production was determined by flow cytometry, first, gating on lymphocytes by light scatter and then, gating on CD4+ T cells. A representative dot plot for each group is shown. Cells cultured with media (inset) are also shown. (C) After 24 h of Mtb-Ag stimulation, protein gel blot detection of LC3-II and GAPDH was performed from the proteins extracts by standard methods. Densitometry of the blots was then determined and the means of the ratio of LC3-II to GAPDH in each experimental condition were expressed as arbitrary units (AU). The results are represented as LC3-II/GAPDH in Mtb-Ag-stimulated cultures vs IFNG levels in Mtb-Ag-stimulated cell culture supernatant fractions. Correlation was analyzed by the Spearman r test.
![Figure 3. The levels of autophagy are directly correlated with the amount of IFNG secreted in response to Mtb-Ag. PBMC from healthy donors (HD) and tuberculosis patients (TB) (High responders [HR TB] and low responders [LR TB]) were incubated at 3 × 106 cells/ml in RPMI with 10% FBS. Cells were cultured for 16 h without stimulus to allow the adherence of monocytes. Then, PBMC were stimulated with sonicated M. tuberculosis (Mtb-Ag) for 24 and/or 48 h. (A) IFNG levels in Mtb-Ag-stimulated cell culture supernatant fractions assayed by ELISA after 24 h of incubation with Mtb-Ag. Bars represent the mean values ± SEM. #P < 0.05, ##P < 0.01, Mann-Whitney Test. (B) PBMC from HR and LR TB patients and HD were stimulated with Mtb-Ag for 24 and 48 h, and then, IFNG production was determined by flow cytometry, first, gating on lymphocytes by light scatter and then, gating on CD4+ T cells. A representative dot plot for each group is shown. Cells cultured with media (inset) are also shown. (C) After 24 h of Mtb-Ag stimulation, protein gel blot detection of LC3-II and GAPDH was performed from the proteins extracts by standard methods. Densitometry of the blots was then determined and the means of the ratio of LC3-II to GAPDH in each experimental condition were expressed as arbitrary units (AU). The results are represented as LC3-II/GAPDH in Mtb-Ag-stimulated cultures vs IFNG levels in Mtb-Ag-stimulated cell culture supernatant fractions. Correlation was analyzed by the Spearman r test.](/cms/asset/c71436bb-66a2-43ba-b757-a7f26361b581/kaup_a_981791_f0003_b.gif)
In agreement with our present and previous findings,Citation18,27 it has been reported that in most M. tuberculosis-infected persons who are healthy tuberculin reactors, their PBMC produce high concentrations of IFNG in response to the bacteria and they do not develop active disease. This clearly indicates that cell-mediated immunity controls the infection in most individuals.Citation29 In contrast, reduced IFNG production by PBMC is a marker of severe disease,Citation30 confirming the role of cell mediated immunity in protection against tuberculosis. As the levels of Mtb-Ag-induced IFNG represent a criterion for patient classification,Citation18 in the present work the probable correlation between the amount of secreted IFNG and the levels of autophagy was investigated. Our data indicated that there was a positive correlation between the levels of IFNG and the levels of LC3-II accumulated upon Mtb-Ag stimulation (). Therefore, the present results indicate that endogenous Mtb-Ag-induced IFNG produced by the individual significantly influences the autophagic process in response to the antigen.
Regulation of autophagy by Mtb-induced IFNG
The results described above (Figs. 1 to 3) demonstrate that: i) Mtb-Ag-induced autophagy in cells from tuberculosis patients directly correlates with IFNG secretion upon antigen simulation; and ii) the autophagic response is lower in cells from the group of tuberculosis patients with weak cellular mediated immunity against the pathogen. To further define the relationship between IFNG production and autophagy, we studied the effect of a neutralizing anti-IFNG monoclonal antibody (mAb) on Mtb-Ag-induced autophagy in subjects that display strong immunity against the pathogen. Therefore, PBMC from HR patients and HD were stimulated with Mtb-Ag in the presence or absence of neutralizing anti-IFNG mAb. LC3 puncta formation was analyzed by immunofluorescence and LC3-II levels were measured by flow cytometry. As shown in , autophagy induction in response to the antigen was markedly inhibited by anti-IFNG, as denoted by the few LC3 puncta detected in antigen-stimulated cells treated with the blocking mAb. Moreover, in agreement with these results, incubation of PBMC with Mtb-Ag in the presence of anti-IFNG significantly decreased the percentage of CD14+ LC3+ monocyte cells in HR tuberculosis patients and HD (). Thus, these findings suggest that Mtb-Ag-induced IFNG participates in the upregulation of the autophagic response against the antigen.
Figure 4 (See previous page). IFNG induced in response to Mtb-Ag participate in the process of autophagy. PBMC from healthy donors (HD), high-responder TB patients (HR TB) and low-responder TB patients (LR TB) were incubated at 2 × 106 cells/ml in RPMI with 10% FBS. Cells were stimulated with or without sonicated M. tuberculosis (Mtb-Ag) ± anti-IFNG (A) or recombinant IFNG (rIFNG) (B) for 24 h. Immunofluorescence and intracellular flow staining for LC3 was then performed and samples were analyzed using a confocal microscope or a FACSAria II flow cytometer, respectively. (A) Bars represent the mean values of the number of LC3 puncta/cell ± SEM. *P < 0.05, Wilcoxon matched-pairs signed rank test. Representative images and representative dot plots of cells obtained from a HD and a HR TB patient are depicted. (B) Representative images of cells obtained from a HD, and HR TB and LR TB patients are shown. Bars represent the mean values of the number of LC3 puncta/cell ± SEM. *P < 0.05, **P < 0.01, Wilcoxon matched-pairs signed rank test; #P < 0.05,##P < 0.01 Mann-Whitney Test.
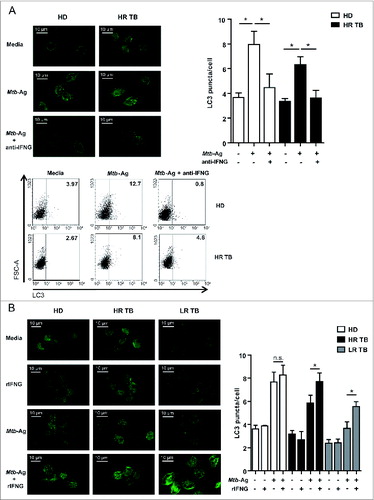
In view of the effect of the blockage of IFNG in HR tuberculosis patients, we next examined whether the levels of autophagy could be enhanced in cells from unresponsive tuberculosis patients (LR) through the addition of recombinant IFNG (rIFNG). To do this, PBMC from HD, HR, and LR patients were stimulated with Mtb-Ag in the presence or absence of rIFNG and, after 24 h, LC3 puncta formation was assayed. When added alone, rIFNG did not alter the distribution pattern of LC3 (). In a previous publication it was demonstrated that addition of rIFNG alone increased the percentage of LC3-II+ human monocytes.Citation31 However, it is important to mention that those studies employed 10 ng/ml of rIFNG, whereas a considerable lower concentration of the cytokine was used in our study (1.8 ng/ml). Interestingly, when cells from LR patients were stimulated with Mtb-Ag, rIFNG significantly increased LC3 puncta formation when compared with cells stimulated with Mtb-Ag alone (). As expected, IFNG treatment also increased the accumulation of LC3 puncta on Mtb-Ag-stimulated cells from HR patients and HD (). Furthermore, we have also observed that infection of PBMC with M. tuberculosis H37Rv strain induced notable levels of autophagy in HD and HR TB patients as compared to uninfected cells, which was further increased by the addition of rIFNG during infection (data not shown, manuscript in preparation). Therefore, our present results, together with the observation that anti-IFNG mAb blocked the ability of Mtb-Ag to upregulate the formation of LC3 puncta, indicate that the increase of the autophagy process in tuberculosis patients in response to Mtb-Ag is mediated by IFNG.
Discussion
In the present report, we investigated the levels of autophagy, a process recognized to be regulated by cytokines,Citation9,32 in the context of tuberculosis, an infectious disease in which a Th1-like response is correlated with protection against M. tuberculosis.Citation33 Indeed, it has been demonstrated that T cells from patients with tuberculosis produce less IFNG than those from persons with latent M. tuberculosis infection.Citation34 Moreover, IFNG production is lower in patients with the most severe manifestation of tuberculosis.Citation6,18 In the present study, we found that M. tuberculosis antigen (Mtb-Ag) increased the conversion of the protein LC3 to the autophagosome-associated form LC3-II in healthy donors and tuberculosis patients. However, low-responder tuberculosis patients displayed the lowest levels of LC3-II, in direct association with the amounts of IFNG secreted by each group of individuals. Moreover, our findings demonstrated that autophagy was specifically induced in CD14+ cells. Besides, we showed that blockage of endogenous Mtb-Ag-induced IFNG decreased the autophagic response in HD and HR tuberculosis patients. Interestingly, when Mtb-Ag was added in the presence of limited amounts of exogenous rIFNG to cells from LR patients, the levels of endogenous LC3-II increased up to the levels detected in Ag-stimulated cells from HR patients. Together, our data indicate that autophagy in Mtb-Ag-stimulated monocytes is influenced and regulated by Th1 responses against the pathogen, denoting the importance of host cell mediated immunity in tuberculosis.
Studies by other authors have suggested that IFNG levels do not correlate with protection. For example, Hoft et al. report a lack of correlation between IFNG production and intracellular killing of mycobacteria.Citation35 Moreover, Elias et al. state that the magnitude of in vitro purified protein derivative-specific IFNG production assessed during the course of tuberculosis infection does not correlate with protection.Citation36 However, the authors clearly indicate that the fact that IFNG is required for protection against M. tuberculosis is not debated, but it is questionable if measuring levels of purified protein derivative-induced IFNG could be used as a surrogate marker of protection, pointing out the need of finding reliable markers of protection.Citation36 In line with that suggestion, we have recently demonstrated that the ratio of Mtb-Ag-expanded CD4+IFNG+IL17+ lymphocytes in peripheral blood and pleural fluid from TB patients is correlated directly with clinical parameters associated with disease severity, indicating that the determination of that lymphocyte population might be an indicator of the clinical outcome of active tuberculosis.Citation23
Several recent studies have demonstrated that autophagy is a crucial weapon in the host cells fight against bacterial and viral invasion.Citation37,38 Thus, accumulating evidence indicates that the outcome of the pathogen interaction with the autophagic pathway is a matter of life or death for the invading microbe.Citation39 Accordingly, it has been previously demonstrated that induction of autophagy suppresses intracellular survival of mycobacteria.Citation10 Besides, autophagy is induced by most of the cytokines produced during Th1-polarized response.Citation40 In fact, activation with IFNG, a physiological stimulus, activates autophagy in phagocytic cells of the immune system.Citation10 By inducing autophagy, IFNG leads to increased maturation of mycobacteria-containing phagosomes and suppresses survival of virulent M. tuberculosis H37Rv in human and murine macrophage cell lines.Citation20 In the present study, we demonstrated the existence of a tight connection between autophagy and IFNG production in cells obtained from patients with active tuberculosis. To our knowledge, this work shows for the first time that the levels of IFNG induced by Mtb-Ag regulate the process of autophagy in cells from tuberculosis patients.
Control of tuberculosis worldwide depends on our understanding of human immune mechanisms to combat the infection. Macrophages, granulocytes, and dendritic cells in the lungs represent the first line of defense against pathogens entering the lungs.Citation41 Then, the collaboration between antigen-presenting cells and T cells culminating in their mutual activation and participation in the granulomatous response is mediated in large part by the release of cytokines, chemokines, and other effector molecules by both phagocytes and T cells. Cytokines important in host defense against M. tuberculosis in both experimental animals and humans include TNF (tumor necrosis factor), IFNG, IL8, IL12, IL17, IL18, and IL23. By contrast, overexpression of Th2 or Th2-like cytokines, such as IL4, IL10, and TGFB1 (transforming growth factor, β 1), is generally considered to predispose the host to M. tuberculosis, although controversies exist.Citation42 Therefore, acquired T-cell responses are critical for host defense against microbial pathogens, although the precise mechanisms by which they act in humans remain unclear. In particular, a key role for IFNG in the immune response to M. tuberculosis infection is corroborated by studies indicating that humans with genetic disorders leading to the decreased production of, or response to, IFNG are highly susceptible to tuberculosis and other mycobacterial diseases.Citation3,43 Nevertheless, it has been difficult to demonstrate that IFNG can activate human monocytes or macrophages to kill intracellular M. tuberculosis. Recently, it has been shown that the acquired T cell response can activate antimicrobial activity in human cells of the monocyte and macrophage lineage.Citation31 The authors demonstrate that T cells, through the release of IFNG, induce autophagy against M. tuberculosis in human macrophages via a vitamin D-dependent pathway.Citation31 In the present study we extended those results to cells from patients with active tuberculosis to further show that Mtb-Ag induced autophagy is in direct correlation with the IFNG levels secreted by the subject against the antigen. Indeed, in the present report we have shown that HR tuberculosis patients displayed significant higher levels of LC3-II than LR patients.
Our findings demonstrated that sonicated Mtb induced the processing of LC3 in tuberculosis patients and HD without altering the autophagic flux (Fig. S1). It is interesting to mention that we have previously demonstrated that M. marinum induces an autophagic response in RAW 264.7-green fluorescent protein-LC3 macrophages although autophagic flux is hampered.Citation44 A similar impairment of autophagic flux is observed when human dendritic cells are infected with M. tuberculosis.Citation45 Thus, this apparent discrepancy might be explained by the fact that in those previous publications cells are infected with live microorganisms, whereas in our present work PBMC are incubated with sonicated Mtb. Thus, it is likely that upon invasion, besides autophagy induction the live pathogen also alters the autophagic machinery in order to prevent a functional autophagic response to eliminate the intruder.
Studies in mice suggest the presence of bacteria in a compartment that cannot be mobilized from the lungs to the lymph node.Citation46 In order to explain the differences in the immune response of HR and LR patients, we hypothesized that variations in the transport of bacteria from the lungs to the local lymph node might impair the complete activation of T cells from LR patients.Citation28 However, additional studies need to be performed to unequivocally explain the weak T cell responses of LR patients by an immunological mechanism. In any case, and even at the site of infection, LR tuberculosis patients produced lower levels of IFNG, the crucial mediator of the protection against M. tuberculosis. We have previously demonstrated that immunological parameters in tuberculosis patients correlate with disease severityCitation18 and that IFNG production from LR tuberculosis patients can be enhanced up to the levels produced by HR individuals after modulation of PDCD1 (programmed cell death 1) and signaling lymphocytic activation molecule (SLAMF1/SLAM) costimulatory pathways.Citation28 Taking into account those previous observations and considering our present findings demonstrating an impaired autophagic response in monocytes from LR patients, we investigated whether the levels of autophagy in LR patients could be increased up to the levels of HR patients. Indeed, when rIFNG was added in combination with Mtb-Ag, we observed a significant augment of autophagy. Interestingly, we used lower concentrations of rIFNG than those previously reported to induce autophagy.Citation31 In fact, we added the cytokine at a concentration that, in combination with the low levels of IFNG secreted by LR tuberculosis patients, would be close to the amounts of IFNG secreted by HR patients in response to the antigen. Under those conditions, IFNG alone did not modify the levels of LC3-II detected. However, in the presence of the Ag, the autophagy process was augmented to levels close to those found in HR patients. Therefore, autophagy in cells from tuberculosis patients would be influenced by the IFNG secreted upon exposure to Mtb-Ag.
While Th1 cytokines play a positive role in autophagy induction, the classical Th2 cytokines IL4 and IL13 have the opposite effect.Citation47 In the context of infection with M. tuberculosis, both IL4 and IL13 inhibit starvation- or IFNG-induced autophagosome formation and lead to decreased phagosome maturation and increased intracellular survival of the bacilli.Citation47 This is dependent on 2 distinct signaling pathways. The inhibition of starvation-induced autophagy is dependent on the AKT1 (v-akt murine thymoma viral oncogene homolog 1) pathway, while inhibition of IFNG-induced autophagy is dependent on signaling via STAT6 (signal transducer and activator of transcription 6, interleukin-4 induced).Citation47 Similarly, IL13 is a potent inhibitor of starvation-induced autophagy and it is also dependent on activation of the AKT1 pathway.Citation47 Furthermore, it has been reported that IL10, a cytokine that displays the unique property of suppressing overall immunity, inhibits autophagy induction in murine macrophages.Citation48 Altogether, these data suggest, in the context of infection with M. tuberculosis, that autophagy is an effector of Th1/Th2 polarization.Citation20 Our present findings showed that LR tuberculosis patients, whose T cells produce low secretion of IFNG against the antigen, displayed low levels of autophagy in CD14+ monocytes. Considering: i) our present results indicating that autophagy is regulated by Th1 cytokines; and ii) that autophagy has an essential role during monocyte differentiation and acquisition of macrophage functions,Citation49 we conclude that the autophagy process might impact the resolution of M. tuberculosis infection.
Given that Th2 cytokines are reported to lead to decreased phagosome maturation and increased intracellular survival of M. tuberculosis,Citation47 we hypothesized that our findings showing an impaired autophagic process in cells from LR patients might be due to the secretion of Th2 cytokines by those individuals. However when we measured IL4 in the supernatant fractions from cells cultured with Mtb-Ag, no detectable levels of the cytokine were found (data not shown). Nevertheless, we have previously demonstrated that both HR and LR patients produce comparable levels of IL10 against Mtb-Ag.Citation27 Moreover, we have reported that Mtb-Ag increases TBX21 (T-box 21) expression and strikingly decreases GATA3 (GATA binding protein 3) levels in HR tuberculosis patients, whereas LR patients display a marked increase in GATA3 expression and no detectable levels of TBX21 after Ag stimulation.Citation27 Then, we proposed that HR patients that secreted IL10, but produced high levels of IFNG in response to Mtb-Ag, might be creating a Th1-like microenvironment, whereas in LR patients, the Mtb-Ag induced IL10 production but with very low levels of IFNG, will generate a predominantly Th2-like environment.Citation27 Together, our previous and present findings suggest that the autophagic process in LR tuberculosis patients might be impaired by effect of Th2 cytokines.
Considering that reduced IFNG production is a marker of severe tuberculosis,Citation30 different amounts of IFNG would be secreted, following Mtb-Ag recognition, by the cells of tuberculosis patients, according to their immune response to the pathogen. Effectively Ag-activated T cells would contribute with IFNG to the cell microenvironment, and consequently, the autophagy process would occur. In contrast, T cells weakly responsive to Mtb-Ag would fail to produce IFNG against the bacteria, but would secrete IL10, generating a predominantly Th2-like environment,Citation27 which would impair the autophagic process. Moreover, whereas only a few LC3 puncta were detected in Mtb-Ag-stimulated cells from LR tuberculosis patients, LC3 puncta accumulated when LPS was added to the culture media of PBMC from these patients (data not shown), further suggesting that the different levels of autophagy observed in tuberculosis patients are specific to Mtb-Ag. Given the key role of autophagy as a defense mechanism, it is tempting to speculate that vaccines that may induce an autophagic response could be more successful in preventing either acquisition of tuberculosis or reactivation of latent tuberculosis. From ours and similar studies by colleagues in the field, it is becoming evident that rigorous analyses of the role of autophagy in pathogen-host interactions, at the molecular and cellular level, are imperative to identify new targets and design novel therapeutic tools to fight against microorganisms such as mycobacteria.Citation39
Materials and Methods
Subjects
HIV-uninfected patients with tuberculosis were diagnosed at the “Dr. F Muñiz” Hospital and at the “Dr. E. Tornú” Hospital (Buenos Aires, Argentina), based on clinical and radiological data, together with the identification of acid-fast bacilli in sputum. All patients had received less than one wk of antituberculosis therapy, and were classified, as previously reported,Citation18 based on in vitro lymphocyte responses to M. tuberculosis antigen. Briefly, high-responder patients are individuals displaying significant proliferative responses, IFNG production and an increased SLAM expression against the antigen; whereas LR patients exhibit low proliferative responses, IFNG production and SLAM expression. LR patients had more severe pulmonary disease, lower leukocyte counts, and a more prolonged illness, compared to HR individuals. In our patient population, no differences regarding age distribution, sex, ethnicity, or frequency of extra pulmonary forms of TB were found between HR and LR tuberculosis patients. However, significant differences were detected regarding X-ray radiography severity, leukocyte count, and time of disease evolution (days previous to hospital admission, during which, the patient displays clinical symptoms). As controls, we enrolled Bacillus Calmette-Guérin (BCG) vaccinated healthy adults lacking a history of tuberculosis. Individuals with latent infection were excluded following assessment using the QuantiFERON TB Gold In-tube test (Cellestis Ltd., T0590-0301 and 0594-0201). All participants provided a written, informed consent for the collection of samples and subsequent analysis. The protocols conducted in this work were approved by the Ethical Committee of the Muñiz Hospital, the Dr. E. Tornú Hospital, and by the International Review Board Fundación Huésped.
Antigen
Cells were stimulated in vitro with a cell lysate from the virulent M. tuberculosis H37Rv strain prepared by probe sonication (Mtb-Ag) (BEI Resources, NIAID, NIH: Mycobacterium tuberculosis, Strain H37Rv, whole cell lysate, NR-14822).
Cell preparation and reagents
PBMC were isolated by centrifugation over Ficoll-Hypaque (GE Healthcare, 17-1440-03) and cultured ± Mtb-Ag with RPMI 1640 (Invitrogen, 22400071) supplemented with 10% fetal bovine serum (FBS; Gibco, 10437028). In different experiments, cells were cultured at 2 × 106 cells/ml for the determination of LC3 puncta formation by confocal microscopy or at 3 × 106 cells/ml for LC3 protein gel blot or LC3 flow cytometry detection. Cell-free supernatant fractions from cultures used for assaying LC3-II levels by western blot were recovered to detect IFNG production by ELISA (BioLegend, 430106). In order to determine the effect of Mtb-Ag on the autophagic flux, the vacuolar-type HC-ATPase inhibitor bafilomycin A1 (100 nM; Fermentek, 88899-55-2) was added for the last 2 h of culture before LC3-II determination by protein gel blot. PBMC were also stimulated with Mtb-Ag in the presence or absence of recombinant IFNG (rIFNG at 1.8 ng/ml) (eBioscience, 14-8319) or an anti-IFNG blocking antibody at 10 μg/ml) (eBioscience, 16-7318) for 24 h, before assaying for LC3 puncta formation by confocal microscopy.
Western blot
Processing of LC3 protein into LC3-II, the phagophore- and autophagosome-associated form was assayed. After 24 h of Mtb-Ag stimulation, nonadherent cells were separated by several washes with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) and protein extracts from both fractions were obtained. Western blotting was performed by standard methods using 15% polyacrylamide gels. Each nitrocellulose membrane was blotted with antibodies to LC3 (Cell Signaling Technology, 2775) and GAPDH (Ambion, AM4300). Bound antibodies were detected with HRP-conjugated antibody (Bio-Rad, anti-rabbit 170-6515, anti-mouse 170-6516) using Amersham ECL PLUS reagent (GE Healthcare, RPN2106). Images were obtained with an Intelligent Dark Box (Fujifilm LAS1000, Tokyo, Japan) and analyzed with ImageJ Analysis software. The intensity of each band was expressed as arbitrary units (AU).
Confocal microscopy
For immunofluorescence, cells were cultured and stimulated on coverslips for 24 h. After incubation under different experimental conditions, cells were washed in order to remove nonadherent cells. Adherent cells were then fixed with 100% cold methanol for 20 sec. Cells were then washed and subsequently permeabilized and blocked with blocking buffer (PBS containing 0.5% saponin (Santa Cruz Biotechnology, sc-280079A) and 1% bovine serum albumin (Santa Cruz Biotechnology, sc-2323A) for 15 min. The buffer was then removed and the LC3 primary antibody was added (Cell Signaling Technology, 2775) and incubated for 16 h at 4°C. Afterwards, the cells were washed with blocking buffer and incubated with the secondary antibody (Alexa Fluor® 488 Goat Anti-Rabbit IgG (H+L); Invitrogen, A11008) for 2 h at room temperature. Finally, nuclei were stained with DAPI (Fig. S2). The coverslips were mounted with PBS-glycerol (Sigma-Aldrich, G2025) and fixed cells were imaged using a Zeiss Spectral LSM 510 confocal microscope (Zeiss, Jena, Germany) using objective 63, numerical aperture (NA) 1.42. The number of LC3 puncta per cell was then quantified using ImageJ image analysis software.
Image processing
All the images were processed using ImageJ software (Wayne Rasband, National Institutes of Health). After the image binarization using a defined threshold, the puncta number was quantified using the Particle Analyzer plugin. Brightness and contrast was adjusted in all images belonging to the same individual, when needed.
Flow cytometry
PBMC from tuberculosis patients and healthy donors were stimulated with Mtb-Ag as described above. Cells were then stained with specific fluorophore-marked antibodies against CD3, CD4 or CD14 (Biolegend, 317336, 317428, 325608, respectively). Intracellular staining of endogenous saponin-resistant LC3 was done as described by Eng KE et al.Citation22 Briefly, PBMC were washed with PBS and then permeabilized with PBS containing 0.05% saponin. In this protocol, the cells are not fixed, therefore LC3-I is washed out of the cell because, unlike LC3-II, it is not anchored to the autophagosome.Citation22 Cells were then incubated with mouse anti-human LC3 antibody (MBL International, M152-3) for 20 min, rinsed with PBS, incubated with anti-mouse secondary antibody conjugated to Phycoerythrin (eBioscience, 12-4012) for 20 min and rinsed twice more with PBS. Afterwards, cells were stained with an anti-CD14 antibody (Biolegend, 325608) to detect the monocyte population. Negative control samples were incubated with irrelevant isotype-matched monoclonal antibody (eBioscience, 12-4717). Intracellular staining to detect IFNG was performed as previously described (IFNG antibody, eBiosciences).Citation23 Samples were analyzed on a FACSAria II flow cytometer (BD Biosciences, California, USA).
Statistics
The Mann-Whitney and the Wilcoxon Rank-Sum tests were used to analyze differences between unpaired or paired samples, respectively. P values of <0.05 were considered statistically significant. Correlation analyses were performed using the nonparametric Spearman correlation test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Supplemental_File_2.pptx
Download MS Power Point (472.3 KB)Supplemental_File_1.pptx
Download MS Power Point (350.5 KB)Acknowledgements
We thank Dr. Peter Barnes for insightful discussions.
Funding
This investigation received financial support from the Agencia Nacional de Promoción Científica y Tecnológica (PAE-PID-2007-00127, PAE-PICT-2007-02332 and PICT-2011-0240 to V. E. G; PICT-2008-0192 and PICT-2011 to M.I.C); the University of Buenos Aires (20020100100221 to V.E.G.); Consejo Nacional de Investigaciones Científicas y Tecnológicas (PIP 2012-2014 to V.E.G.); and Universidad Nacional de Cuyo (SECYPT to M.I.C.). M.I.C and V.E.G. are members of the Researcher Career of CONICET (Argentina).
References
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 1993; 178:2243-7; PMID:8245795; http://dx.doi.org/10.1084/jem.178.6.2243
- Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993; 178:2249-54; PMID:7504064; http://dx.doi.org/10.1084/jem.178.6.2249
- Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med 1996; 335:1941-9; PMID:8960473; http://dx.doi.org/10.1056/NEJM199612263352602
- Holland SM. Interferon gamma, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunol Res 2007; 38:342-6; PMID:17917041; http://dx.doi.org/10.1007/s12026-007-0045-8
- Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect 2006; 8:1179-88; PMID:16513383; http://dx.doi.org/10.1016/j.micinf.2005.10.033
- Sodhi A, Gong J, Silva C, Qian D, Barnes PF. Clinical correlates of interferon gamma production in patients with tuberculosis. Clin Infect Dis: Off Pub Infect Dis Soc Am 1997; 25:617-20; PMID:9314449; http://dx.doi.org/10.1086/513769
- Nathan CF, Kaplan G, Levis WR, Nusrat A, Witmer MD, Sherwin SA, Job CK, Horowitz CR, Steinman RM, Cohn ZA. Local and systemic effects of intradermal recombinant interferon-gamma in patients with lepromatous leprosy. N Engl J Med 1986; 315:6-15; PMID:3086725; http://dx.doi.org/10.1056/NEJM198607033150102
- Kaplan G, Nusrat A, Sarno EN, Job CK, McElrath J, Porto JA, Nathan CF, Cohn ZA. Cellular responses to the intradermal injection of recombinant human gamma-interferon in lepromatous leprosy patients. Am J Pathol 1987; 128:345-53; PMID:3113256
- Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 2007; 27:505-17; PMID:17892853; http://dx.doi.org/10.1016/j.immuni.2007.07.022
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004; 119:753-66; PMID:15607973; http://dx.doi.org/10.1016/j.cell.2004.11.038
- Shi CS, Kehrl JH. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci Signal 2010; 3:ra42; PMID:20501938
- Tanida I, Ueno T, Kominami E. Human light chain 3MAP1LC3B is cleaved at its carboxyl-terminal Met121 to expose Gly120 for lipidation and targeting to autophagosomal membranes. J Biol Chem 2004; 279:47704-10; PMID:15355958; http://dx.doi.org/10.1074/jbc.M407016200
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, et al. A ubiquitin-like system mediates protein lipidation. Nature 2000; 408:488-92; PMID:11100732; http://dx.doi.org/10.1038/35044114
- Feng CG, Zheng L, Lenardo MJ, Sher A. Interferon-inducible immunity-related GTPase Irgm1 regulates IFN gamma-dependent host defense, lymphocyte survival and autophagy. Autophagy 2009; 5:232-4; PMID:19066452; http://dx.doi.org/10.4161/auto.5.2.7445
- Intemann CD, Thye T, Niemann S, Browne EN, Amanua Chinbuah M, Enimil A, Gyapong J, Osei I, Owusu-Dabo E, Helm S, et al. Autophagy gene variant IRGM -261T contributes to protection from tuberculosis caused by Mycobacterium tuberculosis but not by M. africanum strains. PLoS Pathog 2009; 5:e1000577; PMID:19750224; http://dx.doi.org/10.1371/journal.ppat.1000577
- King KY, Lew JD, Ha NP, Lin JS, Ma X, Graviss EA, Goodell MA. Polymorphic allele of human IRGM1 is associated with susceptibility to tuberculosis in African Americans. PLoS One 2011; 6:e16317; PMID:21283700; http://dx.doi.org/10.1371/journal.pone.0016317
- Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science 2011; 332:717-21; PMID:21551061; http://dx.doi.org/10.1126/science.1201711
- Pasquinelli V, Quiroga MF, Martinez GJ, Zorrilla LC, Musella RM, Bracco MM, Belmonte L, Malbran A, Fainboim L, Sieling PA, et al. Expression of signaling lymphocytic activation molecule-associated protein interrupts IFN-gamma production in human tuberculosis. J Immunol 2004; 172:1177-85; PMID:14707094; http://dx.doi.org/10.4049/jimmunol.172.2.1177
- Castillo EF, Dekonenko A, Arko-Mensah J, Mandell MA, Dupont N, Jiang S, Delgado-Vargas M, Timmins GS, Bhattacharya D, Yang H, et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc Natl Acad Sci U S A 2012; 109:E3168-76; PMID:23093667; http://dx.doi.org/10.1073/pnas.1210500109
- Goletti D, Petruccioli E, Romagnoli A, Piacentini M, Fimia GM. Autophagy in Mycobacterium tuberculosis infection: a passepartout to flush the intruder out? Cytokine Growth Factor Rev 2013; 24:335-43; PMID:23395260; http://dx.doi.org/10.1016/j.cytogfr.2013.01.002
- Man N, Chen Y, Zheng F, Zhou W, Wen LP. Induction of genuine autophagy by cationic lipids in mammalian cells. Autophagy 2010; 6:449-54; PMID:20383065; http://dx.doi.org/10.4161/auto.6.4.11612
- Eng KE, Panas MD, Karlsson Hedestam GB, McInerney GM. A novel quantitative flow cytometry-based assay for autophagy. Autophagy 2010; 6:634-41; PMID:20458170; http://dx.doi.org/10.4161/auto.6.5.12112
- Jurado JO, Pasquinelli V, Alvarez IB, Pena D, Rovetta AI, Tateosian NL, Romeo HE, Musella RM, Palmero D, Chuluyan HE, et al. IL-17 and IFN-gamma expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol 2012; 91:991-1002; PMID:22416258; http://dx.doi.org/10.1189/jlb.1211619
- Spellberg B, Edwards JE Jr. Type 1Type 2 immunity in infectious diseases. Clin Infect Dis: Off Pub Infect Dis Soc Am 2001; 32:76-102; PMID:11118387; http://dx.doi.org/10.1086/317537
- Chan ED, Chan J, Schluger NW. What is the role of nitric oxide in murine and human host defense against tuberculosis? Current knowledge. Am J Respir Cell Mol Biol 2001; 25:606-12; PMID:11713103; http://dx.doi.org/10.1165/ajrcmb.25.5.4487
- Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev 2000; 11:321-33; PMID:10959079; http://dx.doi.org/10.1016/S1359-6101(00)00010-1
- Quiroga MF, Pasquinelli V, Martinez GJ, Jurado JO, Zorrilla LC, Musella RM, Abbate E, Sieling PA, Garcia VE. Inducible costimulator: a modulator of IFN-gamma production in human tuberculosis. J Immunol 2006; 176:5965-74; PMID:16670305; http://dx.doi.org/10.4049/jimmunol.176.10.5965
- Jurado JO, Alvarez IB, Pasquinelli V, Martinez GJ, Quiroga MF, Abbate E, Musella RM, Chuluyan HE, Garcia VE. Programmed death (PD)-1:PD-ligand 1PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol 2008; 181:116-25; PMID:18566376; http://dx.doi.org/10.4049/jimmunol.181.1.116
- Samten B, Thomas EK, Gong J, Barnes PF. Depressed CD40 ligand expression contributes to reduced gamma interferon production in human tuberculosis. Infect Immun 2000; 68:3002-6; PMID:10769003; http://dx.doi.org/10.1128/IAI.68.5.3002-3006.2000
- Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, Barnes PF. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun 1996; 64:913-8; PMID:8641800
- Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Trans Med 2011; 3:104ra2; PMID:21998409; http://dx.doi.org/10.1126/scitranslmed.3003045
- Songane M, Kleinnijenhuis J, Netea MG, van Crevel R. The role of autophagy in host defence against Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2012; 92:388-96; PMID:22683183; http://dx.doi.org/10.1016/j.tube.2012.05.004
- Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol 2001; 1:20-30; PMID:11905811; http://dx.doi.org/10.1038/35095558
- Samten B, Ghosh P, Yi AK, Weis SE, Lakey DL, Gonsky R, Pendurthi U, Wizel B, Zhang Y, Zhang M, et al. Reduced expression of nuclear cyclic adenosine 5′-monophosphate response element-binding proteins and IFN-gamma promoter function in disease due to an intracellular pathogen. J Immunol 2002; 168:3520-6; PMID:11907114; http://dx.doi.org/10.4049/jimmunol.168.7.3520
- Hoft DF, Worku S, Kampmann B, Whalen CC, Ellner JJ, Hirsch CS, Brown RB, Larkin R, Li Q, Yun H, et al. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J Infect Dis 2002; 186:1448-57; PMID:12404160; http://dx.doi.org/10.1086/344359
- Elias D, Akuffo H, Britton S. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans Royal Soc Trop Med Hygiene 2005; 99:363-8; PMID:15780343; http://dx.doi.org/10.1016/j.trstmh.2004.08.006
- Marino G, Lopez-Otin C. Autophagy: molecular mechanisms, physiological functions and relevance in human pathology. Cell Mol Life Sci 2004; 61:1439-54; PMID:15197469; http://dx.doi.org/10.1007/s00018-004-4012-4
- Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 2005; 120:159-62; PMID:15680321
- Colombo MI, Gutierrez MG, Romano PS. The two faces of autophagy: coxiella and mycobacterium. Autophagy 2006; 2:162-4; PMID:16874070; http://dx.doi.org/10.4161/auto.2827
- Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE 3rd, Freedman VH, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha beta. Proc Natl Acad Sci U S A 2001; 98:5752-7; PMID:11320211; http://dx.doi.org/10.1073/pnas.091096998
- Gonzalez-Juarrero M, Shim TS, Kipnis A, Junqueira-Kipnis AP, Orme IM. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol 2003; 171:3128-35; PMID:12960339; http://dx.doi.org/10.4049/jimmunol.171.6.3128
- Bai X, Kim SH, Azam T, McGibney MT, Huang H, Dinarello CA, Chan ED. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J Immunol 2010; 184:3830-40; PMID:20190143; http://dx.doi.org/10.4049/jimmunol.0901913
- Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 1998; 280:1432-5; PMID:9603732; http://dx.doi.org/10.1126/science.280.5368.1432
- Lerena MC, Colombo MI. Mycobacterium marinum induces a marked LC3 recruitment to its containing phagosome that depends on a functional ESX-1 secretion system. Cell Microbiol 2011; 13:814-35; PMID:21447143; http://dx.doi.org/10.1111/j.1462-5822.2011.01581.x
- Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, Corazzari M, Falasca L, Goletti D, Gafa V, Simeone R, et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy 2012; 8:1357-70; PMID:22885411; http://dx.doi.org/10.4161/auto.20881
- Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med 2008; 205:105-15; PMID:18158321; http://dx.doi.org/10.1084/jem.20071367
- Harris J, Master SS, De Haro SA, Delgado M, Roberts EA, Hope JC, Keane J, Deretic V. Th1-Th2 polarisation and autophagy in the control of intracellular mycobacteria by macrophages. Vet Immunol Immunopathol 2009; 128:37-43; PMID:19026454; http://dx.doi.org/10.1016/j.vetimm.2008.10.293
- Park HJ, Lee SJ, Kim SH, Han J, Bae J, Kim SJ, Park CG, Chun T. IL-10 inhibits the starvation induced autophagy in macrophages via class I phosphatidylinositol 3-kinase (PI3K) pathway. Mol Immunol 2011; 48:720-7; PMID:21095008; http://dx.doi.org/10.1016/j.molimm.2010.10.020
- Jacquel A, Obba S, Solary E, Auberger P. Proper macrophagic differentiation requires both autophagy and caspase activation. Autophagy 2012; 8:1141-3; PMID:22751215; http://dx.doi.org/10.4161/auto.20367
