Abstract
Autophagy is an integral part of the plant life cycle where it contributes to remodeling of tissues during plant development, and in plant responses to nutrient deficiencies, pathogens, and other environmental stresses. Recently, we reported the involvement of autophagy as part of the self-incompatibility response in the mustard family. Self-incompatibility is a polymorphic genetic system that results in rejection of self-incompatible male pollen by the female pistil, thereby preventing self-fertilization. Our data show that autophagy is part of the cellular rejection response in the underlying pistil cells to prevent vesicle secretion to self-pollen thus causing rejection.
Many flowers are bisexual, containing both the male pollen-producing anthers and female pistil, and various genetic traits have evolved to prevent self-fertilization in bisexual flowers. In the mustard family (Brassicaceae; Arabidopsis, canola, cabbage), successful reproduction involves a number of regulated interactions between the pollen and the pistil (). A pollen grain first comes in contact with a stigmatic papilla on the top surface of a pistil, and a recognition system initiates the delivery of resources to compatible pollen. We have proposed that this delivery system involves exocyst-mediated, polarized secretion in the stigmatic papilla at the compatible pollen contact site (). The compatible pollen then becomes metabolically active and forms a pollen tube, which will grow into the pistil to deliver sperm cells to an egg cell-containing ovule for fertilization. Brassicaceae species with self-incompatibility systems reject self-pollen following stigmatic papillar contact, and we have proposed that this occurs by disrupting polarized secretion in the stigmatic papilla toward the self-pollen. The self-incompatibility response is controlled by 2 polymorphic proteins: the S-locus Protein 11/S-locus Cysteine Rich (SP11/SCR) ligand and the S Receptor Kinase (SRK). The haplotype-specific recognition of the pollen SP11/SCR ligand by pistil SRK triggers a signaling pathway in the stigmatic papilla, which we have proposed to involve the inhibition of the exocyst subunit, EXO70A1, by the ARM-Repeat-Containing-1 (ARC1) E3 ubiquitin ligase. This is predicted to impair vesicle secretion toward the self-incompatible pollen grain, which would prevent pollen germination and pollen tube growth into the pistil. In studying the ultrastructural features of these pollen-stigmatic papillar interactions in 3 different Brassicaceae species, we also discovered the presence of autophagy as an early event in this self-pollen rejection response ().
Figure 1. Models for cellular responses in the stigmatic papilla to compatible vs. self-incompatible pollen. The recognition of compatible pollen activates exocyst-mediated polarized secretion in the stigmatic papilla toward the pollen grain, and the subsequently released vesicle cargo promotes pollen germination. Following self-incompatible pollination, the basal compatibility pathway is again activated, but the simultaneously activated self-incompatibility pathway prevents polarized secretion and induces autophagy. As a result, the cargo needed for pollen germination is not delivered, and the self-pollen is rejected. Abbreviations: CW, cell wall; PM, plasma membrane; v, vesicle; A, ARC1; exo, exocyst.
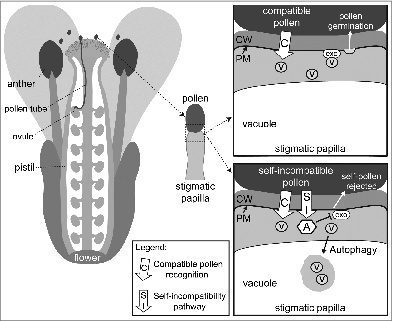
In order to shed light on the mechanistic events involved in the acceptance of compatible pollen versus the rejection of self-incompatible pollen, detailed examinations of the pollen-papilla contact area were conducted using the transmission electron microscope. As predicted from previous studies, vesicle-like structures are observed fusing to the papillar plasma membrane underneath the pollen contact site at 10 min after compatible pollinations in 2 different Arabidopsis species, A. lyrata and A. thaliana. These vesicles are presumably releasing cargo at the pollen-stigma interface for the compatible pollen to germinate and form a pollen tube. For self-incompatible pollinations, we examined naturally-occurring self-incompatible A. lyrata plants and self-incompatible A. thaliana plants expressing A. lyrata SCR, SRK and ARC1 transgenes. Self-incompatible pollinations with A. lyrata flowers display a complete absence of vesicle-like structures at the stigmatic papillar plasma membrane under the pollen grain at 10 min post-pollination. This was not unexpected given that our model predicted a disruption of exocytosis by the self-incompatibility pathway. It has been shown in yeast and animal cells that disruption of the exocyst complex can lead to secretory vesicles accumulating in the cytoplasm; yet, there are no signs of vesicle accumulation in the A. lyrata stigmatic papillae following a self-incompatible pollination. However, correlated with this is the appearance of dense material in the stigmatic papillar vacuole that appears to represent autophagic bodies.
To investigate the potential involvement of autophagy in clearing vesicles, A. lyrata flowers were pre-treated with the E-64 cysteine protease inhibitor to inhibit proteolysis in the vacuole. This treatment allowed the visualization of small vesicle-like structures in the stigmatic papillar vacuole at 10 min post-self-incompatible pollination. Concurrently, the presence of autophagic organelles was examined by monodansylcadaverine (MDC) staining and generating transgenic A. lyrata expressing a GFP:ATG8a fusion protein. Both approaches label punctate structures resembling autophagic organelles in the stigmatic papillae following self-incompatible pollinations, but not in unpollinated stigmatic papillae or with compatible pollinations. For the transgenic self-incompatible A. thaliana plants, the presence of the ARC1 transgene correlates with a stronger self-incompatibility response and appears to promote autophagy with the presence of autophagic organelles in the stigmatic papillar vacuole. Interestingly, unlike the self-incompatible A. lyrata at 10 min post-self-incompatible pollinations, there are still some residual vesicle-like structures observed at the stigmatic papillar plasma membrane and in the cytoplasm for the transgenic self-incompatible A. thaliana plants. This incomplete removal of vesicles supports our working model that vesicle secretion is induced (i.e., compatible pollen response) with self-incompatible pollinations as well. Furthermore, the self-incompatibility response in both Arabidopsis species goes beyond inhibiting vesicle delivery to the stigma-pollen interface, and uncovered a role for autophagy in the destruction of these vesicles ().
Similar TEM analyses with Brassica napus (canola) unexpectedly revealed somewhat different cellular responses. Compatible pollinations are correlated with multivesicular bodies (MVBs) appearing to release exosome-like vesicles at the stigmatic papillar plasma membrane under the pollen attachment site at 10 min post-pollination. Following self-incompatible pollinations, the MVBs are localized in the vacuole, presumably destined for degradation. Thus, the targeting of MVBs to the stigmatic papillar plasma membrane or vacuole is based on whether the pollen is recognized as compatible or self-incompatible. Autophagic organelles were not detected in this study, and so it is not known whether autophagosomes are involved in rounding up MVBs for degradation in the vacuole.
Outstanding questions remain on the general mechanisms of autophagy induction/MVB trafficking by the self-incompatible pathway, and specifically on the connections between the ARC1 E3 ligase and EXO70A1 (exocyst) in regulating these events in the stigmatic papilla following pollination. Recent bioinformatics studies have proposed that the plant exocyst subunits may participate in autophagy through consensus motifs for interacting with the autophagy associated protein ATG8 (through an Atg8 interacting motif or AIM). As well, associations between select exocyst subunits and autophagic organelles have also been demonstrated. Thus, roles are emerging for exocyst subunits as dual regulators in polarized secretion and autophagy. Perhaps ARC1s ubiquitination activity sits at this interface to switch EXO70A1 activity from polarized secretion to autophagy in the stigmatic papillar self-incompatibility response.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Goring lab for critically reading the manuscript.
Funding
Research in the Goring laboratory is supported by a grant from Natural Sciences and Engineering Research Council of Canada, and DS was supported by an Ontario Graduate Scholarship.
