Abstract
Macroautophagy is a degradative pathway that sequesters and transports cytosolic cargo in autophagosomes to lysosomes, and its deterioration affects intracellular proteostasis. Membrane dynamics accompanying autophagy are mostly elusive and depend on trafficking processes. RAB GTPase activating proteins (RABGAPs) are important factors for the coordination of cellular vesicle transport systems, and several TBC (TRE2-BUB2-CDC16) domain-containing RABGAPs are associated with autophagy. Employing C. elegans and human primary fibroblasts, we show that RAB3GAP1 and RAB3GAP2, which are components of the TBC domain-free RAB3GAP complex, influence protein aggregation and affect autophagy at basal and rapamycin-induced conditions. Correlating the activity of RAB3GAP1/2 with ATG3 and ATG16L1 and analyzing ATG5 punctate structures, we illustrate that the RAB3GAPs modulate autophagosomal biogenesis. Significant levels of RAB3GAP1/2 colocalize with members of the Atg8 family at lipid droplets, and their autophagy modulatory activity depends on the GTPase-activating activity of RAB3GAP1 but is independent of the RAB GTPase RAB3. Moreover, we analyzed RAB3GAP1/2 in relation to the previously reported suppressive autophagy modulators FEZ1 and FEZ2 and demonstrate that both reciprocally regulate autophagy. In conclusion, we identify RAB3GAP1/2 as novel conserved factors of the autophagy and proteostasis network.
Abbreviations:
- ATG, autophagy-related
- Bafi, bafilomycin A1
- BSA, bovine serum albumin
- C. elegans, Caenorhabditis elegans
- CALCOCO2, calcium binding and coiled-coil domain 2
- DAPI, 4’, 6-diamidino-2-phenylindole
- DMSO, dimethyl sulfoxide
- DPH, 1, 6-diphenyl-1, 3, 5-hexatriene
- eV, empty vector
- FEZ, fasciculation and elongation protein zeta
- GABARAP, GABA(A) receptor-associated protein
- GEF, guanine nucleotide exchange factor
- GFP, green fluorescent protein
- MAP1LC3, microtubule-associated protein 1 light chain 3
- NBR1, neighbor of BRCA1 gene 1
- PE, phosphatidylethanolamine
- PBS, phosphate-buffered saline
- RABGAP, RAB GTPase activating protein
- siRNA, small interfering RNA
- SQSTM1, sequestosome 1
- TBC domain, TRE2-BUB2-CDC16 domain
Introduction
Macroautophagy (hereafter referred to as autophagy) is a eukaryotic degradative pathway that clears proteins and organelles from the cytosol. As part of the protein homeostasis (proteostasis) network, autophagy supports proteome integrity and its deregulation is associated with various disorders, including cancer and neurodegeneration.Citation1 The initiation of autophagy is achieved by formation of a precursor structure (the phagophore), which elongates to form the characteristic double-layered membrane autophagosome that completely encloses proteins and organelles. After controlled trafficking to the lysosome and subsequent fusion the cargo is degraded.Citation2
Two ubiquitin-like conjugation systems have been identified that are important for the formation of autophagosomes. ATG5 is covalently conjugated to ATG12, a ubiquitin-like protein, and forms a complex with ATG16L1. This complex is present on the phagophore and supports the biogenesis of autophagosomes but is not located on mature autophagosomes.Citation3,4 The small ubiquitin-like Atg8 family members, including MAP1LC3 (henceforth LC3) and GABARAP, are translated with C-terminal extensions that are rapidly removed by ATG4B,Citation5 resulting in LC3-I or GABARAP-I. After activation by ATG7, the proteins are transferred to ATG3 and subsequently the ATG12–ATG5-ATG16L1 complex conjugates phosphatidylethanolamine (PE) to the newly exposed C termini, resulting in the translocation of the lipidated proteins (LC3-II, GABARAP-II) to the growing autophagosomal membrane.Citation6,7 This Atg8 conjugation system is indispensable for autophagosomal maturation, and lipidated Atg8 family members remain associated with mature autophagosomes and get at least partially degraded after lysosomal fusion.Citation8,9 Furthermore, in the selective autophagy pathway Atg8 family members serve as attachment sites for specific cargo receptors, such as SQSTM1/p62, NBR1, and CALCOCO2/NDP52.Citation10-12
Besides the well-defined core machinery of autophagy, the membrane dynamics accompanying this process remains mostly elusive and depends on vesicle trafficking. Small RAB GTPases are essential for the coordination of vesicle budding, transport, and fusion and numerous RAB proteins have been implicated to function in autophagy.Citation13-17 The activity of RAB proteins is regulated by guanine nucleotide exchange factors (GEFs) and RAB GTPase activating proteins (RABGAPs),Citation18 and several TBC (TRE2-BUB2-CDC16) domain-containing RABGAPs have recently been associated with autophagy.Citation17,19-22 The TBC domain facilitates the inactivation of RAB proteins and seems to be a characteristic feature of RABGAPs that regulate more than just single RAB GTPases, but may coordinate different RAB proteins and multiple cellular pathways.Citation23
Here, we show that RAB3GAP1 and RAB3GAP2 are novel factors of the autophagy network. The catalytic subunit RAB3GAP1 and the noncatalytic subunit RAB3GAP2 form the heterodimeric TBC domain-free RAB3GAP complex,Citation24,25 which has been linked to the regulation of the RAB GTPase RAB3 and exocytosis of hormones and neurotransmitters at the neuronal synapse.Citation26,27 Recently, RAB3GAP1 has been associated with LMAN1 (lectin, mannose-binding, 1), which functions in protein trafficking and indicates that the RAB3GAPs might actually be involved in additional trafficking processes.Citation28 Interestingly, mutations in RAB3GAP1 and RAB3GAP2 are known to cause the Warburg Micro and Martsolf Syndrome in humans, which are characterized by severe brain, eye, and endocrine abnormalities.Citation29,30 The molecular mechanisms causing these deteriorations are completely unknown so far. Employing C. elegans and human primary fibroblasts, we show that the RAB3GAPs affect protein aggregation and modulate autophagy at basal and rapamycin-induced conditions. Analyzing these effects in correlation to ATG3 and ATG16L1 and, moreover, focusing also ATG5 punctate structures, we demonstrate that RAB3GAP1/2 influence autophagosome formation. The effect of the RAB3GAPs on autophagy is dependent on the GTPase-activating activity of RAB3GAP1 but independent of the RAB GTPase RAB3. Furthermore, the positive modulation of autophagy by the RAB3GAPs opposes the previously reported suppressive activity of FEZ1Citation31 as well as the so-far uncharacterized homolog FEZ2.
In conclusion, we show that RAB3GAP1/2 influence protein aggregation and are conserved components of the autophagy network, modulating autophagosomal biogenesis.
Results
RBG-1 is a modifier of protein aggregation and autophagy in C. elegans
In an RNAi screen in C. elegans we have selected genes whose knockdown increased the aggregation of a cytosolic reporter protein upon heat stress compared to empty vector (eV) treated control worms. We have used luciferase-GFP expressed in body wall muscle cells as reporter, since we have previously shown that its aggregation is affected by a modified proteostasis networkCitation32 and assayed the aggregation rate by fluorescence microscopy. One of the identified genes that enhanced luciferase-GFP aggregation was rbg-1, which codes for a RABGAP and is orthologous to mammalian RAB3GAP1 (Fig. S1).
To confirm the impact of RBG-1 on protein homeostasis, we employed human amyloid β42-expressing worms that are characterized by an age-dependent paralysis due to the accumulation of amyloid β42 oligomers and high molecular weight aggregates in body wall muscle cells.Citation33 Compared to control worms, reduced RBG-1 levels aggravated the paralysis phenotype and enhanced the number of thioflavine-positive amyloid β42 aggregates (), confirming that RBG-1 is indeed influencing protein aggregation and proteostasis. Applying real-time PCR, we showed that rbg-1 gene expression levels were reduced by the feeding RNAi approach and that amyloid β42 expression was not affected (Fig. S2).
Figure 1. RBG-1 affects proteostasis and autophagy. (A) Paralysis of rbg-1 and eV RNAi-treated CL2006 worms. 316 (eV) and 308 (rbg-1) worms were analyzed. Statistics are depicted as mean ± SD; *P < 0.05, **P < 0.01; n = 6; Oneway Anova. (B) Confocal images of thioflavine stained CL2006 worms and head regions of eV, rbg-1, and atg-7 RNAi-treated worms. Aggregates were counted in 42 (eV), 45 (rbg-1) and 34 (atg-7) head areas. Scale bars = 100 and 20 μm; ***P < 0.001, n = 4, t test. For estimation of background staining, we have employed N2 WT worms and confocal images are depicted in Fig. S2B. (C) Confocal images of seam cells of eV and rbg-1 RNAi-treated GFP-LGG-1 expressing worms and statistical analysis of the total number of autophagosomal structures in at least 20 different cells. Bec-1 RNAi served as control. Scale bars = 50 and 10 μm; statistics are depicted as mean ± SD; ***P < 0.001, n = 3, t test.
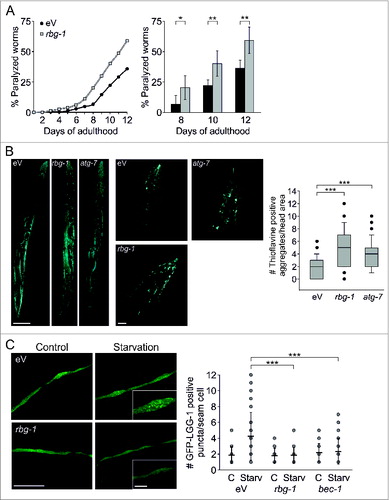
Mammalian RAB3GAPs have recently been associated with ortholog members of the yeast Atg8 family,Citation19 and by RNAi-mediated reduction of atg-7 we confirmed that the deterioration of autophagy is sufficient to increase amyloid β42 aggregation and to affect proteostasis in C. elegans (; Fig. S2). This prompted us to analyze the impact of RBG-1 on autophagy, employing a GFP-LGG-1 reporter worm strain. LGG-1 is a nematode ortholog of the mammalian LC3 family, and the GFP-tagged protein can be used to evaluate the quantity of pre- and autophagosomal structures.Citation34 The knockdown of rbg-1 suppressed the starvation-mediated increase in total numbers of autophagosomal structures (), which indicates that RBG-1 is functionally involved in autophagy. Bec-1 (ortholog of human BECN1), a canonical factor of autophagy induction, served as control.
Thus, employing C. elegans, we associated RBG-1 with the intracellular proteostasis network and assigned this protein a function in the autophagy pathway.
Mammalian RAB3GAP1 and RAB3GAP2 affect Atg8 family protein lipidation and autophagic activity
To investigate the influence of RAB3GAP1 and also RAB3GAP2 on autophagy in more detail, we employed human primary fibroblasts and genetically manipulated both components of the heterodimeric RAB3GAP complex. Notably, manipulating the single RAB3GAPs also displayed effects (not shown), however, they were less reliable than the combined knockdown of RAB3GAP1 and RAB3GAP2. Thus, for the subsequent results, we exclusively manipulated both proteins of the RAB3GAP complex simultaneously.
Reduced levels of RAB3GAP1/2 deteriorated lipidation of Atg8 family members and decreased autophagic activity at basal and rapamycin-induced conditions (). The knockdown of the RAB3GAPs caused an accumulation of unlipidated LC3-I (), which is associated with a disturbed autophagosome formation.Citation8 Since GABARAP was also affected, and GABARAP-I accumulated when the RAB3GAPs were knocked down (Fig. S3), apparently RAB3GAP1/2 are necessary for the efficient lipidation of Atg8 family members and influence autophagy initiation. This was supported by immunostaining of endogenous autophagosomes that displayed reduced amounts of LC3- and SQSTM1-positive vesicles after knockdown of the RAB3GAPs ().
Figure 2. Knockdown of the RAB3GAP complex impairs autophagy. (A) Immunoblot analysis of cells that were manipulated with the indicated siRNA for 48 h and treated with DMSO (−) or bafilomycin A1 (+) for 4 h. Tubulin served as control for equal loading. Statistics are depicted as mean ± SD normalized to nonsense control; *P < 0.05, **P < 0.01, ***P < 0.001, n = 4, t test. (B) Identical to (A) but cells were additionally treated with rapamycin for 4 h. Statistics are depicted as mean ± SD normalized to rapamycin-treated nonsense control; *P < 0.05, **P < 0.01, n = 4, t test. (C) Confocal images of LC3 and SQSTM1 immunostaining. Fibroblasts were manipulated with the indicated siRNA for 48 h and treated with DMSO or bafilomycin A1 for 4 h. DAPI was used to stain nuclei. Scale bar = 50 μm. Autophagosomal structures were counted in 20 to 40 cells of 3 independent experiments; ***P < 0.001, t test. (D) Identical to (C) but cells were additionally treated with rapamycin for 4 h. Scale bar = 50 μm. Autophagosomal structures were counted in 20 to 40 cells of 3 independent experiments; ***P < 0.001, t test.
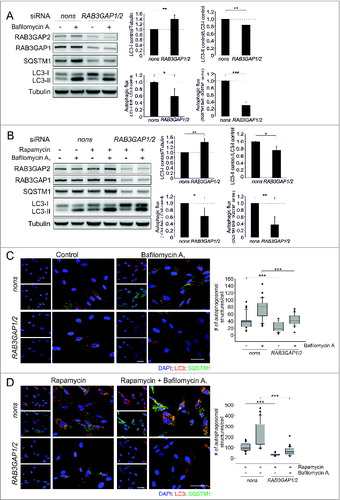
Consistent with the accumulation of unlipidated Atg8 family members and the reduced amount of autophagosomes, autophagic activity was also decreased upon deficiency of the RAB3GAPs. We analyzed LC3-II and SQSTM1, which showed declined fluxes after knockdown of RAB3GAP1/2 (). Since total SQSTM1 protein levels were affected (see below), we additionally analyzed the fluxes of GABARAP-II, NBR1, and CALCOCO2, which confirmed the decreased autophagic activity upon deficiency of the RAB3GAPs (Fig. S3). Furthermore, the immunostaining directly showed the reduced accumulation of autophagosomes after bafilomycin A1-mediated inhibition of lysosomal degradation at basal and rapamycin-induced conditions (). Thus, the disturbance of the lipidation of Atg8 family members culminated in a decreased autophagic activity.
Importantly, the influence of RAB3GAP1/2 on autophagy was observed at basal conditions as well as after treatment with the drug rapamycin. Like starvation, rapamycin is a potent inducer of autophagyCitation35 and strongly and reproducibly affects autophagic activity in IMR-90 fibroblasts. Notably, when applying different paradigms of starvation as autophagy inducer in these human primary cells no satisfying and reproducible effect on autophagy was achievable.
Deficiency of the RAB3GAPs resulted in reduced SQSTM1 protein levels () and employing real-time PCR, we found that this was mediated on gene expression level (Fig. S4). The exact mechanisms causing the decreased SQSTM1 expression are elusive so far. Additionally, we analyzed mRNA levels of MAP1LC3A, MAP1LC3B, ATG3, ATG4B, ATG7, ATG16L1, NBR1, and CALCOCO2, which were unaffected by the RAB3GAP knockdown (Fig. S4), excluding that LC3 lipidation and the evaluation of autophagic activity were influenced by an altered expression of these genes. Importantly, the individual knockdown of RAB3GAP1 and RAB3GAP2 by the use of 2 different siRNAs also caused the reduction in SQSTM1 protein levels (Fig. S5), which emphasizes that this regulation is not an unspecific effect.
In addition to knockdown studies, we analyzed the impact of RAB3GAP overexpression on autophagy and showed that increased levels of RAB3GAP1/2 enhanced autophagic activity at basal conditions (). The fluxes of LC3-II and SQSTM1 were increased compared to control cells as was shown by immunoblotting and immunostaining of endogenous autophagosomes (). This effect was not significantly detectable after rapamycin induction, possibly due to the already substantial increase in autophagic activity in control cells (Fig. S6). Increased RAB3GAP levels neither influenced SQSTM1 protein and mRNA levels, nor did they affect gene expression of MAP1LC3A, MAP1LC3B, ATG3, ATG4B, ATG7, ATG16L1, NBR1, and CALCOCO2 (Fig. S7A and B).
Figure 3. Overexpression of the RAB3GAP complex enhances autophagy. (A) Immunoblot analysis of cells that were manipulated with the indicated plasmids for 48 h and treated with DMSO (−) or bafilomycin A1 (+) for 4 h. Tubulin served as control for equal loading. Statistics are depicted as mean ± SD normalized to eV; *P < 0.05, **P < 0.01, n = 4, t test. (B) Confocal images of LC3 and SQSTM1 immunostaining. Fibroblasts were manipulated with the indicated plasmids for 48 h and treated with DMSO or bafilomycin A1 for 4 h. DAPI was used to stain nuclei. Scale bar = 50 μm. Autophagosomal structures were counted in 20 to 40 cells of 3 independent experiments; ***P < 0.001, t test. (C) Confocal images of RAB3GAP1 and LC3 or GABARAP immunostainings. RAB3GAP1 and RAB3GAP2 were overexpressed for 48 h. DPH was used to stain lipid droplets. Scale bar = 20 and 5 μm.
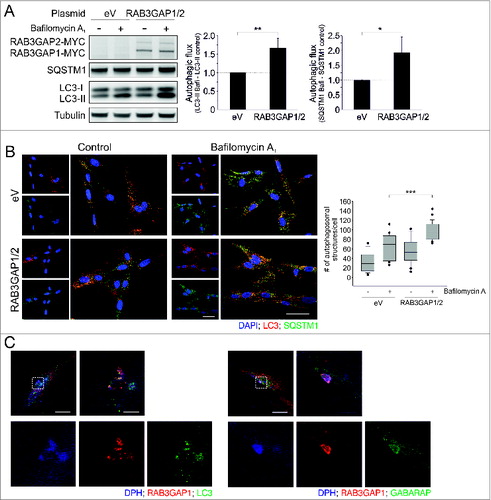
Having established the relevance of the RAB3GAPs for autophagy, we investigated a potential colocalization of RAB3GAP1 and Atg8 family members employing immunostaining. We observed that RAB3GAP1 colocalized with LC3 and GABARAP and the most prominent colocalization appeared at crescent-like structures which we identified as lipid droplets (). Employing the lipid droplet dye DPH and an antibody against the lipid droplet-associated protein PLIN2 (Fig. S8A), we assured the identity of these organelles, which were furthermore inducible by the supplementation of oleic acid (Fig. S8B). Since we analyzed the colocalization of LC3/GABARAP and RAB3GAP1 while overexpressing the latter, we additionally confirmed the localization of LC3 at lipid droplets in untransfected cells to exclude that overexpressed RAB3GAP1 influences the cellular distribution of LC3 (Fig. S8C). Together, these studies associate RAB3GAP1 with Atg8 family members and, interestingly, link significant levels of both factors to lipid droplets.
Thus, as shown by knockdown, overexpression, and colocalization, RAB3GAP1/2 influence autophagy and are novel factors of the autophagy network.
RAB3GAP1/2 function during autophagosome formation
Since we showed that RAB3GAP1/2 affect the lipidation of Atg8 family members, we continued to analyze their effect on autophagosomal biogenesis in more detail and examined their influence in relation to ATG3 and ATG16L1, which are established factors of LC3 conjugation and autophagosome formation (). The knockdown of ATG3 and ATG16L1 resulted in an accumulation of LC3-I and a decreased autophagic activity which resembled the influence of reduced RAB3GAP levels on autophagy. Knocking down ATG3 and RAB3GAP1/2 or ATG16L1 and RAB3GAP1/2 together, respectively, did not significantly enhance these effects. Moreover, RAB3GAP1/2 or ATG3 overexpression increased autophagic activity and this stimulation was counteracted by the concurrent deficiency of ATG16L1 or the RAB3GAPs, respectively.
Figure 4. RAB3GAP1/2 modulate autophagosomal formation. (A) Immunoblot analysis of cells that were manipulated with the indicated siRNAs and plasmids for 48 h and treated with DMSO (−) or bafilomycin A1 (+) for 4 h. Control cells were manipulated with nonsense siRNA and eV. Tubulin served as control for equal loading. Statistics are depicted as mean ± SD normalized to the appropriate control; n.s. = not significant, *P < 0.05, **P < 0.01, n = 3 or 4, t test. (B) Identical to (A) but with different siRNAs and plasmids. Statistics are depicted as mean ± SD normalized to the appropriate control; n.s. = not significant, *P < 0.05, **P < 0.01, n = 3 or 4, t test. (C) Confocal images of ATG5 immunostainings. Fibroblasts were manipulated with the indicated siRNAs for 48 h and thereafter control cells were additionally treated with rapamycin for 4 h. Scale bar = 50 μm. For statistics, cells showing ATG5 punctate structures were counted in relation to cells without puncta (30 to 40 cells per treatment) and statistics are depicted as mean ± SD; **P < 0.01, ***P < 0.001; n = 4, t test.
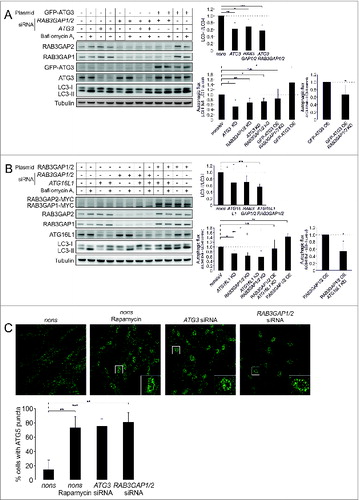
To further elucidate the influence of RAB3GAP1/2 on autophagosomal biogenesis, we conducted immunostaining of ATG5. The ATG5–ATG12-ATG16L1 complex is recruited to the phagophore, and stays attached until autophagosome maturation is complete.Citation3 At basal conditions ATG5 punctate structures are only detectable in a small number of cells, but they are induced by the stimulation of autophagy or by the deterioration of autophagosome maturation.Citation8,9 Indeed, also in the IMR-90 fibroblasts employed here the induction of autophagy by rapamycin as well as the deficiency of ATG3 at basal conditions increased the number of cells with ATG5 punctate structures (). Notably, the knockdown of RAB3GAP1/2 resulted in an accumulation of ATG5 puncta at basal conditions, which demonstrates that the deficiency of the RAB3GAPs deteriorates the maturation of autophagosomes.
Thus, analyzing the function of RAB3GAP1/2 in relation to well-established factors of autophagosome formation mechanistically associates the RAB3GAPs with this process.
The influence of the RAB3GAPs on autophagy is independent of RAB3 but is mediated by the GAP domain
So far the RAB3GAPs have been exclusively linked to the regulation of the RAB GTPase RAB3 and synaptic homeostasis.Citation26,27 To investigate, whether the influence of the RAB3GAPs on autophagy is also mediated by this particular RAB protein, we analyzed the impact of RAB3 on autophagy. We detected the RAB3 isoforms A, B, C, and D in IMR-90 fibroblasts employing real-time PCR. Since the isoforms tend to functionally compensate each other,Citation36 we knocked down all of them at once to exclude these potential compensatory effects. The successful reduction was confirmed by real-time PCR (Fig. S9A). Neither this approach nor the knockdown of each single RAB3 isoforms decreased LC3 conversion or autophagic activity (; Fig. S9B), demonstrating that RAB3, most likely, does not mediate the role of RAB3GAP1/2 in autophagy.
Figure 5. The RAB3GAP complex affects autophagy independent of RAB3 but dependent on its GTPase-activating effect. (A) Immunoblot analysis of cells that were manipulated with the indicated siRNAs for 48 h and treated with DMSO (−) or bafilomycin A1 (+) for 4 h. Tubulin served as control for equal loading. Statistics are depicted as mean ± SD normalized to nonsense control. No significant differences were detected; n = 3, t test. (B) Immunoblot analysis of cells that were manipulated with the indicated plasmids for 48 h and treated with DMSO (−) or bafilomycin A1 (+) for 4 h. Tubulin served as control for equal loading. Statistics are depicted as mean ± SD normalized to eV; n.s. = not significant, **P < 0.01, ***P < 0.001; n = 4, t test.
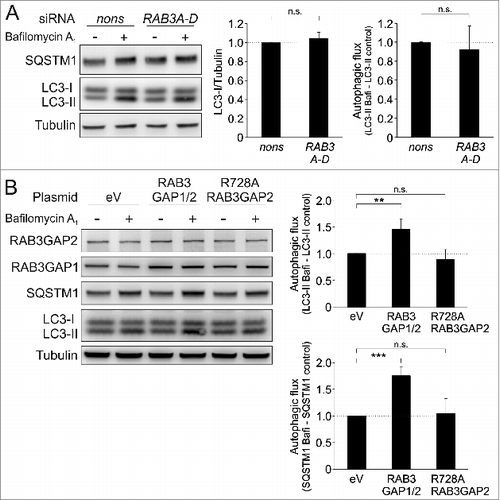
Next, we analyzed whether the GTPase-activating activity of RAB3GAP1 is important for the autophagy modulation and exchanged an arginine (R) of the GAP domain with alanine (A), which strongly reduces its GTPase activating-activity.Citation37 The expression of mutant RAB3GAP1 (R728A) together with wild-type RAB3GAP2 prevented the increased autophagic activity that was observed upon overexpression of both wild-type RAB3GAPs (). This emphasizes the necessity of the GTPase-activating activity of RAB3GAP1 for its influence on autophagy, and shows that this effect is putatively mediated by a so-far unknown downstream RAB GTPase.
The RAB3GAP complex opposes the autophagy suppressing activity of FEZ1 and FEZ2
To further characterize the relevance of the RAB3GAPs for autophagy, we analyzed their function in relation to FEZ1 and FEZ2. FEZ1 was recently identified as a negative regulator of autophagy,Citation31 and in a yeast-2-hybrid study it was shown to interact with the RAB3GAPs.Citation38
To confirm this protein interaction in mammalian cells, we performed co-immunoprecipitation and IP/MS studies, but could not detect a stable interaction of the RAB3GAPs and FEZ1. However, the proteomics analyses yielded interaction networks that included RFWD2 (ring finger and WD repeat domain 2, E3 ubiquitin protein ligase), COP (clathrin-ordered protein) proteins, and VPS51/C11orf2 (vacuolar protein sorting 51 homolog [S. cerevisiae]) for the RAB3GAPs and SCOC (short coiled-coil protein) for FEZ1 (Fig. S10), which were previously linked to autophagy.Citation31,39-41 The protein DNAJC13 that functions in endosomal traffickingCitation42 was identified as a common interaction partner of RAB3GAP1 and FEZ1. We also identified GABARAP and GABARAPL1 as candidate interaction partners of the RAB3GAPs (Table S1). Even though this interaction was not strong enough to pass the stringent threshold for high-confident candidate interacting proteins,Citation43 it supports the association of the RAB3GAPs with the Atg8 family interactome that has been previously provided employing Atg8 family members as bait.Citation19
Although we did not detect a stable interaction of the RAB3GAPs and FEZ1, we analyzed the subcellular distribution of both proteins and observed that RAB3GAP1 and FEZ1 colocalized and, furthermore, that both proteins are associated with the Golgi apparatus (Fig. S11A and B). This prompted us to examine a potential functional relation of autophagy regulation by RAB3GAP1/2 and FEZ1 and also FEZ2. FEZ2 has not been linked to autophagy so far, but we demonstrate that it has the same inhibitory effect as its homolog FEZ1 under basal conditions (). Employing immunoblotting and immunostaining we observed that the knockdown of FEZ1 and FEZ2, as well as the knockdown of both proteins together, increased autophagic activity, whereas the deficiency of RAB3GAP1/2 caused the expected decrease in autophagic activity (). Overexpression of these factors showed the reciprocal effects (). Interestingly, the simultaneous decrease or increase of FEZ1/2 and RAB3GAP1/2 resulted in an autophagic activity that was comparable to control conditions and did not resemble the autophagic flux that was observed by either knocking down or overexpressing the RAB3GAPs or FEZ proteins alone (). Thus, autophagy regulation by RAB3GAP1/2 and FEZ1/2 is contrary and their relative activities might determine cellular autophagic activity. This further emphasizes the relevance of the RAB3GAPs for autophagy as was demonstrated in our study.
Figure 6. The RAB3GAP complex antagonizes the FEZ1/2-mediated suppression of autophagy. (A) Immunoblot analysis of cells that were manipulated with the indicated siRNA for 48 h and treated with DMSO (−) or bafilomycin A1 (+) for 4 h. Tubulin served as control for equal loading. Statistics are depicted as mean ± SD normalized to nonsense control; n.s. = not significant, *P < 0.05, **P < 0.01, n = 4, t test. (B) Identical to (A) but different siRNA treatments. Statistics are depicted as mean ± SD normalized to nonsense control; n.s. = not significant, *P < 0.05, **P < 0.01,n = 4, t test. (C) Confocal images of LC3 immunostainings. Fibroblasts were treated with the indicated siRNA for 48 h and treated with DMSO or bafilomycin A1 for 4 h. DAPI was used to stain nuclei. Scale bar = 50 μm. Autophagosomal structures were counted in 20 to 40 cells of 3 independent experiments; n.s. = not significant, ***P < 0.001, t test. (D) Immunoblot analysis of cells that were manipulated with the indicated plasmids for 48 h and treated with DMSO (−) or bafilomycin A1 (+) for 4 h. Since FEZ1/2 overexpression affected tubulin levels, actin served as control for equal loading. Statistics are depicted as mean ± SD normalized to eV control; n.s. = not significant, *P < 0.05,n = 4, t test.
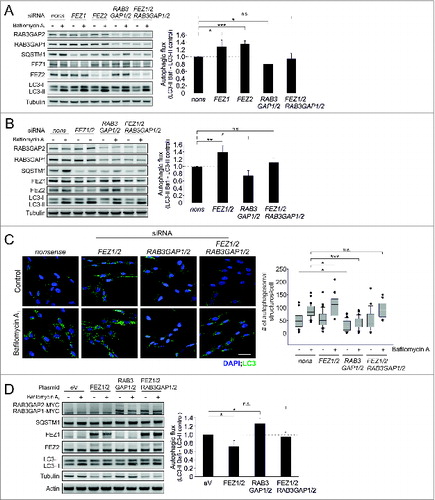
Comparable to the knockdown of the RAB3GAPs, the manipulation of FEZ1/2 affected SQSTM1 protein levels. The molecular mechanisms triggering SQSTM1 levels remain currently unknown, even though we show that this downregulation also appears on the gene expression level (Fig. S12).
Discussion
The heterodimeric RAB3GAP complex is well-acknowledged to regulate the RAB GTPase RAB3 and the release of neurotransmitters at the neuronal synapse.Citation26,27 Here, we show that RAB3GAP1/2 also affects intracellular protein aggregation and modulate autophagy at basal and rapamycin-induced conditions.
Autophagy is dependent on a tightly regulated vesicle transport system and membrane dynamics. RAB GTPases are essential regulators of intracellular trafficking processes and ensure the efficient delivery of vesicles to different compartments and pathways.Citation18 Numerous RAB proteins have been associated with autophagy,Citation13-15,17 and are controlled by GEFs and RABGAPs that coordinate the period and specificity of RAB GTPase activity. Several RABGAPs have been shown to interact with Atg8 family members and affect autophagy.Citation17,20-22 These RABGAPs contain the conserved TBC domain that functions via a dualfinger mechanism which facilitates GTP hydrolysis and inactivation of RAB GTPases.Citation44 Thus, TBC domain-containing RABGAPs are potentially more than sole regulators of distinct RAB GTPases, but seem to be important factors to coordinate intracellular trafficking and autophagy, possibly by recruiting specific proteins or membranes to the autophagic pathway. We now integrate the TBC domain-free RAB3GAP1/2 into this system, which emphasizes that the TBC domain is actually not a prerequisite for the involvement in autophagy regulation. The influence of RAB3GAP1/2 on autophagy is dependent on the GTPase activating activity of RAB3GAP1 and very likely independent of RAB3, highlighting that a yet unknown RAB GTPase might be a novel additional target of the RAB3GAP complex.
Collectively, we show that the RAB3GAPs affect lipidation of mammalian orthologs of yeast Atg8 and influence autophagy during autophagosome formation. ATG3, ATG5, and ATG16L1 are conserved components of LC3 conjugation and autophagosome biogenesis, and analyzing the function of the RAB3GAPs in relation to these ATG proteins emphasizes that RAB3GAP1/2 modulate autophagosomal maturation. ATG16L1 forms a complex with ATG12-ATG5 that localizes to the phagophore membrane and stays attached until autophagosome maturation is complete.Citation3 PE conjugation of Atg8 family members is mediated by the activity of ATG7, an E1-like enzyme, ATG3, an E2-like enzyme, and by the ATG12–ATG5-ATG16L1 complex which acts as an E3-like enzyme.Citation6 The detailed function of lipidated mammalian orthologs of yeast Atg8 is unclear but an atg3 knockout mouse resulted in the accumulation of ATG16L punctate structures and unclosed autophagosomal precursors.Citation8 This suggests that Atg8 family protein PE conjugation is a prerequisite for autophagosome maturation and different mammalian orthologs of yeast Atg8 seem to influence distinct steps of this process.Citation9 The formation of autophagosomes is highly dependent on the sufficient acquisition of membranes and RAB3GAP1/2 might support the transfer of lipids for autophagosome biogenesis. Several RABGAPs that interact with LC3 have previously been shown to affect autophagy initiation,Citation14,17,22 and recently, the RAB3GAPs have also been linked to the Atg8 family interactome.Citation19 The association of the RAB3GAPs with Atg8 family members is supported here by the detection of GABARAP and GABARAPL1 as weak interaction partners in our IP/MS approach, employing RAB3GAP1/2 as bait, and by the colocalization of RAB3GAP1 and LC3 or GABARAP.
Significant levels of RAB3GAP1 and LC3 or GABARAP colocalized at lipid droplets. LC3 and other ATG proteins have been shown to be present there and these cellular lipid stores have been characterized as autophagy substrates whose metabolism is sensitively regulated by autophagy.Citation45,46 Similar to other Warburg Micro Syndrome-associated proteins, mutant RAB3GAP1 has recently been shown to influence the size of lipid droplets,Citation47 and a mouse model of the disorder is characterized by an altered lipid droplet metabolism.Citation48 The localization of RAB3GAP1 at these organelles now points to a potential functional connection of the RAB3GAPs, autophagy, and lipid droplet turn-over and offers a possible molecular pathway that influences this cellular phenotype of the Warburg Micro Syndrome.
The relevance of RAB3GAP1/2 for autophagy is further supported by the comparative analysis of FEZ1/2. Previously, FEZ1 has been shown to suppress autophagy during starvation by interacting with Golgi-located SCOC.Citation31 We show that FEZ1 regulates autophagy also at basal conditions and that the homolog FEZ2 exhibits the same inhibitory activity. Notably, the reciprocal regulation of autophagy by RAB3GAP1/2 and FEZ1/2 is balanced when both factors are modulated at the same time. Thus, the positive autophagy regulation by the RAB3GAPs opposes the inhibitory effect of FEZ1/2 (or vice versa) and their relative activities may be influencing total cellular autophagic activity.
Notably, in yeast 2-hybrid studies analyzing FEZ1Citation38 and FEZ2Citation49 the RAB3GAPs have been assigned as interaction partners for FEZ1 but not for FEZ2. However, both FEZ proteins have overlapping functionsCitation49 and we show that they have identical effects on autophagy in the cell system employed here. Thus, we propose that a direct interaction of FEZ1 and the RAB3GAPs, which we could not transfer into mammalian cells employing co-immunoprecipitation and IP/MS approaches, is actually not a strict prerequisite for the opposing activities of RAB3GAP1/2 and FEZ1/2 on autophagy.
In addition to the association of the RAB3GAPs with autophagy, we also could connect them to the proteostasis network; RBG-1 was initially isolated in an RNAi approach in C. elegans, which was designed to identify novel factors that affect protein aggregation, and the deficiency of RBG-1 enhanced amyloid β42 aggregation and accelerated the paralysis phenotype of amyloid β42-expressing worms. Besides chaperones and the ubiquitin proteasome system, autophagy is a central module of proteostasis and its deterioration is sufficient for increased protein aggregation.Citation50-52 In senescent fibroblasts autophagy is upregulated possibly to compensate an age-associated impairment of proteostasis,Citation53 and in several age-related disorders connected to protein aggregation, such as Alzheimer and Huntington disease, the modulation of autophagy has been shown to affect neurodegeneration.Citation35,54,55
In summary, in addition to the well-acknowledged modulation of synaptic homeostasis, based on our results, we now can integrate RAB3GAP1/2 into the autophagy network and extend their functional spectrum to autophagy and proteostasis.
Materials and Methods
C. elegans methods
C. elegans strains were obtained from the Caenorhabditis Genetics Center and were maintained using standard procedures. RNAi was induced by feeding dsRNA as described previously.Citation32 The large-scale RNAi screen was conducted employing a feeding RNAi library.Citation56 Nematodes expressing the protein-folding reporter Luciferase-GFP in body wall muscle cells were maintained on RNAi conditions for 72 h and heat stressed at 36°C for approx. 1 h. Aggregation of Luciferase-GFP was assayed employing a fluorescence stereomicroscope and was compared to hsp-110 RNAi-treated worms (positive control) and eV treated worms (negative control). Newly identified genes were retested for their potential to influence Luciferase-GFP aggregation. For analysis of paralysis rate, synchronous CL2006 (dvIs2 [pCL12(Punc-54-hAβ)/pRF4]) nematodes were cultivated at 15°C on RNAi plates. Starting at first day of adulthood, worms were transferred to fresh plates daily and were tested for paralysis by tapping their nose with a platinum wire; worms that moved their nose but failed to move their bodies were scored as paralyzed. Dead worms or worms showing other phenotypes were not included into the statistics. Staining of amyloid β42 aggregates using thioflavine were carried out as previously described.Citation57 For analysis of autophagic activity, synchronous nematodes expressing GFP-LGG-1 (ex[Plgg-1-GFP-LGG-1]/pRF4; kind gift of Beth Levine) were cultivated under RNAi conditions at 20°C. At L4 stage, worms were transferred to bacteria-free plates overnight to induce autophagy or kept on bacteria-seeded plates for control. For quantification, fluorescence microscopy images of 2 to 3 seam cells per worm, with a total of at least 30 worms from 3 independent experiments, were obtained and total numbers of pre- and autophagosomal structures were counted.
Cell culture
Primary human fibroblasts (IMR-90) were obtained from the Coriell Institute for Medical Research and were cultured in DMEM (Invitrogen, 41965062) supplemented with 10% fetal bovine serum (PAA, A15-101), 1 mM sodium pyruvate (Invitrogen, 1136-088), 1× nonessential amino acids (Invitrogen, 11140-068), and 1× antibiotic-antimycotic solution (Invitrogen, 15240-112) at 37°C in a 5% CO2-humidified atmosphere. Stock solutions of rapamycin (Enzo, BML-A275-0025) and bafilomycin A1 (LC Laboratories, B-1080) were prepared in DMSO (Roth, A994.2).
Plasmids, siRNAs, and transfections
Expression plasmids for human RAB3GAP1 (pCI neo-Myc Rab3Gap p130) and RAB3GAP2 (pCI neo-Myc Rab3Gap p150) were received as a kind gift from Prof Takuya Sasaki (Department of Biochemistry, University of Tokushima). The RAB3GAP1 R728A mutant was generated employing a site directed mutagenesis kit (Agilent, 210518) following supplier's instructions. Expression plasmids for FEZ1 (pCMV6-FEZ1-Myc) and FEZ2 (pCMV6-FEZ2-Myc) were obtained from OriGene. Generally, cells were transfected with 10 to 15 μg of plasmid and the corresponding amount of eV was transfected as control. siRNAs were purchased from Eurofins MWG Operon as duplexes with 3′-dTdT overhangs. Sequences of siRNAs are listed in Table S2. Cells were transfected with 30 μg of siRNA and the same amounts of nonsense siRNA were used as control. All transfections were carried out employing electroporation as described previously.Citation53
Immunoblotting, co-immunoprecipitation, and immunocytochemistry
Immunoblot analyses were performed as described previously.Citation53 Generally, 15 μg of total protein were subjected to SDS–PAGE using precast NuPAGE 4–12% Bis-Tris gels (Invitrogen, NPO322) or hand-cast 12% Bis-Tris gels. Proteins were detected by chemiluminescence using the Fuji LAS-3000 dark box (Fujifilm, Düsseldorf). Co-immunoprecipitation analyses, employing FEZ1 or RAB3GAP1/2 overexpressing HEK293 cells, were performed as previously described.Citation53 For immunocytochemistry, IMR-90 cells were grown on glass cover slips in 24-well plates. After the indicated treatment, cells were fixed with 4% (w/v) paraformaldehyde (Sigma Aldrich, P6148) for 20 min and incubated with 90% methanol for 6 min. For lipid droplet staining, cells were incubated with 0.1% (v/v) Triton X-100 (Sigma Aldrich, T9284) in PBS (Sigma Aldrich, D8537) for 10 min. Nonspecific binding sites were blocked with 3% (w/v) BSA (Sigma Aldrich, A2153) in PBS, and subsequently cells were incubated with the appropriate primary antibodies (1:200 in 1% [w/v] BSA). Thereafter cells were incubated with fluorophore-conjugated secondary antibodies (1:200 in PBS), and DAPI (Calbiochem, 382061). For induction of lipid droplets, cells were incubated with 400 μM oleic acid (Sigma Aldrich, O1008) for 24 h. Lipid droplets were stained with 4 μM 1,6-diphenyl-1,3,5-hexatriene (DPH; Sigma Aldrich, D208000) for 10 min. Confocal microscopical analyses were performed with the laser scanning microscope LSM 710 (Zeiss, Oberkochen). Antibodies employed in these methods are listed in Table S3.
Interactome analysis
For interactome analysis FEZ1, RAB3GAP1 or RAB3GAP2 were expressed as N-terminal Flag-hemagglutinin (HA) fusion proteins in HEK293 cells, which were cultivated employing standard procedures.Citation19 Proteins eluted from anti-HA immune complexes were subjected to trypsinolysis and analyzed by mass spectrometry (LC-MS/MS) followed by identification of high-confidence candidate interaction proteins (HCIPs) by CompPASS as previously described.Citation43
Quantitative real-time PCR
RNA extraction, reverse transcription, and quantitative real-time PCR for human cellsCitation53 or C. elegansCitation32 were performed as previously described. In IMR-90 cells the gene RPL19 served as loading control, while in C. elegans the gene rpl-21 was employed. Primer sequences are listed in Tables S4 and S5.
Statistical methods
Statistical significance was determined by the Student t test or one-way ANOVA using SIGMA STAT (SPSS Science). Statistical significance was accepted at a level of P < 0.05. The results are expressed as mean ± standard deviation (SD).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
KAUP_A_994359_supplemental_files.zip
Download Zip (3.4 MB)Funding
This work was supported by grants from the Alzheimer Forschung Initiative (to A. K.), DFG/Emmy Noether Research Grant BE 4685/1-1 and European Research Council Starting Grant 282333 (to C. Behrends), the Fritz & Hildegard Berg-Stiftung and the Corona Stiftung (to C. Behl), and DFG/Collaborative Research Center 1080 (to A.M.C. and C. Behl). The initial phase of this project was supported by the Frankfurt-Mainz Autophagy Network (FAN).
References
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132:27-42; PMID:18191218; http://dx.doi.org/10.1016/j.cell.2007.12.018
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000; 290:1717-21; PMID:11099404; http://dx.doi.org/10.1126/science.290.5497.1717
- Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci 2003; 116:1679-88; PMID:12665549; http://dx.doi.org/10.1242/jcs.00381
- Mizushima N, Sugita H, Yoshimori T, Ohsumi Y. A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J Biol Chem 1998; 273:33889-92; PMID:9852036; http://dx.doi.org/10.1074/jbc.273.51.33889
- Fujita N, Hayashi-Nishino M, Fukumoto H, Omori H, Yamamoto A, Noda T, Yoshimori T. An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Mol Biol Cell 2008; 19:4651-9; PMID:18768752; http://dx.doi.org/10.1091/mbc.E08-03-0312
- Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, Inagaki F, Ohsumi Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem 2007; 282:37298-302; PMID:17986448; http://dx.doi.org/10.1074/jbc.C700195200
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19:5720-8; PMID:11060023; http://dx.doi.org/10.1093/emboj/19.21.5720
- Sou YS, Waguri S, Iwata J, Ueno T, Fujimura T, Hara T, Sawada N, Yamada A, Mizushima N, Uchiyama Y, et al. The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol Biol Cell 2008; 19:4762-75; PMID:18768753; http://dx.doi.org/10.1091/mbc.E08-03-0309
- Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 2010; 29:1792-802; PMID:20418806; http://dx.doi.org/10.1038/emboj.2010.74
- Cemma M, Kim PK, Brumell JH. The ubiquitin-binding adaptor proteins p62/SQSTM1 and NDP52 are recruited independently to bacteria-associated microdomains to target Salmonella to the autophagy pathway. Autophagy 2011; 7:341-5; PMID:21079414; http://dx.doi.org/10.4161/auto.7.3.14046
- Kirkin V, Lamark T, Sou YS, Bjorkoy G, Nunn JL, Bruun JA, Shvets E, McEwan DG, Clausen TH, Wild P, et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell 2009; 33:505-16; PMID:19250911; http://dx.doi.org/10.1016/j.molcel.2009.01.020
- Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell 2009; 34:259-69; PMID:19450525; http://dx.doi.org/10.1016/j.molcel.2009.04.026
- Chua CE, Gan BQ, Tang BL. Involvement of members of the Rab family and related small GTPases in autophagosome formation and maturation. Cell Mol Life Sci 2011; 68:3349-58; PMID:21687989; http://dx.doi.org/10.1007/s00018-011-0748-9
- Itoh T, Fujita N, Kanno E, Yamamoto A, Yoshimori T, Fukuda M. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol Biol Cell 2008; 19:2916-25; PMID:18448665; http://dx.doi.org/10.1091/mbc.E07-12-1231
- Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 2004; 117:4837-48; PMID:15340014; http://dx.doi.org/10.1242/jcs.01370
- Lipatova Z, Belogortseva N, Zhang XQ, Kim J, Taussig D, Segev N. Regulation of selective autophagy onset by a Ypt/Rab GTPase module. Proc Natl Acad Sci U S A 2012; 109:6981-6; PMID:22509044; http://dx.doi.org/10.1073/pnas.1121299109
- Longatti A, Lamb CA, Razi M, Yoshimura S, Barr FA, Tooze SA. TBC1D14 regulates autophagosome formation via Rab11- and ULK1-positive recycling endosomes. J Cell Biol 2012; 197:659-75; PMID:22613832; http://dx.doi.org/10.1083/jcb.201111079
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; http://dx.doi.org/10.1038/nrm2728
- Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature 2010; 466:68-76; PMID:20562859; http://dx.doi.org/10.1038/nature09204
- Carroll B, Mohd-Naim N, Maximiano F, Frasa MA, McCormack J, Finelli M, Thoresen SB, Perdios L, Daigaku R, Francis RE, et al. The TBC/RabGAP Armus coordinates Rac1 and Rab7 functions during autophagy. Dev Cell 2013; 25:15-28; PMID:23562278; http://dx.doi.org/10.1016/j.devcel.2013.03.005
- Itoh T, Kanno E, Uemura T, Waguri S, Fukuda M. OATL1, a novel autophagosome-resident Rab33B-GAP, regulates autophagosomal maturation. J Cell Biol 2011; 192:839-53; PMID:21383079; http://dx.doi.org/10.1083/jcb.201008107
- Popovic D, Akutsu M, Novak I, Harper JW, Behrends C, Dikic I. Rab GTPase-activating proteins in autophagy: regulation of endocytic and autophagy pathways by direct binding to human ATG8 modifiers. Mol Cell Biol 2012; 32:1733-44; PMID:22354992; http://dx.doi.org/10.1128/MCB.06717-11
- Frasa MA, Koessmeier KT, Ahmadian MR, Braga VM. Illuminating the functional and structural repertoire of human TBC/RABGAPs. Nat Rev Mol Cell Biol 2012; 13:67-73; PMID:22251903; http://dx.doi.org/10.1038/nrm3364
- Fukui K, Sasaki T, Imazumi K, Matsuura Y, Nakanishi H, Takai Y. Isolation and characterization of a GTPase activating protein specific for the Rab3 subfamily of small G proteins. J Biol Chem 1997; 272:4655-8; PMID:9030515; http://dx.doi.org/10.1074/jbc.272.8.4655
- Nagano F, Sasaki T, Fukui K, Asakura T, Imazumi K, Takai Y. Molecular cloning and characterization of the noncatalytic subunit of the Rab3 subfamily-specific GTPase-activating protein. J Biol Chem 1998; 273:24781-5; PMID:9733780; http://dx.doi.org/10.1074/jbc.273.38.24781
- Muller M, Pym EC, Tong A, Davis GW. Rab3-GAP controls the progression of synaptic homeostasis at a late stage of vesicle release. Neuron 2011; 69:749-62; PMID:21338884; http://dx.doi.org/10.1016/j.neuron.2011.01.025
- Sakane A, Manabe S, Ishizaki H, Tanaka-Okamoto M, Kiyokage E, Toida K, Yoshida T, Miyoshi J, Kamiya H, Takai Y, et al. Rab3 GTPase-activating protein regulates synaptic transmission and plasticity through the inactivation of Rab3. Proc Natl Acad Sci USA 2006; 103:10029-34; PMID:16782817; http://dx.doi.org/10.1073/pnas.0600304103
- Haines DS, Lee JE, Beauparlant SL, Kyle DB, den Besten W, Sweredoski MJ, Graham RL, Hess S, Deshaies RJ. Protein interaction profiling of the p97 adaptor UBXD1 points to a role for the complex in modulating ERGIC-53 trafficking. Mol Cell Proteomics 2012; 11: M111 016444; PMID:22337587; http://dx.doi.org/10.1074/mcp.M111.016444
- Aligianis IA, Johnson CA, Gissen P, Chen D, Hampshire D, Hoffmann K, Maina EN, Morgan NV, Tee L, Morton J, et al. Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nature Genetics 2005; 37:221-3; PMID:15696165; http://dx.doi.org/10.1038/ng1517
- Borck G, Wunram H, Steiert A, Volk AE, Korber F, Roters S, Herkenrath P, Wollnik B, Morris-Rosendahl DJ, Kubisch C. A homozygous RAB3GAP2 mutation causes Warburg Micro syndrome. Human Genetics 2011; 129:45-50; PMID:20967465; http://dx.doi.org/10.1007/s00439-010-0896-2
- McKnight NC, Jefferies HB, Alemu EA, Saunders RE, Howell M, Johansen T, Tooze SA. Genome-wide siRNA screen reveals amino acid starvation-induced autophagy requires SCOC and WAC. EMBO J 2012; 31:1931-46; PMID:22354037; http://dx.doi.org/10.1038/emboj.2012.36
- Kern A, Ackermann B, Clement AM, Duerk H, Behl C. HSF1-controlled and age-associated chaperone capacity in neurons and muscle cells of C. elegans. PloS One 2010; 5:e8568; PMID:20052290; http://dx.doi.org/10.1371/journal.pone.0008568
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science 2006; 313:1604-10; PMID:16902091; http://dx.doi.org/10.1126/science.1124646
- Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003; 301:1387-91; PMID:12958363; http://dx.doi.org/10.1126/science.1087782
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 2004; 36:585-95; PMID:15146184; http://dx.doi.org/10.1038/ng1362
- Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci 2004; 24:6629-37; PMID:15269275; http://dx.doi.org/10.1523/JNEUROSCI.1610-04.2004
- Clabecq A, Henry JP, Darchen F. Biochemical characterization of Rab3-GTPase-activating protein reveals a mechanism similar to that of Ras-GAP. J Biol Chem 2000; 275:31786-91; PMID:10859313; http://dx.doi.org/10.1074/jbc.M003705200
- Assmann EM, Alborghetti MR, Camargo ME, Kobarg J. FEZ1 dimerization and interaction with transcription regulatory proteins involves its coiled-coil region. J Biol Chem 2006; 281:9869-81; PMID:16484223; http://dx.doi.org/10.1074/jbc.M513280200
- Claerhout S, Dutta B, Bossuyt W, Zhang F, Nguyen-Charles C, Dennison JB, Yu Q, Yu S, Balázsi G, Lu Y, et al. Abortive autophagy induces endoplasmic reticulum stress and cell death in cancer cells. PloS One 2012; 7:e39400; PMID:22745748; http://dx.doi.org/10.1371/journal.pone.0039400
- Kobayashi S, Yoneda-Kato N, Itahara N, Yoshida A, Kato JY. The COP1 E3-ligase interacts with FIP200, a key regulator of mammalian autophagy. BMC Biochem 2013; 14:1; PMID:23289756; http://dx.doi.org/10.1186/1471-2091-14-1
- Perez-Victoria FJ, Schindler C, Magadan JG, Mardones GA, Delevoye C, Romao M, Raposo G, Bonifacino JS. Ang2/fat-free is a conserved subunit of the Golgi-associated retrograde protein complex. Mol Biol Cell 2010; 21:3386-95; PMID:20685960; http://dx.doi.org/10.1091/mbc.E10-05-0392
- Girard M, Poupon V, Blondeau F, McPherson PS. The DnaJ-domain protein RME-8 functions in endosomal trafficking. J Biol Chem 2005; 280:40135-43; PMID:16179350; http://dx.doi.org/10.1074/jbc.M505036200
- Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell 2009; 138:389-403; PMID:19615732; http://dx.doi.org/10.1016/j.cell.2009.04.042
- Pan X, Eathiraj S, Munson M, Lambright DG. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature 2006; 442:303-6; PMID:16855591; http://dx.doi.org/10.1038/nature04847
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature 2009; 458:1131-5; PMID:19339967; http://dx.doi.org/10.1038/nature07976
- Velikkakath AK, Nishimura T, Oita E, Ishihara N, Mizushima N. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol Biol Cell 2012; 23:896-909; PMID:22219374; http://dx.doi.org/10.1091/mbc.E11-09-0785
- Liegel RP, Handley MT, Ronchetti A, Brown S, Langemeyer L, Linford A, Chang B, Morris-Rosendahl DJ, Carpanini S, Posmyk R, et al. Loss-of-function mutations in TBC1D20 cause cataracts and male infertility in blind sterile mice and Warburg micro syndrome in humans. Am J Hum Genet 2013; 93:1001-14; PMID:24239381; http://dx.doi.org/10.1016/j.ajhg.2013.10.011
- Carpanini SM, McKie L, Thomson D, Wright AK, Gordon SL, Roche SL, Handley MT, Morrison H, Brownstein D, Wishart TM, et al. A novel mouse model of Warburg Micro Syndrome reveals roles for RAB18 in eye development and organisation of the neuronal cytoskeleton. Dis Mod Mech 2014; 7(6):711-22; PMID:24764192
- Alborghetti MR, Furlan AS, Kobarg J. FEZ2 has acquired additional protein interaction partners relative to FEZ1: functional and evolutionary implications. PloS One 2011; 6:e17426; PMID:21408165; http://dx.doi.org/10.1371/journal.pone.0017426
- Korac J, Schaeffer V, Kovacevic I, Clement AM, Jungblut B, Behl C, Terzic J, Dikic I. Ubiquitin-independent function of optineurin in autophagic clearance of protein aggregates. J Cell Sci 2013; 126:580-92; PMID:23178947; http://dx.doi.org/10.1242/jcs.114926
- Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell 2009; 33:517-27; PMID:19250912; http://dx.doi.org/10.1016/j.molcel.2009.01.021
- Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 2002; 11:1107-17; PMID:11978769; http://dx.doi.org/10.1093/hmg/11.9.1107
- Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 2009; 28:889-901; PMID:19229298; http://dx.doi.org/10.1038/emboj.2009.29
- Morawe T, Hiebel C, Kern A, Behl C. Protein homeostasis, aging and Alzheimer's disease. Mol Neurobiol 2012; 46:41-54; PMID:22361852; http://dx.doi.org/10.1007/s12035-012-8246-0
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 2005; 64:113-22; PMID:15751225
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003; 421:231-7; PMID:12529635; http://dx.doi.org/10.1038/nature01278
- Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci 2006; 26:13102-13; PMID:17167099; http://dx.doi.org/10.1523/JNEUROSCI.3448-06.2006
