Abstract
We have monitored the electrical potential variations (EPV) of sunflower plants illuminated by a high-intensity microwave-frequency (2.5 GHz, 1.5 kV/m) electromagnetic field (EMF). We have designed an appropriate set-up that allows parallel temperature and EPV measurements while part of the plant is being exposed to the field. The results show that the considered EMF does not induce plant EPV directly. This electrophysiological response appears only when the EMF leads to a mechanical injury of the tissues via a thermal effect (dielectric heating). Once the plant inner temperature reached a threshold, we systematically observed burn-like lesions associated with the bending of the stem or leaf-stalks. Theses mechanical constraints were rapidly followed by EPVs, moving through the stem.
Abbreviations
| EPV | = | electrical potential variation |
| EMF | = | electromagnetic field |
| AP | = | action potential |
| SWP | = | slow wave potential |
Introduction
The existence of electrical phenomenon in plants has been suggested from the 18th centuryCitation1 and recorded at the first time in 1973.Citation2 Thanks to the progress in electrophysiology, it is now well described that most plants do sense the fluctuations of their environment (biotic and abiotic stimuli) and use electrical signals to spread systemic informations from organs to other tissues.Citation3,4 Thus, plants can rapidly adapt their metabolismCitation5 and generate distant physiological modificationsCitation6 via the regulation of gene expression.Citation7 The recent years have allowed the determination of the ionic organization of this electrical phenomenonCitation2 in addition to many common features between plants and animals such as the sensitivity of sunflower to the neurotransmitter glutamateCitation8 or ultra-fast action potentials.Citation9 According to their species and physiological conditions, plants display a 50–300 mV resting electrical potential, usually higher than the animal cell resting stage.Citation10 This chemiosmotic phenomenon is based upon unbalanced ions repartition (K+, Na+, Ca2+, Cl−) on both sides of the plasma membrane, due to variable membrane permeability, passive and pH dependent diffusions and active flux such as generated by the H+‑ATPase electrogenic pump.Citation11 Then, following the perception of an environmental signal (initially transduced by the ionic balance modification), very different shaped electrical signals ranging from 5 to 70 mV amplitude, 1 ms to 30 min duration and 0,1 mm.s−1 to 100 m.s−1 velocity, can be measured.Citation9 However, at least 3 types of propagating signals have been well documented:Citation4,5,12 wound potential (WP, or local electrical potential (LEP)) that initiate and disappear around the excitation site, action potential (AP) and slow wave potential (SWP, or variation potential (VP)). APs are self-propagating and amplitude-constant signal initiated by non-damaging internal and environmental stimuli (touch, light, temperature).Citation13 They depend on electro-sensitive channels and share some characteristics with animals APs such as an all-or-nothing law in addition to a fast repolarization (less than 1 minute). SWPs are typically associated with hurting stimuli (cutting, burning, mashing) that induce transient increase of water pressure within the plant. These signals, that show hydraulic propagationCitation14 and imply ATPase shutdown, are unique to plant and display slow repolarization (more than 10 min) in association to an amplitude decrement along their propagation through the stem and organs.Citation12,15 Two other kinds of electrical signals have been recently suggested such as the “system potential”Citation16 that differs from AP and SWP by its electrical characteristics and “solitary waves” that are APs as fast as the mammalian potentials: the plasma membrane continuity of the phloem sieve tube would be the support of these up to 105 m/s propagating APs.Citation9,17 A link has also been demonstrated between a given stimulus (shading, flaming…) and a specific electrophysiological response of the plant.Citation5 However, although AP and SWP have been isolated and well characterized, they frequently overlap and mix together in plantaCitation15 to compose a complex-shaped electrical response to various stimuli.Citation12 This is accentuated by the use of extracellular electrophysiology methods that allow the recording of the whole tissue electrical response. Accordingly, here we use the generic term of “electrical potential variation” (EPV) to describe the considered plant response. Helianthus annus is a well-studied model for electrophysiology. This plant generates EPVs (SWPs with superposed APs) in response to many injurious or not-injurious stimuli (electric stimulation,Citation18 flaming,Citation14 organ excision,Citation19 shadeCitation20) and also spontaneously.Citation21 Plants have been suggested to be used as accurate biosensors.Citation22 For instance on this topic, a recent work has demonstrated the possibility of using cucumber-plants electrophysiological response to precisely detect a few greenhouse climatic variations.Citation23 Due to their high sensitivity to environmental variations, plants would be appropriate sensors of an artificial electromagnetic field (EMF). In addition, flaming has extensively been used to induce EPVs on many plants such as tomato,Citation24 tobacco,Citation25 beanCitation26 and Aloe verra.Citation17
Here we expected to use a high-intensity (1.5 kV/m) microwave-frequency EMF to remotely stimulate the plant. This 2.5 GHz EMF emerged as a good compromise between an inner plant penetration and the excitation of water molecules. The classic “dielectric” (or microwave) heating principle considered here appears when polar molecules (mainly water) re-align themselves to the extremely rapid alternating electric field (dipole rotation), in addition to molecular frictions and electrical constraints that retain these movements.Citation27 Consequently, and due to their high water content, this phenomenon rapidly increases the inner temperature of biological tissues.
The present work aimed to follow the electrophysiological response of sunflower plants illuminated by a high-intensity microwave-frequency electromagnetic field.
Methods
We have used sunflower plants (Helianthus annuus) grown for 4 weeks in a culture chamber (standard compost) allowing a 16 h light (26°C) / 8 h dark (21°C) photoperiod and humidity between 45 and 65%.
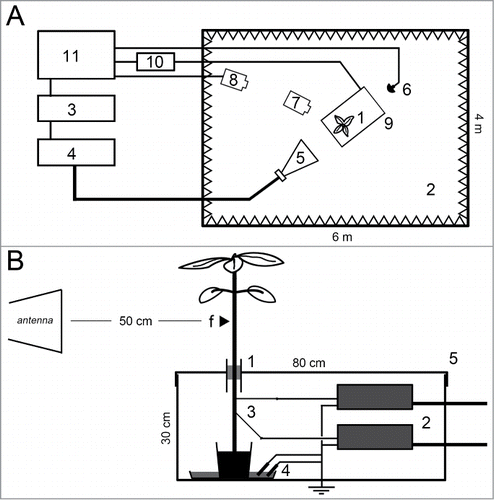
Inside a wide anechoic chamber, we have built a shielded set-up that allows in real-time the monitoring of both the temperature and the electrical potential variations of the plant while being exposed to the EMF (). Temperature of the whole plant was monitored by an accurate thermal video camera (Ti10 Thermal Imager, Fluke, France) with emissivity factor fixed to 0.95 which is acceptable for plant.Citation28 During the whole experiments the room temperature was 28°C ± 0.6 s.e. EPV was monitored by a common electrophysiology set-up: the aim is to record the electrical potential that is naturally being set between a measuring electrode inserted through the plant stem and a reference electrode inserted inside the plant ground substrate. However, the development of an EMF shielding system allowing electrophysiological experiments has required special attentions (). For EPV monitoring we have used 2 very high impedance autonomous amplifiers (DAM50, WPI, Sarasota – USA) connected to a computer via a data acquisition cartridge allowing a 10 Hz sampling rate (DT9816, Data Translation, Marlboro, USA), ultra thin tungsten wire electrodes (TM31/33AXX, WPI) and ref cells (RC3, WPI). The plant was consequently instrumented with 2 measuring electrodes (up and down) spaced from each other by 5–7 cm on the stem. According to previous works,Citation29 the signal amplitude from one electrode to another can vary widely. This could be exacerbated here because electrodes were positioned by hand and then maintained by an insulated holder. For this reason and in order to maximize the insertion to vascular tissues, we have inserted the thin tungsten electrodes through the whole diameter of the stem and perpendicularly from each other as suggested before.Citation18 Electrodes were inserted 40 min before each experiment to allow stabilization of the resting electrical potential. The high impedance amplifiers are very sensitive to all kind of electromagnetic or electrostatic noises. Moreover, by definition, metal micro-electrodes could behave like antennas i.e. they can interact with the EMF and conduct injurious voltage to the inputs of the amplifiers. To face this constraint and protect the electronic equipment we have built a custom-made Faraday box () made of welded stainless steel sheets. Finally, only the upper part (≈30 cm) of the plant stem (with leaves) was exposed to the EMF: the box lid was equipped with a tubular wave-guide that allowed the shielding of the roots and a part of the plant stem in order to protect the metal electrodes and amplifiers from the field. As a consequence, this set-up showed no EMF disturbance of the electrophysiological recording (data not shown). The microwave-frequency electromagnetic field (2.5 GHz, 1.5 kV/m, 50% duty cycle) was emitted inside a 72 m3 anechoic chamber () by an electrical signal generator (MXG, Agilent, Santa Clara – USA) coupled to an amplifier (T7525–500, IFI, Ronkonkoma - USA) and connected to a horn-antenna (EGC-CNP250–100–201, EGC Espace, St Bauzille de Putois, France).
Figure 1. Experimental set-up for real-time monitoring of plant temperature and EPV in response to EMF. Top view of the general arrangement (A): Plants (1) were illuminated inside a wide (72 m3) anechoic chamber (2). The EMF was generated by a signal generator (3) connected to an amplifier (4) and emitted by a horn-antenna (5). The EMF was monitored by a field probe (6). Temperature of the plant was monitored by a thermal video camera (7) itself tracked by a security camera (8). The electrical potential monitoring system was shielded inside a small faraday box (9). Output signals from the electrophysiological amplifiers were transmitted to a data acquisition cartridge (10) through BNC shielded cables. An external computer allowed all signals control and monitoring (11). Detailed profile view of the shielded set-up (B): only the upper part (≈ 30 cm) of the plant stem (with leaves) was EMF exposed. The distance plant-antenna was ≈ 50 cm. The small arrow on the plant stem symbolizes the EMF focalizing point (f). The plant was immobilized inside the small Faraday box through a tubular metallic wave-guide (1) by a polystyrene holder. The rest of the plant, the 2 very high impedance amplifiers (2), the extra-thin tungsten measuring electrodes (3) and the reference cells (4) were all protected from the EMF inside the shielding box (5). The 2 measuring electrodes were hand inserted perpendicularly to each other in the plant stem and spaced by 5 to 7 cm. The references cells and the Faraday box were connected to the ground.
Results and Discussion
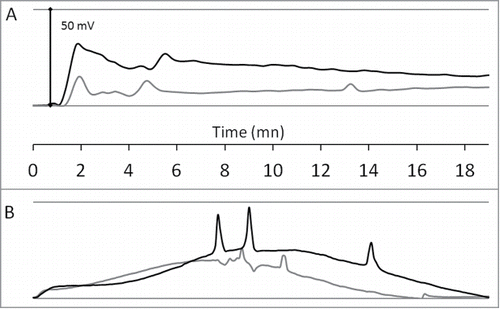
shows the experimental validation of the custom-made set-up and its sufficient accuracy. In order to generate EPVs, we have applied 2 commonly described stimuli to the sunflower plants: leaf flaming () and light-to-dark (200 to 0 μmol/m2/s) switch (). As expected, the overall profiles of the electrophysiological responses were different from flaming to shading. However, the both stress induced rapid EPV made of intense (≈25‑35 mV amplitude) slow wave potential with superposed action potentials (≈5‑20 mV amplitude; ≈7-14 cm.min−1 velocity). The flamed-plant response started to appear ≈1 min after the stimulus on the first electrode (up) and ≈30 sec later on the second electrode (down). In the case of shading (), the EPV recorded by the 2 electrodes increased rapidly and reached a paroxysm ≈7 min after the shading (with the apparition of ≈30–60 sec delayed action potentials between the 2 electrodes). For the both stimuli, the data suggested the movement of mixed basipetal electrical signals which subside from the apex to the roots.
Figure 2. Experimental validation of the electrophysiological set-up. The graphs show the monitoring of EPV with 2 tungsten electrodes (up: black-line; down: gray-line) inserted as described in . Data are baseline-adjusted and expressed in millivolt (the plants resting electrical potentials were 200 to 350 mV). Sunflower leaf flaming (A): one upper leaf of a 4-weeks-old plant was flamed for 1 sec at time 0 min. Shading (B): a sunflower plant was exposed to a light-to-dark switch (200 to 0 μmol/m2/s) at time 0 min.
Thanks to these low-noise data we next illuminated the plants with a high-intensity microwave-frequency EMF (2.5 GHz, 1.5 kV/m) to induce the plant EPV response. Moreover, the EMF was vertically polarized in order to get a maximum field-coupling to the plant stem. displays the sunflower responses to the considered EMF, for 4 experiments. As expected, and due to microwave heating, we monitored a rapid increase of the sunflower stem temperature (≈30°C in 3 min) that started from a 5 mm-diameter focalizing point () and progressively propagates up and down. When the temperature reached a threshold, the plant stem rapidly bent down (, black bars) due to the appearance of burn-like lesions (mashing of stem tissues). Soon after this movement we registered a rapid and complex EPV (wide SWP with superposed APs) from the upper electrode. This response was time-delayed and less intense on the second (down) electrode indicating a basipetal moving electrical signal. In these conditions (), the average time at which the stem bending appeared was 04 min 00 sec ± 0.5 sec after the start of the EMF emission and the average temperature was 59.5°C ± 2.4. In addition, the various stem shapes (more or less straight) from a plant to another seemed to strongly affect the temperature threshold as illustrated by the high standard error value. The overall shapes of the EPV were also very variable form a plant to another probably due to the plant-to-plant biological variability and the fact that the electrodes were positioned by hand. However, as we repeated this experiment, we noticed a concomitance (although time-delayed) of the movement (bending) of the stem with the initiation of plant EPVs.
Table 1. Bending time points and temperature thresholds
Figure 3. Sunflower exposition to high-intensity microwave-frequency EMF. Height sunflower plants were exposed to a 2.5 GHz ‑ 1.5 kV/m EMF (dashed timeline). The graphs show the monitoring of EPV with 2 tungsten electrodes (up: black-line; down: gray-line) inserted as described in . Data are baseline-adjusted and expressed in millivolt (the plants resting electrical potentials were 200 to 350 mV). Black bars symbolize the time point of stem or petiole bending. Free apex (A): Four sunflower plants were exposed to EMF as described in . Tied apex (B): To avoid stem bending, the sunflower plants apexes were tied with a non conductive plastic string, before exposure to EMF.
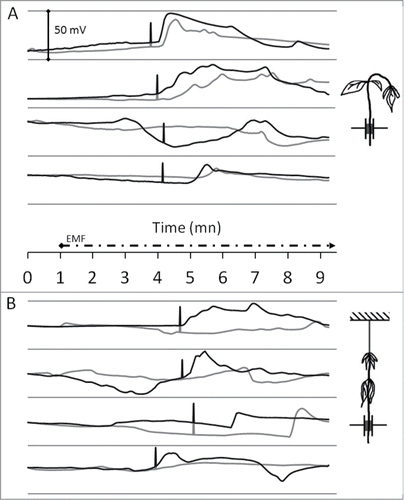
Accordingly, to get a precise value of the temperature threshold which induced the plant EPV (as a consequence of burn-like lesion on inner tissues), we tied the plant apexes with a non conductive plastic string in order to prevent the bending of the stem (). In this case, EPV were only recorded soon after the falling down of the petioles (, black bars), i.e., the plant response showed the same mechanical-injury dependent behavior. Moreover, the average temperature threshold was higher and less variable: 65.4°C ± 0.5 (). We can explain the required higher temperature to initiate bending (and consequently EPV) by the fact that the heating effect has to reach the leafstalks before they fall down: the inner temperature still increased from the focalizing point on the stem but had to diffuse to the petioles. This is confirmed by the delay: the leafstalks bending appeared 4 min 36 sec ± 14 sec after the EMF started (). In addition, there was a lower variability of limbs shapes between a plant to another (in contrast to the stems straightness), explaining the better precision of the temperature threshold (smaller standard error).
These results show that a mechanical injury (bending or falling down of the stem or petioles following inner burning of tissues) is required to generate an electrophysiological response in sunflower plants illuminated by a high-intensity microwave-frequency EMF. This is consistent with the suggested role of the water column pressureCitation14 in the EPV spreading. Here, the mechanical constraints were in the form of stem or petiole bending that may induce direct water pressure (hydraulic tension) modification. In this way some specific cellular structure (based on cytoskeleton, membrane and ion channels) have been suggested for plant mechanoperceptionCitation5,30 and the nucleus itself seems to be sensitive to mechanical stimuli.Citation31 The concept of the “hydraulic wave” was firstly proposed as the vector of the systemic signal in plant.Citation24 It is now demonstrated that this hydraulic mechanism (surge of water pressure) is required to propagate the slow wave potentials observed here.Citation12,14 Although pressure measurements are needed to robustly validate this conclusion, the present work brings new data that tend to confirm this principle.
It is well described that plants do have electrical response to a fast temperature increase such as a leaf burning.Citation17,24-26 However, here we were able to remotely heat the plant from the inside (microwave dielectric heating) without any physical contact. However, it appeared that neither the temperature nor the EMF themselves directly induce the plant EPV: the production of SWPs with superposed APs only appeared after the falling down of the plant stem or the leaf stalks, indicating that this was the forerunner event. In addition, the timing displayed by the data show that it took 4 to 6 minutes for the EPV to appear after the start of EMF emission. Conversely, only 30 sec to 2 min is required for the EPV to cover the inter-electrode distance (5–7 cm): EPVs would have appeared sooner if the EMF itself was sufficient to induce it.
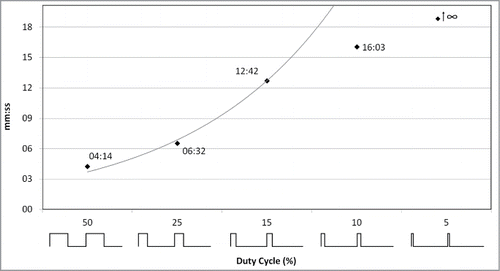
To reinforce the finding that the considered high-intensity EMF was not able by itself to induce EPV directly, we have progressively decreased the EMF signal duty cycle (without changing neither the field-amplitude nor the 2.5 GHz carrier frequency). The duty cycle corresponds to the percentage of one period in which the EMF is emitted. shows that reducing the average radiated energy proportionally delayed the bending of the stem and consequently the plant EPV initiation (more time needed by the temperature to reach the threshold). A 5% duty cycle never induced stem bending (and EPV). This indicates that the quantity of thermal energy laid by the considered EMF was readily spread by the plant so that it could not induce burn-like lesion and its subsequent EPV.
Figure 4. Effect of the radiated energy decrease on the plant response delay. Sunflower plants were exposed to a 2.5 GHz ‑ 1.5 kV/m EMF showing various duty cycles (50 to 5 %). As illustrated on the x-axis, the duty cycle is the percentage of emission during one period. Black squares display the time point of stem bending (followed by EPV).
However, it has recently been shown that plants do sense EMF (at very low field-amplitude i.e. 5 V.m−1 and lower frequency 900 MHz) in terms of modulation of stress-related genes expression.Citation32,33 Although EPV in response to this lower amplitude and frequency EMF has not been studied, this field would still be interpreted by the plant as an environmental stimulusCitation34 without necessarily the need to induce an electrophysiological response. Since the year 1900, it has been suggested and later demonstrated that plants are able to detect the electric field induced by storm.Citation35 This would help them to predict the presence of water and pre-adapt their metabolism to potentiate the imminent watering. One of the underlying mechanisms consists of electrically induced calcium (Ca2+) influx into the cytoplasm. This preliminary signal then being transduced into gene expression modifications and metabolic changes.Citation35 In this way, a few works have focused on plants such as Arabidopsis thalianaCitation36, duckweedCitation37, onionCitation38 and mung beanCitation39 facing various EMF (0.4–1.9 GHz; 10–275 V/m) and have shown some molecular and physiological responses (gene expression modulations, oxidative stress, mitotic aberrations, plant growth decrease). At higher frequencies, a 105 GHz EMF has modulated the meristem production of flax,Citation40 while a 2.45 GHz EMF had no effect on a few alfalfa growth variables.Citation41 Finally, a recent work has stated that 2, 2.5 and 5.5 GHz EMFs have modulated the resting potential of parrot feather.Citation42 However, the authors have also highlighted that the measurement itself was influenced by the EMF due to the lack of an appropriate electrode shielding. Although the details of the interaction bridge remain to be dissected, all these examples highlight the sensitiveness of plants to artificial EMFs. However, all these works were focused on non-heating (low intensity) EMFs and none of them has studied the EMF induction of AP or VP. The present paper brings new data concerning the induction of plant EPVs by the consequences of a microwave-frequency EMF treatment.
In conclusion, we found here that the illumination of sunflower plant by a high-intensity microwave-frequency EMF (2.5 GHz, 1.5 kV/m) indirectly induces EPV via the mechanical consequences of an inner tissues burning (dielectric heating). No EPV was recorded below a ≈60–65°C plant temperature threshold and EPV initiation was proportionally delayed when decreasing the radiated energy (EMF duty cycle). This is consistent with the “SWP-looking” EPV we recorded which is commonly associated with injurious environmental signals.Citation5 This experiment illustrates the need of tissue mashing (stem or leaf-stalks) for the plant to respond to a rapid and intense heating, and reinforce the hydraulic character of the plant electrophysiological response in this context.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the common CEA-Gramat / Institut Blaise Pascal BIOEM laboratory (France).
References
- Bertholon P. De l’electricite des vegetaux. PF Didot jeune. Paris: 1783.
- Stahlberg R. Historical Overview on Plant Neurobiology. Plant Signal Behav 2006; 1:6-8; PMID:19521469; http://dx.doi.org/10.4161/psb.1.1.2278
- Brenner ED, Stahlberg R, Mancuso S, Vivanco J, Baluška F, Van Volkenburgh E. Plant neurobiology: an integrated view of plant signaling. Trends Plant Sci 2006; 11:413-9; PMID:16843034; http://dx.doi.org/10.1016/j.tplants.2006.06.009
- Yan X, Wang Z, Huang L, Wang C, Hou R, Xu Z, Qiao X. Research progress on electrical signals in higher plants. Prog Nat Sci 2009; 19:531-41; http://dx.doi.org/10.1016/j.pnsc.2008.08.009
- Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant, Cell Environ 2007; 30:249-57; http://dx.doi.org/10.1111/j.1365-3040.2006.01614.x
- Graham JS, Hall G, Pearce G, Ryan CA. Regulation of synthesis of proteinase inhibitors I and II mRNAs in leaves of wounded tomato plants. Planta 1986; 169:399-405; PMID:24232653; http://dx.doi.org/10.1007/BF00392137
- Davies E. Electrical Signals in Plants: Facts and Hypotheses. In: Volkov AG, editor. Plant Electrophysiology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006; 407-422.
- Stolarz M, Król E, Dziubińska H, Kurenda A. Glutamate induces series of action potentials and a decrease in circumnutation rate in Helianthus annuus. Physiol Plantarum 2010; 138:329-38; PMID:20051031; http://dx.doi.org/10.1111/j.1399-3054.2009.01330.x
- Lang RD, Volkov AG. Solitary waves in soybean induced by localized thermal stress. Plant Signal Behav 2008; 3:224-8; PMID:19513218; http://dx.doi.org/10.4161/psb.3.4.5586
- Stahlberg R. Historical Introduction to Plant Electrophysiology. In: Volkov PDAG, editor. Plant Electrophysiology. Springer Berlin Heidelberg; 2006; 3-14.
- Spanswick RM. Electrogenic Pumps. In: Volkov PDAG, editor. Plant Electrophysiology. Springer Berlin Heidelberg; 2006; 221-246.
- Stahlberg R, Cleland RE, Volkenburgh E. Slow Wave Potentials – a Propagating Electrical Signal Unique to Higher Plants. In: Baluška F, Mancuso S, Volkmann D, editors. Communication in Plants. Springer Berlin Heidelberg; 2006; 291-308.
- Zawadzki T, Davies E, Dziubinska H, Trebacz K. Characteristics of action potentials in Helianthus annuus. Physiol Plantarum 1991; 83:601-4; http://dx.doi.org/10.1111/j.1399-3054.1991.tb02475.x
- Stankovic B, Zawadzki T, Davies E. Characterization of the Variation Potential in Sunflower. Plant Physiol 1997; 115:1083-8; PMID:12223859
- Ilík P, Hlaváčková V, Krchňák P, Nauš J. A low-noise multi-channel device for the monitoring of systemic electrical signal propagation in plants. Biol Plantarum 2010; 54:185-90; http://dx.doi.org/10.1007/s10535-010-0032-0
- Zimmermann MR, Maischak H, Mithöfer A, Boland W, Felle HH. System Potentials, a Novel Electrical Long-Distance Apoplastic Signal in Plants, Induced by Wounding. Plant Physiol 2009; 149:1593-1600; PMID:19129416; http://dx.doi.org/10.1104/pp.108.133884
- Volkov AG, Lang RD, Volkova-Gugeshashvili MI. Electrical signaling in Aloe vera induced by localized thermal stress. Bioelectrochemistry 2007; 71:192-7; PMID:17544342; http://dx.doi.org/10.1016/j.bioelechem.2007.04.006
- Dziubińska H, Trębacz K, Zawadzki T. Transmission route for action potentials and variation potentials in Helianthus annuus L. J Plant Physiol 2001; 158:1167-72; http://dx.doi.org/10.1078/S0176-1617(04)70143-1
- Stahlberg R, Cleland RE, Van Volkenburgh E. Decrement and amplification of slow wave potentials during their propagation in Helianthus annuus L. shoots. Planta 2005; 220:550-8; PMID:15365838; http://dx.doi.org/10.1007/s00425-004-1363-x
- Stahlberg R, Stephens NR, Cleland RE, Van Volkenburgh E. Shade-Induced Action Potentials in Helianthus annuus L. Originate Primarily from the Epicotyl. Plant Signal Behav 2006; 1:15-22; PMID:19521471; http://dx.doi.org/10.4161/psb.1.1.2275
- Zawadzki T, Dziubiska H, Davies E. Characteristics of action potentials generated spontaneously in Helianthus annuus. Physiol Plantarum 1995; 93:291-7; http://dx.doi.org/10.1111/j.1399-3054.1995.tb02231.x
- Volkov AG, Ranatunga DRA. Plants as Environmental Biosensors. Plant Signal Behav 2006; 1:105-15; PMID:19521490; http://dx.doi.org/10.4161/psb.1.3.3000
- Wang Z-Y, Leng Q, Huang L, Zhao L-L, Xu Z-L, Hou R-F, Wang C. Monitoring system for electrical signals in plants in the greenhouse and its applications. Biosystems Eng 2009; 103:1-11; http://dx.doi.org/10.1016/j.biosystemseng.2009.01.013
- Malone M, Alarcon J-J, Palumbo L. An hydraulic interpretation of rapid, long-distance wound signalling in the tomato. Planta 1994; 193:181-185; http://dx.doi.org/10.1007/BF00192528
- Hlavácková V, Krchnák P, Naus J, Novák O, Spundová M, Strnad M. Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta 2006; 225:235-44; http://dx.doi.org/10.1007/s00425-006-0325-x
- Dziubinska H, Filek M, Koscielniak J, Trebacz K. Variation and action potentials evoked by thermal stimuli accompany enhancement of ethylene emission in distant non-stimulated leaves of Vicia faba minor seedlings. J Plant Physiol 2003; 160:1203-10; PMID:14610889; http://dx.doi.org/10.1078/0176-1617-00914
- Piyasena P, Dussault C, Koutchma T, Ramaswamy HS, Awuah GB. Radio frequency heating of foods: principles, applications and related properties-a review. Crit Rev Food Sci Nutr 2003; 43:587-606; PMID:14669879; http://dx.doi.org/10.1080/10408690390251129
- Cole M, Sabins FF. Remote Sensing: Principles and Interpretation. The Geographical Journal 1987; 153:423; http://dx.doi.org/10.2307/633704
- Stankovic B, Witters DL, Zawadzki T, Davies E. Action potentials and variation potentials in sunflower: An analysis of their relationships and distinguishing characteristics. Physiologia Plantarum 1998; 103:51-58; http://dx.doi.org/10.1034/j.1399-3054.1998.1030107.x
- Telewski FW. A unified hypothesis of mechanoperception in plants. Am J Bot 2006; 93:1466-76; PMID:21642094; http://dx.doi.org/10.3732/ajb.93.10.1466
- Xiong TC, Jauneau A, Ranjeva R, Mazars C. Isolated plant nuclei as mechanical and thermal sensors involved in calcium signalling. Plant J 2004; 40:12-21; PMID:15361137; http://dx.doi.org/10.1111/j.1365-313X.2004.02184.x
- Roux D, Vian A, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G. Electromagnetic fields (900 MHz) evoke consistent molecular responses in tomato plants. Physiologia Plantarum 2006; 128:283-8; http://dx.doi.org/10.1111/j.1399-3054.2006.00740.x
- Vian A, Faure C, Girard S, Davies E, Hallé F, Bonnet P, Ledoigt G, Paladian F. Plants Respond to GSM-Like Radiation. Plant Signal Behav 2007; 2:522-4; PMID:19704547; http://dx.doi.org/10.4161/psb.2.6.4657
- Roux D, Vian A, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G. High frequency (900 MHz) low amplitude (5 V m-1) electromagnetic field: a genuine environmental stimulus that affects transcription, translation, calcium and energy charge in tomato. Planta 2008; 227:883-91; PMID:18026987; http://dx.doi.org/10.1007/s00425-007-0664-2
- Goldsworthy A. Effects of Electrical and Electromagnetic Fields on Plants and Related Topics. In: Volkov PDAG, editor. Plant Electrophysiology. Springer Berlin Heidelberg; 2006; 247-67.
- Engelmann JC, Deeken R, Müller T, Nimtz G, Roelfsema MRG, Hedrich R. Is gene activity in plant cells affected by UMTS-irradiation? A whole genome approach. Adv Appl Bioinform Chem 2008; 1:71-83; PMID:21918607
- Tkalec M, Malarić K, Pevalek-Kozlina B. Exposure to radiofrequency radiation induces oxidative stress in duckweed Lemna minor L. Sci Total Environ 2007; 388:78-89; PMID:17825879; http://dx.doi.org/10.1016/j.scitotenv.2007.07.052
- Tkalec M, Malarić K, Pavlica M, Pevalek-Kozlina B, Vidaković-Cifrek Z. Effects of radiofrequency electromagnetic fields on seed germination and root meristematic cells of Allium cepa L. Mutat Res 2009; 672:76-81; PMID:19028599; http://dx.doi.org/10.1016/j.mrgentox.2008.09.022
- Sharma VP, Singh HP, Kohli RK, Batish DR. Mobile phone radiation inhibits Vigna radiata (mung bean) root growth by inducing oxidative stress. Sci Total Environ 2009; 407:5543-7; PMID:19682728; http://dx.doi.org/10.1016/j.scitotenv.2009.07.006
- Tafforeau M, Verdus M-C, Norris V, White GJ, Cole M, Demarty M, Thellier M, Ripoll C. Plant sensitivity to low intensity 105 GHz electromagnetic radiation. Bioelectromagnetics 2004; 25:403-7; PMID:15300725; http://dx.doi.org/10.1002/bem.10205
- Skiles JW. Plant response to microwaves at 2.45 GHz. Acta Astronautica 2006; 58:258-63; http://dx.doi.org/10.1016/j.actaastro.2005.12.007
- Senavirathna MDHJ, Asaeda T. Radio-frequency electromagnetic radiation alters the electric potential of Myriophyllum aquaticum. Biol Plant 2014; 58:355-62; http://dx.doi.org/10.1007/s10535-013-0384-3
