Abstract
Blood vessels and the lymphatic vasculature are extensive tubular networks formed by endothelial cells that have several indispensable functions in the developing and adult organism. During growth and tissue regeneration but also in many pathological settings, these vascular networks expand, which is critically controlled by the receptor EphB4 and the ligand ephrin-B2. An increasing body of evidence links Eph/ephrin molecules to the function of other receptor tyrosine kinases and cell surface receptors. In the endothelium, ephrin-B2 is required for clathrin-dependent internalization and full signaling activity of VEGFR2, the main receptor for vascular endothelial growth factor. In vascular smooth muscle cells, ephrin-B2 antagonizes clathrin-dependent endocytosis of PDGFRβ and controls the balanced activation of different signal transduction processes after stimulation with platelet-derived growth factor. This review summarizes the important roles of Eph/ephrin molecules in vascular morphogenesis and explains the function of ephrin-B2 as a molecular hub for receptor endocytosis in the vasculature.
Abbreviations
| Ang | = | angiopoietin |
| aPKC | = | atypical protein kinase C |
| CHC | = | clathrin heavy chains |
| CLASP | = | clathrin-associated-sorting protein |
| CV | = | cardinal vein |
| DA | = | dorsal aorta |
| EC | = | endothelial cell |
| EEA1 | = | early antigen 1 |
| Ephrin-B2ΔV | = | ephrin-B2 deletion of C-terminal PDZ binding motif |
| HSPG | = | heparan sulfate proteoglycan |
| JNK | = | c-Jun N-terminal kinase |
| LEC | = | lymphatic endothelial cells |
| LRP1 | = | Low density lipoprotein receptor-related protein 1 |
| MVB | = | multivesicular body |
| NRP | = | neuropilin |
| PC | = | pericytes |
| PDGF | = | platelet-derived growth factor |
| PDGFR | = | platelet-derived growth factor receptor |
| PlGF | = | placental growth factor |
| PTC | = | peritubular capillary |
| RTK | = | receptor tyrosine kinase |
| VEGF | = | Vascular endothelial growth factor |
| VEGFR | = | Vascular endothelial growth factor receptor |
| VSMC | = | vascular smooth muscle cells |
Introduction
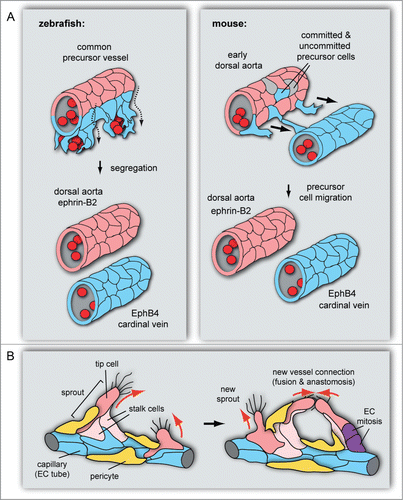
Blood vessels are essential during development and throughout adult life. They are among the first structures that form in the early vertebrate embryo and extend rapidly to match the increasing metabolic needs of the growing organism. Before the onset of heart beat, mesodermal precursor cells give rise to hemangioblast precursors, which will migrate, differentiate into endothelial cells and assemble the first vessels in a process called vasculogenesis.Citation1 This early step in vascular development generates the dorsal aorta (DA), cardinal vein (CV) () and the primitive capillary plexus of the yolk sac. For the morphogenesis of the DA, endothelial cells align in 2 lateral cords that adhere and form an extracellular lumen at their cell-cell contact junctions.Citation2 Next, the resulting 2 lateral endothelial tubes fuse and thereby generate a single aorta at the embryonic midline.Citation3 The CV arises later than the DA and is followed by the formation of highly branched interconnected capillary networks.Citation4-6 The latter is achieved through angiogenesis, which is the expansion of an existing vessel network through endothelial cell (EC) proliferation, migration and vessel sprouting. During sprouting, specialized tip cells migrate and lead the sprout towards the avascular space by emitting filopodia and lamellipodia that sense the growth factor gradient ().Citation7-9 More proliferative stalk cells follow the invasive tip cells and form the base of endothelial sprouts, but ECs can also actively compete for the leading position.Citation8,10 Such cell shuffling events during sprouting angiogenesis are promoted by heterogeneous adhesion interactions between endothelial cells.Citation11 When stable EC-EC connections are formed, newly assembled vessel branches get lumenized and stabilized by mural cells.Citation12
Figure 1. Roles of EphB4 and ephrin-B2 in vascular morphogenesis. (A) Dorsal aorta and cardinal vein formation in zebrafish and mouse embryos. In the zebrafish embryo (left), the DA (red, ephrin-B2+) and CV (blue, EphB4+) segregate from a common precursor vessel by ventral migration of venous-fated cells, which requires ephrin-B2a and EphB4a. In early mouse development, CVs form by sprouting of venous-fated (EphB4+) endothelial cells from an early dorsal aorta, which contains precursors with venous or no detectable arteriovenous commitment in addition to ephrin-B2+ arterial cells. (B) In angiogenic sprouting, invasive tip cells, which are motile, lead sprouts and emit filopodia, are followed by more proliferative stalk cells. Ephrin-B2 (red) expression in tip cells and other angiogenic ECs partially overlaps with EphB4 in stalk cells at the sprout base and capillaries (blue). Pericytes (yellow) stabilize the sprout. Following the extension of sprouts, tip cells establish contact with other sprouts or existing vessels. This leads to the formation of a new vessel branch, which subsequently gets lumenized and stabilized by mural cells.
There is also a second endothelial network, the lymphatic vasculature, which unidirectionally transports interstitial liquid, proteins, lipids and immune cells from distal tissues through collecting ducts and lymph nodes into the venous branch of the circulation.Citation13 In the embryo, the first lymphatic endothelial cells (LEC) differentiate from the roof of the CV and from additional venous sources, bud off and migrate as streams of connected cells between the CV and superficial venous plexus before they form the first lumenized lymphatic vessels.Citation14-16 Further rounds of LEC sprouting and mitosis lead to the formation of peripheral lymphatic vasculature in a process termed lymphangiogenesis.Citation13,16,17 After the completion of developmental growth, blood vessels and the lymphatic vasculature in most tissues acquire a mature and quiescent phenotype that is characterized by the absence of endothelial sprouting and proliferation.Citation18,19 However, vascular networks retain plasticity that permits growth in response to tissue damage and in a range of disease processes.Citation20-23
Apart from endothelial cells, mural cells of the mesenchymal lineage, namely pericytes and vascular smooth muscle cells (VSMC), are another essential component of vascular networks. Pericytes, which associate with the abluminal (basal) surface of endothelial tubes in capillaries and post-capillary venules, are thought to stabilize blood vessels, prevent vascular leakage, and contribute to vascular maturation and quiescence.Citation24 VSMCs, which cover arteries and veins, are highly plastic and help the vessel wall to resist against the laterally acting forces in the circulatory system. In mature blood vessels, VSMCs differentiate and express markers important for the regulation of contraction and, upon injury, can dedifferentiate by losing the expression of these contractile markers.Citation25 Loss or dedifferentiation of VSMCs is associated with aneurysms, i.e., the local ballooning of vessels.Citation26,27 VSMCs are also found on collecting lymphatic ducts and their rhythmic contraction contributes to tissue drainage and the transport of lymph.Citation28-30
Many studies have focused on the signaling processes controlling angiogenesis and lymphangiogenesis. This has led to the identification of numerous key signals with conserved roles in physiological and pathological vessel growth. Among those signals, many secreted growth factors and the corresponding receptors of the tyrosine kinase superfamily have particularly important functions.
Receptor Tyrosine Kinases in the Vascular System
Growth, migration and differentiation processes in the developing vascular system are critically controlled by receptor tyrosine kinases (RTKs). Vascular endothelial growth factors (VEGFs) activate their receptors on endothelial cells and thereby promote the expansion of the blood vessel and lymphatic vessel networks.Citation17,31,32 Within the VEGF family, VEGF-A (also known as vascular permeability factor) is the most important regulator of angiogenesis and levels of this growth factor need to be tightly regulated.Citation17,31-33 Accordingly, loss of only one copy of the Vegfa gene led to early embryonic lethality of the heterozygous mice.Citation34 The related growth factor VEGF-C is essential for many aspects of lymphangiogenesis.Citation17,31,32 Three other family members, VEGF-B, VEGF-D and placental growth factor (PlGF), have distinct biological activities.Citation31,33-36 Additional VEGF homologues, VEGF-E and VEGF-F, were identified in viruses and snake venom, respectively.Citation31,37 Alternative splice variants and processed forms of VEGFs increase the complexity of this pathway.Citation38,39 VEGFs, in particular VEGF-A, are produced by many cell types and act mainly as paracrine factors on endothelial cells, but autocrine expression of VEGF-A has been also shown to be essential for endothelial cell survival.Citation40
Different VEGF family growth factors can bind with high affinities to one or several of the cognate receptor tyrosine kinases, namely VEGFR1/Flt1, VEGFR2/Flk1/KDR and VEGFR3/Flt4.Citation34 VEGF-A binds VEGFR1 and VEGFR2.Citation34,38 While the latter mediates the important pro-angiogenic functions of VEGF-A, VEGFR1 in ECs acts predominantly as a high affinity trap that prevents VEGF-A binding to and signaling through VEGFR2.Citation34,38 VEGFR3 is the main receptor for VEGF-C and, accordingly, is indispensable for lymphangiogenesis.Citation17,34 Expression of VEGFR3 during angiogenesis and the phenotypes of knockout mice and zebrafish mutants indicate that this receptor is also important for blood vessel growth.Citation41-46 In addition to VEGF receptors, VEGFs can bind to heparan sulfate proteoglycans (HSPGs), neuropilins (NRPs) and integrins, which are important for VEGF presentation or complex formation with VEGFRs.Citation38,47 All VEGF receptors are structurally similar and consist of extracellular Ig-like domains, a single transmembrane region, an intracellular kinase domain, and a less conserved C-terminal tail.Citation34 VEGF ligand binding to VEGFRs can occur in cis or trans (via HSPGs expressed on adjacent cells) and thereby induces receptor homo- or heterodimerization, activation of the kinase domain and signaling through various downstream pathways.Citation38
The angiopoietins (Ang) ligands and Tie receptors, which are expressed by vascular and haematopoietic cells, are important for morphogenesis and homeostasis of blood vessels and lymphatic vessels.Citation48 While inactivation of the Tek gene encoding Tie-2 in mice led to midgestation lethality due to defects in capillary plexus remodeling and maturation, hematopoiesis and heart development, loss of Tie-1, an orphan receptor without known ligand, resulted in later embryonic lethality due to impaired vascular integrity without defects in hematopoiesis.Citation48 Four angiopoietins (Ang-1-4) can bind to the receptor Tie-2. Although Ang-1 and Ang-2 have similar structures and bind with similar affinities to Tie-2, they have distinct functions.Citation49 Ang-1 promotes vascular growth, maintenance and maturation regulating endothelial cell survival, prevents apoptosis and inhibits inflammation, whereas Ang-2 can promote EC death and vascular regression.Citation48,50 While Ang-1 deficient mice die in utero with similar phenotype to Tie-2 deficient embryos, Ang-2 KO mice are born normally and die postnatally due to lymphatic defects.Citation51,52 Although it was thought that Ang-2 has an antagonistic role by binding Tie-2 and preventing Ang-1-induced receptor activation, some evidence indicates that Ang-2 can bind and signal through Tie-2 and integrins in certain settings.Citation50,53
The receptors for platelet-derived growth factors (PDGFs), PDGFRα and PDGFRβ, are evolutionary and structurally related to VEGFRs. They are bound by one or more isoforms of 5 different disulphide-linked dimers PDGF growth factors (PDGF-AA, -BB, -AB, -CC, and -DD).Citation54 Ligand-receptor interaction leads to receptor homo- or heterodimerization, conformational change, and kinase activation, which triggers auto- and transphosphorylation processes.Citation54 This is followed by binding of various signaling and docking molecules leading to the activation of several signaling pathways, which are required for cellular proliferation, migration and survival (for a review see ref.Citation55). In the vasculature, PDGF-B is expressed by endothelial cells and enriched in the tip cell area.Citation8,56 PDGF-B signals in a paracrine fashion to PDGFRβ-expressing mural cells and thereby promotes their proliferation, directional migration and vessel wall assembly. Both Pdgfb and Pdgfrb KO mice are lethal during late embryonic gestation due to capillary dilation and rupture of microaneurisms resulting in widespread hemorrhaging.Citation57 Mutants showed increased pericyte/VSMC detachment and reduced mural cell coverage of the endothelium, which led to renal, vascular, cardiac and placental defects. In the adult, activation of PDGFRβ expressed by fibroblasts and myofibroblasts regulates interstitial fluid pressure of tissues and prevents edema formation by inducing contraction of these cell types.Citation55
Eph/ephrin interactions play important roles in numerous morphogenetic processes including angiogenesis and lymphangiogenesis. While there is little evidence for a major role of A-class receptors and their GPI-anchored ephrin ligands in the developing vasculature, several important functions have been identified for the transmembrane protein ephrin-B2 and the receptor EphB4.Citation4-6,29,58-66 In classical forward signaling, ephrin ligands activate Eph receptors on neighboring cells (in trans), which frequently translates into cell-cell repulsion that leads to cell sorting, the segregation of ligand-expressing and receptor-expressing cells into distinct tissue domains, and boundary formation ().Citation67,68 In reverse signaling, Eph receptors induce signaling processes in ephrin-presenting cell, which often promotes cell-cell adhesion and cell-substrate adhesion.Citation69-71 Ephrin-B reverse signaling relies on its scaffolding activity and involves the phosphorylation of several conserved tyrosine residues in the cytoplasmic domain, the recruitment of cytoplasmic adapters and binding of PDZ domain-containing proteins to the C-terminal PDZ motif.Citation71,72 It is currently unknown whether the outcome of Eph-ephrin interactions in vascular cells can be explained through presentation of ligands and receptors in distinct membrane microdomains of the same cell, as has been proposed for EphA and ephrin-A molecules.Citation73 Distinct signaling outputs downstream of the B-class ligand ephrin-B3 are mediated by the SH2/SH3 adaptor Grb4 and the PDZ domain proteins PICK1 and syntenin, respectively.Citation74
Figure 2. Structure and function of Eph/ephrin molecules. (A) Schematic representation of Eph receptor and ephrin ligand interactions and structural domain organization. Eph/ephrin interaction in trans leads to bidirectional signaling between neighboring cells. The following protein domains and motifs are indicated: ligand binding, cysteine (Cys)-rich, fibronectin (FN) type III, kinase, SAM and PDZ binding domains, transmembrane (TM) region, and tyrosine phosphorylation sites (Y). Binding (arrows) takes place predominantly within the same class: Eph-A receptors bind GPI-anchored ephrin-A ligands and EphBs interact with ephrin-B transmembrane proteins. There are also exceptions for interactions involving proteins from different subclasses (dashed arrows). (B) Eph/ephrin interactions frequently lead to repulsion, which is important for cell migration, cell guidance and cell sorting. The latter can promote cell segregation events that minimize interactions between Eph and ephrin-expressing cells, which facilitates tissue patterning processes during developmental morphogenesis. (C) Eph and ephrin monomers, homo- and heterodimers are inactive. Heterotetramers have weak signaling activity, which is increased by cluster oligomerization and assembly of larger receptor clusters that can expand laterally through Eph–Eph cis interactions that do not require interaction with ephrins.
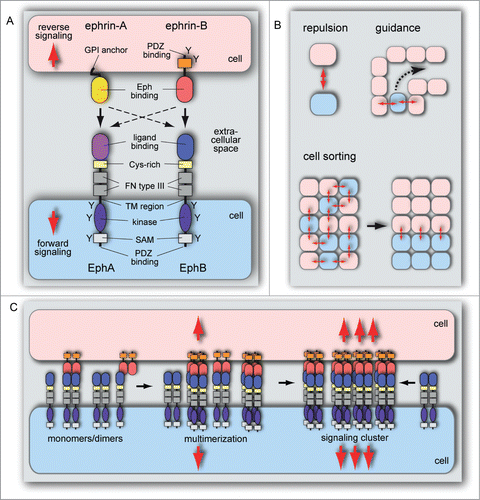
Eph/ephrin interactions require direct cell-cell contact because Eph receptors are only efficiently activated by membrane-bound ligands and not by the soluble forms.Citation75 These interactions and signaling outputs can also depend on differential receptor ectodomain binding affinity for a specific ligand.Citation76-78 The analysis of EphA4 ectodomain structure showed that interactions with ephrin can induce conformational changes in the ligand-binding domain of the receptor besides its ability to increase the local concentration of the receptor above the critical value required for Eph/ephrin clustering.Citation79 This clustering is necessary for the initiation of further signaling processes at cell-cell contacts ().Citation76,80-82 Gradually, through a multistep process, higher-order clusters will assemble.Citation77,83 The extent of receptor clustering was shown to determine the strength of the signaling in living cells.Citation77,84 While trimeric or tetrameric small-sized EphB2 receptor clusters can induce cellular signaling, cellular responses got stronger the more active, multimeric (trimeric or greater) EphB2 clusters complexed with ephrins dominated over inactive dimeric clusters ().Citation84 Interestingly, even ligand-unbound receptors have the ability to interact with Eph clusters that thereby expand beyond the interface of ephrin ligand interactions ().Citation79,85,86 Receptor clustering is also modulated by other Eph interacting proteins, which bind to the EphB cytoplasmic part.Citation84 Accordingly, deletion of SAM and PDZ binding motifs in EphB2 increased clustering and signaling of this receptor.
Regulation of Blood Vessel Morphogenesis by Eph/ephrin Signaling
As mentioned above, EphB4 and its ligand ephrin-B2 constitute the only receptor/ligand pair within the Eph/ephrin family with essential and well-defined roles in angiogenesis ().Citation87 Mice deficient for Efnb2 or Ephb4 were embryonically lethal at midgestation and showed no defects in vasculogenesis but compromised angiogenic blood vessel growth and remodeling.Citation4,58-60 From zebrafish to human and from early embryos to adults, ephrin-B2 expression dominates in arterial domains, while EphB4 is more prominent in the venous compartment.Citation88 Another receptor, EphB1, shows venous restricted expression in the skin after the capillary plexus has formed, which is maintained into adulthood.Citation89 However, venous expression of EphB1 is lost in other adult organs.
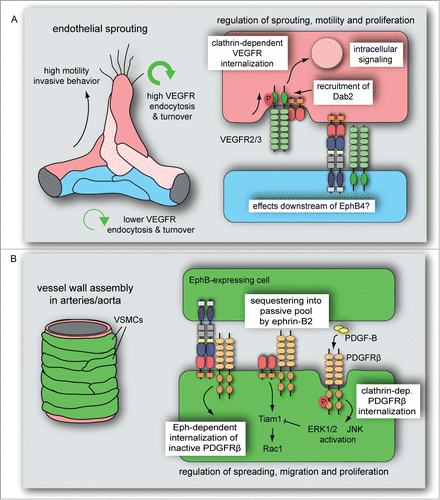
Figure 3. Ephrin-B2 modulates VEGF- and PDGF receptors endocytosis in vascular cells. (A) VEGFR endocytosis and turnover is higher in sprouting cells compared to established vessels. Ephrin-B2 and its interacting partners, the clathrin-associated sorting protein Dab2 and the cell polarity regulator PAR-3, control VEGFR activity, clathrin-dependent VEGFR internalization and downstream intracellular signal transduction. These interactions and activities dynamically regulate vascular endothelial cell sprouting, motility and proliferation. It is currently unknown whether VEGF signaling is also modulated by processes downstream of EphB4. (B) PDGF-B-induced PDGFRβ activation leads to clathrin-dependent PDGFRβ internalization followed by ERK1/2 and JNK activation in VSMCs. This process is limited by ephrin-B2, which sequesters PDGFRβ into a caveolin-dependent, passive pool and positively regulates Tiam1 expression and Rac1 activation. Eph-dependent ephrin-B2 internalization in interacting VSMCs induces the endocytosis of inactive (unphosphorylated) PDGFRβ. PDGF-induced ERK1/2 activation counteracts Tiam1 expression, which reduces VSMC spreading, migration and proliferation.
In contrast, ephrin-B2 and EphB4 are widely perceived as markers for arterial and venous territories, respectively, throughout development and in adult life. In mouse, their expression starts as early as embryonic day 8.25 and marks large regions of the primary vascular plexus prior to the formation of arteries and veins.Citation4,58-60,90 It is well possible that a cell-cell repulsion mechanism helps at this stage to establish clear borders between the developing arterial and venous vascular compartments.
VEGF and Notch signaling have been shown to positively control ephrin-B2 expression.Citation91-95 In arterial angioblast precursors, VEGF-A/VEGFR2 act together with the neuropilin-1 (NRP1) co-receptor to activate Notch signaling.Citation96 Notch promotes the arterial and suppresses the venous fate of ECs, and thereby orchestrates the arterial-venous differentiation program. VEGF regulates also the early arterial expression of Dll4, a key ligand of Notch receptors in the endothelium, via ETS family transcription factors.Citation97 SOXF transcription factors interact in a combinatorial fashion with Rbpj, a key transcriptional regulator downstream of active Notch, which upregulates Dll4 expression and promotes arterial differentiation.Citation98 Following the initial VEGF-dependent induction of Dll4, Notch activity is required to maintain expression of the Delta ligand and also promotes the expression of target genes such as ephrin-B2.Citation97,99 In venous endothelium, COUP-TFII, a nuclear orphan receptor transcription factor, and PI3K-Akt signaling suppress Notch activity, NRP1 expression and MAP kinase signaling, which results in the repression of arterial differentiation.Citation96 Consequently, Notch down-regulation led to reduced expression of ephrin-B2 and upregulation of EphB4.Citation100
Ephrin-B2 and EphB4 expression is detected in the 2 first axial vessels of the embryonic body, the dorsal aorta and the cardinal vein (). Although the balanced formation of these big vessels was compromised after the inactivation of these genes, arterial-venous differentiation itself was not affected.Citation4,6,101 In zebrafish, the DA assembles first and more dorsal, which is followed by the assembly of the CV more ventral to the midline.Citation102 Accordingly, arterial and venous angioblasts precursors are localized in separate stripes along the midline and migrate in separate waves, in temporal relation to expression of the Etv2 transcription factor.Citation102,103 Interestingly, in zebrafish, the DA and the CV are thought to be derived from a common precursor vessel by ventral sprouting, which is controlled by repulsive EphB4/ephrin-B2 signaling ().Citation5 Although cells of venous origin were not formally shown to populate this precursor vessel, it was proposed that venous-fated angioblasts segregate, sprout and migrate ventrally to assemble the CV, which is thereby segregated from the DA. While expression of ephrin-B2a in endothelial cells or angioblasts limited their ability to move ventrally into the CV, expression of EphB4a, one of the 2 paralogous gene products in zebrafish, promoted this migration process. Cells injected with ephrin-B2a-targeting morpholinos or overexpressing a C-terminally truncated (reverse signaling-incompetent) version of the ligand were more abundant in the CV and reduced in the DA. The opposite effect was obtained with morpholinos targeting EphB4a.Citation5
Similar to zebrafish, longitudinal connections between the DA and the CV that could correspond to migrating endothelial cells have been reported in chick and mouse embryos ().Citation4,101,104-106 Recently, the existence of an arterial-venous segregation process through endothelial cell migration was demonstrated in mouse via time-lapse imaging of early embryos.Citation6 These data indicate that the mouse DA contains a mixed population of precursor cells with venous or arterial signature as well as a small number of cells expressing both markers, which might reflect that arterio-venous commitment is not yet specified. Similar to the processes in zebrafish, EphB4/eprin-B2 signaling regulates CV formation from the DA by promoting ventral sprouting and by repelling venous ECs away from those with an arterial fate. Venous endothelial cells were enriched in the DA and strongly reduced in the CV of EphB4-deficient mice, while the total number of cells was not changed.Citation6
The sorting of lymphatic endothelial cells from the venous ECs during the venous-lymphatic segregation process and formation of the first lymphatic vessel structures resembles aspects of arterial-venous segregation events.Citation13,15,16 Although it is clear that ephrin-B2 and EphB4 are expressed by lymphatic vessels and play important roles in lymphangiogenesis, further studies are needed to address whether they promote cell segregation similar to the processes in the DA/CV.Citation29,64 It has been shown that ephrin-B2 reverse signaling is necessary for the lymphatic sprouting from the primitive plexus, a process that gives rise to the dermal lymphatic vasculature.Citation29 The remodeling of the primary lymphatic vessels into a more organized network was also affected by loss of the C-terminal ephrin-B2 PDZ motif but not by the impairment of ephrin-B2 tyrosine phosphorylation.Citation29 EphB4/ephrin-B2 signaling also controls valve formation and maintenance in the lymphatic vasculature.Citation29,66 Somewhat unexpectedly, ephrin-B2 was found to be highly enriched in the venous valves where it is also required for valve formation and maintenance.Citation107
Besides its expression in endothelial cells, ephrin-B2 was also found in mural cells ().Citation4,29,62,108 Whereas endothelial specific inactivation of the Efnb2 gene led to lethality during early embryo development due to impaired angiogenesis and heart morphogenesis, mural cell-specific Efnb2 mutants developed to term and died perinatally.Citation4,62 This finding most likely reflects the essential roles of endothelial cells in the growth of the embryonic vasculature, whereas mural cell function in mice only becomes indispensable after birth.Citation24
Regulation of VEGF Receptor Endocytosis by Eph/ephrin Signaling
Eph/ephrin signaling regulates endothelial sprouting angiogenesis, which has been studied best in the postnatal murine retina ().Citation64,65,109 Ephrin-B2 and EphB4 show partially overlapping expression in the angiogenic growth front and are presumably also active in the neovascular sprouts and blind-ended capillaries during hypoxia-induced angiogenesis in oxygen-induced retinopathy, a model for pathological vessel growth in the eye.Citation64,110 Ephrin-B2 overexpression stimulated endothelial motility and invasiveness in cultured cells and transgenic mice.Citation64,65,111 Conversely, ephrin-B2 conditional deletion in endothelium or deletion of the C-terminal PDZ binding motif (in ephrin-B2ΔV mutants) impaired sprouting angiogenesis in physiological and pathological conditions.Citation64,65 When the ephrin-B2 cytoplasmic domain was replaced by β-galactosidase, retina vascularization and filopodia formation were reduced.Citation110 Likewise, revascularization and the formation of vascular malformations, so-called neovascular tufts, were attenuated when these mutants were analyzed under conditions of oxygen-induced retinopathy.Citation110
The 2 main signaling pathways known to reciprocally regulate and cooperate in the specification of tip-stalk cell phenotypes during angiogenesis are VEGF and Notch.Citation32 VEGF promotes Dll4 ligand expression in tip cells.Citation43,44,112,113 Dll4 activates its Notch receptor in the neighboring stalk cells, which is thought to further repress VEGF receptor expression in these cells and inhibit sprouting.Citation44,113,114 Surprisingly, Notch is a weak regulator of VEGFR2 expression, while being a potent inhibitor of VEGFR3 protein expression.Citation46 Accordingly, when Notch was inhibited in postnatal mice, VEGFR3 was found to promote unproductive, excessive angiogenesis in a VEGF-independent fashion.Citation46
One of the critical molecular events involved in VEGF signaling regulation is endocytosis.Citation115,116 While the traditional view was that endocytosis leads to receptor degradation and the termination of signaling, there is increasing evidence for RTK signaling from endocytic vesicles after internalization.Citation40,117,118 Within the plasma membrane, VEGFR2 co-localizes with caveolin-1 or is found at the endothelial cell-cell contacts in adherens junctions.Citation117,119,120 During angiogenesis, these junctions get less stable and VEGFR2 is no longer blocked by interaction with VE-cadherin or several phosphatases.Citation11,117,121-123 In response to VEGF binding, VEGFR2 is rapidly internalized, which occurs mainly in a clathrin and dynamin-dependent fashion, and is trafficked to Rab5+ vesicles. VEGFR2 in endosomes was found in a complex with Nrp1, dynamin-binding protein synectin/GIPC and myosin-VI, a reverse transport motor.Citation124,125 VEGFR2 next moves from early antigen 1 (EEA1)/Rab5+ endosomes either into recycling (Rab11+ or Rab4+) endosomes or into multivesicular bodies (MVBs) and late endosomes (Rab7+) prior to recycling or degradation respectively.Citation116,118,126
High rates of VEGF uptake, VEGF receptor endocytosis and turnover have been detected in sprouting endothelial cells of the early postnatal retina ().Citation109 Here, ephrin-B2 regulates VEGF signaling by promoting clathrin-mediated endocytosis of VEGFR2 and VEGFR3.Citation64,65,109 Ephrin-B2 recruits Dab2, a clathrin-associated-sorting protein (CLASP), which associates with the cell polarity factor Par3 in clathrin-coated vesicles.Citation64,65,109 Accordingly, endothelial specific deletion of Dab2 or Par3 impaired VEGF receptor internalization and phenocopied sprouting defects seen in EC-specific Efnb2 mutants.Citation64,65,109
Ligand-dependent VEGFR2 and VEGFR3 endocytosis is spatially regulated and predominantly takes place at the leading angiogenic front, which is also a site of high VEGFR2 transcription.Citation8,109 Accordingly, while VEGFR2/R3 protein steady state levels were surprisingly low in sprouts at the leading edge of the growing retinal vasculature, levels of the 2 receptors were profoundly increased in sprouts after blockade of protein degradation or inhibition of clathrin-mediated endocytosis.Citation109 These experiments also indicated that VEGF receptor internalization and turnover in the more mature vessels of the central retina is much lower, which was attributed to negative modulation of Dab2-dependent internalization by atypical protein kinase C (aPKC). aPKC-λ and aPKC-ζ can phosphorylate Dab2 in proximity of its cargo-binding PTB domain, which inhibited the interaction with VEGFR2 and VEGFR3.Citation109 Immunostaining of phosphorylated (active) aPKC was weak in sprouts at the angiogenic growth front (i.e, the area with high VEGF receptor turnover and activity), while strong phospho-aPKC staining marked more mature vessels with low VEGFR internalization.Citation109 Accordingly, postnatal inactivation of the gene encoding aPKC-λ in ECs increased the uptake of fluorescently labeled VEGF through VEGFR2 in the central retina, and the abundance of filopodial protrusions was greatly increased in mutant vessels.
Interestingly, not only endocytosis but also VEGFR signaling activity is regulated by ephrin-B2, Dab2, Par3, and aPKC.Citation109 In the absence of ephrin-B2 signaling, stimulated VEGF receptors were retained on the cell surface, which was accompanied by reduced tyrosine phosphorylation of these molecules and impaired downstream activation of Rac1, Akt and MAP kinase Erk1/2 ().Citation64,65,88 Inhibition of aPKC in cultured cells led to accelerated VEGFR2 and VEGFR3 internalization and strongly increased VEGF-A and VEGF-C-dependent MAP kinase activation.Citation109 While the stimulation of EphB4 forward or ephrin-B2 reverse signaling triggered some VEGF receptor internalization even in the absence of VEGF stimulation, this process failed to induce significant activation of the VEGF pathway.Citation64,65
Regulation of PDGF Receptor B Endocytosis by Eph/ephrin Signaling
The recruitment of mural cells strongly depends on PDGF-B/PDGFRβ signaling. Activation of this pathway suppresses the differentiation and promotes the proliferation of VSMCs, pericytes and other mesenchymal cell types.Citation127 Upon ligand binding, PDGF receptors accumulate in coated pits at the cell membrane and internalize mainly in a clathrin- and dynamin-dependent fashion, but they can also chose a caveolin-dependent pathway or macropinosomes.Citation54,128,129 Caveolin-1 has been also shown to interfere with PDGF-induced signal transduction suggesting that lipid rafts or caveolae might harbor an inactive (passive) pool of receptors.Citation130,131 Following endocytosis, PDGF receptors continue to signal from endosomes before they get degraded or recycled to the plasma membrane.Citation54,132 Signaling processes downstream of PDGFRβ include the activation of Ras-mitogen-activated protein kinase (Ras-MAP kinase), phosphoinositide 3-kinase, the small GTPase Rac1, and c-Jun N-terminal kinase (JNK), which control specific cellular functions.Citation127 Remarkably, point mutation of 7 cytoplasmic tyrosine phosphorylation sites in PDGFRβ, which allow the docking of various signaling and adapter molecules, led to a strong reduction in the number of mural cells but was still compatible with survival of the resulting mutants.Citation57 In contrast, mice carrying an inactivating mutation in the kinase activity showed perinatal lethality and thereby phenocopied the full gene knockout.
Gene targeting experiments in mice have shown that ephrin-B2 is also required for normal mural cell function.Citation62 As a consequence of the inactivation of the Efnb2 gene in pericytes and VSMCs, these cells were only loosely attached to the endothelium and could not form a normal vessel wall, which resulted in altered vessel architecture, hemorrhaging and perinatal lethality reminiscent of defects in Pdgfb/Pdgfrb knockout mice.Citation57,62 The identity of the EphB receptors controlling mural cell function requires further investigation, but expression of EphB2, EphB3 and EphB4 was observed in cultured murine VSMCs.Citation62 Cell contact-dependent interactions between pericytes and endothelial Eph/ephrin molecules are also possible.
In cultured ephrin-B2-deficient VSMCs, signaling of the SH2-containing adaptor proteins Crk and Crk associated substrate, p130(CAS), which control cell migration and cell-matrix adhesion, were altered.Citation62 Accordingly, the cells showed defects in focal adhesion formation, cell adhesion, spreading, and directional migration, whereas cell motility was increased. Interestingly, many of these defects did not require cell-cell contact arguing that ephrin-B2 can control mural cell function in a cell-autonomous fashion and without interacting with receptors in trans.Citation62
The molecular function of ephrin-B2 in mural cells is linked to PDGFRβ plasma membrane distribution, endocytosis and signaling.Citation108 Efnb2 mutant aorta lysates or PDGF-B-stimulated ephrin-B2-deficient VSMCs showed increased activation of PDGFRβ, MAP kinase Erk1/2 and JNK, which was linked to more rapid, clathrin-dependent internalization of PDGFRβ. While ephrin-B2 co-localized with caveolin and PDGFRβ in the membrane fractions from control VSMCs, the loss of ephrin-B2 led to redistribution of PDGFRβ into clathrin-containing membrane compartments, which preferentially contained the active, phosphorylated form of the receptor.Citation108 These findings indicated that ephrin-B2 is a negative regulator of clathrin-mediated PDGFRβ endocytosis and thereby MAP kinase and JNK signaling. At the same time, aortas from mice lacking ephrin-B2 in VSMCs or cultured Efnb2 KO smooth muscle cells also showed impaired activation of small GTPase Rac1 and strongly reduced expression of the Rac GTP Exchange Factor Tiam1 (). Re-expression of full-length Tiam1 in Efnb2 KO cells partially rescued VSMC spreading, migration, focal adhesion formation, and proliferation.Citation62,108 Thus, ephrin-B2 modulates not only the internalization of PDGFRβ but is required for the balanced activation of different signaling transduction cascades in the PDGF pathway.
Stimulation of ephrin-B2 internalization with soluble, recombinant EphB4 fusion protein also triggered the clustering and endocytosis of surface PDGFRβ, but only from the inactive, unphosphorylated pool (). Under these conditions, residual PDGFRβ at the cell surface was more readily activated by PDGF-B leading to enhanced autophosphorylation and MAP kinase signaling similar to ephrin-B2-deficient VSMCs.Citation108 These results suggest that the presence/absence of ephrin-B2 or its interactions with EphB-expressing cells can strongly modulate PDGFRβ activity and downstream signaling in mural cells. Future work should integrate these findings with the role of other known co-receptors and interaction partners of PDGFRβ. Low density lipoprotein receptor-related protein 1 (LRP1), a transmembrane protein that can bind lipoprotein, proteases, growth factors, cytokines and matrix proteins, has been shown to associate with PDGFRβ and modulates its expression, internalization and signaling.Citation133-136 LRP6, another member of the LDL receptor-related protein family, can also bind PDGFRβ and enhance the degradation of the RTK, which led to reduced PDGF-dependent VSMC proliferation.Citation137 As a final example, the expression of Neuropilin-1 in VSMCs was shown to promote PDGF-stimulated smooth muscle cell migration.Citation138
Role of Ephrin-B2 in Kidney Damage and Fibrosis
Imbalance of receptor endocytosis due to the absence or increased activation of ephrin-B2 can lead to disease-like conditions in different tissues. In kidney, microvascular disease causes peritubular capillary (PTC) rarefaction.Citation139 Here, pericytes migrate away from the capillary wall and differentiate into myofibroblasts, which results in kidney fibrosis.Citation139 As a consequence, peritubular capillaries become less stable and regress. After inactivation of the Efnb2 gene in pericytes, mutant capillaries in embryonic skin were less stable due to impaired pericyte association, a phenomenon that mimics processes during the onset of fibrosis in kidney.Citation62 During kidney injury, ephrin-B2 reverse signaling protected against PTC rarefaction by promoting angiogenesis and vascular stability.Citation140 Pericytes were protected against myofibroblasts conversion and activation, which limited fibrogenesis.Citation140 Cultured primary kidney microvascular endothelial cells from ephrin-B2ΔV mice showed impaired migration and proliferation after VEGF-A stimulation, which was linked to reduced VEGFR2 activation and internalization.Citation140 Interestingly, in these mice, inhibition of VEGFR2 or PDGFRβ with blocking antibodies or soluble receptor ectodomains was shown to ameliorate microvascular rarefaction, pericyte detachment and differentiation into myofibroblasts, which led to reduced fibrotic scar formation.Citation141-143 However, future work will have to address whether this role of ephrin-B2 in pericytes is directly linked to the function of PDGFRβ or other receptors. These findings point at important disease-promoting roles of deregulated VEGFR2 or PDGFRβ signaling, which might be linked to Eph/ephrin molecules and/or other regulators of RTK endocytosis and signaling.
The examples above highlight how ephrin-B2 controls the function of RTKs in different cell types, which has fundamental impact on processes such as cell migration, proliferation, adhesion, cell-cell communication, or the assembly of functional tissue compartments. Important roles of Eph/ephrin molecules in trafficking, surface presentation and internalization have been also reported outside the vascular system and for receptors without tyrosine kinase activity.Citation144-151 Thus, the regulation of a diverse set of cell surface receptors might be one of the general principles underlying Eph/ephrin function in tissue morphogenesis. Such a versatile role might also explain how a substantial but nevertheless limited set of Eph/ephrin molecules is able to control a very large number of highly diverse biological processes involving very different cell types and tissue environments. Future work will undoubtedly uncover additional receptors and cell surface molecules that are functionally modulated by Ephs and ephrins. At the same time, many other crucial details remain to be unraveled. What is the exact mechanistic role of ephrin-B2 and other Eph/ephrin molecules in receptor trafficking and endocytosis? What other molecules, adapters and interaction partners are involved? What is the precise nature of the endosomal compartments enabling receptor tyrosine kinase signaling after internalization? What are the exact contributions of ephrin reverse and Eph forward signaling in these processes and how do they affect the clathrin machinery and other endocytic pathways as well as the trafficking, recycling or degradation of interacting RTKs and surface receptors? Understanding these key questions will be undoubtedly challenging but, given the many important roles of Eph/ephrin regulation in almost every cell type and organ system, also worthwhile. As there is more and more evidence for roles of Eph receptors and ephrin ligands in disease processes, better insight into the underlying mechanism might also provide important leads for the development of novel therapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Max Planck Society, the University of Muenster, the German Research Foundation (SFB 629), and the DFG cluster of excellence ‘Cells in Motion’ for financial support, and members of the Department of Tissue Morphogenesis for helpful discussions.
References
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 1995; 11:73-91; PMID:8689573; http://dx.doi.org/10.1146/annurev.cb.11.110195.000445
- Strilic B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara N, Lammert E. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell 2009; 17:505-15; PMID:19853564; http://dx.doi.org/10.1016/j.devcel.2009.08.011
- Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 2000; 95:1671-9; PMID:10688823
- Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development 2002; 129:1397-410; PMID:11880349
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 2009; 326:294-8; PMID:19815777; http://dx.doi.org/10.1126/science.1178577
- Lindskog H, Kim YH, Jelin EB, Kong Y, Guevara-Gallardo S, Kim TN, Wang RA. Molecular identification of venous progenitors in the dorsal aorta reveals an aortic origin for the cardinal vein in mammals. Development 2014; 141:1120-8; PMID:24550118; http://dx.doi.org/10.1242/dev.101808
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 2002; 16:2684-98; PMID:12381667; http://dx.doi.org/10.1101/gad.242002
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 2003; 161:1163-77; PMID:12810700; http://dx.doi.org/10.1083/jcb.200302047
- Phng LK, Stanchi F, Gerhardt H. Filopodia are dispensable for endothelial tip cell guidance. Development 2013; 140:4031-40; PMID:24046319; http://dx.doi.org/10.1242/dev.097352
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 2010; 12:943-53; PMID:20871601; http://dx.doi.org/10.1038/ncb2103
- Bentley K, Franco CA, Philippides A, Blanco R, Dierkes M, Gebala V, Stanchi F, Jones M, Aspalter IM, Cagna G, et al. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol 2014; 16:309-21; PMID:24658686; http://dx.doi.org/10.1038/ncb2926
- Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development 2011; 138:4569-83; PMID:21965610; http://dx.doi.org/10.1242/dev.062323
- Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J Clin Invest 2014; 124:888-97; PMID:24590273; http://dx.doi.org/10.1172/JCI71609
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 2007; 21:2422-32; PMID:17908929; http://dx.doi.org/10.1101/gad.1588407
- Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 2012; 120:2340-8; PMID:22859612; http://dx.doi.org/10.1182/blood-2012-05-428607
- Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J 2013; 32:629-44; PMID:23299940; http://dx.doi.org/10.1038/emboj.2012.340
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 2007; 8:464-78; PMID:17522591; http://dx.doi.org/10.1038/nrm2183
- Jain RK. Molecular regulation of vessel maturation. Nat Med 2003; 9:685-93; PMID:12778167; http://dx.doi.org/10.1038/nm0603-685
- Ehling M, Adams S, Benedito R, Adams RH. Notch controls retinal blood vessel maturation and quiescence. Development 2013; 140:3051-61; PMID:23785053; http://dx.doi.org/10.1242/dev.093351
- Risau W. Mechanisms of angiogenesis. Nature 1997; 386:671-4; PMID:9109485; http://dx.doi.org/10.1038/386671a0
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407:249-57; PMID:11001068; http://dx.doi.org/10.1038/35025220
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011; 146:873-87; PMID:21925313; http://dx.doi.org/10.1016/j.cell.2011.08.039
- Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014; 14:159-72; PMID:24561443; http://dx.doi.org/10.1038/nrc3677
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21:193-215; PMID:21839917; http://dx.doi.org/10.1016/j.devcel.2011.07.001
- Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg 2007; 45 Suppl A:A25-32; PMID:17544021; http://dx.doi.org/10.1016/j.jvs.2007.03.001
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84:767-801; PMID:15269336; http://dx.doi.org/10.1152/physrev.00041.2003
- Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 2012; 95:194-204; PMID:22467316; http://dx.doi.org/10.1093/cvr/cvs135
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 2004; 10:974-81; PMID:15322537; http://dx.doi.org/10.1038/nm1094
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev 2005; 19:397-410; PMID:15687262; http://dx.doi.org/10.1101/gad.330105
- Lutter S, Xie S, Tatin F, Makinen T. Smooth muscle-endothelial cell communication activates Reelin signaling and regulates lymphatic vessel formation. J Cell Biol 2012; 197:837-49; PMID:22665518; http://dx.doi.org/10.1083/jcb.201110132
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 2004; 56:549-80; PMID:15602010; http://dx.doi.org/10.1124/pr.56.4.3
- Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med 2013; 3:a006569; PMID:23085847; http://dx.doi.org/10.1101/cshperspect.a006569
- Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 2008; 4:241-6; PMID:19337404; http://dx.doi.org/10.4161/org.4.4.7414
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling-in control of vascular function. Nat Rev Mol Cell Biol 2006; 7:359-71; PMID:16633338; http://dx.doi.org/10.1038/nrm1911
- Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 2010; 464:917-21; PMID:20228789; http://dx.doi.org/10.1038/nature08945
- Haiko P, Makinen T, Keskitalo S, Taipale J, Karkkainen MJ, Baldwin ME, Stacker SA, Achen MG, Alitalo K. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol 2008; 28:4843-50; PMID:18519586; http://dx.doi.org/10.1128/MCB.02214-07
- Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Mol Divers 2006; 10:515-27; PMID:16972015; http://dx.doi.org/10.1007/s11030-006-9027-3
- Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2012; 2:a006502; PMID:22762016; http://dx.doi.org/10.1101/cshperspect.a006502
- Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer 2008; 8:880-7; PMID:18923433; http://dx.doi.org/10.1038/nrc2505
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell 2007; 130:691-703; PMID:17719546; http://dx.doi.org/10.1016/j.cell.2007.06.054
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A 1995; 92:3566-70; PMID:7724599; http://dx.doi.org/10.1073/pnas.92.8.3566
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 1998; 282:946-9; PMID:9794766; http://dx.doi.org/10.1126/science.282.5390.946
- Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008; 454:656-60; PMID:18594512; http://dx.doi.org/10.1038/nature07083
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 2007; 445:781-4; PMID:17259972; http://dx.doi.org/10.1038/nature05577
- Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol 2011; 13:1202-13; PMID:21909098; http://dx.doi.org/10.1038/ncb2331
- Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature 2012; 484:110-4; PMID:22426001; http://dx.doi.org/10.1038/nature10908
- Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer 2008; 8:604-17; PMID:18497750; http://dx.doi.org/10.1038/nrc2353
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 2009; 10:165-77; PMID:19234476; http://dx.doi.org/10.1038/nrm2639
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277:55-60; PMID:9204896; http://dx.doi.org/10.1126/science.277.5322.55
- Koh GY. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med 2013; 19:31-9; PMID:23182855; http://dx.doi.org/10.1016/j.molmed.2012.10.010
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996; 87:1171-80; PMID:8980224; http://dx.doi.org/10.1016/S0092-8674(00)81813-9
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell 2002; 3:411-23; PMID:12361603; http://dx.doi.org/10.1016/S1534-5807(02)00217-4
- Thomas M, Felcht M, Kruse K, Kretschmer S, Deppermann C, Biesdorf A, Rohr K, Benest AV, Fiedler U, Augustin HG. Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalization and degradation. J Biol Chem 2010; 285:23842-9; PMID:20519501; http://dx.doi.org/10.1074/jbc.M109.097543
- Heldin CH, Lennartsson J. Structural and functional properties of platelet-derived growth factor and stem cell factor receptors. Cold Spring Harb Perspect Biol 2013; 5:a009100; PMID:23906712; http://dx.doi.org/10.1101/cshperspect.a009100
- Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal 2013; 11:97; PMID:24359404; http://dx.doi.org/10.1186/1478-811X-11-97
- del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, et al. Identification and functional analysis of endothelial tip cell-enriched genes. Blood 2010; 116:4025-33; PMID:20705756; http://dx.doi.org/10.1182/blood-2010-02-270819
- Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev 2004; 15:205-13; PMID:15207812; http://dx.doi.org/10.1016/j.cytogfr.2004.03.003
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 1999; 13:295-306; PMID:9990854; http://dx.doi.org/10.1101/gad.13.3.295
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell 1999; 4:403-14; PMID:10518221; http://dx.doi.org/10.1016/S1097-2765(00)80342-1
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998; 93:741-53; PMID:9630219; http://dx.doi.org/10.1016/S0092-8674(00)81436-1
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell 2001; 104:57-69; PMID:11163240; http://dx.doi.org/10.1016/S0092-8674(01)00191-X
- Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 2006; 124:161-73; PMID:16413489; http://dx.doi.org/10.1016/j.cell.2005.10.034
- Salvucci O, de la Luz Sierra M, Martina JA, McCormick PJ, Tosato G. EphB2 and EphB4 receptors forward signaling promotes SDF-1-induced endothelial cell chemotaxis and branching remodeling. Blood 2006; 108:2914-22; PMID:16840724; http://dx.doi.org/10.1182/blood-2006-05-023341
- Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010; 465:483-6; PMID:20445537; http://dx.doi.org/10.1038/nature09002
- Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 2010; 465:487-91; PMID:20445540; http://dx.doi.org/10.1038/nature08995
- Katsuta H, Fukushima Y, Maruyama K, Hirashima M, Nishida K, Nishikawa S, Uemura A. EphrinB2-EphB4 signals regulate formation and maintenance of funnel-shaped valves in corneal lymphatic capillaries. Invest Ophthalmol Vis Sci 2013; 54:4102-8; PMID:23696610; http://dx.doi.org/10.1167/iovs.12-11436
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell 2008; 133:38-52; PMID:18394988; http://dx.doi.org/10.1016/j.cell.2008.03.011
- Batlle E, Wilkinson DG. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb Perspect Biol 2012; 4:a008227; PMID:22214769
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 2005; 6:462-75; PMID:15928710; http://dx.doi.org/10.1038/nrm1662
- Singh A, Winterbottom E, Daar IO. Eph/ephrin signaling in cell-cell and cell-substrate adhesion. Front Biosci (Landmark Ed) 2012; 17:473-97; PMID:22201756; http://dx.doi.org/10.2741/3939
- Daar IO. Non-SH2/PDZ reverse signaling by ephrins. Semin Cell Dev Biol 2012; 23:65-74; PMID:22040914; http://dx.doi.org/10.1016/j.semcdb.2011.10.012
- Lee HS, Daar IO. EphrinB reverse signaling in cell-cell adhesion: is it just par for the course? Cell Adh Migr 2009; 3:250-5; PMID:19276658; http://dx.doi.org/10.4161/cam.3.3.8211
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell 2005; 121:127-39; PMID:15820684; http://dx.doi.org/10.1016/j.cell.2005.01.020
- Xu NJ, Sun S, Gibson JR, Henkemeyer M. A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses. Nat Neurosci 2011; 14:1421-9; PMID:21964490; http://dx.doi.org/10.1038/nn.2931
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science 1994; 266:816-9; PMID:7973638; http://dx.doi.org/10.1126/science.7973638
- Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, Daniel TO. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev 1998; 12:667-78; PMID:9499402; http://dx.doi.org/10.1101/gad.12.5.667
- Seiradake E, Schaupp A, del Toro Ruiz D, Kaufmann R, Mitakidis N, Harlos K, Aricescu AR, Klein R, Jones EY. Structurally encoded intraclass differences in EphA clusters drive distinct cell responses. Nat Struct Mol Biol 2013; 20:958-64; PMID:23812375; http://dx.doi.org/10.1038/nsmb.2617
- Klein R, Kania A. Ephrin signalling in the developing nervous system. Curr Opin Neurobiol 2014; 27C:16-24; http://dx.doi.org/10.1016/j.conb.2014.02.006
- Xu K, Tzvetkova-Robev D, Xu Y, Goldgur Y, Chan YP, Himanen JP, Nikolov DB. Insights into Eph receptor tyrosine kinase activation from crystal structures of the EphA4 ectodomain and its complex with ephrin-A5. Proc Natl Acad Sci U S A 2013; 110:14634-9; PMID:23959867; http://dx.doi.org/10.1073/pnas.1311000110
- Himanen JP, Rajashankar KR, Lackmann M, Cowan CA, Henkemeyer M, Nikolov DB. Crystal structure of an Eph receptor-ephrin complex. Nature 2001; 414:933-8; PMID:11780069; http://dx.doi.org/10.1038/414933a
- Huynh-Do U, Stein E, Lane AA, Liu H, Cerretti DP, Daniel TO. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. EMBO J 1999; 18:2165-73; PMID:10205170; http://dx.doi.org/10.1093/emboj/18.8.2165
- Himanen JP, Nikolov DB. Eph signaling: a structural view. Trends Neurosci 2003; 26:46-51; PMID:12495863; http://dx.doi.org/10.1016/S0166-2236(02)00005-X
- Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, et al. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A 2010; 107:10860-5; PMID:20505120; http://dx.doi.org/10.1073/pnas.1004148107
- Schaupp A, Sabet O, Dudanova I, Ponserre M, Bastiaens P, Klein R. The composition of EphB2 clusters determines the strength in the cellular repulsion response. J Cell Biol 2014; 204:409-22; PMID:24469634; http://dx.doi.org/10.1083/jcb.201305037
- Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PI, Lackmann M. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol 2004; 164:661-6; PMID:14993233; http://dx.doi.org/10.1083/jcb.200312001
- Singla N, Goldgur Y, Xu K, Paavilainen S, Nikolov DB, Himanen JP. Crystal structure of the ligand-binding domain of the promiscuous EphA4 receptor reveals two distinct conformations. Biochem Biophys Res Commun 2010; 399:555-9; PMID:20678482; http://dx.doi.org/10.1016/j.bbrc.2010.07.109
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer 2010; 10:165-80; PMID:20179713; http://dx.doi.org/10.1038/nrc2806
- Pitulescu ME, Adams RH. Eph/ephrin molecules–a hub for signaling and endocytosis. Genes Dev 2010; 24:2480-92; PMID:21078817; http://dx.doi.org/10.1101/gad.1973910
- Li W, Mukouyama YS. Tissue-specific venous expression of the EPH family receptor EphB1 in the skin vasculature. Dev Dyn 2013; 242:976-88; PMID:23649798; http://dx.doi.org/10.1002/dvdy.23985
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature 2001; 414:216-20; PMID:11700560; http://dx.doi.org/10.1038/35102599
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proc Natl Acad Sci U S A 2002; 99:8219-24; PMID:12048246; http://dx.doi.org/10.1073/pnas.122109599
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 2002; 109:693-705; PMID:12086669; http://dx.doi.org/10.1016/S0092-8674(02)00757-2
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 2002; 3:127-36; PMID:12110173; http://dx.doi.org/10.1016/S1534-5807(02)00198-3
- Torres-Vazquez J, Kamei M, Weinstein BM. Molecular distinction between arteries and veins. Cell Tissue Res 2003; 314:43-59; PMID:14505031; http://dx.doi.org/10.1007/s00441-003-0771-8
- Lamont RE, Childs S. MAPping out arteries and veins. Sci STKE 2006; 2006:pe39; PMID:17018851; http://dx.doi.org/10.1126/stke.3552006pe39
- Lin FJ, Tsai MJ, Tsai SY. Artery and vein formation: a tug of war between different forces. EMBO Rep 2007; 8:920-4; PMID:17906673; http://dx.doi.org/10.1038/sj.embor.7401076
- Wythe JD, Dang LT, Devine WP, Boudreau E, Artap ST, He D, Schachterle W, Stainier DY, Oettgen P, Black BL, et al. ETS factors regulate Vegf-dependent arterial specification. Dev Cell 2013; 26:45-58; PMID:23830865; http://dx.doi.org/10.1016/j.devcel.2013.06.007
- Sacilotto N, Monteiro R, Fritzsche M, Becker PW, Sanchez-Del-Campo L, Liu K, Pinheiro P, Ratnayaka I, Davies B, Goding CR, et al. Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci U S A 2013; 110:11893-8; PMID:23818617; http://dx.doi.org/10.1073/pnas.1300805110
- Iso T, Maeno T, Oike Y, Yamazaki M, Doi H, Arai M, Kurabayashi M. Dll4-selective Notch signaling induces ephrinB2 gene expression in endothelial cells. Biochem Biophys Res Commun 2006; 341:708-14; PMID:16430858; http://dx.doi.org/10.1016/j.bbrc.2006.01.020
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 2005; 435:98-104; PMID:15875024; http://dx.doi.org/10.1038/nature03511
- Kim YH, Hu H, Guevara-Gallardo S, Lam MT, Fong SY, Wang RA. Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development 2008; 135:3755-64; PMID:18952909; http://dx.doi.org/10.1242/dev.022475
- Kohli V, Schumacher JA, Desai SP, Rehn K, Sumanas S. Arterial and venous progenitors of the major axial vessels originate at distinct locations. Dev Cell 2013; 25:196-206; PMID:23639444; http://dx.doi.org/10.1016/j.devcel.2013.03.017
- Williams C, Kim SH, Ni TT, Mitchell L, Ro H, Penn JS, Baldwin SH, Solnica-Krezel L, Zhong TP. Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Dev Biol 2010; 341:196-204; PMID:20193674; http://dx.doi.org/10.1016/j.ydbio.2010.02.028
- Sabin FR. Origin and development of the primitive vessels of the chick and of the pig. Washington: Carnegie Institution of Washington, 1917
- Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, Messina LM, Capobianco AJ, Werb Z, Wang R. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci U S A 2005; 102:9884-9; PMID:15994223; http://dx.doi.org/10.1073/pnas.0504391102
- Benedito R, Trindade A, Hirashima M, Henrique D, da Costa LL, Rossant J, Gill PS, Duarte A. Loss of Notch signalling induced by Dll4 causes arterial calibre reduction by increasing endothelial cell response to angiogenic stimuli. BMC Dev Biol 2008; 8:117; PMID:19087347; http://dx.doi.org/10.1186/1471-213X-8-117
- Bazigou E, Lyons OT, Smith A, Venn GE, Cope C, Brown NA, Makinen T. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest 2011; 121:2984-92; PMID:21765212; http://dx.doi.org/10.1172/JCI58050
- Nakayama A, Nakayama M, Turner CJ, Hoing S, Lepore JJ, Adams RH. Ephrin-B2 controls PDGFRbeta internalization and signaling. Genes Dev 2013; 27:2576-89; PMID:24298057; http://dx.doi.org/10.1101/gad.224089.113
- Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HC, Itoh N, Hirose T, Breier G, Vestweber D, et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol 2013; 15:249-60; PMID:23354168; http://dx.doi.org/10.1038/ncb2679
- Taylor AC, Mendel TA, Mason KE, Degen KE, Yates PA, Peirce SM. Attenuation of ephrinB2 reverse signaling decreases vascularized area and preretinal vascular tuft formation in the murine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2012; 53:5462-70; PMID:22789927; http://dx.doi.org/10.1167/iovs.11-8599
- Bochenek ML, Dickinson S, Astin JW, Adams RH, Nobes CD. Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J Cell Sci 2010; 123:1235-46; PMID:20233847; http://dx.doi.org/10.1242/jcs.061903
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A 2007; 104:3225-30; PMID:17296941; http://dx.doi.org/10.1073/pnas.0611177104
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The Notch Ligands Dll4 and Jagged1 Have Opposing Effects on Angiogenesis. Cell 2009; 137:1124-35; PMID:19524514; http://dx.doi.org/10.1016/j.cell.2009.03.025
- Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. Bioessays 2008; 30:303-13; PMID:18348190; http://dx.doi.org/10.1002/bies.20736
- Horowitz A, Seerapu HR. Regulation of VEGF signaling by membrane traffic. Cellular Signalling 2012; 24:1810-20; PMID:22617029; http://dx.doi.org/10.1016/j.cellsig.2012.05.007
- Nakayama M, Berger P. Coordination of VEGF receptor trafficking and signaling by coreceptors. Exp Cell Res 2013; 319:1340-7; PMID:23499743; http://dx.doi.org/10.1016/j.yexcr.2013.03.008
- Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol 2006; 174:593-604; PMID:16893970; http://dx.doi.org/10.1083/jcb.200602080
- Simons M. An inside view: VEGF receptor trafficking and signaling. Physiology (Bethesda) 2012; 27:213-22; PMID:22875452; http://dx.doi.org/10.1152/physiol.00016.2012
- Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell 2003; 14:334-47; PMID:12529448; http://dx.doi.org/10.1091/mbc.E02-07-0379
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 1999; 98:147-57; PMID:10428027; http://dx.doi.org/10.1016/S0092-8674(00)81010-7
- Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol 2003; 161:793-804; PMID:12771128; http://dx.doi.org/10.1083/jcb.200209019
- Mattila E, Auvinen K, Salmi M, Ivaska J. The protein tyrosine phosphatase TCPTP controls VEGFR2 signalling. J Cell Sci 2008; 121:3570-80; PMID:18840653; http://dx.doi.org/10.1242/jcs.031898
- Hayashi M, Majumdar A, Li X, Adler J, Sun Z, Vertuani S, Hellberg C, Mellberg S, Koch S, Dimberg A, et al. VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nature Communications 2013; 4:1672; PMID:23575676
- Zhang X, Lanahan AA, Simons M. VEGFR2 trafficking Speed doesn't kill. Cell Cycle 2013; 12:2163-4; PMID:23803732; http://dx.doi.org/10.4161/cc.25536
- Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev Cell 2010; 18:713-24; PMID:20434959; http://dx.doi.org/10.1016/j.devcel.2010.02.016
- Pasula S, Cai X, Dong Y, Messa M, McManus J, Chang B, Liu X, Zhu H, Mansat RS, Yoon SJ, et al. Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling. J Clin Invest 2012; 122:4424-38; PMID:23187125; http://dx.doi.org/10.1172/JCI64537
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008; 22:1276-312; PMID:18483217; http://dx.doi.org/10.1101/gad.1653708
- Sorkin A, Westermark B, Heldin CH, Claesson-Welsh L. Effect of receptor kinase inactivation on the rate of internalization and degradation of PDGF and the PDGF beta-receptor. J Cell Biol 1991; 112:469-78; PMID:1846866; http://dx.doi.org/10.1083/jcb.112.3.469
- Sadowski L, Jastrzebski K, Kalaidzidis Y, Heldin CH, Hellberg C, Miaczynska M. Dynamin inhibitors impair endocytosis and mitogenic signaling of PDGF. Traffic 2013; 14:725-36; PMID:23425318; http://dx.doi.org/10.1111/tra.12061
- Fujita Y, Maruyama S, Kogo H, Matsuo S, Fujimoto T. Caveolin-1 in mesangial cells suppresses MAP kinase activation and cell proliferation induced by bFGF and PDGF. Kidney Int 2004; 66:1794-804; PMID:15496150; http://dx.doi.org/10.1111/j.1523-1755.2004.00954.x
- Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell 2008; 15:37-48; PMID:18606139; http://dx.doi.org/10.1016/j.devcel.2008.04.015
- Wang Y, Pennock SD, Chen X, Kazlauskas A, Wang Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J Biol Chem 2004; 279:8038-46; PMID:14660565; http://dx.doi.org/10.1074/jbc.M311494200
- Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science 2003; 300:329-32; PMID:12690199; http://dx.doi.org/10.1126/science.1082095
- Zhou L, Takayama Y, Boucher P, Tallquist MD, Herz J. LRP1 regulates architecture of the vascular wall by controlling PDGFRbeta-dependent phosphatidylinositol 3-kinase activation. Plos One 2009; 4:e6922; PMID:19742316; http://dx.doi.org/10.1371/journal.pone.0006922
- Lehti K, Rose NF, Valavaara S, Weiss SJ, Keski-Oja J. MT1-MMP promotes vascular smooth muscle dedifferentiation through LRP1 processing. J Cell Sci 2009; 122:126-35; PMID:19066283; http://dx.doi.org/10.1242/jcs.035279
- Muratoglu SC, Mikhailenko I, Newton C, Migliorini M, Strickland DK. Low density lipoprotein receptor-related protein 1 (LRP1) forms a signaling complex with platelet-derived growth factor receptor-beta in endosomes and regulates activation of the MAPK pathway. J Biol Chem 2010; 285:14308-17; PMID:20220145; http://dx.doi.org/10.1074/jbc.M109.046672
- Keramati AR, Singh R, Lin A, Faramarzi S, Ye ZJ, Mane S, Tellides G, Lifton RP, Mani A. Wild-type LRP6 inhibits, whereas atherosclerosis-linked LRP6R611C increases PDGF-dependent vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A 2011; 108:1914-8; PMID:21245321; http://dx.doi.org/10.1073/pnas.1019443108
- Pellet-Many C, Frankel P, Evans IM, Herzog B, Junemann-Ramirez M, Zachary IC. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem J 2011; 435:609-18; PMID:21306301; http://dx.doi.org/10.1042/BJ20100580
- Kida Y, Duffield JS. Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Physiol 2011; 38:467-73; PMID:21517936; http://dx.doi.org/10.1111/j.1440-1681.2011.05531.x
- Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol 2013; 24:559-72; PMID:23492730; http://dx.doi.org/10.1681/ASN.2012080871
- Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 2011; 178:911-23; PMID:21281822; http://dx.doi.org/10.1016/j.ajpath.2010.10.012
- Pan SY, Chang YT, Lin SL. Microvascular pericytes in healthy and diseased kidneys. Int J Nephrol Renovasc Dis 2014; 7:39-48; PMID:24465134
- Campanholle G, Ligresti G, Gharib SA, Duffield JS. Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol 2013; 304:C591-603; PMID:23325411; http://dx.doi.org/10.1152/ajpcell.00414.2012
- Bethani I, Skanland SS, Dikic I, Acker-Palmer A. Spatial organization of transmembrane receptor signalling. EMBO J 2010; 29:2677-88; PMID:20717138; http://dx.doi.org/10.1038/emboj.2010.175
- Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, Acker-Palmer A. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci 2008; 11:1035-43; PMID:19160501; http://dx.doi.org/10.1038/nn.2171
- Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, Ethell IM. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J Cell Biol 2003; 163:1313-26; PMID:14691139; http://dx.doi.org/10.1083/jcb.200306033
- Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat Cell Biol 2005; 7:501-9; PMID:15821731; http://dx.doi.org/10.1038/ncb1252
- Marler KJ, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci 2008; 28:12700-12; PMID:19036963; http://dx.doi.org/10.1523/JNEUROSCI.1915-08.2008
- Huai J, Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J Biol Chem 2001; 276:6689-94; PMID:11053419; http://dx.doi.org/10.1074/jbc.M008127200
- Noren NK, Yang NY, Silldorff M, Mutyala R, Pasquale EB. Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem J 2009; 422:433-42; PMID:19552627; http://dx.doi.org/10.1042/BJ20090014
- Yamazaki T, Masuda J, Omori T, Usui R, Akiyama H, Maru Y. EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J Cell Sci 2009; 122:243-55; PMID:19118217; http://dx.doi.org/10.1242/jcs.036467
