Abstract
Although the epidermal growth factor receptor (EGFR), also known as HER1, has been studied for over a decade, it continues to be a molecule of great interest and focus of investigators for development of targeted therapies. The marketed monoclonal antibody cetuximab binds to HER1, and thus might serve as the basis for creation of imaging or therapies that target this receptor. The potential of cetuximab as a vehicle for the delivery of α-particle radiation was investigated in an intraperitoneal tumor mouse model. The effective working dose of 10 μCi of 212Pb-cetuximab was determined from a dose (10–50 μCi) escalation study. Toxicity, as indicated by the lack of animal weight loss, was not evident at the 10 μCi dose of 212Pb-cetuximab. A subsequent study demonstrated 212Pb-cetuximab had a therapeutic efficacy similar to that of 212Pb-trastuzumab (p = 0.588). Gemcitabine given 24 h prior to 212Pb-cetuximab increased the median survival from 174 d to 283 d, but carboplatin suppressed the effectiveness of 212Pb-cetuximab. Notably, concurrent treatment of tumor-bearing mice with 212Pb-labeled cetuximab and trastuzumab provided therapeutic benefit that was greater than either antibody alone. In conclusion, cetuximab proved to be an effective vehicle for targeting HER1-expressing tumors with α-radiation for the treatment of disseminated intraperitoneal disease. These studies provide further evidence that the multimodality therapy regimens may have greater efficacy and benefit in the treatment of cancer patients.
Abbreviations
| mAb | = | monoclonal antibody |
| RIT | = | radioimmunotherapy |
| EGFR | = | epidermal growth factor receptor |
| HulgG | = | human immunoglobulin |
| TCMC | = | 1,4,7,10-tetraaza-1,4,7,10-tetra-(2-carbamoyl methyl)-cyclododecane |
| BSA | = | bovine serum albumin |
| %ID/g | = | percent injected dose per gram |
| i.p. | = | intraperitoneal |
| s.c | = | subcutaneous |
| PBS | = | phosphate-buffered saline |
| PET | = | positron emission tomography |
| MS | = | median survival |
Introduction
To date, studies from this laboratory have focused on targeting human epidermal growth factor receptor 2 (HER2)-expressing tumors with 212Pb and 213Bi using trastuzumab as the delivery vehicle. Targeting HER2 with α-particle radiation has proven successful in treating disseminated peritoneal disease. Trastuzumab labeled with either 213Bi or 212Pb extended the median survival of mice bearing intraperitoneal (i.p.) tumors by 3- to 4-fold.Citation1,Citation2 This therapeutic benefit can be potentiated by incorporating chemotherapeutics such as gemcitabine, paclitaxel or carboplatin into the treatment regimen.Citation3-Citation5
213Bismuth, a decay product of 225Ac, has a T1/2 of 45.7 min whereas 212Pb is a 10.6 h half-life β−-emitter that decays to 212Bi, an α-emitter with a T1/2 of 60.6 min. The strategy of exploiting 212Pb as an in vivo generator of 212Bi, overcomes the disadvantages of a shorter half-life radionuclide. In fact, a 212Pb-labeled monoclonal antibody (mAb) can deliver >10 times the dose of a mAb labeled with a bismuth radioisotope. Most of the dose delivered to the cell comes from the α-decays of the 212Pb daughters, 212Bi and 212Po.Citation6 When 212Pb decays, up to 36% of the 212Bi could be lost from the chelate as a result of recoil. Loss of the 212Bi appears not to be particularly problematic if the radioimmunoconjugate is injected intraperitoneally.Citation7
The success of the investigations with 212Pb has led to their translation into a clinical study at the University of Alabama. Initiated in July 2011, this study (NCT01384253) is assessing the safety of 22Pb-1,4,7,10-tetraaza-1,4,7,10-tetra-(2-carbamoyl methyl)-cyclododecane–trastuzumab (22Pb-TCMC-trastuzumab) radioimmunotherapy (RIT) administered by i.p. injection. Patients under recruitment are those with HER2-positive peritoneal neoplasms of ovarian, pancreatic and gastric origin. At this juncture, no adverse reactions or toxicities have been reported for patients receiving 212Pb-trastuzumab.Citation8 This clinical study has now been extended to a second site at the University of California at San Diego.
More recent studies within the laboratory have focused on elucidating the mechanism(s) by which the α-targeted therapy invokes effective tumor cell death. Targeting LS-174T tumors with 212Pb-trastuzumab increases DNA double-stranded breaks, impairs DNA damage repair, persistent G2/M arrest and chromatin remodeling.Citation9,Citation10
HER2 is expressed by an array of epithelial cancers such as pancreas (35–45%), breast (25–30%), ovarian (25–100%) and colorectal (up to 90%).Citation11–Citation14 Conversely, one might also state that HER2, for example, is not well expressed in 55–65% of pancreatic cancers, and therefore identification of additional molecular targets is warranted to expand the repertoire of α-targeted RIT. The epidermal growth factor receptor (EGFR; HER1) is one such possibility. HER1 is over-expressed in pancreatic, head and neck, breast, renal, colorectal, prostate, ovarian, bladder and breast cancer. This target is also overexpressed in malignancies such as glioma and mesothelioma.Citation15–Citation17
First approved in 2004, cetuximab is indicated for the treatment of patients with HER1-positive metastatic colorectal cancer, either in combination with irinotecan, or without, dependent on the patient's tolerance. Cetuximab therapy has been found to reduce tumor burden and delay tumor growth in some patients, but does not necessarily extend patients’ lives.Citation18-Citation20 The advantage of RIT is that its effectiveness is not dependent on the pharmacological effect of the targeting vector's biological interaction with its target, i.e., cetuximab's interaction with HER1. Furthermore, RIT does not require high expression of the target molecule. Nevertheless, cetuximab has potential as a radiotherapeutic agent. Several imaging investigations have explored the practicality of using cetuximab for monitoring disease, assaying EGFR expression, determining patient selection as well as for performing dosimetric calculations for RIT.Citation21-Citation26 The possibility that cetuximab might be appropriate for RIT applications was demonstrated in a set of studies published by this laboratory.Citation23 Tumor targeting of 111In-cetuximab was validated in 5 tumor models by direct quantitation of tumor tissue and with γ-scintigraphy. The usefulness of cetuximab was also confirmed in a study in which cetuximab labeled with 86Y was studied for positron-emission tomography (PET) imaging.Citation24,Citation25
The studies described herein are both exploratory and confirmatory to the above hypothesis, evaluating the potential of cetuximab for α-targeted therapy of disseminated intraperitoneal disease. Consistent with previous studies from this laboratory, this investigation also includes studies combining chemotherapeutics (gemcitabine and carboplatin) with RIT to assess potential therapeutic efficacy enhancement of HER1 targeting α-emitting high-LET 212Pb-labeled cetuximab. The ultimate goal is to establish a multimodality regimen, using multiple targeting vehicles, for the management of cancer patients with disseminated intraperitoneal disease.
Results
In vitro analysis of cetuximab-TCMC and 212Pb-cetuximab
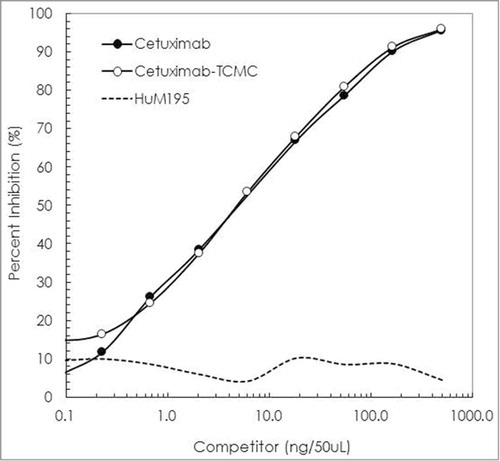
Conjugations of cetuximab with TCMC proved routine. When performed at a 10:1 molar ratio of TCMC:cetuximab, a final chelate-to-cetuximab ratio of 4.2 ± 2.1 was obtained. The immunoreactivity of the cetuximab-TCMC conjugate was maintained compared to unmodified cetuximab in a competition radioimmunoassay (). Radiolabeling the TCMC-cetuximab conjugate was likewise consistent with results that are obtained with trastuzumab. The cetuximab labeling was efficient (65%), achieving an average specific activity of 4 mCi/mg. The immunoreactivity of the 212Pb-cetuximab was evaluated in a radioimmunoassay using purified recombinant human EGFR. After a 4 h incubation at 37°C, the percent bound of 212Pb-cetuximab was 78%. Addition of unlabeled cetuximab to one set of wells reduced the amount bound to 0.9%, thus demonstrating retention of specificity by the 212Pb-cetuximab.
Figure 1. Evaluation of cetuximab-TCMC immunoreactivity. The imunoreactivity of cetuximab-TCMC (○) was evaluated in a competition radioimmunoassay using purified human EGFR. The immunoconjugate was compared to unmodified cetuximab (•). HuM195 (----), a mAb that recognizes CD-33, was used as a negative control.
Validation of tumor targeting of radiolabeled cetuximab in an i.p. tumor xenograft model
Previous publications from this laboratory demonstrated the feasibility of targeting HER1 with radiolabeled cetuximab (111In or 86Y) for the management of a range of cancers, included ovarian, colon, prostate, pancreatic and mesothelioma.Citation23–Citation25 These studies were performed with mice bearing subcutaneous (s.c.) tumor xenografts and the radiolabeled cetuximab given by an intravenous (i.v.) route. The first step in the current series of studies, therefore, was to appraise the targeting qualities of cetuximab in the i.p. model to determine if further pursuit of this mAb for targeted α-radiation therapy of disseminated peritoneal disease was valid.
Athymic mice with i.p. LS-174T xenografts (n = 5) were injected (i.p.) with 111In-cetuximab to establish and define tumor targeting as well as normal organ distribution of the radioimmunoconjugate. Detailed in , the 111In-CHX-A”-cetuximab demonstrates excellent targeting of the i.p. tumors with a tumor percent injected dose per gram (%ID/g) of 51.8 ± 26.4 at 24 hr. The tumor%ID/g then decreases through the 1 week study period to 37.8 ± 20.5, 34.8 ± 31.8, 21.1 ± 2.2 and 8.3 ± 3.2 at 48, 72, 144 and 168 h, respectively. This decrease in the tumor%ID/g reflects the aggressive nature of the rapidly growing LS-174T tumor. At 24 h, the average weight of the tumor tissue harvested from the mice was 147 ± 102 mg. By the end of the study, at 168 h, the average amount of tumor collected was 1,362 ± 802 mg. When the amount of radioactivity in the tumor is calculated and plotted (), the cpm (decay corrected) was found to be relatively constant through the study, demonstrating a decreasing trend that is not as dramatic as that perceived in the %ID/g vs. time plot.
Figure 2. Tumor and normal tissue distribution of 111In-cetuximab.(A) Athymic mice bearing 5 d LS-174T i.p. tumor xenografts were injected i.p. with 111In-cetuximab (∼7.5 μCi). The mice (n = 5) were euthanized at 24 (![]()
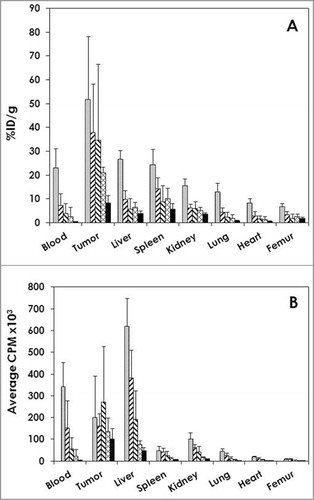
Of the normal organs, the highest %ID/g was observed in the liver (26.8 ± 3.4) and the spleen (24.5 ± 6.2) at 24 h; both decreased by 168 h (4.0 ± 1.1 and 5.8 ± 2.2, respectively). The high %ID/g of these normal organs at 24 h and subsequent decrease corresponds with the trend observed in the blood. The blood presented with the next highest %ID/g at 24 h with a value of 23.1 ± 7.86 and ended with %ID/g of 0.5 ± 0.22 at 168 h.
Determination of effective therapeutic dose of 212Pb-cetuximab
Having validated i.p. injected cetuximab as a vehicle for targeting i.p. tumor xenografts, a therapy study was then designed and performed to establish an effective therapeutic dose. When combining cetuximab RIT with chemotherapeutics, the lower range of the maximum effective therapeutic dose is desired to avoid obscuring any potentiation of therapy.
Athymic mice bearing a 3 d i.p. tumor (LS-174T) burden were injected i.p. with increasing doses (10–40 μCi) of 212Pb-cetuximab. As illustrated in , therapeutic efficacy was observed at all doses. A median survival (MS) of >294, 148, 235 and 223 d was achieved with 10, 20, 30 and 40 μCi of 212Pb-cetuximab, respectively (p = < 0.001). A MS, in fact, could not be determined for the 10 μCi treatment group. At 294 d, when the experiment was terminated, 6 of 10 mice were still alive. Compared to the set of mice that were not treated (28 d MS), this represents a therapeutic index (treatment MS divided by the untreated MS) of >10.5, 5.3, 8.4 and 8.0 for the mice treated with 10, 20, 30 and 40 μCi of 212Pb-cetuximab, respectively. In contrast, the mice treated with 20 and 40 μCi of 212Pb-HuIgG, experienced a MS of 33 and 50 d, corresponding to only 1.2 (p = 0.936) and 1.8-fold (p = 0.796) greater than that of the untreated group. There was a significant difference between the groups that received 20 μCi (p = 0.004) or 40 uCi (p = 0.022) of 212Pb-labeled cetuximab and HuIgG.
Figure 3. Effect of increasing doses of 212Pb-cetuximab. Kaplan-Meier survival curves of mice (n = 10) bearing i.p. LS-174T tumor xenografts and treated with increasing doses of i.p. injected 212Pb-cetuximab (10, 20, 30 and 40 μCi) or the control antibody, 212Pb-HuIgG (20 and 40 μCi) and compared to a group of mice that were not treated.
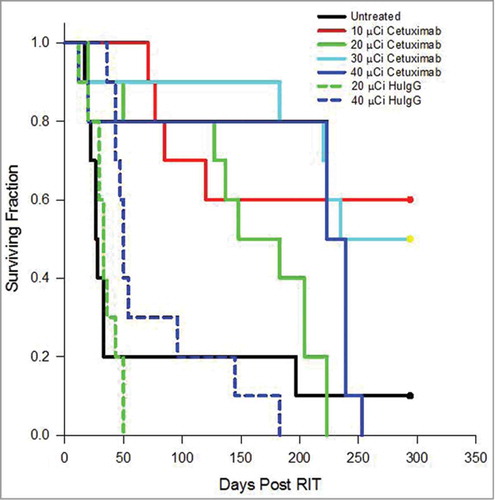
Examination of () the animals’ weights, used as an indicator of toxicity, showed that, among the groups injected with 212Pb-cetuximab, the 30 μCi and 40 μCi groups lost 8.8% and 9.1% of their body weight, respectively, 9 d after treatment. However, after 4 weeks, the mice appear to have recovered because they attained the body weight recorded at the beginning of the therapy study. The 20 μCi group experienced a modest weight loss of 2.8% after 9 d and the group treated with 10 μCi of 212Pb-cetuximab showed no weight loss. There was no statistical difference between the weights of the untreated group and the groups receiving either 10 μCi (p = 0.4656) or 20 μCi (p = 0.1008) of 212Pb-cetuximab. In contrast, the weight loss was more dramatic in the mice receiving the 212Pb-HuIgG; at 11 d there was a weight loss of 24% for the 20 μCi dose (p = 0.0060) and 36% for the 40 μCi (p = 0.0029). This latter group of mice did not regain weight. Simply based on the superior therapeutic index along with the lack of “toxicity,” 10 μCi was chosen as the effective therapeutic dose for subsequent studies with 212Pb-cetuximab, thereby also making a direct comparison with 212Pb-trastuzumab possible.
Table 1. Effect of increasing doses 212Pb-cetuximab i.p. therapy on the weights of athymic mice bearing LS-174T i.p. tumor xenografts
Confirmation of 212Pb-cetuximab dose for subsequent RIT studies
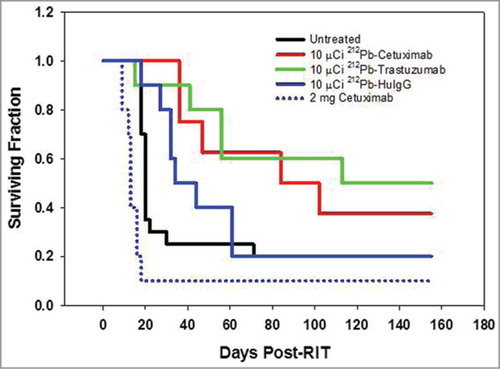
An experiment to validate the dose of 212Pb-cetuximab was then performed. In the same study, the therapeutic efficacy of targeting HER1 with 212Pb-cetuximab was compared to that of targeting HER2 with 212Pb-trastuzumab. Cohorts of 10 mice each, bearing i.p. LS-174T tumor xenografts, were treated with 10 μCi of 212Pb-labeled cetuximab, trastuzumab or the non-specific polyclonal 212Pb-HuIgG. While, 212Pb-cetuximab was effective with a MS of 84 d and a therapeutic index of 4.2 (), greater therapeutic efficacy was observed with 212Pb-trastuzumab with a MS of 113 d and a therapeutic index of 5.7. The differences in survival of the two groups, however, were not significant (p = 0.588). As is usually observed, the 212Pb-HuIgG also provided some therapy with a MS of 34 d. All groups are compared to the untreated group of mice with a MS of 20 d. To determine whether or not unlabeled cetuximab alone had any therapeutic effect on the LS-174T tumor xenografts, one set of mice (n = 10) was treated with 2 mg of cetuximab. With a MS of 13 d, cetuximab alone at this protein dose and scheduling does not appear to have any therapeutic effect on the LS-174T tumor xenografts. Consistent with the previous experiment, no weight loss was observed in the tumor-bearing mice treated with 10 μCi of 212Pb-cetuximab. There was also no weight loss in the groups that were injected with 212Pb-trastuzumab, HuIgG or the unlabeled cetuximab (data not presented).
Figure 4. Validation of the effective working dose of 212Pb-cetuximab therapy. Kaplan-Meier survival curves of mice (n = 10) bearing i.p. LS-174T tumor xenografts were injected i.p. with 10 μCi 212Pb-labeled cetuximab and compared to 212Pb-trastuzumab. Additional groups of mice included untreated and 212Pb-HuIgG. Another group of mice received a single dose of unlabeled cetuximab (2 mg).
Combination of chemotherapeutics with 212Pb-cetuximab therapy
The next round of studies focused on the potentiation of HER1-targeted RIT by chemotherapeutics. Previous studies from this laboratory combining gemcitabine (GEM), paclitaxel or carboplatin with 213Bi- or 212Pb-labeled trastuzumab have demonstrated augmentation of RIT efficacy. The pretreatment of mice bearing i.p. tumor (LS-174T) xenografts (), 24 h prior to injection of 212Pb-cetuximab (10 μCi), with 1 mg of GEM improved the median survival (283 d) by 109 d compared to mice that received 212Pb-cetuximab only (MS of 174 d). This increase in MS translates to a therapeutic index of 11.8, but it was not significant when compared to the group treated with 212Pb-cetuximab alone (p = 0.724). Consistent with previous reports from this laboratory, the GEM pretreatment also resulted in an increase in the therapeutic efficacy of the 212Pb-HuIgG group. In this instance, the MS was 49 d, a 2-fold increase in survival compared to the mice treated with only the 212Pb-HuIgG.
Table 2. Median survival (days) of athymic mice bearing i.p. LS-174T xenografts following i.p. administration of 212Pb-cetuximab and chemotherapeutics
Each of the treatment groups experienced weight loss 1 week after RIT administration (). The greatest weight loss (2.9 g) was observed in the group that received the combination of GEM and 212Pb-cetuximab; this weight loss was statistically significant compared to the control group (p = 0.0058). The next greatest weight loss was observed in the group of mice treated with GEM alone (2.4 g; p = 0.341). Recovery from the weight, in all treatment groups, was evident 11–14 d post-RIT. Complete recovery from the weight loss occurred 25–28 d post RIT.
Table 3. Effect of chemotherapeutics in combination with 212Pb-radioimmunotherapy on the weights of athymic mice bearing LS-174T i.p. tumor xenografts
The combination of carboplatin with 212Pb-cetuximab proved less successful. Following the same i.p. administration schedule as was determined with 212Pb-trastuzumab, carboplatin (1.25 mg) was injected concurrently with the 212Pb-RIT ().Citation3 Carboplatin alone resulted in a modest therapeutic effect on the LS-174T i.p. tumor xenografts with a MS of 31 d and a therapeutic index of 1.3. When combined with 212Pb-cetuximab, a MS of 80 d with a therapeutic index of 3.3 was realized. The carboplatin combined with 212Pb-HuIgG also provided some therapeutic benefit with a MS of 43 d, but this result does not compare well to the MS of 174 d in the group of tumor-bearing mice that received just the 212Pb-cetuximab.
Again, weight loss, which ranged from 1.3 to 3.2 g, occurred by 7 d post-RIT in each of the treatment groups, with the exception of the group treated with only carboplatin (). The greatest loss was observed in the mice treated with the carboplatin and 212Pb-cetuximab (p = 0.034). Recovery from the weight loss was detected as early as 11 d, and was evident in all of the affected groups by 21 d.
Targeting of multiple antigens to augment efficacy of 212Pb RIT
The targeting of multiple distinct molecules in tumors is another strategy being pursued by investigators in the field of RIT.Citation27-Citation31 A pilot study was conducted to explore the potential of dual targeting of HER2 and HER1 with 212Pb-labeled mAbs as a natural extension of the studies described herein.
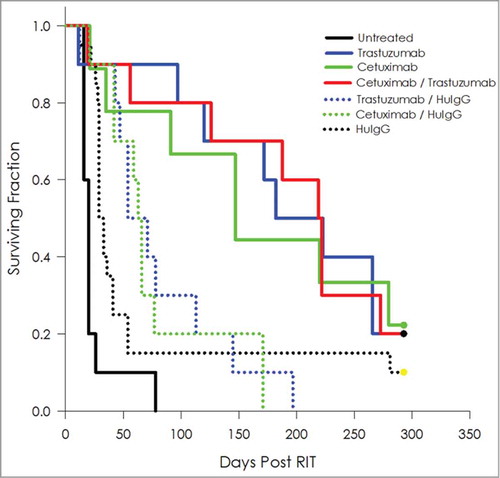
Tumor-bearing mice treated with 10 μCi of 212Pb-labeled cetuximab or trastuzumab experienced a MS of 147 and 182 d, respectively, an improvement in survival of 7.4- and 9.1-fold compared to the untreated group (, ). When tumor-bearing mice were treated with a formulation of 5 μCi 212Pb-cetuximab and 5 μCi 212Pb-trastuzumab, the MS was 219 d, which translates into a therapeutic index of 11. Some therapeutic benefit was derived from the combination of 212Pb-labeled cetuximab/HuIgG (MS = 63 d) and trastuzumab/HuIgG (MS = 71 d).
Table 4. Median survival (days) of athymic mice bearing i.p. LS-174T xenografts following dual administration (i.p.) of 212Pb-labeled cetuximab and trastuzumab targeting HER1 and HER2
Figure 5. Targeting of multiple antigens to augment efficacy of 212Pb RIT. Kaplan-Meier survival curves of mice (n = 10) bearing i.p. LS-174T tumor xenografts were co-injected i.p. with 5 μCi each of 212Pb-cetuximab and 212Pb-trastuzumab. These were compared to groups injected with the combination of 212Pb-cetuximab / 212Pb-HuIgG and 212Pb-trastuzumab / 212Pb-HuIgG. Additional groups of mice included untreated, 212Pb-cetuximab, 212Pb-trastuzumab and 212Pb-HuIgG. A total of 10 μCi 212Pb was administered to each mouse.
The greatest weight loss was observed in the groups that received 212Pb-cetuximab/212Pb-HuIgG (8.2% loss) and 212Pb-trastuzumb/212Pb-HuIgG (6.8% loss). The mice receiving the combination of 212Pb-cetuximab and 212Pb-trastuzumab maintained their weight following the treatment ().
Table 5. Effect of dual targeting using dual targeted 212Pb-radioimmunotherapy on the weights of athymic mice bearing LS-174T i.p. tumor xenografts
Discussion
The ultimate objective of studies within this laboratory is to develop a multi-modality treatment regimen for cancer patients with residual tumor tissue following surgical debulking/resection, micrometastic tumor or disseminated peritoneal disease. Having established that α-particle radiation has great potential in the treatment of cancers such as pancreatic, ovarian and others that can present as disseminated intraperitoneal disease, using 213Bi- and 212Pb-labeled trastuzumab to target HER2, subsequent studies have concentrated on exploring the potential of integration of chemotherapeutics (gemcitabine, paclitaxel and carboplatin) to enhance the efficacy of α-RIT. Adding cetuximab, which targets HER1, would provide another possibility or combination for the treatment and management of cancer patients.
The epidermal growth factor receptor, EGFR/HER1, continues to be a molecule of great interest and focus of investigators for the development of targeted therapies. Primarily, the strategy has been to inhibit or block the activation of tyrosine kinase (TK) by using small molecule inhibitors or large molecules such as mAbs. The former exert their effect intracellularly, preventing TK phosphorylation, while the latter interact with the extracellular domains of the receptor, blocking ligand binding. When combined with other chemotherapeutics or radiation, EGFR inhibitors have been shown to potentiate therapeutic efficacy. Two HER1-binding mAbs, cetuximab (Erbitux®) and panitumumab (Vectibix®), have been approved by the US Food and Drug Administration. Both mAbs have demonstrated sensitization of tumor cells to chemotherapy and radiation in pre-clinical and clinical studies.Citation32–Citation34
Studies from this laboratory and others have reported on the potential of HER1 as a target for molecular imaging and for targeted radiation therapy.Citation21-Citation24,Citation26,Citation35-Citation38 Cetuximab has been the focus of several imaging studies with the objective of assessing the value of this mAb for disease monitoring, HER1 expression and distribution, patient selection and for performing dosimetric calculations.Citation23-Citation26 The preclinical studies from this laboratory demonstrated excellent targeting of s.c. tumor by 111In-cetuximab given intravenously.Citation23 Targeting was obtained in 5 tumor models (2 colorectal, 1 prostate, 1 pancreatic and 1 ovarian), determined by direct quantitation of tissues and by planar γ-scintigraphy. Citation23 Meanwhile, minimal normal tissue uptake was also observed in these models, as well as in a melanoma tumor model (A375) in which tumor uptake of the radioimmunoconjugate was also minimal. The studies were extended to evaluating cetuximab for positron-emissions tomography (PET) imaging. PET imaging was performed with the models mentioned above using cetuximab radiolabeled with 86Y using the 2-(p-isothiocyanatobenzyl)-cyclohexyl-diethylenetriaminepentaacetic acid (CHX-A”-DTPA) chelate along with 3 additional models for mesothelioma.Citation24,Citation25 The results from all of these studies suggest that cetuximab not only has potential as a diagnostic agent, but also for RIT applications.
This hypothesis is corroborated by the available literature. Imaging has been demonstrated using either 64Cu- or 89Zr-cetuximab, while therapy has been conducted with 90Y- or 177Lu-cetuximab.Citation21,Citation22,Citation26,Citation37 Unfortunately, close inspection of these reports reveals a range of problems with study design. In the case of 64Cu, the chelate/Cu complex was not stable and 64Cu was steadily released from the radioimmunoconjugate to be sequestered in normal tissue. The use of 89Zr for PET imaging currently presents with the same problem.Citation39,Citation40 Cetuximab radiolabeled with 177Lu appears promising. The treatment of mice bearing s.c. head and neck tumor xenografts with a single dose of 177Lu-cetuximab resulted in a ∼3 week delay of tumor growth.Citation21 In this instance however, a negative 177Lu-labeled control was not included in the study to account for the effect of non-specific radiation. At this juncture, to the best knowledge of the authors, this study represents the first report in which cetuximab is used to target HER1-expressing tumors with α-particle radiation.
Although studies from this laboratory had shown tumor targeting of 111In-cetuximab in a s.c. model, it was deemed necessary to establish that i.p. administration of 111In-cetuximab would be just as effective. Indeed, the locoregional delivery of the radioimmunoconjugate proved as successful, attaining a tumor%ID/g at 24 h that was comparable to that published for a s.c. tumor xenograft at 72 h.Citation23 With this encouraging result, radioimmunotherapy studies were conducted.
As mentioned, the conjugation of cetuximab with the TCMC chelate and subsequent labeling with 212Pb was routine and were well tolerated by the mAb. The chelate:mAb ratio were consistent with prior results obtained with the conjugation of trastuzumab with the TCMC ligand. Mice tolerated the 10 or 20 μCi of 212Pb-cetuximab with minimal indications of toxicity. Anticipating that 212Pb-cetuximab would be assessed in combination with chemotherapeutic agents, the 10 μCi dose was selected as the effective “working” dose. As stated earlier, the lower range of the maximum effective therapeutic dose is desired to avoid obscuring any potentiation of therapy by the chemotherapeutic. A direct comparison of 212Pb-cetuximab with 212Pb-trastuzumab established 212Pb-cetuximab as a viable radioimmunotherapeutic agent. Differences in the therapeutic efficacy of the 2 radioimmunoconjugates were not significant.
Pre-treatment of tumor-bearing mice with gemcitabine prior to 212Pb-cetuximab augmented the therapeutic efficacy of the radioimmunoconjugate, increasing the therapeutic index from 7.3 to 11.8. This overall tumor response was similar to mice pretreated with GEM prior to injection with 212Pb-trastuzumab.4 GEM increases the survival of mice receiving either 212Pb-labeled cetuximab or trastuzumab by 1.6-fold. Treatment of tumor-bearing mice with carboplatin appears to inhibit or suppress the therapeutic efficacy of 212Pb-cetuximab. This result is contrary to what was observed when tumor-bearing mice were treated with carboplatin in combination with 212Pb-trastuzumab.Citation3 An evaluation of paclitaxel in combination with 212Pb-cetuximab has yet to be performed.
The heterogeneous nature of tumors presents an obstacle to delivering a therapeutic radiation dose throughout a tumor mass.Citation41 Fortunately, an advantage of RIT is that not all cells in a tumor need express the target molecule nor does that molecule need to be expressed in high numbers. Neighboring cells may receive cytotoxic doses, courtesy of the omnidirectional decay of radioactivity. α-Particle radiation provides an additional benefit. Estimates are that only 3 to 6 traversals of a cell nucleus by an α-particle brings about cell death; the dose rate is estimated to be as low as 1 centigray per hour.Citation42-Citation45 Targeting of multiple antigens in tumors is another strategy for overcoming heterogeneity and thus improving the therapeutic efficacy of RIT.Citation27-Citation31 A report from this laboratory demonstrated that this approach is feasible for α-particle-targeted radiation therapy.Citation30 The concurrent administration of 213Bi-labeled trastuzumab and the 213Bi-labeled humanized ΔCH2 variant of CC49 resulted in an enhanced, additive, therapeutic benefit. Co-injection of these 2 213Bi-labeled antibodies extended the MS of mice bearing i.p. tumors by almost 5-fold compared to mice treated with either antibody alone.Citation30 Although the approach of combining antibodies to target multiple antigens was not without precedence, the studies had been conducted with β− -emitting radionuclides. Prior to the present study, and the earlier report from this laboratory, the therapeutic efficacy of α-radiation using 213Bi-labeled antibodies, has been evaluated in vitro with prostate cancer cells, grown in monolayer or as spheroids.Citation46-Citation48 In the present study, the extension of the MS of mice treated with the combination of 212Pb-labeled cetuximab and trastuzumab added an additional 37 and 72 d, respectively. This improved therapy was clearly specific because mice treated with a combination of 212Pb-labeled cetuximab and HuIgG or trastuzumab and HuIgG experienced only modest improvements in the MS. To better understand the therapeutic effect of dual targeting with cetuximab and trastuzumab, studies are currently underway investigating the expression and distribution of both HER2 and HER1 in the LS-174T i.p. tumor xenografts.
As these studies with 212Pb-cetuximab may be translated to a clinical trial, as was 212Pb-trastuzumab, the evaluation of this radioimmunoconjugate will move forward. Future studies will include those required for the filing of an investigational new drug application, e.g., evaluation of the stability of both cetuximab-TCMC and 212Pb-cetuximab. Studies are also proposed to elucidate mechanisms associated with 212Pb-cetuximab therapy, as well as the dual targeting 212Pb RIT and the combination therapy with chemotherapeutics.
In summary, as a vehicle for the targeting of α-particle radiation, cetuximab was effective in extending the median survival of mice bearing i.p. tumor xenografts. This therapeutic efficacy was enhanced by chemotherapeutics, as well as by the targeting of 2 antigens expressed by the i.p. LS-174T tumor xenografts. The studies demonstrate that, with careful consideration of cancer cell targets and targeting vehicles along with methodical evaluation, 212Pb-RIT has enormous potential to provide effective therapies for cancer. It remains clear that despite the exquisite therapeutic impact delivered by a single dose of a singularly-targeted α-therapeutic agent, that integration of this modality with both chemotherapeutics as well as delivery to multiple molecular targets, e.g., HER2, HER1, should prove most efficacious for treating cancer. The studies described herein represent another step toward providing choices of treatment for cancer patients.
Materials and Methods
Ethics statements
All animal protocols were approved by the National Cancer Institute Animal Care and Use Committee.
Cell lines
Media and supplements were purchased from Lonza unless otherwise indicated. Therapy studies were conducted using the LS-174T, a human colon carcinoma cell line, grown in Dulbecco's minimum essential medium (12–614Q). The medium was supplemented with 1 mM glutamine 17–605E), 10% FetalPlex (Gemini Bioproducts, Inc.; 100–602) and 1 mM non-essential amino acids 13–114E) as previously described.Citation49,Citation50
Chelate synthesis and mAb conjugation
Cetuximab (Erbitux®; Amgen, Inc.), was purchased through the National Institute of Health (NIH), Division of Veterinary Resources Pharmacy. Conjugation of cetuximab with the bifunctional ligands, TCMC and CHX-A”-DTPA, was performed according to established methods that have been previously described in detail.Citation51,Citation52 The final concentration of cetuximab was determined by the Lowry method using a BSA standard.Citation53 The number of CHX-A”-DTPA or TCMC molecules linked to cetuximab was quantitated using spectrophotometric assays based on the titration of either ytrrium- or lead-Arsenazo(III) complex, respectively.Citation54,Citation55 Polyclonal HuIgG (MP Biochemicals; 64145), chosen to serve as a negative control in these studies, was similarly conjugated with CHX-A″-DTPA or TCMC in parallel and evaluated as described above. The HuIgG is purified from human serum, and to date no known antigen has been described with which it reacts. Trastuzumab, conjugated with TCMC, was utilized in one study to allow a direct comparison of the therapeutic efficacy of cetuximab to that of trastuzumab when radiolabeled with 212Pb.
Radiolabeling
Radiolabeling of CHX-A”-cetuximab (50 μg in 100 μL of 0.15 M NH4OAc buffer, pH 7.0) with 111In (PerkinElmer) has been detailed elsewhere.Citation23 For these studies, 212Pb was obtained from a 224Ra/212Pb generator (AlphaMed, Inc..). Elution of the 212Pb and radiolabeling of cetuximab-, trastuzumab- and HuIgG-TCMC″ was performed as previously described.Citation2,Citation7
Radioimmunoassays
The immunoreactivity of the cetuximab-TCMC conjugate was evaluated in a competition radioimmunoassay as outlined in an earlier publication using purified human epidermal growth factor receptor (EGFR; Sigma-Aldrich, E3641–500UN).Citation56 Briefly, EGFR was allowed to adsorb to the wells of a 96-well plate, the EGFR was removed and 1% bovine serum albumin in phosphate buffered saline (BSA/PBS; 100 μL) was added to each well. Following a 0.5–1 h incubation at room temperature, the solution was removed and serial dilutions of the immunoconjugate (500 to 0.01 ng in 25 μL) in BSA/PBS were added to the wells in triplicate. Following the addition of 125I-cetuximab (50,000 cpm/25 μL) to each of the wells, the plates were incubated 4 h at 37°C. The wells were washed, the radioactivity dissociated from the wells with 0.1 M NaOH (100 μL), adsorbed to cotton filters and counted in a γ-scintillation counter. The immunoconjugate was compared to unmodified cetuximab. The percent inhibition was calculated using the buffer control and plotted. HuM195, a mAb that reacts with human CD33, served as a negative control.
The immunoreactivity of the 111In- and 212Pb-cetuximab was assessed in a radioimmunoassay using purified EGFR (100 ng per well). After absorption of EGFR to the wells of a 96-well plate, the EGFR was removed and the wells treated as described above. Serial dilutions of radiolabeled cetuximab (∼200,000 cpm to 12,500 cpm in 50 μL of BSA/PBS) were added to the wells and incubated for 4 h incubation at 37°C. The wells were washed, the radioactivity harvested and counted in a γ-scintillation counter again as just described. The percentage binding was calculated for each dilution and averaged. The specificity of the radiolabeled cetuximab was confirmed by incubating one set of wells with radiolabeled cetuximab and 10 μg of unlabeled cetuximab.
In Vivo Studies
All in vivo studies were performed using 5 - 6 week old female athymic (NCr-nu/nu) mice (NCI-Frederick, Cat#01B70).
Tumor targeting
Mice were injected intraperitoneally (i.p.) with 1×108 LS-174T cells in 1 mL of medium and utilized in tumor targeting studies 5 d later. Mice (n = 5) were injected i.p. with 111In-CHX-A”-cetuximab (∼7.5 μCi on 0.6 μg) and euthanized 24 to 168 h by exsanguination. The blood, tumor and major organs were collected, wet-weighed, and counted in a γ-scintillation counter. The percent injected dose per gram (%ID/g) and standard deviation were calculated.
Therapy
RIT studies detailed below were initiated at 2–3 d following i.p. injection with LS-174T as described above. 212Pb-trastuzumab (10 μCi), was administered i.p. to mice in 0.5 mL PBS. 212Pb-HuIgG served as a non-specific control in these studies. The mice were monitored daily and body weight was measured and recorded 1–2 times per week for 4–6 weeks as a measure of toxicity due to therapy. Progression of disease was observed either as an extension of the abdomen, development of ascites or noticeable, palpable, nodules in the abdomen or, conversely, as weight loss. Mice were euthanized if found to be in distress, moribund, or cachectic. Euthanasia was also performed when a 10–20% weight loss occurred, or when disease progression was evident as cited above.
Study 1 was conducted to establish the effective working dose of 212Pb-cetuximab for the therapy of HER1-positive i.p. tumor xenografts. Tumor-bearing mice (n = 10) were given increasing doses of 212Pb-cetuximab (10, 20, 30 and 40 μCi) by i.p. injection, 212Pb-HuIgG (20 and 40 μCi) or no RIT.
A subsequent experiment (Study 2) was conducted to confirm the effective working dose of 10 uCi. This study was also performed to directly compare the therapeutic efficacy of targeting HER1 with 212Pb-cetuximab to that of 212Pb-trastuzumab targeting of HER2. Mice (n = 10) bearing i.p. LS-174T xenografts were injected (i.p.) with 10 μCi of 212Pb-labeled cetuximab, trastuzumab or HuIgG. An additional set of mice were injected with 2 mg of unlabeled cetuximab to assess the potential contribution that the mAb as a stand-alone therapeutic would have toward therapy of the LS-174T tumor xenografts.
The studies with 212Pb-cetuximab were then extended to investigate potential enhancement of therapeutic efficacy by the inclusion of chemotherapeutics in the treatment regimen. Studies were conducted with gemcitabine (GEMZAR; Eli Lilly and Company) and carboplatin (Hospira, Inc.). Both chemotherapeutics were purchased through the NIH, Division of Veterinary Resources Pharmacy. In Study 3, mice (n = 10) bearing i.p. LS-174T tumors were injected i.p. with 1 mg of gemcitabine (GEM) or 1.25 mg of carboplatin, 2 d post tumor cell implantation, followed 24 h later with 212Pb-cetuximab (10 μCi). The decision to administer the carboplatin 24 h before 212Pb-cetuximab was based on data obtained with 212Pb-trastuzumab.3 These treatment groups were compared to mice pre-treated with GEM or carboplatin followed by 212Pb-HuIgG. Control groups included mice receiving no treatment, 212Pb-cetuximab, 212Pb-HuIgG, carboplatin or GEM only.
Lastly, a pilot study (Study 4) was conducted to determine whether or not the concurrent targeting of HER2 and HER1 expressed on a tumor would provide greater therapeutic benefit. Tumor-bearing mice were given a single i.p. administration of a solution containing 5 μCi of each antibody (10 μCi total) 212Pb-cetuximab / 212Pb-trastuzumab, 212Pb-cetuximab / 212Pb-HuIgG, or 212Pb-HuIgG / 212Pb-trastuzumab. Other treatment groups included each of the 212Pb-labled antibodies at 10 μCi and one group of mice that were not treated.
Statistical analyses
Kaplan-Meier survival (time to sacrifice or natural death) analysis was conducted using SigmaPlot 12.5; groups were compared using a log-rank test. A pairwise comparison was performed to test for differences between treatment groups (Holm-Sidak method). All reported p-values correspond to 2-sided tests.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin Cancer Res 2004; 10:7834-41; PMID:15585615; http://dx.doi.org/10.1158/1078-0432.CCR-04-1226
- Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. Alpha-particle radioimmunotherapy of disseminated peritoneal disease using a (212)Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm 2005; 20:557-68; PMID:16248771; http://dx.doi.org/10.1089/cbr.2005.20.557
- Milenic DE, Baidoo KE, Shih JH, Wong KJ, Brechbiel MW. Evaluation of platinum chemotherapy in combination with HER2-targeted alpha-particle radiation. Cancer Biother Radiopharm 2013; 28:441-9; PMID:23758610; http://dx.doi.org/10.1089/cbr.2012.1423
- Milenic DE, Garmestani K, Brady ED, Albert PS, Abdulla A, Flynn J, Brechbiel MW. Potentiation of high-LET radiation by gemcitabine: targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clin Cancer Res 2007; 13:1926-35; PMID:17363549; http://dx.doi.org/10.1158/1078-0432.CCR-06-2300
- Milenic DE, Garmestani K, Brady ED, Baidoo KE, Albert PS, Wong KJ, Flynn J, Brechbiel MW. Multimodality therapy: potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin Cancer Res 2008; 14:5108-15; PMID:18698028; http://dx.doi.org/10.1158/1078-0432.CCR-08-0256
- Howell RW, Azure MT, Narra VR, Rao DV. Relative biological effectiveness of alpha-particle emitters in vivo at low doses. Radiat Res 1994; 137:352-60; PMID:8146279; http://dx.doi.org/10.2307/3578710
- Baidoo KE, Milenic DE, Brechbiel MW. Methodology for labeling proteins and peptides with lead-212 (212Pb). Nucl Med Biol 2013; 40:592-9; PMID:23602604; http://dx.doi.org/10.1016/j.nucmedbio.2013.01.010
- Meredith RF, Torgue J, Azure MT, Shen S, Saddekni S, Banaga E, Carlise R, Bunch P, Yoder D, Alvarez R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother Radiopharm 2014; 29:12-7; PMID:24229395; http://dx.doi.org/10.1089/cbr.2013.1531
- Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. (212)Pb-radioimmunotherapy induces G(2) cell-cycle arrest and delays DNA damage repair in tumor xenografts in a model for disseminated intraperitoneal disease. Mol Cancer Ther 2012; 11:639-48; PMID:22238365; http://dx.doi.org/10.1158/1535-7163.MCT-11-0671
- Yong KJ, Milenic DE, Baidoo KE, Kim YS, Brechbiel MW. Gene expression profiling upon (212) Pb-TCMC-trastuzumab treatment in the LS-174T i.p. xenograft model. Cancer Med 2013; 2:646-53; PMID:24403230
- Agus DB, Bunn PA, Jr., Franklin W, Garcia M, Ozols RF. HER-2/neu as a therapeutic target in non-small cell lung cancer, prostate cancer, and ovarian cancer. Semin Oncol 2000; 27:53-63; discussion 92-100.
- Natali PG, Nicotra MR, Bigotti A, Venturo I, Slamon DJ, Fendly BM, Ullrich A. Expression of the p185 encoded by HER2 oncogene in normal and transformed human tissues. Int J Cancer 1990; 45:457-61; PMID:1968437; http://dx.doi.org/10.1002/ijc.2910450314
- Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest 2001; 19:554-68; PMID:11458821; http://dx.doi.org/10.1081/CNV-100103852
- Lanitis E, Dangaj D, Hagemann IS, Song DG, Best A, Sandaltzopoulos R, Coukos G, Powell DJ, Jr. Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically-detectable levels. PLoS One 2012; 7:e49829; PMID:23189165
- Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer 2004; 11:689-708; PMID:15613446; http://dx.doi.org/10.1677/erc.1.00600
- Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer 2002; 94:1593-611; PMID:11920518; http://dx.doi.org/10.1002/cncr.10372
- Kim ES, Khuri FR, Herbst RS. Epidermal growth factor receptor biology (IMC-C225). Curr Opin Oncol 2001; 13:506-13; PMID:11673692; http://dx.doi.org/10.1097/00001622-200111000-00014
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351:337-45; PMID:15269313; http://dx.doi.org/10.1056/NEJMoa033025
- Humblet Y. Cetuximab: an IgG(1) monoclonal antibody for the treatment of epidermal growth factor receptor-expressing tumours. Expert Opin Pharmacother 2004; 5:1621-33; PMID:15212612; http://dx.doi.org/10.1517/14656566.5.7.1621
- Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007; 357:2040-8; PMID:18003960; http://dx.doi.org/10.1056/NEJMoa071834
- Guo Y, Parry JJ, Laforest R, Rogers BE, Anderson CJ. The role of p53 in combination radioimmunotherapy with 64Cu-DOTA-cetuximab and cisplatin in a mouse model of colorectal cancer. J Nucl Med 2013; 54:1621-9; PMID:23873478; http://dx.doi.org/10.2967/jnumed.112.118539
- Liu Z, Ma T, Liu H, Jin Z, Sun X, Zhao H, Shi J, Jia B, Li F, Wang F. Lu-Labeled Antibodies for EGFR-Targeted SPECT/CT Imaging and Radioimmunotherapy in a Preclinical Head and Neck Carcinoma Model. Mol Pharm 2014; 11: 800-807; http://dx.doi.org/10.1021/mp4005047
- Milenic DE, Wong KJ, Baidoo KE, Ray GL, Garmestani K, Williams M, Brechbiel MW. Cetuximab: preclinical evaluation of a monoclonal antibody targeting EGFR for radioimmunodiagnostic and radioimmunotherapeutic applications. Cancer Biother Radiopharm 2008; 23:619-31; PMID:18999934; http://dx.doi.org/10.1089/cbr.2008.0493
- Nayak TK, Garmestani K, Milenic DE, Baidoo KE, Brechbiel MW. HER1-targeted 86Y-panitumumab possesses superior targeting characteristics than 86Y-cetuximab for PET imaging of human malignant mesothelioma tumors xenografts. PLoS One 2011; 6:e18198; PMID:21464917; http://dx.doi.org/10.1371/journal.pone.0018198
- Nayak TK, Regino CA, Wong KJ, Milenic DE, Garmestani K, Baidoo KE, Szajek LP, Brechbiel MW. PET imaging of HER1-expressing xenografts in mice with 86Y-CHX-A’’-DTPA-cetuximab. Eur J Nucl Med Mol Imaging 2010; 37:1368-76; PMID:20155263; http://dx.doi.org/10.1007/s00259-009-1370-z
- Perk LR, Visser GW, Vosjan MJ, Stigter-van Walsum M, Tijink BM, Leemans CR, van Dongen GA. (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med 2005; 46:1898-906; PMID:16269605
- Gaffar SA, Pant KD, Shochat D, Bennett SJ, Goldenberg DM. Experimental studies of tumor radioimmunodetection using antibody mixtures against carcinoembryonic antigen (CEA) and colon-specific antigen-p (CSAp). Int J Cancer 1981; 27:101-5; PMID:7251228; http://dx.doi.org/10.1002/ijc.2910270116
- Hillairet de Boisferon M, Raguin O, Dussaillant M, Rostene W, Barbet J, Gruaz-Guyon A. Enhanced targeting specificity to tumor cells by simultaneous recognition of two antigens. Bioconjug Chem 2000; 11:452-60; PMID:10898565; http://dx.doi.org/10.1021/bc9901090
- Hillairet De Boisferon M, Raguin O, Thiercelin C, Dussaillant M, Rostene W, Barbet J, Pelegrin A, Gruaz-Guyon A. Improved tumor selectivity of radiolabeled peptides by receptor and antigen dual targeting in the neurotensin receptor model. Bioconjug Chem 2002; 13:654-62; PMID:12009958; http://dx.doi.org/10.1021/bc015585g
- Milenic DE, Brady ED, Garmestani K, Albert PS, Abdulla A, Brechbiel MW. Improved efficacy of alpha-particle-targeted radiation therapy: dual targeting of human epidermal growth factor receptor-2 and tumor-associated glycoprotein 72. Cancer 2010; 116:1059-66; PMID:20127951; http://dx.doi.org/10.1002/cncr.24793
- Munz DL, Alavi A, Koprowski H, Herlyn D. Improved radioimmunoimaging of human tumor xenografts by a mixture of monoclonal antibody F(ab’)2 fragments. J Nucl Med 1986; 27:1739-45; PMID:3772507
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567-78; PMID:16467544; http://dx.doi.org/10.1056/NEJMoa053422
- Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010; 11:21-8; PMID:19897418; http://dx.doi.org/10.1016/S1470-2045(09)70311-0
- Kruser TJ, Armstrong EA, Ghia AJ, Huang S, Wheeler DL, Radinsky R, Freeman DJ, Harari PM. Augmentation of radiation response by panitumumab in models of upper aerodigestive tract cancer. Int J Radiat Oncol Biol Phys 2008; 72:534-42; PMID:18793955; http://dx.doi.org/10.1016/j.ijrobp.2008.06.1490
- Koi L, Bergmann R, Bruchner K, Pietzsch J, Pietzsch HJ, Krause M, Steinbach J, Zips D, Baumann M. Radiolabeled anti-EGFR-antibody improves local tumor control after external beam radiotherapy and offers theragnostic potential. Radiother Oncol 2014; 110:362-9; PMID:24440046; http://dx.doi.org/10.1016/j.radonc.2013.12.001
- Nayak TK, Garmestani K, Baidoo KE, Milenic DE, Brechbiel MW. Preparation, biological evaluation, and pharmacokinetics of the human anti-HER1 monoclonal antibody panitumumab labeled with 86Y for quantitative PET of carcinoma. J Nucl Med 2010; 51:942-50; PMID:20484421; http://dx.doi.org/10.2967/jnumed.109.071290
- Niu G, Sun X, Cao Q, Courter D, Koong A, Le QT, Gambhir SS, Chen X. Cetuximab-based immunotherapy and radioimmunotherapy of head and neck squamous cell carcinoma. Clin Cancer Res 2010; 16:2095-105; PMID:20215534; http://dx.doi.org/10.1158/1078-0432.CCR-09-2495
- Song H, Hedayati M, Hobbs RF, Shao C, Bruchertseifer F, Morgenstern A, Deweese TL, Sgouros G. Targeting aberrant DNA double-strand break repair in triple-negative breast cancer with alpha-particle emitter radiolabeled anti-EGFR antibody. Mol Cancer Ther 2013; 12:2043-54; PMID:23873849; http://dx.doi.org/10.1158/1535-7163.MCT-13-0108
- Guerard F, Lee YS, Brechbiel MW. Rational design, synthesis, and evaluation of tetrahydroxamic acid chelators for stable complexation of zirconium(IV). Chemistry 2014; 20:5584-91; PMID:24740517; http://dx.doi.org/10.1002/chem.201304115
- Guerard F, Lee YS, Tripier R, Szajek LP, Deschamps JR, Brechbiel MW. Investigation of Zr(IV) and 89Zr(IV) complexation with hydroxamates: progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem Commun (Camb) 2013; 49:1002-4; PMID:23250287; http://dx.doi.org/10.1039/c2cc37549d
- Hand PH, Nuti M, Colcher D, Schlom J. Definition of antigenic heterogeneity and modulation among human mammary carcinoma cell populations using monoclonal antibodies to tumor-associated antigens. Cancer Res 1983; 43:728-35; PMID:6848188
- Baidoo KE, Yong K, Brechbiel MW. Molecular pathways: targeted alpha-particle radiation therapy. Clin Cancer Res 2013; 19:530-7; PMID:23230321; http://dx.doi.org/10.1158/1078-0432.CCR-12-0298
- Hei TK, Wu LJ, Liu SX, Vannais D, Waldren CA, Randers-Pehrson G. Mutagenic effects of a single and an exact number of alpha particles in mammalian cells. Proc Natl Acad Sci U S A 1997; 94:3765-70; PMID:9108052; http://dx.doi.org/10.1073/pnas.94.8.3765
- Pugliese M, Durante M, Grossi GF, Monforti F, Orlando D, Ottolenghi A, Scampoli P, Gialanella G. Inactivation of individual mammalian cells by single alpha-particles. Int J Radiat Biol 1997; 72:397-407; PMID:9343105; http://dx.doi.org/10.1080/095530097143176
- Soyland C, Hassfjell SP. Survival of human lung epithelial cells following in vitro alpha-particle irradiation with absolute determination of the number of alpha-particle traversals of individual cells. Int J Radiat Biol 2000; 76:1315-22; PMID:11057739; http://dx.doi.org/10.1080/09553000050151583
- Li Y, Abbas Rizvi SM, Blair nee Brown JM, Cozzi PJ, Qu CF, Ow KT, Tam PN, Perkins AC, Russell PJ, Allen BJ. Antigenic expression of human metastatic prostate cancer cell lines for in vitro multiple-targeted alpha-therapy with 213Bi-conjugates. Int J Radiat Oncol Biol Phys 2004; 60:896-908; PMID:15465208; http://dx.doi.org/10.1016/j.ijrobp.2004.04.035
- Li Y, Cozzi PJ, Qu CF, Zhang DY, Abbas Rizvi SM, Raja C, Allen BJ. Cytotoxicity of human prostate cancer cell lines in vitro and induction of apoptosis using 213Bi-Herceptin alpha-conjugate. Cancer Lett 2004; 205:161-71; PMID:15036648; http://dx.doi.org/10.1016/j.canlet.2003.10.035
- Wang J, Abbas Rizvi SM, Madigan MC, Cozzi PJ, Power CA, Qu CF, Morgenstern A, Apostolidis C, Russell PJ, Allen BJ, et al. Control of prostate cancer spheroid growth using 213Bi-labeled multiple targeted alpha radioimmunoconjugates. Prostate 2006; 66:1753-67; PMID:16955401; http://dx.doi.org/10.1002/pros.20502
- Milenic DE, Wong KJ, Baidoo KE, Nayak TK, Regino CA, Garmestani K, Brechbiel MW. Targeting HER2: a report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs 2010; 2:550-64; PMID:20716957; http://dx.doi.org/10.4161/mabs.2.5.13054
- Tom BH, Rutzky LP, Jakstys MM, Oyasu R, Kaye CI, Kahan BD. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro 1976; 12:180-91; PMID:1262041; http://dx.doi.org/10.1007/BF02796440
- Garmestani K, Yao Z, Zhang M, Wong K, Park CW, Pastan I, Carrasquillo JA, Brechbiel MW. Synthesis and evaluation of a macrocyclic bifunctional chelating agent for use with bismuth radionuclides. Nucl Med Biol 2001; 28:409-18; PMID:11395314; http://dx.doi.org/10.1016/S0969-8051(00)00203-1
- Wu C, Kobayashi H, Sun B, Yoo TM, Paik CH, Gansow OA, Carrasquillo JA, Pastan I, Brechbiel MW. Stereochemical influence on the stability of radio-metal complexes in vivo. Synthesis and evaluation of the four stereoisomers of 2-(p-nitrobenzyl)-trans-CyDTPA. Bioorg Med Chem 1997; 5:1925-34; PMID:9370037; http://dx.doi.org/10.1016/S0968-0896(97)00130-2
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193:265-75; PMID:14907713
- Dadachova E, Chappell LL, Brechbiel MW. Spectrophotometric method for determination of bifunctional macrocyclic ligands in macrocyclic ligand-protein conjugates. Nucl Med Biol 1999; 26:977-82; PMID:10708314; http://dx.doi.org/10.1016/S0969-8051(99)00054-2
- Pippin CG, Parker TA, McMurry TJ, Brechbiel MW. Spectrophotometric method for the determination of a bifunctional DTPA ligand in DTPA-monoclonal antibody conjugates. Bioconjug Chem 1992; 3:342-5; PMID:1390990; http://dx.doi.org/10.1021/bc00016a014
- Ray GL, Baidoo KE, Wong KJ, Williams M, Garmestani K, Brechbiel MW, Milenic DE. Preclinical evaluation of a monoclonal antibody targeting the epidermal growth factor receptor as a radioimmunodiagnostic and radioimmunotherapeutic agent. Br J Pharmacol 2009; 157:1541-8; PMID:19681874; http://dx.doi.org/10.1111/j.1476-5381.2009.00327.x
