Abstract
Heat shock proteins (HSP) represent important antigenic targets for the immune response, playing an important role in the pathology and infectious diseases control. The purpose of this work was to investigate the levels of HSP60 and HSP70 specific antibodies in the bloodstream of patients with different bacterial infections and cancer, in order to evaluate their potential role as diagnosis markers of different infectious diseases. Detection of specific anti-HSP 60 and HSP 70 serum levels was performed by ELISA. Statistical analysis of data by multivariate logistic regression was performed using GraphPadPrism software and statistical tests based on chi-square and Student t-test. High levels of anti-HSP60 were found in patients with localized infections, while the levels of anti- HSP70 were higher in the group with generalized infections. The serum levels of both anti-HSP 60 and anti-HSP70 were significantly increased in patients with Gram-negative bacterial infections, as compared with patients harbouring infections produced by Gram-positive and fungal strains, demonstrating their potential use as additional diagnosis and prognosis markers in infections with this etiology.
Introduction
Stress or heat shock proteins (HSPs), one of the most phylogenetically conserved superfamily of chaperonesCitation1-3 could be expressed at very low levels under non-stressing conditions, including different stages of the cellular cycle, development, and differentiation,Citation4-7 but are highly induced by environmentalCitation8 and patho-physiological stress factors.Citation9-15
During carcinogenesis, HSPs have been reported to show alteration of their expression levels, either increasing or decreasing. Although HSP expression was recognized as a factor of prognostic value in certain tumors, the data are limited and the results often are contradictory. HSP60 and its co-chaperone HSP10 are expressed early during the development of a malignant phenotype. For example, in colon and uterine cervix cancers, HSP10 and HSP60 expression levels are increased as cells progress from their normal state to dysplasia and cancer.Citation16 HSP 60 over expression was associated with a poor prognosis in the ovarian cancer,Citation17 but HSP10 over-expression was associated with a lower risk of progression in patients exposed to chemotherapy.Citation18 HSP70 basal level is unusually high in cells or tissues from a wide range of tumors, contributing to tumor genesis through their pleiotropic activities on proteins that influence tumor cell growth or blocking the apoptosis at different levels, using caspase dependentCitation19 or independent pathways.Citation20,21
Recognition of peptides derived from HSPs by the immune system can have an anti-inflammatory effect and down-regulate the chronic state of inflammation via modulation of cytokine secretion.Citation22,23 So, despite their ubiquitous and high homology among different species, they also represent important antigenic targets of the cellular and humoral immune response, playing thus an important but yet, unclarified role in the pathology and infectious diseases control, as well as in the survival and virulence of pathogenic bacteria.
In this context, our aim was to investigate the serum levels of antibodies to HSP60 and HSP70 in patients with different localized and generalized infections, by comparison with cancer patients, used as positive control group, in order to evaluate their potential role as an aid in the diagnosis of different infectious diseases.
Results
The highest levels of serum HSP60 and HSP70 antibodies were found in patients with laryngo-pharyngeal cancer (p = 0.002, Kruskal-Wallis test, Gaussian Approximation), probably due to increasing of plasma levels of intracellular proteins through cell cytolysis. Also, high levels of anti-HSP60 were found in patients with localized infections. The levels of anti- HSP70 were higher in the group with generalized infections ().
Table 1. The average of the HSP60 and HSP70 antibodies titers, according to the pathological stratification of the patients
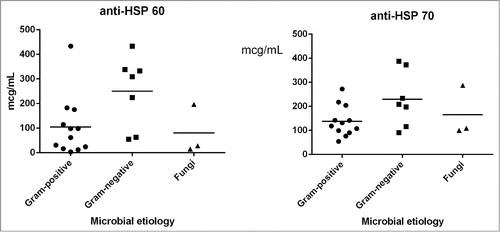
Concerning the levels of the investigated antibodies inside the group of patients with generalized and localized infections with different aetiologies, it could be noticed that the levels of both anti-HSP60 and anti-HSP70 were significantly increased in patients with infections produced by Gram-negative microorganisms, as compared with those produced by Gram-positive and fungal strains ().
Discussion
In order to evaluate the potential role of antibodies to HSP60 and HSP70 in different infectious diseases, we have investigated the levels of these antibodies in the bloodstream of patients with different infections, comparatively with those occurred in cancer patients. Our results demonstrate that the patients with infectious diseases exhibited high levels of HSP 60, especially when the infections were caused by Gram-negative bacteria.
Cross-reactivity between microbial and HSP supports the involvement of HSP in the autoimmune diseases pathogenesis. Zlacka et al. (2006) investigated the anti-HSP60 and anti-HSP70 serum levels in juvenile patients with idiopathic arthritis.Citation24 While the anti-HSP60 levels were similar to those obtained for the control group, the anti-HSP70 level was much higher in patients versus controls, suggesting their implication in this disease.Citation24 An altered immune response to HSP60 in rheumatoid arthritis has been also signaled by vanHalm et al. (2006).Citation25
The viral, bacterial, parasitic and fungal infections were incriminated to increase the levels of HSP antibodies; therefore we investigated their potential to be used as biomarkers for the severity of bacterial diseases. In our study, we have found a positive correlation between bacterial infections with Gram negative etiology and increased levels of anti-HSP antibodies, especially anti-HSP60. During infection, the host cells and microbial agent are engaged in a stressful relationship, requiring the rapid and increased synthesis of HSP. Some studies report the increased concentrations of serum anti- Helicobacter pylori HSP60 in patients diagnosed with H. pylori infection and peptic ulcers,Citation26,27 the measurement of these antibodies proving to be useful for the monitoring of eradication therapy efficiency.Citation27 It has been shown that the HSP60 of Gram-negative bacteria are involved in the synthesis of cellular bacterial wall, suggesting their location at the surface of bacterial cells, accessible to antibodies.Citation28,29 This could explain also the high levels of anti-HSP60 obtained in patients with Gram-negative infections. Another possible explanation for the increased anti-HSP60 serum levels in Gram-negative infections could be related to the fact that HSP60 stimulates the macrophage activity by specific LPS bounding, through a HSP60 epitope region.Citation30 Therefore, mammalian HSP60, specialized for the recognition and binding of microbial structures, may be involved in Gram-negative motifs recognition by phagocytic cells.Citation30 Thus, the anti-HSP60 immune response activation is potentiated by the presence of bacterial LPS, as it happens in Gram-negative infections. To our knowledge, this is the first report showing the specific association between the anti-HSP increased serum levels, particularly of the anti-HSP60 and the presence of Gram negative bacterial infections.
Conclusion
The increased levels of both HSP60 and HSP70 antibodies could be used as diagnosis markers in patients with cancers, but also in those with different microbial infections. The serum level of anti-HSP60 was increased in patients with localized infections, while anti-HSP70 in those bearing generalized infections. Also, both tested antibodies were produced with high levels in patients with Gram –negative infections, demonstrating their potential use as additional diagnosis and prognosis markers in infectious diseases with this etiology.
Material and Methods
Patients
A total number of 42 patients were analyzed, being distributed in different groups: i) patients with generalized infections (15 patients with positive blood cultures with Staphylococcus sp., Acinetobacter sp, Pseudomonas sp., Candida sp); ii) patients with localized infections (17 patients); iii) 10 patients with laryngo-pharyngeal cancers.
Quantification of HSPs antibodies serum levels
ELISA was performed to measure the amount of the human anti-HSP60-IgG, A, M and anti-HSP70-IgG, A, M antibody patients sera using anti-HSP60-IgG, A, M and anti-HSP70-IgG, A, M ELISA kit (Enzo Life Sciences BVBA, Belgium) according with producer instructions.
Statistical analysis
Data analysis by multivariate logistic regression was performed using GraphPadPrism software. Other statistical tests based on chi-square and Student t-test were also applied to the obtained results. Level of significance was considered when P<0.05 or P <0.01.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr Otilia Banu, Dr Vlad Claudia and Dr Alina Iancu for providing the serum samples.
Funding
This work was supported by 2 grants of the Romanian National Authority for Scientific Research, type CNCSIS Human Resources, IDEAS (project nos. 296/2007 and 154/2013).
References
- Ritossa P. Problems of prophylactic vaccinations of infants. Riv Ist Sieroter Ital 1962; 37:79-108; PMID:14492506
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet 1988; 22:631-677; PMID:2853609; http://dx.doi.org/10.1146/annurev.ge.22.120188.003215
- Morimoto RI, Tisseres A, Georgopolous C. Stress proteins in biology and medicine. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1990.
- Schlesinger MJ. Heat shock proteins. J Biol Chem 1990; 265:12111-12224; PMID:2197269
- Wu TC, Tanguay RM, Wu Y, He HZ, Xu DG, Feng JD, Shi WX, Zhang GG. Presence of antibodies to heat stress proteins and its possible significance in workers exposed to high temperature and carbon monoxide. Biomed Environ Sci 1996; 9:370-379; PMID:8988805.
- van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci 2007; 1113: 217-237; PMID:17584980; http://dx.doi.org/10.1196/annals.1391.020
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science 1993; 259:1409-1410; PMID:8451637; http://dx.doi.org/10.1126/science.8451637
- Hohfeld J, Hartl F. Role of the chaperonin cofactor HSP10 in protein folding and sorting in yeast mitochondria. JCB 1994; 126: 305-315; PMID:7913473; http://dx.doi.org/10.1083/jcb.126.2.305
- Jin GB, Nakayama H, Shmyhlo M, Inoue S, Kondo M, Ikezawa Z, Ouchi Y, Cyong JC. High positive frequency of antibodies to metallothionein and heat shock protein 70 in sera of patients with metal allergy. Clin Exp Immunol 2003; 131:275-279; PMID:12562388; http://dx.doi.org/10.1046/j.1365-2249.2003.02074.x
- Wang ZZ, Wang CL, Wu T, Pan HN, Wang SK, Jiang JD. Autoantibody response to heat shock protein 70 in patients with heatstroke. Am J Med 2001; 111:654-657; PMID:11755509; http://dx.doi.org/10.1016/S0002-9343(01)00974-3
- Wu T, Chen S, Xiao C, Wang C, Pan Q, Wang Z, Xie M, Mao Z, Wu Y, Tanguay RM. Presence of antibody against the inducible Hsp71 in patients with acute heat-induced illness. Cell Stress Chaperones 2001; 6:113-120; PMID:11599572; http://dx.doi.org/10.1379/1466-1268(2001)006%3c0113:POAATI%3e2.0.CO;2
- Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM: Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones 1998; 3:161-167; PMID:9764756; http://dx.doi.org/10.1379/1466-1268(1998)003%3c0161:POATHS%3e2.3.CO;2
- Wu T, Ma J, Chen S, Sun Y, Xiao C, Gao Y, Wang R, Poudrier J, Dargis M, Currie RW, et al. Association of plasma antibodies against the inducible Hsp70 with hypertension and harsh working conditions. Cell Stress Chaperones 2001; 6:394-401; PMID:11795477; http://dx.doi.org/10.1379/1466-1268(2001)006%3c0394:AOPAAT%3e2.0.CO;2
- Yang M, Zheng J, Yang Q, Yao H, Chen Y, Tan H, Jiang C, Wang F, He M, Chen S, et al. Frequency-specific association of antibodies against heat shock protein 60 and 70 with noise-induced hearing loss in workers. Cell Stress Chaperones 2004; 9:206-213; http://dx.doi.org/10.1379/CSC-12R.1
- Yuan J, Yang M, Yao H, Zheng J, Yang Q, Chen S, Wei Q, Tanguay RM, Wu T. Plasma antibodies to heat shock protein 60 and heat shock protein 70 are associated with increased risk of electrocardiograph abnormalities in automobile workers exposed to noise. Cell Stress Chaperones 2005, 10:126-136; http://dx.doi.org/10.1379/CSC-95R.1
- Cappello F, Bellafiore M, David S, Anzalone R, Zummo G. Ten kilodalton heat shock protein (HSP10) is overexpressed during carcinogenesis of large bowel and uterine exocervix. Cancer Lett 2003; 196:35-41; PMID:12860287; http://dx.doi.org/10.1016/S0304-3835(03)00212-X
- Kimura E, Enns RE, Alcaraz JE, Arboleda J, Slamon DJ, Howell SB. Correlation of the survival of ovarian cancer patients with mRNA expression of the 60-kD heat-shock protein HSP-60. J Clin Oncol 1993; 11:891-898; PMID:8098058.
- Têtu B, Popa I, Bairati I, L'Esperance S, Bachvarova M, Plante M, Harel F, Bachvarov D. Immunohistochemical analysis of possible chemoresistance markers identified by micro-arrays on serous ovarian carcinomas. Modern Pathol 2008; 21:1002-1010; http://dx.doi.org/10.1038/modpathol.2008.80
- Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, Anderson RL. HSP72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem 2004; 279:51490-51499; PMID:15371421; http://dx.doi.org/10.1074/jbc.M401314200
- Creagh EM, Carmody RJ, Cotter TG. Heat shock protein 70 inhibits caspase-dependent and -independent apoptosis in Jurkat T cells. Exp Cell Res 2000; 257:58-66; PMID:10854054; http://dx.doi.org/10.1006/excr.2000.4856
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 2001; 3:839-843; PMID:11533664; http://dx.doi.org/10.1038/ncb0901-839
- Kamphuis S, Kuis W, de Jager W, Teklenburg G, Massa M, Gordon G, Boerhof M, Rijkers GT, Uiterwaal CS, Otten HG, et al. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet 2005; 366:50-56; PMID:15993233; http://dx.doi.org/10.1016/S0140-6736(05)66827-4
- Massa M, Passalia M, Manzoni SM, Campanelli R, Ciardelli L, Yung GP, Kamphuis S, Pistorio A, Meli V, Sette A, et al. Differential recognition of heat-shock protein dnaJ-derived epitopes by effector and Treg cells leads to modulation of inflammation in juvenile idiopathic arthritis. Arthritis Rheum 2007; 56:1648-1657; PMID:17469159; http://dx.doi.org/10.1002/art.22567
- Zlacka D, Vavrincova P, Hien Nguyen TT, Hromadnikova I. Frequency of anti-hsp60, -65 and -70 antibodies in sera of patients with juvenile idiopathic arthritis. J Autoimmun 2006, 27:81-88
- van Halm VP, Slot MC, Nurmohamed MT, Tervaert JW, Dijkmans BA, Voskuyl AE. Antibodies against human 60 kDa heat shock protein are not associated with cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis 2006; 65:590-594; PMID:16249230; http://dx.doi.org/10.1136/ard.2005.038828
- Kalabay L, Fekete B, Czirják L, Horváth L, Daha MR, Veres A, Fónyad G, Horváth A, Viczián A, Singh M, et al. Helicobacter pylori infection in connective tissue disorders is associated with high levels of antibodies to mycobacterial hsp65 but not to human hsp60. Helicobacter 2002; 7:250-256; PMID:12165033; http://dx.doi.org/10.1046/j.1523-5378.2002.00092.x
- Yunoki N, Yokota K, Mizuno M, Kawahara Y, Adachi M, Okada H, Hayashi S, Hirai Y, Oguma K, Tsuji T. Antibody to heat shock protein can be used for early serological monitoring of Helicobacter pylori eradication treatment. Clin Diagn Lab Immunol 2000; 7:574-577; PMID:10882654
- McLennan N, Masters M. GroE is vital for cell-wall synthesis. Nature 1998; 392:139; PMID:9515958; http://dx.doi.org/10.1038/32317
- Zügel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev 1999; 12:19-39; PMID:9880473
- Habich C, Kempe K, van der Zee R, Rümenapf R, Akiyama H, Kolb H, Burkart V. Heat shock protein 60: specific binding of lipopolysaccharide. J Immunol 2005; 174:1298-1305; PMID:15661886; http://dx.doi.org/10.4049/jimmunol.174.3.1298