Abstract
Probiotic strains of Lactobacillus have been studied for their inhibitory effects on Candida albicans. However, few studies have investigated the effect of these strains on biofilm formation, filamentation and C. albicans infection. The objective of this study was to evaluate the influence of Lactobacillus acidophilus ATCC 4356 on C. albicans ATCC 18804 using in vitro and in vivo models. In vitro analysis evaluated the effects of L. acidophilus on the biofilm formation and on the capacity of C. albicans filamentation. For in vivo study, Galleria mellonella was used as an infection model to evaluate the effects of L. acidophilus on candidiasis by survival analysis, quantification of C. albicans CFU/mL, and histological analysis. The direct effects of L. acidophilus cells on C. albicans, as well as the indirect effects using only a Lactobacillus culture filtrate, were evaluated in both tests. The in vitro results showed that both L. acidophilus cells and filtrate were able to inhibit C. albicans biofilm formation and filamentation. In the in vivo study, injection of L. acidophilus into G. mellonella larvae infected with C. albicans increased the survival of these animals. Furthermore, the number of C. albicans CFU/mL recovered from the larval hemolymph was lower in the group inoculated with L. acidophilus compared to the control group. In conclusion, L. acidophilus ATCC 4356 inhibited in vitro biofilm formation by C. albicans and protected G. mellonella against experimental candidiasis in vivo.
Abbreviations
| ATCC | = | American type culture collection |
| YNB | = | Yeast nitrogen base |
| MRS | = | Man, Rogosa and Sharpe |
| PBS | = | phosphate buffered saline |
| BHI | = | Brain heart infusion |
| CFU | = | colony-forming unit |
| SEM | = | Scanning electron microscopy |
| PAS | = | periodic acid-Schiff |
| HE | = | hematoxylin-eosin |
| pH | = | potential hydrogen ion |
| NIH | = | National Institutes of Health |
Introduction
Fungal infections are one of the most common diseases caused by microorganisms, especially in hospitalized and immunocompromised patients. Among species of the genus Candida, C. albicans is the pathogen most frequently isolated from the human body, including the oral cavity and gastrointestinal and genitourinary tract. This species can cause infections that range from superficial lesions of the mucosa or skin to severe systemic infections.Citation1,2 C. albicans shows a great capacity of biofilm formation on oral structures and its presence in the oral cavity may serve as a reservoir of this fungus for infections in other parts of the body.Citation3
Current treatment for candidiasis consists of the administration of topical antifungal agents such as nystatin, amphotericin B and clotrimazole, or systemic antifungal agents such as fluconazole, ketoconazole and itraconazole. However, the use of these drugs can cause side effects and lead to microbial development of resistance.Citation4,5 The increase in the resistance of microorganisms to conventional antifungal drugs has encouraged studies designed to discover new treatments for infections caused by Candida spp.Citation6,7 The use of probiotics to prevent or treat Candida infections may be an interesting strategy. In this respect, certain Lactobacillus strains have been shown to exert anti-Candida activity by producing antimicrobial molecules, such as organic acids, hydrogen peroxide and bacteriocins.Citation8
Probiotics are defined by the World Health Organization as live microorganisms that confer health benefits on the host when administered in adequate amounts. They are included in a variety of products such as foods, dietary supplements and medications. In addition to Lactobacillus, other microorganisms have been used as probiotics, including Bifidobacterium, Saccharomyces cerevisiae, Escherichia coli and Bacillus.Citation9
The anti-Candida effects of Lactobacillus have been investigated in several in vitro studies using different strains of L. plantarum, L. rhamnosus, L. paracasei, L. reuteri and L. acidophilus. All of these strains were able to inhibit the growth of C. albicans, but the inhibitory effects were dependent on the strain tested, dose administered and duration of treatment.Citation10-12 Therefore, further studies are needed to elucidate the inhibitory activity of Lactobacillus strains against C. albicans, particularly in terms of biofilm formation, filamentation capacity and infection potential.
In addition to the antifungal activity of probiotic strains, some Lactobacillus strains have been shown to stimulate the host immune response in C. albicans infections by interacting with intestinal epithelial cells and cells of the immune system, releasing cytokines involved in the regulation of the immune response.Citation13 These data suggest that the effect of Lactobacillus on C. albicans needs to be studied in vivo using host models of experimental infection.
Although mice are the gold standard for the study of experimental candidiasis, economic, logistic and ethical issues limit the use of mammals in these experiments, especially when a large number of strains need to be analyzed.Citation14 In the last decades, invertebrate models have been used to study the molecular basis of microbial pathogenicity and pathogen-host interactions, which provided considerable insight into different aspects of microbial infection.Citation15 In this respect, Galleria mellonella has been found to be an interesting invertebrate model for the study of experimental candidiasis.Citation16-23 This larvae has a sufficient size for injection of a standard inoculum of the microorganism and its hemolymph contains various types of hemocytes that play an important role in the defense against pathogens.Citation16,17 In these models, the experiments are conducted at temperatures ranging from 25 to 37°C, conditions that simulate the natural environment of mammalian hosts.Citation18 In addition, C. albicans produces filaments and can form a biofilm structure inside the G. mellonella larvae that are useful tools in evaluating the pathogenicity of C. albicans and new anti-fungal therapies.Citation24
So far, no studies have used G. mellonella to evaluate the effects of probiotic bacteria on experimental infection, which would permit to standardize an in vivo model for future studies on the antimicrobial and immunomodulatory activity of probiotics in experimental candidiasis.
Therefore, the objective of the present study was to evaluate the in vitro effects of L. acidophilus ATCC 4356 on the biofilm formation and filamentation capacity of C. albicans ATCC 18804, as well as to determine its activity on experimental candidiasis in the G. mellonella model.
Results
Effects of L. acidophilus ATCC 4356 on in vitro biofilm formation by C. albicans: CFU/mL count
First, we determined the best growth phase of the L. acidophilus culture (4, 6, 18 and 24 h) capable of inhibiting C. albicans cells in the biofilms formed in vitro. A reduction in the number of C. albicans CFU/mL compared to the control group was observed at all time points tested. The highest inhibition (57.52%) was observed after 24 h of culture of L. acidophilus ().
Figure 1. Candida albicans biofilm formed in vitro: (A) Percentage of reduction, expressed as mean values (CFU/mL), in the viability of C. albicans in the groups treated with L. acidophilus cells obtained from cultures in different phases of growth (4, 6, 18 and 24 h) in relation to the control group (PBS). (B) Percentage of reduction, expressed as mean values (CFU/mL), in the viability of C. albicans in the groups treated with L. acidophilus cells or culture filtrate obtained from 24-h cultures in relation to the control groups (PBS or MRS broth). (C) Number of C. albicans CFU/mL (log) in biofilms of the control group (PBS or MRS broth) and groups treated with L. acidophilus cells or culture filtrate. *Significant difference between the control group (PBS) and C. albicans + L. acidophilus cell group (p = 0.0001). **Significant difference between the control group (MRS broth) and C. albicans + L. acidophilus culture filtrate group (p = 0.0001). Student t-test, P ≤ 0.05.
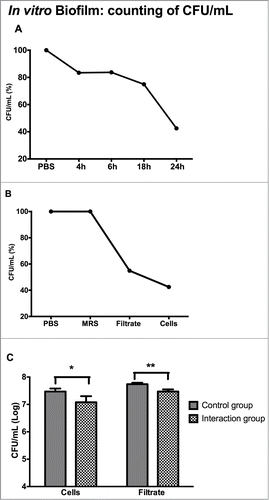
In addition to the effects of L. acidophilus cells on C. albicans biofilms, the indirect effects of L. acidophilus were also analyzed using only the culture filtrate of Lactobacillus obtained after growth for 24 h. In order to determine whether the MRS broth of the L. acidophilus culture exerts an effect on C. albicans, and hence interferes with the culture filtrate experiment, a control group consisting of C. albicans and MRS broth was included. The results showed that L. acidophilus culture filtrate reduced the growth of C. albicans cells by 45.10% (), suggesting that L. acidophilus produced substances with anti-Candida activity.
Next, the C. albicans CFU/mL results obtained for biofilms treated with L. acidophilus cells or culture filtrate obtained from 24-h cultures were log transformed and analyzed statistically. A significant reduction in the number of CFU/mL (log) of C. albicans was observed for both biofilms treated with L. acidophilus cells and biofilms treated only with the culture filtrate (). Therefore, a culture period of L. acidophilus of 24 h was adopted for all subsequent tests of this study.
Effects of L. acidophilus ATCC 4356 on in vitro C. albicans filamentation
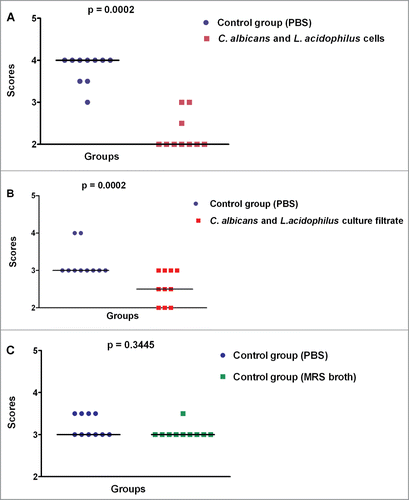
The induction of filamentation was tested in vitro to determine whether L. acidophilus is able to inhibit hyphal formation by C. albicans. The light microscopy images showed a smaller number of hyphae in the interaction groups (C. albicans + L. acidophilus cells and C. albicans + L. acidophilus culture filtrate) when compared to the control groups (PBS and MRS broth). The light microscopy images and the results of hyphal quantification are shown in and , respectively.
Figure 2. Light microscopy photomicrographs of in vitro Candida albicans filamentation. (A) Control group (PBS): intense formation of hyphae. (B) C. albicans + L. acidophilus cells group: presence of some hyphae. (C) Control group (MRS broth): intense formation of hyphae. (D) C. albicans + L. acidophilus culture filtrate group: note the presence of few hyphae. Original magnification: 400×.

Figure 3. Quantification of hyphae in the in vitro C. albicans filamentation assays. Scores were attributed to the number of C. albicans hyphae formed in each group. (A) Comparison between the control group (PBS) and C. albicans + L. acidophilus cells group. (B) Comparison between the control group (PBS) and C. albicans + L. acidophilus culture filtrate group. (C) Comparison between the PBS and MRS control groups. Mann-Whitney test, p ≤ 0.05.
Effects of L. acidophilus ATCC 4356 on experimental candidiasis: Galleria mellonella survival curve
Since there are no studies in the literature about inoculating L. acidophilus into G. mellonella used as an experimental model, the susceptibility of G. mellonella to infection with L. acidophilus was evaluated prior to the study of experimental candidiasis in order to determine the sub-lethal concentration for these animals.
Standard suspensions of L. acidophilus at 104 to 108 cells/larva concentrations were inoculated into G. mellonella and survival curves were constructed. The results showed that L. acidophilus was not pathogenic for G. mellonella, since none of the larvae died during the experiment at any of the concentrations tested. On the basis of these results, a concentration of 105 cells/larva was adopted for all subsequent assays, since it is the same concentration as that used for infection of G. mellonella with C. albicans.
Next, G. mellonella larvae were used to study the interaction between C. albicans and L. acidophilus. In some groups, called therapeutic, the standard suspension of C. albicans was first injected into G. mellonella, followed 1 h later by the inoculation of L. acidophilus cells or culture filtrate. In other groups, called prophylactic, the L. acidophilus cells or culture filtrate was inoculated 1 h before the inoculation of C. albicans.
In the control group with no infected larvae and inoculated with MRS broth was observed 100% of the survival rate indicating that the MRS broth was innocuous to G. mellonella larvae (data not shown). In the control groups infected with C. albicans and treated with PBS or MRS broth, 100% of the larvae died within 48 h. The interaction of C. albicans with L. acidophilus cells or culture filtrate significantly increased the survival of the insects, with approximately 20% of the larvae being still alive at the end of the experiment. The increase in the survival of G. mellonella larvae infected with C. albicans was observed both in the groups treated therapeutically with L. acidophilus and in the groups treated prophylactically with L. acidophilus ().
Figure 4. Effects of L. acidophilus on experimental candidiasis based on the analysis of survival curves of G. mellonella larvae (A) Therapeutic groups: significant differences were observed between the C. albicans + L. acidophilus cells group and PBS control group (p=0.0001) and between the C. albicans + L. acidophilus culture filtrate group and MRS control group (p=0.0002) (B) Prophylactic groups: significant differences were observed between the C. albicans + L. acidophilus cells group and PBS control group (p=0.0001) and between the C. albicans + L. acidophilus culture filtrate group and MRS control group (p=0.0490) Log-rank test, p ≤ 0.05.
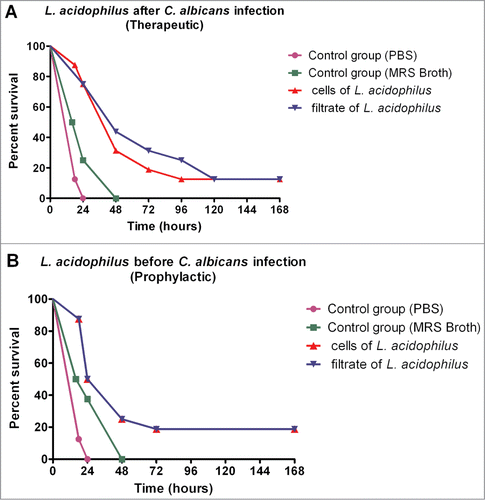
In the study of the interaction between C. albicans and L. acidophilus, although no significant difference in the survival curve of G. mellonella was observed between the prophylactic and therapeutic groups, the latter presented greater median survival (48 h) when compared to the prophylactic groups (36 h). Therefore, the therapeutic groups were studied in the subsequent assays.
Effects of L. acidophilus ATCC 4356 on experimental candidiasis: CFU/mL count of C. albicans in the hemolymph of Galleria mellonella
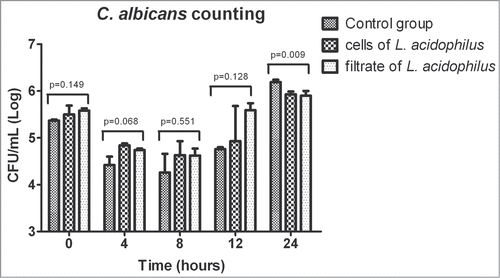
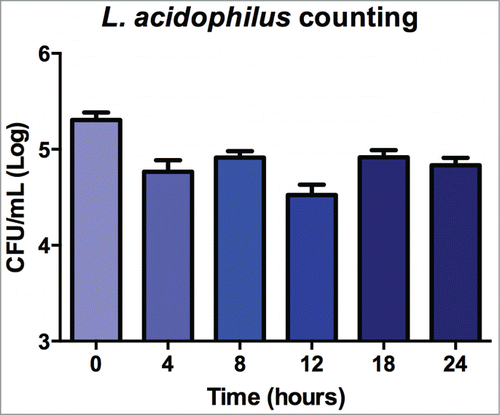
For quantification of the presence of C. albicans in infected G. mellonella, CFU/mL count in the hemolymph was measured at 0, 4, 8, 12, 18 and 24 h after infection. Quantification of C. albicans CFU/mL during the first 24 h of infection of G. mellonella showed a similar growth of the fungus in all experimental groups. A significant difference between groups was only observed at 24 h of infection, with higher growth of C. albicans in the MRS control group compared to the groups treated with L. acidophilus ().
Figure 5. Mean and standard deviation of C. albicans counts (CFU/mL) in the hemolymph of Galleria mellonella immediately after inoculation and after 4, 8, 12 and 24 h of experimental infection. The following groups were compared at each time of infection: control group (MRS broth), C. albicans + L. acidophilus cells group, and C. albicans + L. acidophilus culture filtrate group. A significant difference between groups was only observed after 24 h of infection (ANOVA, P ≤ 0.05), with a larger number of CFU/mL in the control group compared to the C. albicans + L. acidophilus cells (p = 0.0205) and C. albicans + L. acidophilus culture filtrate groups (p = 0.0135). No significant difference was observed between the last 2 groups (p = 0.9251). Tukey test, P ≤ 0.05.
In the C. albicans and L. acidophilus cells group, the number of Lactobacillus was also counted and similar results of CFU/mL were observed at all times tested. Statistical analysis revealed no significant difference between the times ().
Figure 6. Mean and standard deviation of L. acidophilus counts (CFU/mL) in the hemolymph of G. mellonella at times 0, 4, 8, 12, 18 and 24 h for the group formed by interaction of C. albicans + L. acidophilus cells. No significant difference between the times was observed (p = 0.840). ANOVA, P ≤ 0.05).
Effects of L. acidophilus ATCC 4356 on experimental candidiasis: C. albicans filamentation in Galleria mellonella tissues
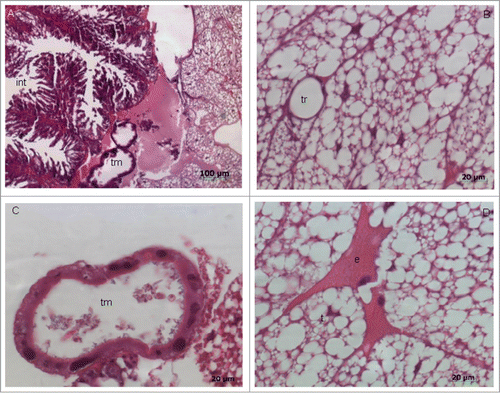
Microscopic analysis was used to evaluate the effects of L. acidophilus on C. albicans filamentation in G. mellonella. Prior to this analysis, the histomorphological structures found in the perivisceral portion of the fat body of uninfected G. mellonella larvae were analyzed. The fat body of the larva basically consists of 2 types of cells: trophocytes (or adipocytes) and oenocytes. In the present study, the trophocytes had a globular appearance and nucleus was present in the center of the cells. Oenocytes were found in smaller numbers scattered throughout the fat body and contained a round or slightly elliptical nucleus ().
Figure 7. Photomicrographs of a histologically normal fat body of Galleria mellonella larvae not infected with the microorganisms. (A) Note the presence of Malpighi tubules (tm), part of the intestine (int), and trophocytes (t). HE; original magnification: 100x. (B) Presence of the trachea (TR) surrounded by trophocytes of the fat body. HE; original magnification: 630×. (C) Malpighi tubule (tm) responsible for the removal of excreta from the hemolymph. HE; original magnification: 630×. (D) Presence of trophocytes (t) with irregular nuclei and oenocytes (e). HE; original magnification: 630×.
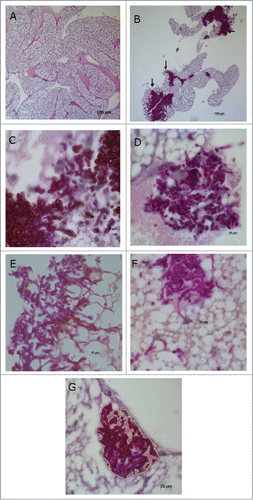
Next, the effects of L. acidophilus on experimental candidiasis were studied. In all groups, the hyphae and yeast cells were not uniformly spread throughout the fat body, but rather formed clusters in certain areas of the histological sections. These clusters of hyphae and yeast cells were located close to the organs or scattered throughout the adipose tissue ().
Figure 8. Histological sections of the fat body of Galleria mellonella.(A) Normal appearance of the fat body of G. mellonella not infected with Candida albicans. (B) C. albicans and PBS control group: observe the presence of clusters of hyphae and yeast cells (arrow). PAS; original magnification: 100×. (C) C. albicans and MRS control group. (D) C. albicans + L. acidophilus culture filtrate group. (E) C. albicans and PBS control group. (F) C. albicans + L. acidophilus cells group. (G) C. albicans + L. acidophilus cells group: with demarcation taken by Image J program for obtaining occupied by hyphae and yeasts area. PAS; original magnification: 1000×.
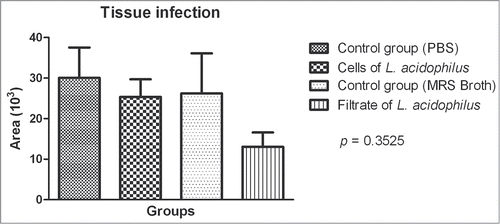
For analysis of tissue infection with C. albicans, all areas containing hyphae and yeast cells were photographed at 1,000x magnification and the area occupied by these structures (in μm) was measured in each image. The areas of each section were summed and the results were analyzed statistically. The mean size of the areas occupied by yeast cells/hyphae was smaller in the groups treated with L. acidophilus (cells or culture filtrate) when compared to the control groups (PBS or MRS broth), but no significant differences were observed between the 4 groups studied ().
Figure 9. Mean and standard deviation of the area occupied by Candida albicans yeast cells and hyphae in histological sections of Galleria mellonella with experimental candidiasis. No significant difference was observed between groups: control group (PBS), C. albicans + L. acidophilus cells group, control group (MRS broth), and C. albicans + L. acidophilus culture filtrate group. ANOVA, P ≤ 0.05.
Discussion
Some strains of Lactobacillus are cited in the literature as microorganisms that confer probiotic effects when incorporated into fermented food products, such as L. acidophilus ATCC 4356, L. casei ATCC 393 and L. paracasei subsp. paracasei ATCC BAA52.Citation25-26 The prevention of biofilm formation by such natural lactobacilli-derived agents is one possible therapy currently tested.Citation27 The present study showed a reduction of C. albicans CFU/mL counts in in vitro polymicrobial biofilms with L. acidophilus compared to the control group at all times tested; however, this difference was only significant for the 24-h culture of L. acidophilus (reduction of 57.5%). Similar results have been reported by Smith et al.Citation12 who showed a reduction of C. albicans cell counts in biofilms during application of a synbiotic containing L. acidophilus.
Interaction of the L. acidophilus culture filtrate with the biofilm formed by C. albicans also led to a significant reduction in the CFU/mL count of this fungus. This finding demonstrates a competitive relationship between the 2 species determined by the presence of substances that render the medium hostile for the development of the fungus. In certain interactions, molecules secreted by bacteria into the external medium can inhibit the growth of a second species as demonstrated in different studies.Citation27-29 Walencka et al.Citation27 investigated the effect of a substance obtained from the culture filtrate of L. acidophilus on S. aureus and S. epidermidis biofilms. Confocal microscopy revealed that this substance resulted in the formation of biofilms that covered a smaller surface area, with a reduction in biofilm volume and total thickness. Pascual et al.Citation30 showed that a bacteriocin present in the culture filtrate of L. fermentum L23 was responsible for a broad spectrum of inhibition when inoculated together with Gram-negative and Gram-positive pathogenic bacteria and Candida species.
In the present study, analysis of in vitro filamentation showed that the interaction between C. albicans and L. acidophilus reduced the number of hyphae when compared to the control group inoculated only with C. albicans. The formation of hyphae seems to be a critical step in the development of C. albicans biofilms since studies have shown that mutations in the genes related to hyphal formation cause severe defects in biofilm formation in vitro.Citation21,31,32 Metabolites released by Lactobacillus species, such as sodium butyrate, have been shown to inhibit biofilm formation, to potentiate the effect of antifungal agents, and to suppress C. albicans filamentation, reducing fungal pathogenicity.Citation33 Studying the interactions between C. albicans and P. aeruginosa, Morales et al.Citation34 demonstrated that phenazines produced by P. aeruginosa can modulate the metabolism of C. albicans. The presence of low concentrations of these substances permitted the growth of C. albicans, but affected biofilm formation and inhibited the transition from the yeast to the hyphal form. Noverr and HuffnagleCitation10 investigated the effect of live cultures, culture supernatants and dead cultures of probiotic bacteria on the morphogenesis of C. albicans. The authors observed that supernatants obtained from 2-h cultures of these bacteria inhibited germ tube formation in C. albicans and the addition of 24-h cultures completely inhibited germination, suggesting that the accumulation of a soluble compound in the culture supernatant is responsible for this inhibition.
Another factor that plays a role in filamentation is the pH of the medium. In the case of C. albicans, pH serves as a strong signal for morphological differentiation. Acid conditions favor the growth of the yeast form, whereas alkaline conditions favor the growth of hyphae.Citation35 In this respect, the production of lactic acid and other organic acids by Lactobacillus can substantially decrease the pH, which plays an important role in fungal growth.Citation36 Köhler et al.Citation36 investigated the potential of L. rhamnosus and L. reuteri to control C. albicans. The reduction in cell viability was greater when C. albicans was incubated with the Lactobacillus culture filtrate in MRS broth at pH 4.5. Furthermore, the incubation of C. albicans with MRS broth alone did not reduce cell viability.
Analysis of the virulence of L. acidophilus for G. mellonella larvae showed that the bacterium did not cause the death of the animals tested, thus demonstrating its lack of pathogenicity in this experimental model. Studies in the literature have only used strains of Lactococcus lactis, which is used as a control since it does not cause infection in this experimental model.Citation37-38 Similarly, L. acidophilus is not pathogenic for Caenorhabditis elegans, another invertebrate model, with the bacterium being unable to colonize the intestine of this species. Furthermore, inoculation of L. acidophilus significantly reduced infection with Enterococcus faecalis and prolonged survival of the nematode when exposed to strains of E. faecalis and S. aureus.Citation39
In the in vivo study, experiments were conducted to determine whether the presence of L. acidophilus injected prophylactically or therapeutically into G. mellonella has an effect on systemic infection with C. albicans. We showed that both prophylactic and therapeutic inoculation significantly increased the survival of the infected larvae. These results were obtained for interactions with cells and with the culture filtrate of L. acidophilus, demonstrating that the metabolites produced by the bacterium also exert an effect on C. albicans virulence in vivo.
Similar results have been reported by Matsubara et al.Citation40 who evaluated oral colonization with C. albicans in immunocompromised mice. Candida counts were significantly reduced in the groups treated with probiotic bacteria (L. acidophilus and L. rhamnosus) compared to untreated animals. Furthermore, treatment with the probiotic resulted in greater reduction of C. albicans than treatment with nystatin. McCann et al.Citation41 treated G. mellonella larvae infected with C. albicans with ketoconazole antifungal modified by silver nanoparticles which were administered prophylactically or therapeutically. Similar to the present study, survival of the larvae increased in the presence of the compound with survival rates of up to 90% after 72 h in prophylactic treatment and of 75% in therapeutic treatment after 1 h of infection with C. albicans.
Studies have demonstrated the involvement of probiotic bacteria in the regulation of the host immune system.Citation39 Immune system of insects basically comprises 2 types of immune responses, a humoral and a cellular response. The immune responses are triggered through the recognition of microorganisms by specific host cell receptors. These immune reactions have been shown to be similar to those that occur in vertebrates, and insect models are therefore useful for the study of fungal and bacterial virulence.Citation42
The results of the in vivo study of C. albicans cell counts (CFU/mL) in the hemolymph of G. mellonella showed the occurrence of an immune response of the larva after infection with the fungus. The number of microorganisms recovered from the larval hemolymph immediately after inoculation (time 0) did not differ from the number used at the beginning of infection. However, a reduction in the number of recovered cells was noted after 8 h. After this period, the larval immune system was probably no longer able to combat the infection and the number of C. albicans cells gradually increased, with the highest count being observed after 24 h. The same trend of microbial growth was seen in the interaction and control groups. However, growth of C. albicans was significantly lower in the interaction groups after 24 h when compared to control. These findings suggest that L. acidophilus renders the larva more resistant to infection when compared to the control group. Another interesting observation was the constant presence of bacteria throughout the period analyzed, demonstrating again that L. acidophilus is not pathogenic in this experimental model and that the bacterium was interacting with C. albicans in vivo.
Evans and RozenCitation43 evaluated the growth of Streptococcus pneumoniae strains that differed in virulence in the G. mellonella model after 0, 0.5, 1, 4 and 24 h of infection. As observed in the present experiment, the number of bacteria recovered from the larval hemolymph during the first hours did not differ from the number used at the beginning of infection. However, the mean number of cells of the most virulent strain increased after 4 h, whereas the bacterial count of the least virulent strain decreased significantly within the first 30 min of infection. A small number of bacteria were observed in some larvae after 4 h. These results suggest that high mortality may be associated with the proliferation of bacteria inside the host as a result of a deficient immune response.
In relation to the study of C. albicans filamentation in G. mellonella tissues, the results from microscopic analysis showed that the areas occupied by hyphae were smaller in the groups treated with L. acidophilus (cells or culture filtrate) when compared to the control groups (PBS or MRS broth), however, no significant differences were observed between the groups. Probably, the lack of a significant reduction in the C. albicans filamentation can be attributed to the selected time for observation (18 h after infection) and not 24 h as in the previous experiments. The time of observation for microscopic analysis was limited to 18 h because we wanted to remove the body fat with the larvae still alive and the results from survival curve assays showed death of larvae 24 h post infection in the control group (PBS).
The interactions between bacteria and fungi are highly complex and a series of factors need to be analyzed in conjunction, such as the virulence of microorganisms associated with environmental factors and the interactions between different species present in a given niche. The development of appropriate in vitro and in vivo models is necessary to characterize these interactions. In this study, we verified that G. mellonella is an adequate model for the study of the interaction between C. albicans and L. acidophilus. Here, we conducted a large number of in vitro and in vivo experiments in order to determine the conditions of Lactobacillus inoculation (growth phase, sub-lethal concentration, cells and culture filtrate, and prophylactic or therapeutic) required to achieve a significant reduction of C. albicans and experimental candidiasis. Since this model is established, future studies can be conducted for evaluating other strains and dosages of Lactobacillus, as well as the molecular changes and dynamics of the immune response in this interaction to better understand human disease and to develop new therapies for candidiasis.
In summary, in the present study both cells and culture supernatant of L. acidophilus ATCC 4356 were able to inhibit biofilm formation and filamentation by C. albicans in vitro, with 24-h cultures of L. acidophilus showing the greatest inhibitory effect on C. albicans biofilm formation. The prophylactic or therapeutic inoculation of L. acidophilus into G. mellonella infected with C. albicans reduced the number of yeast cells in the larval hemolymph and increased the survival of these animals.
Material and Methods
Interaction between L. acidophilus ATCC 4356 and C. albicans ATCC 18804 - in vitro assays
In the in vitro study, the direct and indirect effects of Lactobacillus on biofilm formation by C. albicans and its filamentation capacity were evaluated.
First, the direct effect was evaluated during different phases of growth of the bacterial culture (4, 6, 18 and 24 h). Ten assays were performed per experimental group and repeated twice on different occasions.
Reference strains of Candida albicans ATCC 18804 and Lactobacillus acidophilus ATCC 4356 were used in the experiments. C. albicans was cultured for 18 h at 37°C in yeast nitrogen base broth (YNB; Difco, Detroit, USA) supplemented with 100 mM glucose. L. acidophilus was grown in Lactobacillus MRS Broth (Himedia, Mumbai, India) for 4, 6, 18 and 24 h at 37°C in a bacteriological incubator under microaerophilic conditions. The number of cells in suspension was determined with a spectrophotometer (B582, Micronal, São Paulo, Brazil) at a concentration of 107 cells/mL. The optical density and wavelength used were 0.381 and 530 nm for C. albicans, respectively, and 0.296 and 600 nm for L. acidophilus. Cells densities of the inoculum were confirmed by CFU/mL counting after plating in Sabouraud dextrose agar for C. albicans and Rogosa agar for L. acidophilus.
For preparation of the L. acidophilus culture filtrate, 1 mL of the standard suspension was transferred to a Falcon tube containing 6 mL MRS broth and incubated for 24 h at 37ºC in a bacteriological incubator under microaerophilic conditions. After this period, the broth was centrifuged (2000 x g for 10 min) and filtered through a membrane with a 0.22-μm pore size (MFS, Dublin, USA).
Biofilm Formation
The method described by Thein et al.,Citation44 with some modifications, was used for the study of biofilm formation. Briefly, 100 μL aliquot of the standard C. albicans suspension was inoculated into each well of a 96-well flat-bottom microtiter plate (Costar Corning, New York, USA) and the plate was incubated for 90 min at 37°C under shaking at 75 rpm. Each well was then washed twice with PBS, 100 μL fetal bovine serum was added, and the plate was again incubated for 2 h. The plates were then washed twice and 50 μL of the standard suspension or the L. acidophilus culture filtrate was added. In the control group, 50 μL PBS was added. For promotion of biofilm growth, 70 μL YNB supplemented with 100 mM glucose and 30 μL BHI broth were added to each well and the plate was incubated for 48 h at 37°C under shaking at 75 rpm. The broth was partially changed at intervals of 24 h. After this period, the wells were washed and the biofilm adhered to the bottom of the plate was scraped off with a sterile wooden toothpick. One hundred μL of this inoculum was transferred to a Falcon tube containing 6 mL PBS and homogenized for 30 s. Next, decimal dilutions were prepared and seeded onto Petri dishes containing selective culture media. The plates were incubated for 48 h at 37°C. After this period, the number of colonies was counted for the calculation of colony-forming units per mL (CFU/mL). The study was supported by 2 experiments at different times with 10 biofilms per group.
Study of L. acidophilus effects of on C. albicans filamentation
The following groups were formed for the study of in vitro filamentation: PBS control group, MRS control group, C. albicans + L. acidophilus cell group, and C. albicans + L. acidophilus culture filtrate group. One mL distilled water supplemented with 10% fetal bovine serum and 100 μL of the standard C. albicans suspension were added to the wells of a 24-well cell culture plate (Costar Corning, New York, USA). Next, 50 μL of the standard suspension or the L. acidophilus culture filtrate was inoculated into each well. In the control groups, 50 μL PBS or MRS broth was used. The plates were incubated for 24 h at 37°C under microaerophilic conditions.
After this period, 50 μL of the inoculum was spread on glass slides and observed under a light microscope at 400x magnification. The images were analyzed regarding morphological features and quantification of the number of hyphae. For the latter, 10 microscopic fields were analyzed per slide and a score (0 to 4) was attributed to each field according to the number of hyphae present: score 0: no hyphae; score 1: 1 to 3 hyphae; score 2: 4 to 10 hyphae; score 3: 11 to 20 hyphae; and score 4: more than 20 hyphae.
Interaction between L. acidophilus ATCC 4356 and C. albicans ATCC 18804 - in vivo model
For this study, the methodology described by Cowen et al.Citation45 was used with some modifications.
G. mellonella (Embrapa Gado de Leite, Juiz de Fora, MG 36038-330, Brazil) in the final larval stage were stored in the dark and used within 7 days from shipment. Sixteen randomly chosen G. mellonella larvae with similar weight and size (250-350mg) were used in each group in all assays. Two control groups with no infected larvae were included for all assays: one group was inoculated with PBS to observe physical trauma, and the other received no injection as a control for general viability.
Before the study of the interaction between C. albicans and L. acidophilus, the susceptibility of G. mellonella to infection with L. acidophilus was tested to determine the sub-lethal concentration to be administered to these animals. For this purpose, 5 μL of different concentrations (104 to 108 cells/mL) of the standard L. acidophilus suspension was inoculated into G. mellonella. A group of 16 larvae was used per concentration.
G. mellonella survival assay
The cell densities of C. albicans were adjusted to 105 cells/mL with a hemocytometer. The standard L. acidophilus suspension (105 cells/mL) was obtained with a spectrophotometer as described above.
An inoculum of 5 μL of the standard C. albicans suspension was injected into the hemolymph of each larva through the last left proleg and 5 μL of the standard cell suspension or the L. acidophilus culture filtrate was inoculated into the last right proleg. For the groups infected with only one microorganism, 5 μL of the microbial suspension was inoculated into the last left proleg and the same volume of PBS or MRS broth into the last right proleg. In order to verify the toxicity of MRS broth for G. mellonella larvae, a control group with no infected larvae and inoculated with MRS broth was included in this study.
Sixteen larvae were used per experimental group. The interaction groups were subdivided into prophylactic and therapeutic. In the prophylactic group, the L. acidophilus cells or supernatant were injected 1 h before the inoculation of C. albicans cells, whereas in the therapeutic group C. albicans cells were inoculated 1 h before the inoculation of L. acidophilus cells or supernatant. The larvae were kept on Petri dishes and incubated at 37°C in a bacteriological oven. The number of dead larvae was recorded daily for 7 days.
Colony-Forming Unit Count
For quantification of the presence of C. albicans in infected G. mellonella, the larvae were euthanized 0, 4, 8, 12, 18 and 24 h after infection in the following groups: MRS control group, C. albicans + L. acidophilus cell group, and C. albicans + L. acidophilus culture filtrate group. A pool of 6 larvae was used per group and time. The experiment was carried out in triplicate using 18 larvae per group, for a total of 324 infected larvae. A control group was included for each time point, which was injected with 5 μL PBS into the last right and left proleg.
At each time point, the larvae were cut in the cephalocaudal direction with a scalpel blade and squeezed to remove the hemolymph, which was transferred to an Eppendorf tube. Serial dilutions were prepared from the hemolymph pool, seeded onto Petri dishes containing Sabouraud dextrose agar (Difco, Detroit, USA) supplemented with chloramphenicol (100 μg/mL), and incubated for 48 h at 37°C. In the C. albicans + L. acidophilus cell group, dilutions were also seeded on Rogosa agar (Himedia, Mumbai, India) and incubated under microaerophilic conditions (48 h at 37°C) to count the number of Lactobacillus in the hemolymph of larvae. After this period, the colonies were counted for the calculation of CFU/mL.
G. mellonella histological analysis
Histology was used to analyze histological structures present in the fat body of the larva and to evaluate the effects of L. acidophilus on C. albicans filamentation in G. mellonella. The following groups were formed: PBS control group, MRS control group, C. albicans + L. acidophilus cell group, C. albicans + L. acidophilus culture filtrate group, and control group not inoculated with any of the microorganisms. Five larvae were used per group.
Eighteen hours after infection, an incision was made in the midline of the ventral part of the animal, the hemolymph was discarded, and the fat body was removed. The fat body was placed in 10% formalin and stored for 24 h at 4°C. The tissue was then immersed in different alcohol concentrations (50, 70 and 90% for 1 h and 100% for 3 h), incubated in xylene for 3 h, and mounted in paraffin blocks.
The blocks were cut and the sections were stained with hematoxylin-eosin (HE) and periodic acid Schiff (PAS). Hyphae and yeast cells were visualized under a light microscope at 100, 630 and 1,000x magnification. For analysis of filamentation, all areas of the histological section that contained hyphae and yeast cells were photographed at an original magnification of 1,000× with a Cyber Shot DSC-585 digital camera (Sony Corporation) coupled to a Zeiss Axiophot 2 light microscope (Carl Zeiss, Oberkochen, Germany). The area occupied by hyphae and yeast cells (in μm) was determined for each image using the ImageJ program (version 1.32 for Windows), a public domain image processing program developed at the National Institutes of Health (NIH), Bethesda, USA. All areas of hyphae and yeast cells in each section were summed and the result was log10 transformed.
Statistical analysis
The CFU/mL results of in vitro biofilm formation were analyzed by the Student t-test. Analysis of variance and the Tukey test were used for the analysis of CFU/mL recovered from G. mellonella and microscopic analysis of the presence of hyphae. The Mann-Whitney test was applied to compare the scores obtained in the analysis of in vitro filamentation. For survival analysis of G. mellonella, survival curves were constructed and differences were estimated by the log-rank method (Mantel-Cox test) using the GraphPad Prism program. A level of significance of 5% was adopted for all tests.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We acknowledge Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support (grants 2012/02184-9 and 2012/19915-6) and scholarship (process 2011/15194-0).
References
- Harriott MM, Noverr MC. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol 2011; 19:557-63; PMID:21855346; http://dx.doi.org/10.1016/j.tim.2011.07.004
- Silverman RJ, Nobbs AH, Vickerman MM, Barbour ME, Jenkinson HF. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect Immun 2010; 78:4644-52; PMID:20805332; http://dx.doi.org/10.1128/IAI.00685-10
- Ten Cate JM, Klis FM, Pereira-Cenci T, Crielaard W, de Groot PW. Molecular and cellular mechanisms that lead to Candida biofilm formation. J Dent Res 2009; 88:105-15; PMID:19278980; http://dx.doi.org/10.1177/0022034508329273
- Farah CS, Lynch N, McCullough MJ. Oral fungal infections: an update for the general practitioner. Aust Dent J 2010; 55:48-54; PMID:20553244; http://dx.doi.org/10.1111/j.1834-7819.2010.01198.x
- Niimi M, Firth NA, Cannon RD. Antifungal drug resistance of oral fungi. Odontology 2010; 98:15-25; PMID:20155503; http://dx.doi.org/10.1007/s10266-009-0118-3
- Thompson GR, Patel PK, Kirkpatrick WR, Westbrook SD, Berg D, Erlandsen J, Redding SW, Patterson TF. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109:488-95; PMID:20156694; http://dx.doi.org/10.1016/j.tripleo.2009.11. 026
- Spampinato C, Leonardi D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed Res Int 2013; 2013:204237; PMID:23878798; http://dx.doi.org/10.1155/2013/204237
- Sharma A, Srivastava S. Anti-Candida activity of spent culture filtrate of Lactobacillus plantarum strain LR/14.J Mycol Med 2014; 24:e25-34; PMID:24316318; http://dx.doi.org/10.1016/j.mycmed.2013.11.001
- FAO/WHO. Food and Agriculture Organization of the United Nations, World Health Organization. Guidelines for the Evaluation of Probiotics in Food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. London (Ontario), 2002. Available from: ftp://ftp.fao.org/docrep/fao/009/a0512e00.pdf
- Noverr MC, Huffnagle GB. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun 2004; 72:6206-10; PMID:15501745; http://dx.doi.org/10.1128/IAI.72.11.6206-6210.2004
- Falagas ME, Gregoria I, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother 2006; 58:266-72; PMID:16790461; http://dx.doi.org/10.1093/jac/dkl246
- Smith AR, Macfarlane GT, Reynolds N, O’May GA, Bahrami B, Macfarlene S. Effect of a synbiotic on microbial community structure in a continuous culture model of the gastric microbiota in enteral nutrition patients. FEMS Microbiol Ecol 2012; 80:135-45; PMID:22176141; http://dx.doi.org/10.1111/j.1574-6941.2011.01279.x
- Amdekar S, Dwivedi D, Roy P, Kushwah S, Singh V. Probiotics: multifarious oral vaccine against infectious traumas. FEMS Immunol Med Microbiol 2010; 58:299-306; PMID:20100178; http://dx.doi.org/10.1111/j.1574-695X.2009.00630.x
- Jacobsen ID. Galleria mellonella as a model host to study virulence of Candida. Virulence 2014; 5:237-9; PMID:24384470; http://dx.doi.org/10.4161/viru.27434
- Fedhila S, Buisson C, Dussurget O, Serror P, Glomski IJ, Liehl P, Lereclus D, Nielsen-LeRoux C. Comparative analysis of the virulence of invertebrate and mammalian pathogenic bacteria in the oral insect infection model Galleria mellonella. J Invertebr Pathol 2010; 103:24-9; PMID:19800349; http://dx.doi.org/10.1016/j.jip.2009.09.005
- Fuchs BB, Mylonakis E. Using non-mammalian hosts to study fungal virulence and host defense. Curr Opin Microbiol 2006; 9:346-51; PMID:16814595; http://dx. doi.org/10.1016/j.mib.2006.06.004
- Mylonakis E. Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathol 2008; 165:1-3; PMID:18060516; http://dx.doi.org/10.1007/s11046-007-9082-z
- Fuchs BB, O’brien E, Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010; 1:475-82; PMID:21178491; http://dx.doi.org/10.4161/viru.1.6.12985
- Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenity testing of yeasts. FEMS Immunol Med Microbiol 2000; 27:163-9; PMID:10640612; http://dx.doi.org/10.1111/j.1574-695X.2000.tb01427.x
- Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella. FEMS Immunol Med Microbiol 2002; 34:153-7; PMID:12381467; http://dx.doi.org/10.1111/j.1574-695X.2002.tb00617.x
- Fuchs BB, Eby J, Nobile CJ, El Khoury JB, Mitchell AP, Mylonakis E. Role of filamentation in Galleria mellonella killing by Candida albicans. Microb Infect 2010; 12:488-96; PMID:20223293; http://dx.doi.org/10.1016/j.micinf.2010.03.001
- Junqueira JC, Fuchs BB, Muhammed M, Coleman JJ, Suleiman JM, Vilela SF, Costa AC, Rasteiro VM, Jorge AO, Mylonakis E. Oral Candida albicans isolates from HIV-positive individuals have similar in vitro biofilm-forming ability and pathogenicity as invasive Candida isolates. BMC Microbiol 2011; 11:247; PMID:22053894; http://dx. doi.org/10.1186/1471-2180-11-247
- Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ, Zaragoza O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS One 2013; 8:e60047; PMID:23555877; http://dx.doi.org/10.1371/journal.pone.0060047
- Borghi E, Romagnoli S, Fuchs BB, Cirasola D, Perdoni F, Tosi D, Braidotti P, Bulfamante G, Morace G, Mylonakis E. Correlation between Candida albicans biofilm formation and invasion of the invertebrate host Galleria mellonella. Future Microbiol 2014; 9:163-73. PMID:24571071; http://dx.doi.org/10.2217/fmb.13.159
- Campana R, Federici S, Ciandrini E, Baffone W. Antagonistic activity of Lactobacillus acidophilus ATCC 4356 on the growth and adhesion/invasion characteristics of human Campylobacter jejuni. Curr Microbiol 2012; 64:371-8; PMID:22271268; http://dx.doi.org/10.1007/s00284-012-0080-0
- Sah BN, Vasiljevic T, McKechnie S, Donkor ON. Effect of probiotics on antioxidant and antimutagenic activities of crude peptide extract from yogurt. Food Chem 2014; 156:264-70; PMID:24629967, http://dx.doi.org/10.1016/j.foodchem.2014.01.105
- Walencka E, Rózalska S, Sadowska B, Rózalska B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol 2008; 53:61-6; PMID:18481220; http://dx.doi.org/10.1007/s12223-008-0009-y
- Martins M, Henriques M, Azeredo J, Rocha SM, Coimbra MA, Oliveira R. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot Cell 2007; 6:2429-36; PMID:17981993; http://dx.doi.org/10.1128/EC.00252-07
- Sadowska B, Walencka E, Wieckowska-Szakiel M, Rózalska B. Bacteria competing with the adhesion and biofilm formation by Staphylococcus aureus. Folia Microbiol 2010; 55:497-501; PMID:20941586; http://dx.doi.org/10.1007/s12223-010-0082-x
- Pascual LM, Daniele MB, Giordano W, Pájaro MC, Marberis IL. Purification and partial characterization of novel bacteriocin L23 produced by Lactobacillus fermentum L23. Curr Microbiol 2008; 56:397-402; PMID:18172715; http://dx.doi.org/10.1007/s00284-007-9094-4
- Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol 2005; 15:1150-5; PMID:15964282; http://dx.doi.org/10.1016/j.cub.2005.05.047
- Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol 2006; 8:1382-91; PMID:16848788; http://dx.doi.org/10.1111/j.1462-5822.2006.00761.x
- Nguyen LN, Lopes LCL, Cordero RJB, Nosanchuk JD. Sodium butyrate inhibits pathogenic yeast growth and enhances the functions of macrophages. J Antimicrob Chemother 2011; 66:2573-80; PMID:21911344; http://dx.doi.org/10.1093/jac/dkr358
- Morales DK, Grahl N, Okegbe C, Dietrich LE, Jacobs NJ, Hogan DA. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. MBio 2013; 4:e00526-12; PMID:23362320; http://dx.doi.org/.10.1128/mBio.00526-12
- Davis D. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr Genet 2003; 44:1-7; PMID:12819929; http://dx.doi.org/10.1007/s00294-003-0431-2
- Köhler GA, Assefa S, Reid G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect Dis Obstet Gyneco 2012; 2012:636474; PMID:22811591; http://dx.doi.org/10.1155/2012/636474
- Joyce SA, Gahan CG. Molecular pathogenesis of Listeria monocytogenes in the alternative model host Galleria mellonella. Microbiology 2010; 156:3456-68; PMID:20688820; http://dx.doi.org/10.1099/mic.0.040782-0
- Michaux C, Martini C, Hanin A, Auffray Y, Hartke A, Giard JC. SlyA regulator is involved in bile salts stress response of Enterococcus faecalis. FEMS Microbiol Lett 2011; 324:142-6; PMID:22092815; http://dx.doi.org/10.1111/j.1574-6968.2011.02390.x
- Kim Y, Mylonakis E. Caenorhabditis elegans immune conditioning with the probiotic bacterium Lactobacillus acidophilus NCFM enhances gram-positive immune responses. Infect Immun 2012; 80:2500-8; PMID:22585961; http://dx.doi.org/10.1128/IAI.06350-11
- Matsubara VH, Silva EG, Paula CR, Ishikawa KH, Nakamae AE. Treatment with probiotics in experimental oral colonization by Candida albicans in murine model (DBA/2). Oral Dis 2012; 18:260-4; PMID:22059932; http://dx.doi.org/10.1111/j.1601-0825.2011.01868.x
- McCann M, Curran R, Bem-Shoshan M, McKee V, Tahir AA, Devereux M, Kavanagh K, Creaven BS, Kellett A. Silver(i) complexes of 9-anthracenecarboxylic acid and imidazoles: synthesis, structure and antimicrobial activity. Dalton Trans 2012; 41:6516-27; PMID:22476383; http://dx.doi.org/10.1039/c2dt12166b
- Roma GC, Mathias MI, Bueno OC. Fat body in some genera of leaf-cutting ants (Hymenoptera: Formicidae). Proteins, lipids and polysaccharides detection. Micron 2006; 37:234-42; PMID:16388950; http://dx.doi.org/10.1016/j.micron.2005.10.012
- Evans BA, Rozen DE. A Streptococcus pneumoniae infection model in larvae of the wax moth Galleria mellonella. Eur J Clin Microbiol Infect Dis 2012; 31:2653-60; PMID:22466968; http://dx.doi.org/10.1007/s10096-012-1609-7
- Thein ZM, Samaranayake YH, Samaranayake LP. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch Oral Biol 2006; 51:672-80; PMID:16620775; http://dx.doi.org/10.1016/j.archoralbio.2006.02.005
- Cowen L, Singh SD, Köhler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acady Sci 2009; 106:2818-23; PMID:19196973; http://dx.doi.org/10.1073/pnas.0813394106
