Abstract
The establishment and maintenance of epithelial cell-cell junctions is crucially important to regulate adhesion, apico-basal polarity and motility of epithelial cells, and ultimately controls the architecture and physiology of epithelial organs. Junctions are supported, shaped and regulated by cytoskeletal filaments, whose dynamic organization and contractility are finely tuned by GTPases of the Rho family, primarily RhoA, Rac1 and Cdc42. Recent research has identified new molecular mechanisms underlying the cross-talk between these GTPases and epithelial junctions. Here we briefly summarize the current knowledge about the organization, molecular evolution and cytoskeletal anchoring of cell-cell junctions, and we comment on the most recent advances in the characterization of the interactions between Rho GTPases and junctional proteins, and their consequences with regards to junction assembly and regulation of cell behavior in vertebrate model systems. The concept of “zonular signalosome” is proposed, which highlights the close functional relationship between proteins of zonular junctions (zonulae occludentes and adhaerentes) and the control of cytoskeletal organization and signaling through Rho GTPases, transcription factors, and their effectors.
Abbreviations
| AJ | = | adherens junction |
| AMOT | = | angiomotin |
| AMPK | = | Adenosine Monophosphate-Activated Protein Kinase |
| APC | = | adenomatous poliposis coli |
| Cdc42 | = | cell division cycle 42 |
| CD2AP | = | CD2-associated protein |
| CGN | = | cingulin |
| CGNL1 | = | paracingulin |
| Dbl | = | diffuse B-cell lymphoma |
| DLC | = | deleted in liver cancer |
| EPLIN | = | epithelial protein lost in neoplasm |
| ERK | = | extracellular regulated kinase |
| FERM | = | four.point.one, ezrin, radixin, moesin |
| FGD5 | = | FYVE, RhoGEF and PH domain containing 5 |
| GEF | = | guanine nucleotide exchange factor |
| GAP | = | GTPase activating protein |
| GST | = | glutathione -S- transferase; JAM = junctional adhesion molecule |
| MCF-7 | = | Michigan Cancer Foundation - 7 |
| MDCK | = | Madin Darby Canine Kidney |
| MgcRacGAP | = | male germ cell racGAP |
| MKLP1 | = | mitotic kinesin-like protein-1 |
| MRCK | = | myotonic dystrophy-related Cdc42-binding kinase |
| PA | = | puncta adhaerentia |
| PAK | = | p21-activated kinase; PATJ, Pals1 associated tight junction protein |
| PCNA | = | proliferating cell nuclear antigen |
| PDZ | = | Post synaptic density protein (PSD95), Drosophila, disc large tumour suppressor (DlgA), and zonula occludens-1 |
| PLEKHA7 | = | pleckstrin homology domain containing, family A member 7 |
| RICH-1 | = | RhoGAP interacting with CIP4 homologues |
| ROCK | = | Rho-associated protein kinase |
| SH3BP1 | = | (SH3 domain 490 binding protein-1) |
| Tbx-3 | = | T-box-3 |
| Tiam | = | Tumor invasion and metastasis |
| TJ | = | tight junction |
| WASP | = | Wiskott-Aldrich Syndrome Protein |
| WAVE | = | WASP family Verprolin-homologous protein |
| ZA | = | zonula adhaerens |
| ZO | = | zonula occludens |
| ZONAB | = | (ZO-1)–associated nucleic acid binding protein |
Introduction
Cell-cell junctions provide epithelial tissues with mechanical and functional integrity, by playing an essential role in cell-cell adhesion and formation of barriers between distinct body compartments. One key feature of junctions is their association with highly ordered cytoskeletal networks of actin, microtubules and intermediate filaments. Since Rho GTPases are major regulators of the polymerization, organization and mechanics of the cytoskeleton, the interplay between Rho GTPase activity and the organization of junctions is of fundamental importance in epithelial morphogenesis and physiology. In this review we attempt to address the complexity of this regulation, going from basic concepts about the organization, evolution and cytoskeletal anchoring of cell-cell junctions, to the most recent exciting findings about the role of GEFs and GAPs at junctions, and their mechanisms of regulation.
The Organization and Molecular Evolution of the Epithelial Apical Junctional Complex
Epithelial tissues are at the boundary between the organism and the external environment, and form the first barrier to the entry of pathogens and toxins.Citation1,2 In addition, they separate internal body compartments, thus allowing the maintenance of homeostatic specialized functions, which depend on polarized secretion and absorption, and maintenance of gradients across epithelia.
To form efficient barriers, epithelial tissues must display specific architectural characteristics, such as being formed by at least one continuous layer of closely packed cells, and show a topological asymmetry, paralleled in the structural and functional apico-basal polarity of their individual units. Furthermore, they must ensure the maintenance of a strong adhesion between cells, to prevent their mechanical separation when tensile forces are applied. Finally, they must establish and maintain continuous seals, to prevent the free diffusion of solutes, molecules and pathogens through the paracellular space. The adhesion and barrier functions in vertebrate epithelia are carried out by specialized intercellular junctions: tight junction (TJ), zonula adhaerens (ZA), and desmosomes. Tight junctions (TJ, also called zonulae occludentes) () provide the paracellular permeability seal, through 4-pass membrane proteins such as occludin and claudins, which are anchored to the actin cytoskeleton via scaffolding complexes of PDZ-containing proteins.Citation3,4 ZAs can be viewed as a highly specialized and topologically unique form of adherens junction (AJ): a continuous, linear circumferential belt (zonula) around the apex of polarized epithelial cells. AJ are present both in non-epithelial cells (e.g., intercalated disks of cardiac myocites, sites of adhesion between fibroblasts, neurons and others), and epithelial and endothelial cells, and are characterized by the presence of a member of the classical cadherin family (E-cadherin for epithelia, VE-cadherin for endothelia, and so on).Citation5,6 The epithelial ZA is located immediately below the TJ, and contains not only E-cadherin, α-catenin, β-catenin, and p120ctn, but also PLEKHA7 and afadin, whereas lateral contacts with spot-like adhesions (puncta adhaerentia) lack PLEKHA7 and afadin.Citation7 Transmembrane Ig-like adhesion molecules such as JAM and nectin are also present both in TJ and AJ, where they perform adhesion and signaling functions. Desmosomes are essential to provide tissue integrity and strength to the cell-cell junctions, and they contain, as transmembrane proteins, desmocollin and desmoglein, which belong to the cadherin superfamily of proteins. Although there is evidence for a cross-talk between desmosomes and Rho GTPases,Citation8-11 here we will focus primarily on Rho GTPase regulation at the apical, zonular junctional complex of vertebrates (ZA and TJ). The structure, function, and molecular composition of vertebrate cell-cell junctions have been described in several excellent reviews.Citation3,4,6,12,13
Figure 1. Evolution of junctional architecture, and the molecular complexity of vertebrate junctions. Simplified schemes showing the organization of the apical junctional complexes of polarized epithelial cells in insects (as an example of invertebrates) and vertebrate organisms. The canonical functions (polarity, barrier, adhesion) of each type of junction (SAC = sub-apical complex/marginal zone, zonula adhaerens (ZA), septate junction, tight junction, desmosome) are indicated on the left of the respective junction. E-cadherin based junctions along the lateral contacts of epithelial cells (puncta adhaerentia) have a composition similar to that of punctate junctions between filopodial tips, e.g they contain a classical cadherin, and catenins (p120ctn, β-catenin, α-catenin), but not PLEKHA7 and afadin. On the right, a non-exhaustive list of proteins associated with vertebrate junctions is shown. Proteins, which have so far been identified exclusively in vertebrate organisms, are highlighted in bold character.
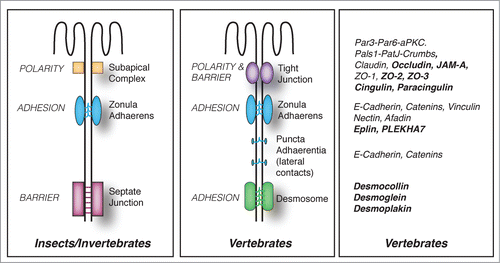
Morphological and genomic analyses show that during evolution from lower Eukaryotes to Metazoans, and from invertebrates to vertebrates, junctions have undergone dramatic changes with regards to architectural organization, molecular composition, regulatory mechanisms, and, in some cases, the functions of individual molecular constituents (). For example, the barrier function in vertebrates is fulfilled by TJ, which are located immediately apical to the cadherin-based zonula adhaerens (). In contrast, the barrier function in invertebrates is carried out by septate junctions, which are located basally, with respect to cadherin-based adherens junctions.Citation14 Ultrastructurally, vertebrate TJ are characterized by the intimate apposition of claudins on adjoining plasma membranes, which appear as a network of fibrils upon freeze fracture. Insect septate junctions show extracellular electron-dense “septa” bridging the opposite plasma membranes, rather than claudin-based fibrils.Citation14 In vertebrates, TJ correspond topologically to the physical “fence” separating apical from lateral plasma membrane domains, which maintains apico-basal polarity (). Instead, the fence in invertebrates is not the septate junction, but the subapical complex (SAC)/marginal zone, which is apical to the ZA, and morphologically distinct from TJ (). Evolutionarily conserved polarity complexes confer either apical identity (Par3-Par6-apKC and Crumbs-Pals1-PatJ complexes), or basolateral identity (Scribble-Dlg-Lgl complex) to the plasma membrane, and are segregated at the level of the TJ in vertebrates and the subapical complex (SAC)/marginal zone in invertebrates ().Citation3,14,15 At the molecular level, the number of isoforms and/or family members for most junctional proteins is considerably larger in vertebrates, providing for increased molecular complexity and redundancy. For example, although cadherin and catenins are shared between insect and vertebrate AJ, invertebrates do not express many classical cadherin isoforms, and lack desmosomal cadherins, desmosomes and intermediate filaments.Citation15,16 Strikingly, epithelial cells of lower Eukaryotes, such as the amoeba Dyctiostelium discoideum, achieve adhesion and polarity in the absence of any cadherin, whereas in metazoans E-cadherin is critically required for cell-adhesion, embryonic development, and the generation of apico-basal polarity.Citation17,18 Claudins, the transmembrane proteins responsible for the barrier of TJ to ions, are highly divergent in their sequence from invertebrates to vertebrates, and the family includes over 20 members in vertebrates, whereas only 5 and 3 members, respectively, have been described so far in C. elegans or Drosophila.Citation14 A ZO-1 homolog has been identified in Drosophila, but the ZO family in vertebrates comprises also ZO-2 and ZO-3, which have partially redundant functions with ZO-1.Citation19,20 Knock-out of the components of the Par3-Par6-aPKC complex in invertebrates has dramatic consequences on epithelial and neuronal morphogenesis, but can have only tissue-specific and more subtle effects in mice.Citation21 The lateral polarity protein Lgl acts as a canonical tumor suppressor protein in Drosophila, but not in mice.Citation22 Over 70 GEFs and 60 GAPs have been described in Vertebrates, whereas only about 10 Rho GEFs and GAPs combined have so far been identified in Drosophila.Citation23 In summary, although invertebrate model systems are useful to establish some general principles, vertebrate cells and organisms are required to understand the remarkably more complex organization of vertebrate junctions and their signaling and regulatory mechanisms. In this review, we focus on vertebrate model systems.
Assembly and Anchoring of the Cytoskeleton at Epithelial Apical Junctional Complexes
To understand the relationships between Rho GTPase regulation and assembly of vertebrate junctions, it is necessary to examine how the actomyosin and microtubule cytoskeletons, which are major targets of Rho GTPase effectors, functionally and structurally interact with AJ and TJ.
The circumferential, junction-associated bundle of actin microfilaments and nonmuscle myosin in the brush border of polarized epithelial cells was described three decades ago.Citation24 The actomyosin cytoskeleton regulates the distribution, stability, clustering and endocytosis of cadherin at the cell membrane,Citation25 and the thickness of the bundle is related to the greater mechanical tensions applied to the ZA, compared to weaker forces applied to lateral AJ complexes.Citation26 Contraction of the ZA-associated actomyosin ring causes apical constriction, which is crucial to support morphogenetic changes in developing embryos.Citation27 In addition, the contractile apical actomyosin ring is critical for the regulation of TJ integrity and barrier function.Citation28
How actin and myosin are structurally connected to junctions, and how they are regulated in their polymerization, assembly and activities by junctional molecules are crucial questions. Several actin-binding proteins are localized at AJ and TJ ().Citation12,29 E-cadherin, although not directly binding to actin, acts as a scaffold for cytoskeleton-associated protein complexes, and plays an instructive role by marking the sites of de novo actin filament polymerization.Citation30,31 E-cadherin directly interacts with cortactin, which in the presence of N-WASP can recruit Arp2/3 and its activator WAVE2 to the ZA, thus promoting actin nucleation at junctions.Citation30,32 This process also requires α-actinin.Citation33 The WAVE2-Arp2/3 complex is activated by Rac1, and is necessary for junctional integrity and contractile tension at the ZA.Citation34 At the ZA, N-WASP is also involved in a putative “non-canonical," Arp2/3-independent pathway, to stabilize newly formed actin filaments, and promote their incorporation into apical rings.Citation35 This is an example of a new role played at zonular junctions by a protein, beyond its classical activity. Nucleation of actin filaments by the Arp2/3 complex gives rise to an extensive array of branched actin filaments, but mature apical junctions are characterized by the presence of bundled actin filaments. Several actin-binding proteins can influence microfilament organization and dynamics at the ZA. Formins, for example, have been implicated in the formation of junctional actin bundles in some cell types.Citation36,37 α-catenin, which has an evolutionarily conserved role in organizing the cortical actin cytoskeleton,Citation17,38,39 suppresses actin polymerization by the Arp2/3 complex, while stabilizing and bundling actin filaments. The affinities of interaction of monomeric α-catenin with actin and vinculin are dramatically increased when tensile forces are applied to junctions, through a molecular stretching mechanism, indicating that monomeric α-catenin bound to β-catenin can directly link F-actin to the cadherin complex in vivo,Citation40,41 although this is not observed in vitro.Citation42 EPLIN (Epithelial Protein Lost In Neoplasm) is recruited to junctions by α-catenin, and it inhibits actin depolymerization, and crosslinks actin filaments.Citation43,44 Afadin associates with the cytoplasmic domain of nectins and JAM,Citation45 is recruited to the ZA through an interaction with α-catenin, and directly interacts with actin filaments.Citation46 Afadin is a major organizer of the apical junctional complex, and is essential for the development of apico-basal polarity in vertebrate embryogenesis.Citation47,48 Finally, myosin II is an essential component of the contractile bundle associated with the ZA, and its positioning is regulated by Shroom, and actin-binding protein which interacts with the Rho effector kinase ROCK,Citation49 and is regulated by the FERM domain protein Lulu.Citation50 Recent studies have addressed the role of different actin and myosin isoforms at epithelial junctions. Depletion studies show that both β- and γ- actin isoforms, though differently distributed, are essential for TJ barrier function and junction assembly, whereas β-actin is selectively involved in the establishment of apico-basal cell polarity.Citation51 Concerning myosins, myosin IIA is the most important in regulating cell morphology and cell-cell adhesion, whereas myosin IIB has more subtle roles in actin filament dynamics.Citation52,53
Figure 2. Proteins implicated in the organization and junctional anchoring of cytoskeletal filaments. For each type of cytoskeletal filament (actin and microtubules) the proteins shown are involved either in their polymerization, bundling, and anchoring to junctions, based on biochemical and/or cell biological evidence.
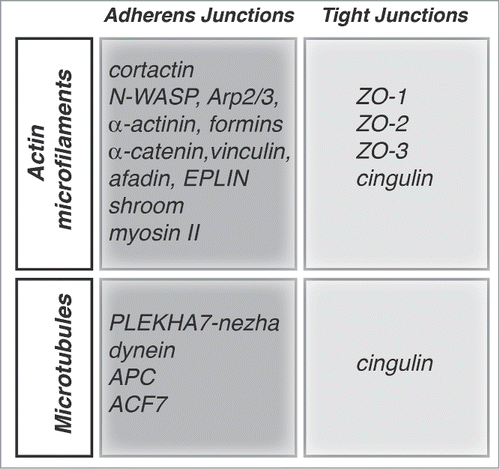
Besides AJ, TJ are also structurally and functionally linked to the actin cytoskeleton (). Actin has multiple potential partners at TJ, including the ZO proteins (ZO-1, ZO-2, ZO-3), occludin and cingulin ().Citation54-56 Cells depleted of ZO-1 show defects in the barrier to larger solutes, and changes in the junction-associated actin, indicating that ZO-1 forms a stabilizing link between the barrier and the junctional actomyosin.Citation57,58 In contrast, depletion of ZO-2 does not lead to either actin reorganization or altered permeability to larger molecules,Citation59 whereas depletion of both ZO-1 and ZO-2 leads to a dramatic expansion of the actomyosin belt associated with AJ.Citation60 Since ZO-1 interacts directly or indirectly with several actin-binding proteins, including α-catenin and cortactin,Citation19,61,62 and with GEFs for Rac1 and RhoA,Citation63,64 some of the phenotypes of these knock-down models may be dependent on these interactions, although this remains to be determined. Cingulin is so far the only TJ protein for which an actin-bundling activity has been described in vitro.Citation56 However, cingulin depletion or overexpression in MDCK cells does not result in dramatic changes in actin organization or barrier function,Citation65-67 suggesting functional redundancies with other proteins.
Microtubules show a polarized distribution in epithelial cells, and associate with the apical junctional complex.Citation68 Recent studies show that E-cadherin is connected to the minus ends of microtubules through a complex containing p120ctn, PLEKHA7, paracingulin and nezha (CAMSAP3)Citation69,70 (). Microtubule anchoring confers stability to apical junctions,Citation69,71 and also indirectly stabilizes TJ barrier function, by enhancing the accumulation of E-cadherin and associated proteins at the ZA.Citation72 Exogenous PLEKHA7 can accumulate at lateral contacts puncta adhaerentia, probably through its interaction with p120ctn, but this does not result in increased recruitment of microtubule minus ends, suggesting that microtubule anchoring requires a specialized molecular environment that occurs only at the ZA.Citation72 Interaction of microtubule plus ends with cadherin-based junctions involves dynein, which interacts with β-catenin,Citation73 APC,Citation74 and the spectroplakin ACF7.Citation75 Recent experiments indicate that a planar apical network of microtubules is anchored to TJ through cingulin, and this interaction is regulated by adenosine monophosphate protein kinase (AMPK)-mediated phosphorylation of cingulin Citation76 (). There is an important cross-talk between the actin and microtubule cytoskeletons. For example, the formin mDia is involved both in linear actin polymerization and microtubule stabilization,Citation77 and microtubules can both sequester Rho GEFs that control actin organization,Citation78,79 and associate with the centralspindlin complex, which plays roles not only in mitotic spindle organization and cytokinesis, but also in the control of Rho and Rac activity at junctions.Citation29,80 In summary, cell-cell junctions are critical sites of anchoring and organization of cytoskeletal filaments, through specific adaptor and regulatory molecules.
The Involvement of RhoA, Rac1 and Cdc42 in Epithelial Junction Assembly and Regulation
The cytoskeleton is essential for the establishment, maintenance, remodeling and disassembly of apical junctions, and this process is regulated by Rho family GTPases and their effectors. The first studies addressed the role of Rho GTPases in junction regulation by exogenously expressing either the Rho inhibitor C3 transferase, or dominant negative (DN) or constitutively active (CA) mutants of Rho GTPases. This lead to loss of barrier and fence functions of TJ, inhibition or perturbation of junction assembly, and was in some cases associated with disrupted localization of junctional proteins, depending on expression levels of mutant proteins.Citation81-85 The observation that DN and CA mutants have similar effects is consistent with the notion that catalytic cycling between active and inactive states, rather than a permanent “on” or “off” state, is essential for the proper functioning of Rho GTPases. Thus, mutant phenotypes may similarly affect the Rho GTPases functional output, by binding to and sequestering targets and effectors. In summary, correct junction assembly and function requires a finely tuned balance in the activities of RhoA, Rac1 and Cdc42.
Rac1 and Cdc42 are essential in the initial formation of junctions, following engagement of adhesion receptors at primordial junctions,Citation31,86,87 by promoting the polymerization of actin filaments in lamellipodia and filopodia, through activation of the Arp2/3 complex by WAVE2.Citation29 A second crucial role of Cdc42 is to promote the formation of the Par6-aPKC-Par3 complex, thus allowing the establishment of apico-basal polarity and segregation of apical TJ.Citation3 The role of Cdc42 in polarity was first discovered in the yeast Saccharomyces cerevisiae, where a Cdc42 mutation resulted in inhibition of polarized budding.Citation88 In vertebrate cells, aPKC activity is required for the establishment of TJ, but not for their maintenance, whereas the role of Cdc42 in the regulation of mature TJ appears to depend on cell type. For example, in endothelial cells Cdc42 does not play a significant role in regulating junctional actin organization and barrier function,Citation89 but in other, non-endothelial cells, regulation of Cdc42 is necessary for TJ maintenance.Citation90,91 Evidence from invertebrate models indicates that at steady state the Cdc42-Par6-aPKC axis acts by limiting RhoA activity, and thus junctional tension at AJ.Citation92 The role of N-WASP, a target of Cdc42 and Rac1, in regulating junction architecture and cortical tension has also been demonstrated in vertebrate model systems.Citation63 N-WASP can also be targeted by pathogens, to promote cell-to-cell spreading.Citation93
RhoA plays a fundamental role both in the establishment and maintenance of AJ and TJ through 2 major effectors: Rho-associated protein kinase (ROCK) and Diaphanous-related formin-1 (Dia).Citation94 mDia nucleates linear actin polymerization at the AJ,Citation36 and can sense and generate mechanical forces on actin filaments,Citation95 whereas ROCK promotes the bundling of actin filaments and the contractility of actomyosin, by enhancing the phosphorylation of nonmuscle myosin light chains.Citation96 These functions are critical to maintain tension at apical junctions, inhibit cadherin endocytosis, and establish and maintain TJ barriers.Citation92,97,98 A physiological balance between mDia and ROCK activities is required to maintain ZAs, since decreased Dia or increased ROCK activation can induce the transition from belt-like ZA to punctate PA.Citation94,99 Indeed, ROCK activation can also be a major mechanism of junction disruption, triggered by cytokines and other exogenous stimuli.Citation100 Therefore the fine-tuning of the activation of RhoA effectors in space and time is a critical factor in the regulation of junction assembly and stability.
New Insights Into the Molecular Mechanisms Underlying the Spatio-temporal Regulation of Rho Family GTPases at Junctions
The biological impact of Rho family GTPases critically depends on the precise site and timing of their activation. Thus, understanding how Rho GTPases control junction assembly requires the identification of the molecular mechanisms that regulate Rho GTPase activity at junctions.
The spatial and temporal control of Rho GTPases is coordinated by GEFs and GAPs, which activate and deactivate Rho GTPases by promoting either the exchange of GDP for GTP, or GTP hydrolysis, respectively. GEFs and GAPs interact with adaptor proteins, which recruit them to defined subcellular sites, and/or modulate their activity, for example by phosphorylation (see section on “Regulation of regulators”). In a previous review, we summarized the interactions of vertebrate junctional proteins with GEFs and GAPs implicated in the regulation of RhoA, Rac1, Rap1 and Cdc42.Citation101 Here, we will focus on more recent studies, which have provided additional insights into the cross-talk between junctional proteins and Rho family GTPases, and frame them into a dynamic view of the involvement of GEFs and GAPs in the different steps of junction formation.
RhoA
New roles in the regulation of RhoA activation at junctions have recently been identified for both TJ and ZA proteins. ARHGEF11 (also known as PDZ-RhoGEF), a Rho-GEF containing a regulator of G protein signaling (RGS) domain, was found to interact with ZO-1, and to be important for the efficient assembly and remodeling of apical junctions Citation64 (). Genomic studies identify ARHGEF11 as a susceptibility locus for intracranial aneurysms Citation102 and kidney injury in the Dahl salt-sensitive rat model,Citation103 suggesting that ARHGEF11 is also involved in cardiovascular and renal physiology and pathology, possibly through its activity at endothelial and/or epithelial junctions. Two additional RhoGEFs were recently found to be associated with the E-cadherin-catenin junctional complex. TEM4 (ARHGEF17, also known as p164-RhoGEF) localizes at stress fibers in sparse cells, and at junctions in confluent epithelial cells.Citation104 TEM4 depletion leads to decreased RhoA activation, decreased myosin light chain phosphorylation, defective endothelial junctions, and attenuated angiogenesis.Citation104 Second, the E-cadherin-α-catenin complex was found to mediate the retention of the RhoGEF ECT2 (Epithelial Cell Transforming gene 2, also known as ARHGEF31) at the ZA in breast cancer (MCF7) cells, resulting in spatially restricted RhoA activation, and generation of junctional tension, to maintain junction integrity Citation29,105 (). During cytokinesis ECT2 plays an important regulatory role in furrow contractility, and is associated with the centralspindin complex, which comprises MgcRacGAP (RACGAP1), and the kinesin family member MKLP1 (KIF23).Citation106 Centralspindin not only regulates ECT2-Rho signaling at junctions, but also inhibits the junctional recruitment of p190RhoGAP (ARHGAP35),Citation29 which functionally interacts with p120-catenin.Citation107,108 In addition to TEM4 and ECT2, a new junctional RhoA GEF which has been identified is p114RhoGEF (ARHGEF18), which interacts with cingulin to promote junctional tension in some, but not all types of epithelial cellsCitation98 (). Recently it was found that p114RhoGEF also binds to the FERM domain protein Lulu2, the polarity protein PatJ,Citation109 and the Ser/Thr kinase LKB Citation110 (), suggesting that different adaptor proteins can recruit p114RhoGEF to cellular sites where it must be localized. GEF-H1 is another prominent junctional Rho GEF, which interacts with cingulin and paracingulin, resulting in its inactivation, and thus decreased RhoA activation and stress fiber formation in the cytoplasm (reviewed in Citation101). GEF-H1 has been implicated in diverse cellular activities, and recently it was also shown to regulate apical constriction and cell intercalation to regulate neural tube closure in Xenopus development.Citation111 Additional RhoGEFs which have been implicated in epithelial apical constriction during morphogenesis are Trio,Citation112 and ARHGEF11.Citation113
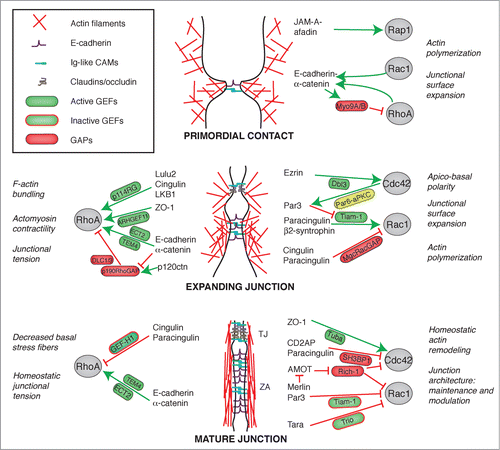
Figure 3. Crosstalk between junctions and Rho GTPases during the biogenesis of epithelial junctions. Simplified schemes showing sequential steps in the formation and maturation of the apical junctional complex (TJ and ZA) in epithelial cells, from primordial contact (top) to mature junction (bottom), and the proteins involved. Legends for graphical objects are shown in box (top left). Green and red arrows/lines indicate activation and inhibition, respectively. The main effects of Rho GTPase regulation on cytoskeletal organization and function are summarized on the sides of each scheme. Proteins and protein interactions depicted here are derived from studies on different model systems, so they do not necessarily occur together, but are grouped in one scheme for the sake of summarizing them. See text for additional details.
Regarding Rho GAPs, indirect roles in regulating junctions have been found for the unconventional myosins Myo9a and Myo9b, large single-headed motor molecules that comprise a N-terminal actin binding domain, and a tail with a Rho GAP domain.Citation114,115 Depletion and overexpression studies show that both Myo9a and Myo9b regulate collective epithelial cell migration and wound healing, by down-regulating RhoA activity, and thus reducing localized cytoskeletal tension at the leading edge of lamellipodia, thus stabilizing nascent cell-cell contacts. However, assembly of junctions in non-migrating cells is not affected by Myo9a-depletion, suggesting that this myosin may be important only for dynamic junctions.Citation114 In another study, knockdown of Myo9a was reported to disrupt TJ,Citation116 similarly to what observed following Myo9b depletion in Caco2 intestinal cells.Citation115 Interestingly, polymorphisms in the gene encoding the Myo9b heavy chain are linked to several forms of inflammatory bowel disease,Citation117,118 and Myo9b function may be implicated in pathogenesis both through defective cell migration of sub-mucosal immune cells, and a leaky TJ barrier. Another Rho GAP that has recently been implicated in the maintenance of cell adhesion is DLC1 (Deleted in Liver Cancer 1), which acts as a GAP for RhoA, RhoC, and, to a lesser extent, for Cdc42. Exogenous DLC1 interacts with α-catenin at AJ, and suppresses invasion and metastasis by up-regulating E-cadherin expression, in a Rho-dependent manner.Citation119 Another member of the DLC family of RhoGAP proteins, DLC3, is localized at AJ in breast cancer cells when exogenously expressed, and is essential for E-cadherin-mediated maintenance of cell-cell contactsCitation120 ().
Rac1
A key regulator of Rac1 activity at epithelial junctions is Tiam1, which is required for the efficient formation of TJ, and is inhibited in confluent cells by Par3,Citation121 suggesting a negative feedback mechanism upon junction maturation. The junctional adaptor paracingulin interacts with Tiam1 and is involved in its recruitment to junctions in MDCK cells,Citation122 whereas β2-syntrophin regulates apico-basal positioning of Rac1 activity at junctions, by counteracting the Par3-Tiam1 inhibitory interactionCitation123 (). A new E-cadherin associated Rac GEF, Trio, was recently localized at AJ, and its Rac1-activating activity is down-regulated by the F-actin binding protein Tara in confluent epithelial monolayers Citation124 (). Interestingly, the regulation of Rac1 activity by Tara is implicated in the modulation of expression of E-cadherin, through a pathway involving the transcription factor Tbx3,Citation124 highlighting the link between junction assembly and Rho family GTPase-mediated regulation of gene expression.Citation125 A new functional interaction of AMOT with merlin was reported to regulate Rac signaling, through the Cdc42/Rac1 GAP RICH1 (ARHGAP17).Citation126 Merlin is a FERM-domain protein encoded by the NF2 (neurofibromatosis-2) tumor suppressor gene, and it regulates cell proliferation in response to adhesive signaling.Citation127 In confluent cells, junctionally localized merlin relieves the inhibition of AMOT over Rich-1, thereby allowing Rich-1 to inhibit Rac1, and thus inhibit downstream MAPK and PAK signaling.Citation126 Thus merlin functions to block mitogenic signaling, by inhibiting Rac1 activity at TJ. Recent studies also demonstrate that Rac1 activity during junction assembly is regulated by the centralspindlin complex protein MgcRacGAP, which is recruited to TJ by cingulin and paracingulin.Citation80 Since cingulin and paracingulin do not affect the localization of the RhoGEF ECT2, it appears that there are 2 pools of MgcRacGAP at apical junctions, one which is recruited by cingulin and paracingulin (at TJ), and a second one which is recruited by the E-cadherin/ α-catenin complex, and interacts with ECT2, at least in MCF7 cells.Citation29,80
It should be emphasized that not all GEFs and GAPs act locally at junctions, but they may contribute to junction assembly through their action on different steps of junctions biogenesis, as shown in the case of the Rac1/Cdc42 GAP PX-RICS (ARHGAP32), which is involved in the transport of N-cadherin and β-catenin from the endoplasmic reticulum to the junctional surface, but is not localized at junctions.Citation128
Cdc42
Cdc42 is a third Rho family GTPase member that has been implicated in regulation of junctions, albeit not in all cell types.Citation129 In MDCK cells, for example, activation of Cdc42 is crucial for regulation of membrane traffic, biogenesis of cell polarity, and formation of junctions, primarily through the activation of the Par6-aPKC-Par3 apical polarity complex.Citation3 In addition to the previously characterized regulation by the Cdc42 GAP Rich-1,Citation101 a new protein complex, comprising paracingulin and CD2AP (CD2-associated protein), was found to regulate Cdc42 activity at junctions of intestinal carcinoma cells, through its interaction with the Cdc42 GAP SH3BP1 (SH3 domain binding protein-1).Citation130 SH3BP1 is implicated both in the maturation of cell-cell junctions, and in homeostatic actin remodeling at mature junctions.Citation130 CD2AP is a scaffolding protein that has been implicated in the maintenance of cell-cell contacts in the slit diaphragms of glomerular podocytes, as well as the function of cortactin and actin-capping proteins.Citation131,132 Regarding specific Cdc42 GEFs, genetic experiments in Drosophila embryos indicate that multiple GEFs, including the Rho GEF ECT2, contribute to cortical activation of Cdc42 during contact-induced cell polarization.Citation133 However in mammalian epithelia only the ZO-1-interacting Cdc42 GEF Tuba has so far been implicated in the maintenance of junctional architecture, but not in junction assembly.Citation63,93 Recently, it was shown that the Cdc42 GEF Dbl (MCF2, also known as ARHGEF21) regulates apical differentiation and apical junction positioning, but not junction assembly, through enhancing the accumulation and activity the Par6-aPKC complex, and the expansion of the apical membrane.Citation134
Importantly, Cdc42 and RhoA activities can also be modulated by the cross-talk with Rap1, a member of the Ras family of GTPases, which is associated with cadherin-, JAM- and nectin-based complexes in epithelial and endothelial cells.Citation101,135 In endothelial cells the physiological restoration of the TJ barrier requires the activation of the Rap1-afadin axis, through phosphorylation of the Rap1 GEF C3G (RAPGEF1), and leads to the down-regulation of RhoA signaling, and enhanced AJ assembly.Citation136 In addition, Rap1 induces FGD5 (FYVE, RhoGEF and PH domain containing 5)-dependent Cdc42 activation, leading to MRCK (myotonic dystrophy-related Cdc42-binding kinase)-dependent circumferential accumulation of nonmuscle myosin II at junctions, while at the same time suppressing the Rho-ROCK pathway, leading to dissolution of radial stress fibers.Citation137 In summary, different molecular pathways are employed, in a cell-context-dependent manner, to orchestrate junction assembly/disassembly through Rho GTPase-dependent modulation of the actomyosin cytoskeleton. Moreover, the finely tuned antagonism between different Rho GTPases (typically Rac1/Cdc42 versus RhoA) sets the position of the border between apical and lateral plasma membrane domain, and thus apico-basal polarity, through the spatially restricted accumulation of cytoskeletal and polarity complex proteins.
Regulation of Regulators
Several mechanisms have been characterized, which regulate the activity and stability of GEFs and GAPs, including phosphorylation, lipid binding, intra-molecular auto-inhibition, and protein-protein interactions.
The Rho GEF GEF-H1 can be sequestered either by binding to microtubules in the cytoplasm,Citation78,138 or by binding to cingulin and paracingulin at epithelial junctions.Citation66,122,139 Phosphorylation of GEF-H1 occurs at several different sites and has multiple effects on GEF-H1 activity. In Jurkat cells, phosphorylation by the Rac1 effector PAK1 leads to GEF-H1 binding to 14-3-3, and association of the complex with microtubules, resulting in inhibition of GEF-H1 activity.Citation140 Another member of the PAK kinase family, PAK4, induces dissociation of GEF-H1 from microtubules in fibroblasts, and switching of substrate specificity, from Rho to Rac1.Citation141 In COS cells, phosphorylation by Par1b, a member of the conserved Par/MARK serine/threonine kinase family, leads to dissociation of GEF-H1 from microtubules, and microtubule destabilization.Citation142,143 GEF-H1 phosphorylation can be cell-cycle dependent, since at early stages of mitosis in Hela cells GEF-H1 is phosphorylated by Aurora A kinase, whereas at telophase it is dephosphorylated, to allow RhoA activation, cleavage furrow formation, and ingression during cytokinesis.Citation144 In HT1080 (fibrosarcoma) and LK2 (lung squamous cell carcinoma) cells phosphorylation by ERK enhances the guanidine exchange activity of GEF-H1.Citation145 In LLC-PK1 kidney tubular cells, GEF-H1 is involved in the sequential activation of Rac1 and RhoA, through TNF-α induced phosphorylation, which activates Rac1, followed by a signaling cascade that results in ERK phosphorylating GEF-H1, leading to RhoA activation.Citation146 ERK signaling also leads to inhibition of GEF-H1 through phosphorylation in MDA-MB-231 breast cancer cells, thus regulating cell motility and invasiveness.Citation147 In summary, the specific mechanisms of GEF-H1 regulation appear largely determined by the cell context-dependent expression of interacting partners.
The Rho activator ECT2 is a key regulator of cytokinesis, and is subjected to cell-cycle-dependent regulation. ECT2 first becomes active in prophase, when it is phosphorylated by Cdk1, and exported from the nucleus into the cytoplasm, to activate RhoA and induce the formation of a mechanically stiff and rounded metaphase cortex.Citation148 Phosphorylation on a different site is required for catalytic activity and interaction with polo-like kinase, leading to stimulation of RhoA activity and SRE-regulated transcription.Citation149 In anaphase ECT2 associates with the centralspindlin complex, and is targeted to the equatorial membrane through a mechanism that requires a pleckstrin homology domain and a polybasic cluster that bind to phosphoinositide lipids.Citation150 Targeting of ECT2 to the equatorial membrane is the key step to initiate cleavage furrow formation during cytokinesis. Upon completion of mitosis, ECT2 undergoes ubiquitin-dependent degradation, indicating that ECT2 is a bona fide cell-cycle-regulated protein.Citation151
Another GEF for which a putative phosphoinositide lipid-mediated recruitment to the membrane has been proposed is Tiam1, a Rac1-specific GEF.Citation152 The guanine nucleotide exchange activity of Tiam1 is enhanced by different inositol phospholipids and other lipids.Citation153,154 Tiam1 is a substrate for the Src kinase, and phosphorylation of residue Y384 of Tiam1, which occurs preferentially at AJ, triggers its degradation, leading to AJ disruption and increased cell migration.Citation155 Tiam1 also interacts with 14-3-3 proteins when phosphorylated on serine residues, and although phosphorylation does not affect Tiam1 activity, it is required for Tiam1 proteolytic degradation.Citation155,156 Tiam1 phosphorylation by protein kinase C and by calcium-calmodulin kinase II have been described in activated fibroblasts, this latter leading to increased guanidine exchange activity toward Rac1 in vitro.Citation157 Finally, protein kinase-D-mediated phosphorylation of the Rho GEF Syx reduces its junctional targeting, through binding to 14-3-3 proteins.Citation158
Phosphorylation also regulates GAPs, to activate or inhibit their activity, or affect their stability. p190RhoGAP is regulated by phosphorylation on Tyr, Ser and Thr residues, and by binding to phospholipids. Activation of Src by different pathways (EGF, integrin, PKC, and cadherin engagement) leads to phosphorylation of p190RhoGAP on Y1105, resulting in enhanced GAP activity, inhibition of RhoA and stress fiber disassembly.Citation159,160 Recruitment of active p190RhoGAP to cadherin through p120ctn leads to local suppression of RhoA activity, which is essential for AJ formation.Citation107,161 ERK mediated phosphorylation on different Ser and Thr residues in the C-terminal part of the protein suppresses the GAP activity of p190RhoGAP during focal adhesion formation.Citation162 Interaction of a polybasic region (PBR) of p190RhoGAP with phospholipids can switch substrate specificity of p190RhoGAP, from RhoA to Rac1, and this interaction is antagonized by phosphorylation on Ser1221 and Thr1226.Citation163 Substrate specificity of RICH-1 is regulated in platelets by Src-mediated phosphorylation, either to inhibit activity on Rho/Rac or to activate GAP activity toward Cdc42.Citation164 MgcRacGAP is regulated by binding to PRC1, which inhibits GAP activity toward Cdc42, thus allowing spindle formation during mitosis, and by Aurora B-mediated phosphorylation and PP2A-mediated dephosphorylation, which affect substrate specificity and interaction with ECT2.Citation165-167 The Rho GAP activity of DLC1 can be inhibited either by phosphorylation, which favors interaction with 14-3-3 and exclusion from focal adhesions,Citation168 or by intramolecular autoinhibition, which is mediated by a SAM domain, and modulated by EGF signaling, through tensin3.Citation169
A Dynamic View of the Cross-talk Between Rho GTPases and Junction Assembly
The process that leads to the formation of epithelial cell-cell junctions is highly regulated in space and time, and results from the coordinated interactions between Rho GTPases, their GEF and GAP regulators, and junctional molecules (). Upon formation of primordial contacts, accumulation of E-cadherin and Ig-like adhesion molecules (JAMs, nectins) is driven by and, at the same time, stimulates Rac1-dependent actin polymerization at the submembrane cortex, in positive feedback loop that further promotes the accumulation of adhesion molecules at new junctions (). In this initial phase, the activities of Rac1/Cdc42, and the Ras-like GTPase Rap1 play a key role, both to generate the cytoskeletal scaffold upon which to build the new junction, and to expand the junctional surface, through cortactin/N-WASP/WAVE2/Arp2/3-mediated actin polymerization, and directed targeting of membrane vesicles. In order for junctions to be expanded and stabilized, RhoA must also be deactivated at sites of cell-substratum interaction, for example through the Rho GAP activity of myosin-9, and activated at junctions (). Junctional RhoA activation is stimulated by GEFs such as p114RhoGEF, ARHGEF11 and ECT2, which interact with different ZA and TJ molecules (), and lead to positioning, assembly and contractility of myosin filaments. Actomyosin contraction at junctions generates the tension required to strengthen adhesion, and helps to cluster and stabilize adhesion molecules (). Since excessive RhoA activation can lead to junction disruption, a process which occurs also during epithelial-mesenchymal transition or in response to pathogens and injury, RhoA activity must also be downregulated, a process which depends at least in part on the p120-dependent recruitment of p190RhoGAP to AJ, and on the antagonism between RhoA and Rac1. Activation of the Rac1/Cdc42 axis of Rho GTPases is essential both for junction expansion, through actin polymerization, and to set apico-basal polarity, through the precise segregation of apical from basolateral determinants at TJ. This is achieved through ezrinDbl3-mediated localized activation of Cdc42, which in turns activates the Par6-aPKC complex, to expand the apical membrane.Citation134 In developing embryos most junctional proteins are targeted to the new junctional membrane through incorporation of membrane vesicles along the basal/lateral plasma membrane, whereas some junctional proteins are recruited to new junctions from a cortical, apical pool.Citation170-174 Once junctions are mature, the dynamic remodelling and functional modulation of the actin and microtubule cytoskeletons is supported by the steady-state equilibrium between activation and inhibition of GEFs, which maintains homeostatic junction architecture and tension ().
A Zonular Signalosome at the Crossroads of Junction Assembly, Rho GTPase Activities, Cytoskeletal Organization, and Nuclear Signaling
Junction assembly and maturation is a dynamic process where different inter-related events are coordinated in space and time: a) accumulation and stabilization of transmembrane adhesion proteins at TJ and ZA; b) accumulation of cytoplasmic plaque proteins of ZA and TJ in the submembrane cortical cytoplasm, and their linkage to cytoskeletal filaments; c) actin polymerization, actin filament bundling and actomyosin contractility; d) microtubule reorganization; e) spatial segregation of junctional protein complexes into apical zonular TJ, subapical zonular ZA, and lateral adherens junction (puncta adhaerentia). This process is mediated by and culminates in the formation of a “zonular signalosome” (), defined as a complex of apical adaptor and signaling proteins associated with zonular (circumferential, belt-like) epithelial junctions (TJ and ZA). The zonular signalosome therefore includes: 1) zonular junctional proteins which function as adaptors for GEFs, GAPs (see ), transcription factors and other signaling molecules (for example ZO proteins, cingulin, paracingulin, AMOT, Par3, afadin, etc); 2) the interacting zonular signaling partners of zonular adaptors (GEFs, GAPs, transcription factors, and other signaling molecules); 3) potentially, additional zonular structural proteins for which no or little direct role in regulation of signaling has yet been described (PLEKHA7, for example), and their interacting signaling partners. The targets and/or effectors of the zonular signalosome are: 1) RhoGTPases and their effectors (kinases and phosphatases for example), which in turn affect signalosome assembly and disassembly, through feedback loops (); 2) genes whose expression is modulated by zonular signalosome-regulated pathways; 3) cytoskeletal proteins (actins, myosins, microtubules and associated proteins): 4) transmembrane and adaptor proteins which are clustered at zonulae through interactions with signalosome adaptors, but can also be distributed at lateral contacts (claudins, occludin, Ig-like CAMs, E-cadherin, catenins, and others). Zonular clustering creates a molecular environment which may confer new or different functions to these proteins, for example only at TJ claudins assemble into continuous fibrils, and are thus able to form ionic pores or barriers.
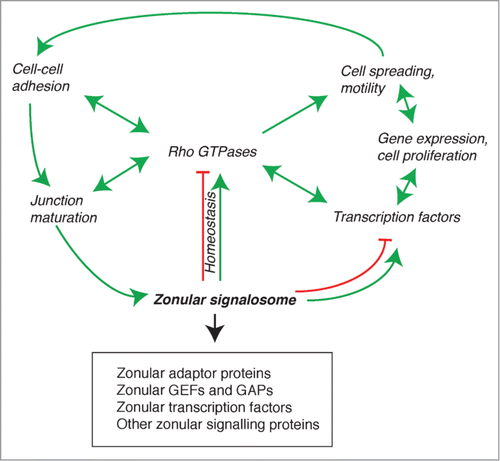
Figure 4. The zonular signalosome. The zonular signalosome is composed of zonular adaptor proteins, GEFs and GAPs, transcription factors and other signaling proteins (see text). Rho GTPases which functionally interact with the signalosome are at the center of a regulatory network that controls adhesion, junction assembly and maturation, regulation of gene expression, cell differentiation and survival, and motile behavior of cells. Transcription factors and other signaling molecules can either exist as part of the signalosome, or are cytoplasmic and regulated indirectly by the signalosome (for example, through RhoA regulation). Arrows indicate functional interactions (unidirectional or reciprocal activation, inhibition, homeostatic balance). See text for additional details.
The composition and properties of the zonular signalosome depend on cell and tissue type, as well as on cell-cycle stages. For example, the Rho GEF ECT2 is zonular in cultured MCF7 mammalian carcinoma cells,Citation29 partially zonular in keratinocytes, not zonular in intestinal carcinoma cells, and not detectable in kidney cells.Citation80 MgcRacGAP is zonular in interphase kidney cells, and excluded from junctions during mitosis.Citation80 Different members of the large GEFs and GAPs families probably show cell- and tissue-type specific expression, however we are far from a precise immunohistochemical mapping of their distribution in different normal and diseased tissues. Similarly, structural adaptor proteins are not identically distributed in all cell types. For example paracingulin is not detected in differentiated intestinal epithelial cells,Citation175 and ZO-3 shows a narrower tissue distribution than ZO-1,Citation176 and is not expressed in cultured mammary epithelial cells.Citation20
What are the functions of the zonular signalosome? One is to fine-tune in space and time the activation of Rho family GTPases, and thus the organization of the cytoskeleton, during the dynamic processes of junction assembly and disassembly, and at steady-state, to ensure the correct remodelling and turnover of cytoskeletal and junctional proteins (). This equilibrium can be perturbed by mechanical stresses, and pathological cues (cytokines, pathogens, toxins), leading to junction disruption, perturbation of the signalosome complex, and hence dramatically altered spatio-temporal regulation of Rho GTPases. As such, the signalosome can be viewed as a sensor, or signal transducer, of extra- and intra-cellular signals, to control cell behavior, including adhesion, motility, and junctional membrane integrity and dynamics. By sequestering and/or stabilizing at junctions GEFs and GAPs, the zonular signalosome also indirectly controls activation of signaling at cell-substratum adhesions, and thus cell spreading and motility, for example through the Par3-Tiam1,Citation177,178 p114RhoGEF,Citation179 and GEF-H1 Citation139 modules. At the tissue and organ level, perturbation of the zonular signalosome can elicit dramatic consequences on epithelial or endothelial cell cell-adhesion and barrier functions, resulting for example in loss of skin or mucosal barrier integrity, jaundice, edema, and loss of proteins or ions across tissue barriers.
A second important function of the zonular signalosome is to regulate transcription factors, cell-cycle regulators, and other signaling molecules, thus controlling gene expression, proliferation, differentiation, and survival. One mechanism of this regulation involves the direct or indirect sequestration of the signaling molecules at junctions, as shown for example by the role of ZO proteins in the junctional retention and stability of the transcription factor DbpA/ZONAB,Citation20,180 and the cell cycle regulator cyclinD1.Citation181 Moreover, ZO-2, α-catenin and AMOT control the nucleo-cytoplasmic shuttling of YAP/TAZ transcription factors by different mechanisms, including direct or indirect interaction, stabilization at junctions, and cytoplasmic retention through modulation of phosphorylation (reviewed in Citation125). Another mechanism involves the modulation of RhoA activation, by zonular GEFs, and interacting adaptor proteins. For example GEF-H1 activation is critical in Dbpa/ZONAB activation and nuclear shuttling Citation182 and RhoA activation is also critically involved in the mechanotransduction-dependent regulation of the activity of YAP transcription factors.Citation183 RhoA is a central molecule in the cross-talk between cytoskeletal organization and nuclear signaling, and the integrity of the zonular signalosome, by regulating not only Rho, but also Rac and Cdc42 activities, is crucial to coordinate regulation of cytoskeletal organization with cell-cell adhesion, motility and nuclear signaling.
Concluding Remarks
In the past decade there have been striking advances in clarifying the identity of junctional proteins, GEFs and GAPs, and their functional interactions. The general picture that has emerged is one whereby epithelial morphogenesis and physiology are regulated by the carefully tuned balance between the activities of antagonistic Rho GTPases, through modulation of the expression, subcellular localization and activities of GEFs and GAPs. However, many important questions remain open. First of all, the majority of studies have been carried out on only one or a few experimental models (cells, tissues, species), and should be validated on additional vertebrate models. Junctions are remarkably heterogeneous in architecture, composition and function,Citation12 and each cell type is thus likely to be characterized by a unique configuration of junctional adaptors and Rho GTPase regulating molecules. So, results obtained in one model system cannot be extrapolated to other cells and tissues. Systematic transcriptomic and proteomic approaches, and the generation of new and improved reagents for the subcellular localization of GEFs and GAPs will be critical to map sets of regulatory modules in their correct context. In addition, detailed characterization of vertebrate knockout models will be essential to understand the role of GEFs and GAPs, and their interacting partners, in tissue and organ physiology and pathology. Special attention should be devoted to elucidating the 3-dimensional structures and affinities of interaction between the adaptor molecules, GEFs and GAPs, and how post-translational modifications can modulate them. Addressing these questions will help to define the composition and functions of zonular signalosomes in different epithelial and endothelial cells and tissues, and develop strategies for their experimental and therapeutic modulation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The authors gratefully acknowledge the funding agencies that sponsor research in the Citi laboratory: the Swiss National Foundation, the Swiss Cancer League, and the State of Geneva.
References
- Gonzalez-Mariscal L, Garay E, Lechuga S. Virus interaction with the apical junctional complex. Front Biosci 2009; 14:731-68; http://dx.doi.org/10.2741/3276
- Vogelmann R, Amieva MR, Falkow S, Nelson WJ. Breaking into the epithelial apical-junctional complex–news from pathogen hackers. Curr Opin Cell Biol 2004; 16:86-93; PMID:15037310; http://dx.doi.org/10.1016/j.ceb.2003.12.002
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 2006; 22:207-35; PMID:16771626; http://dx.doi.org/10.1146/annurev.cellbio.22.010305.104219
- Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 2009; 1:a002584: 1-16; PMID:20066090; http://dx.doi.org/10.1101/cshperspect.a002584
- Franke WW, Rickelt S, Barth M, Pieperhoff S. The junctions that don't fit the scheme: special symmetrical cell-cell junctions of their own kind. Cell Tissue Res 2009; 338:1-17; PMID:19680692; http://dx.doi.org/10.1007/s00441-009-0849-z
- Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 2014; 15:397-410; PMID:24824068; http://dx.doi.org/10.1038/nrm3802
- Pulimeno P, Bauer C, Stutz J, Citi S. PLEKHA7 is an adherens junction protein with a tissue distribution and subcellular localization distinct from ZO-1 and E-cadherin. PLoS One 2010; 5: 10.1371journal.pone.0012207; PMID:20808826; http://dx.doi.org/10.1371/journal.pone.0012207
- Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK). Genes Cells 2002; 7:91-7; PMID:11895474; http://dx.doi.org/10.1046/j.1356-9597.2001.00504.x
- Spindler V, Waschke J. Role of Rho GTPases in desmosomal adhesion and pemphigus pathogenesis. Ann Anat = Anat Anz: Off Organ Anat Ges 2011; 193:177-80; PMID:21441018; http://dx.doi.org/10.1016/j.aanat.2011.02.003
- Tsang SM, Brown L, Gadmor H, Gammon L, Fortune F, Wheeler A, Wan H. Desmoglein 3 acting as an upstream regulator of Rho GTPases, Rac-1Cdc42 in the regulation of actin organisation and dynamics. Exp Cell Res 2012; 318:2269-83; PMID:22796473; http://dx.doi.org/10.1016/j.yexcr.2012.07.002
- Koetsier JL, Amargo EV, Todorovic V, Green KJ, Godsel LM. Plakophilin 2 affects cell migration by modulating focal adhesion dynamics and integrin protein expression. J Invest Dermatol 2014; 134:112-22; PMID:23884246; http://dx.doi.org/10.1038/jid.2013.266
- Franke WW. Discovering the molecular components of intercellular junctions-a historical view. Cold Spring Harbor Perspect Biol 2009; 1:a003061; PMID:20066111; http://dx.doi.org/10.1101/cshperspect.a003061
- Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta 2008; 1778:572-87; PMID:17854763; http://dx.doi.org/10.1016/j.bbamem.2007.07.014
- Simske JS. Claudins reign: the claudinEMPPMP22gamma channel protein family in C. elegans. Tissue Barriers 2013; 1:e25502; PMID:24665403; http://dx.doi.org/10.4161/tisb.25502
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 2010; 11:502-14; PMID:20571587; http://dx.doi.org/10.1038/nrm2927
- Hulpiau P, Gul IS, van Roy F. New insights into the evolution of metazoan cadherins and catenins. Prog Mol Biol Translational Sci 2013; 116:71-94; PMID:23481191; http://dx.doi.org/10.1016/B978-0-12-394311-8.00004-2
- Dickinson DJ, Nelson WJ, Weis WI. A polarized epithelium organized by beta- and alpha-catenin predates cadherin and metazoan origins. Science 2011; 331:1336-9; PMID:21393547; http://dx.doi.org/10.1126/science.1199633
- Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 2007; 18:189-200; PMID:17093058; http://dx.doi.org/10.1091/mbc.E06-05-0471
- Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci 2009; 1165:113-20; PMID:19538295; http://dx.doi.org/10.1111/j.1749-6632.2009.04440.x
- Spadaro D, Tapia R, Jond L, Sudol M, Fanning AS, Citi S. ZO proteins redundantly regulate the transcription factor DbpAZONAB. J Biol Chem 2014; 289:22500-11; PMID:24986862; http://dx.doi.org/10.1074/jbc.M114.556449
- Yamanaka T, Tosaki A, Kurosawa M, Akimoto K, Hirose T, Ohno S, Hattori N, Nukina N. Loss of aPKClambda in differentiated neurons disrupts the polarity complex but does not induce obvious neuronal loss or disorientation in mouse brains. PLoS One 2013; 8:e84036; PMID:24391875; http://dx.doi.org/10.1371/journal.pone.0084036
- Sripathy S, Lee M, Vasioukhin V. Mammalian Llgl2 is necessary for proper branching morphogenesis during placental development. Mol Cell Biol 2011; 31:2920-33; PMID:21606200; http://dx.doi.org/10.1128/MCB.05431-11
- Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta 2003; 1603:47-82; PMID:12618308
- Mooseker MS. Organisation, chemistry and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol 1985; 1:209-41; PMID:3916317; http://dx.doi.org/10.1146/annurev.cb.01.110185.001233
- Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell 2001; 12:847-62; PMID:11294891; http://dx.doi.org/10.1091/mbc.12.4.847
- Budnar S, Yap AS. A mechanobiological perspective on cadherins and the actin-myosin cytoskeleton. F1000prime Rep 2013; 5:35; PMID:24049639; http://dx.doi.org/10.12703/P5-35
- Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn 2005; 232:685-94; PMID:15712202; http://dx.doi.org/10.1002/dvdy.20334
- Turner JR. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation. Semin Cell Dev Biol 2000; 11:301-8; PMID:10966864; http://dx.doi.org/10.1006/scdb.2000.0180
- Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol 2012; 14:818-28; PMID:22750944; http://dx.doi.org/10.1038/ncb2532
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: e-cadherin physically associates with the Arp23 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 2002; 12:379-82; PMID:11882288; http://dx.doi.org/10.1016/S0960-9822(02)00661-9
- Ehrlich JS, Hansen MD, Nelson WJ. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev Cell 2002; 3:259-70; PMID:12194856; http://dx.doi.org/10.1016/S1534-5807(02)00216-2
- Han SP, Gambin Y, Gomez GA, Verma S, Giles N, Michael M, Wu SK, Guo Z, Johnston W, Sierecki E, et al. Cortactin scaffolds Arp23 and WAVE2 at the epithelial zonula adherens. J Biol Chem 2014; 289:7764-75; PMID:24469447; http://dx.doi.org/10.1074/jbc.M113.544478
- Tang VW, Brieher WM. alpha-Actinin-4FSGS1 is required for Arp23-dependent actin assembly at the adherens junction. J Cell Biol 2012; 196:115-30; PMID:22232703; http://dx.doi.org/10.1083/jcb.201103116
- Verma S, Han SP, Michael M, Gomez GA, Yang Z, Teasdale RD, Ratheesh A, Kovacs EM, Ali RG, Yap AS. A WAVE2-Arp23 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol Biol Cell 2012; 23:4601-10; PMID:23051739; http://dx.doi.org/10.1091/mbc.E12-08-0574
- Kovacs EM, Verma S, Ali RG, Ratheesh A, Hamilton NA, Akhmanova A, Yap AS. N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nat Cell Biol 2011; 13:934-43; PMID:21785420; http://dx.doi.org/10.1038/ncb2290
- Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 2004; 6:21-30; PMID:14647292; http://dx.doi.org/10.1038/ncb1075
- Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci 2007; 120:3870-82; PMID:17940061; http://dx.doi.org/10.1242/jcs.014365
- Ozono K, Komiya S, Shimamura K, Ito T, Nagafuchi A. Defining the roles of alpha-catenin in cell adhesion and cytoskeleton organization: isolation of F9 cells completely lacking cadherin-catenin complex. Cell Struct Funct 2011; 36:131-43; PMID:21685705; http://dx.doi.org/10.1247/csf.11009
- Desai R, Sarpal R, Ishiyama N, Pellikka M, Ikura M, Tepass U. Monomeric alpha-catenin links cadherin to the actin cytoskeleton. Nat Cell Biol 2013; 15:261-73; PMID:23417122; http://dx.doi.org/10.1038/ncb2685
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. Alpha-catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 2010; 12:533-42; PMID:20453849; http://dx.doi.org/10.1038/ncb2055
- le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol 2010; 189:1107-15; PMID:20584916; http://dx.doi.org/10.1083/jcb.201001149
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005; 123:903-15; PMID:16325583; http://dx.doi.org/10.1016/j.cell.2005.09.021
- Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A 2008; 105:13-9; PMID:18093941; http://dx.doi.org/10.1073/pnas.0710504105
- Maul RS, Song Y, Amann KJ, Gerbin SC, Pollard TD, Chang DD. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J Cell Biol 2003; 160:399-407; PMID:12566430; http://dx.doi.org/10.1083/jcb.200212057
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, et al. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol 1997; 139:517-28; PMID:9334353; http://dx.doi.org/10.1083/jcb.139.2.517
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol 2000; 150:1161-76; PMID:10974003; http://dx.doi.org/10.1083/jcb.150.5.1161
- Zhadanov AB, Provance DW, Speer CA, Coffin JD, Goss D, Blixt JA, Reichert CM, Mercer JA. Absence of the tight junctional protein AF-6 disrupts epithelial cell- cell junctions and cell polarity during mouse development. Curr Biol 1999; 9:880-8; PMID:10469590; http://dx.doi.org/10.1016/S0960-9822(99)80392-3
- Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, et al. Afadin: a key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol 1999; 146:1117-32; PMID:10477764; http://dx.doi.org/10.1083/jcb.146.5.1117
- Simoes Sde M, Mainieri A, Zallen JA. Rho GTPase and Shroom direct planar polarized actomyosin contractility during convergent extension. J Cell Biol 2014; 204:575-89; PMID:24535826; http://dx.doi.org/10.1083/jcb.201307070
- Chu CW, Gerstenzang E, Ossipova O, Sokol SY. Lulu regulates Shroom-induced apical constriction during neural tube closure. PLoS One 2013; 8:e81854; PMID:24282618; http://dx.doi.org/10.1371/journal.pone.0081854
- Baranwal S, Naydenov NG, Harris G, Dugina V, Morgan KG, Chaponnier C, Ivanov AI. Nonredundant roles of cytoplasmic beta- and gamma-actin isoforms in regulation of epithelial apical junctions. Mol Biol Cell 2012; 23:3542-53; PMID:22855531; http://dx.doi.org/10.1091/mbc.E12-02-0162
- Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One 2007; 2:e658; PMID:17668046; http://dx.doi.org/10.1371/journal.pone.0000658
- Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 2010; 12:696-702; PMID:20543839; http://dx.doi.org/10.1038/ncb2072
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 1998; 273:29745-53; PMID:9792688; http://dx.doi.org/10.1074/jbc.273.45.29745
- Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and zo-1 forms independent complexes with zo-2 and zo-3. J Biol Chem 1999; 274:35179-85; PMID:10575001; http://dx.doi.org/10.1074/jbc.274.49.35179
- D’Atri F, Citi S. Cingulin interacts with F-actin in vitro. FEBS Lett 2001; 507:21-4; PMID:11682052; http://dx.doi.org/10.1016/S0014-5793(01)02936-2
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell 2009; 20:3930-40; PMID:19605556; http://dx.doi.org/10.1091/mbc.E09-04-0320
- Tokuda S, Higashi T, Furuse M. ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape. PLoS One 2014; 9:e104994; PMID:25157572; http://dx.doi.org/10.1371/journal.pone.0104994
- Fanning AS, Van Itallie C, Anderson JM. Zonula occludens (ZO)-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell 2011; 23:577-90; PMID:22190737; http://dx.doi.org/10.1091/mbc.E11-09-0791
- Fanning AS, Van Itallie CM, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell 2012; 23:577-90; PMID:22190737; http://dx.doi.org/10.1091/mbc.E11-09-0791
- Maiers JL, Peng X, Fanning AS, DeMali KA. ZO-1 recruitment to alpha-catenin–a novel mechanism for coupling the assembly of tight junctions to adherens junctions. J Cell Sci 2013; 126:3904-15; PMID:23813953; http://dx.doi.org/10.1242/jcs.126565
- Ooshio T, Kobayashi R, Ikeda W, Miyata M, Fukumoto Y, Matsuzawa N, Ogita H, Takai Y. Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J Biol Chem 2010; 285:5003-12; PMID:20008323; http://dx.doi.org/10.1074/jbc.M109.043760
- Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol 2006; 175:135-46; PMID:17015620; http://dx.doi.org/10.1083/jcb.200605012
- Itoh M, Tsukita S, Yamazaki Y, Sugimoto H. Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc Natl Acad Sci U S A 2012; 109:9905-10; PMID:22665792; http://dx.doi.org/10.1073/pnas.1115063109
- Paschoud S, Citi S. Inducible overexpression of cingulin in stably transfected MDCK cells does not affect tight junction organization and gene expression. Mol Membr Biol 2008; 25:1-13; PMID:18097951; http://dx.doi.org/10.1080/09687680701474009
- Guillemot L, Citi S. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol Biol Cell 2006; 17:3569-77; PMID:16723500; http://dx.doi.org/10.1091/mbc.E06-02-0122
- Guillemot L, Schneider Y, Brun P, Castagliuolo I, Pizzuti D, Martines D, Jond L, Bongiovanni M, Citi S. Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury. J Cell Sci 2012; 125:5005-14; in press; PMID:22946046; http://dx.doi.org/10.1242/jcs.101261
- Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harbor Perspect Biol 2009; 1:a002899; PMID:20457565; http://dx.doi.org10.1101cshperspect.a002899
- Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 2008; 135:948-59; PMID:19041755; http://dx.doi.org/10.1016/j.cell.2008.09.040
- Pulimeno P, Paschoud S, Citi S. A role for ZO-1 and PLEKHA7 in recruiting paracingulin to tight and adherens junctions of epithelial cells. J Biol Chem 2011; 286:16743-50; PMID:21454477; http://dx.doi.org/10.1074/jbc.M111.230862
- Ivanov AI, McCall IC, Babbin B, Samarin SN, Nusrat A, Parkos CA. Microtubules regulate disassembly of epithelial apical junctions. BMC Cell Biol 2006; 7:12; PMID:16509970; http://dx.doi.org/10.1186/1471-2121-7-12
- Paschoud S, Jond L, Guerrera D, Citi S. PLEKHA7 modulates epithelial tight junction barrier function. Tissue Barriers 2014; 2:e28755; PMID:24843844; http://dx.doi.org/10.4161/tisb.28755
- Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic 2007; 8:808-19; PMID:17550375; http://dx.doi.org/10.1111/j.1600-0854.2007.00574.x
- Rosin-Arbesfeld R, Ihrke G, Bienz M. Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells. EMBO J 2001; 20:5929-39; PMID:11689433; http://dx.doi.org/10.1093/emboj/20.21.5929
- Karakesisoglou I, Yang Y, Fuchs E. An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J Cell Biol 2000; 149:195-208; PMID:10747097; http://dx.doi.org/10.1083/jcb.149.1.195
- Yano T, Matsui T, Tamura A, Uji M, Tsukita S. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J Cell Biol 2013; 203:605-14; PMID:24385485; http://dx.doi.org/10.1083/jcb.201304194
- Bartolini F, Ramalingam N, Gundersen GG. Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol Biol Cell 2012; 23:4032-40; PMID:22918941; http://dx.doi.org/10.1091/mbc.E12-05-0338
- Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol 2002; 4:294-301; PMID:11912491; http://dx.doi.org/10.1038/ncb773
- Nagae S, Meng W, Takeichi M. Non-centrosomal microtubules regulate F-actin organization through the suppression of GEF-H1 activity. Genes Cells 2013; 18:387-96; PMID:23432781; http://dx.doi.org/10.1111/gtc.12044
- Guillemot L, Guerrera D, Spadaro D, Tapia R, Jond L, Citi S. MgcRacGAP interacts with cingulin and paracingulin to regulate Rac1 activation and development of the tight junction barrier during epithelial junction assembly. Mol Biol Cell 2014; 25:1995-2005; PMID:24807907; http://dx.doi.org/10.1091/mbc.E13-11-0680
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carmes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA 1995; 92:10629-33; PMID:7479854; http://dx.doi.org/10.1073/pnas.92.23.10629
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol 1997; 137:1421-31; PMID:9182672; http://dx.doi.org/10.1083/jcb.137.6.1421
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol 1997; 139:1047-59; PMID:9362522; http://dx.doi.org/10.1083/jcb.139.4.1047
- Jou TS, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol 1998; 142:85-100; PMID:9660865; http://dx.doi.org/10.1083/jcb.142.1.85
- Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol 1998; 142:101-15; PMID:9660866; http://dx.doi.org/10.1083/jcb.142.1.101
- Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem 2002; 277:6708-18; PMID:11744701; http://dx.doi.org/10.1074/jbc.M109640200
- Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol 2001; 13:76-84; PMID:11163137; http://dx.doi.org/10.1016/S0955-0674(00)00177-0
- Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol 1990; 111:131-42; PMID:2195038; http://dx.doi.org/10.1083/jcb.111.1.131
- Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxiareoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 2005; 288:L749-60; PMID:15591411; http://dx.doi.org/10.1152/ajplung.00361.2004
- Fukuhara A, Shimizu K, Kawakatsu T, Fukuhara T, Takai Y. Involvement of nectin-activated Cdc42 small G protein in organization of adherens and tight junctions in Madin-Darby canine kidney cells. J Biol Chem 2003; 278:51885-93; PMID:14530286; http://dx.doi.org/10.1074/jbc.M308015200
- Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, et al. A Rich1Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 2006; 125:535-48; PMID:16678097; http://dx.doi.org/10.1016/j.cell.2006.02.045
- Warner SJ, Longmore GD. Cdc42 antagonizes Rho1 activity at adherens junctions to limit epithelial cell apical tension. J Cell Biol 2009; 187:119-33; PMID:19805632; http://dx.doi.org/10.1083/jcb.200906047
- Rajabian T, Gavicherla B, Heisig M, Muller-Altrock S, Goebel W, Gray-Owen SD, Ireton K. The bacterial virulence factor InlC perturbs apical cell junctions and promotes cell-to-cell spread of Listeria. Nat Cell Biol 2009; 11:1212-8; PMID:19767742; http://dx.doi.org/10.1038/ncb1964
- Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol 2002; 4:408-15; PMID:11992112; http://dx.doi.org/10.1038/ncb796
- Jegou A, Carlier MF, Romet-Lemonne G. Formin mDia1 senses and generates mechanical forces on actin filaments. Nat Commun 2013; 4:1883; PMID:23695677; http://dx.doi.org/10.1038/ncomms2888
- Amano M, Nakayama M, Kaibuchi K. Rho-kinaseROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskel (Hoboken) 2010; 67:545-54; PMID:20803696; http://dx.doi.org/10.1002/cm.20472
- Levayer R, Pelissier-Monier A, Lecuit T. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat Cell Biol 2011; 13:529-40; PMID:21516109; http://dx.doi.org/10.1038/ncb2224
- Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol 2011; 13:159-66; PMID:21258369; http://dx.doi.org/10.1038/ncb2156
- Ngok SP, Anastasiadis PZ. Rho GEFs in endothelial junctions: effector selectivity and signaling integration determine junctional response. Tissue Barriers 2013; 1:e27132; PMID:24790803; http://dx.doi.org/10.4161/tisb.27132
- Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta 2009; 1788:864-71; PMID:18952050; http://dx.doi.org/10.1016/j.bbamem.2008.08.027
- Citi S, Spadaro D, Schneider Y, Stutz J, Pulimeno P. Regulation of small GTPases at epithelial cell-cell junctions. Mol Membr Biol 2011; 28:427-44; PMID:21781017; http://dx.doi.org/10.3109/09687688.2011.603101
- Akiyama K, Narita A, Nakaoka H, Cui T, Takahashi T, Yasuno K, Tajima A, Krischek B, Yamamoto K, Kasuya H, et al. Genome-wide association study to identify genetic variants present in Japanese patients harboring intracranial aneurysms. J Hum Genet 2010; 55:656-61; PMID:20613766; http://dx.doi.org/10.1038/jhg.2010.82
- Williams JM, Johnson AC, Stelloh C, Dreisbach AW, Franceschini N, Regner KR, Townsend RR, Roman RJ, Garrett MR. Genetic variants in Arhgef11 are associated with kidney injury in the Dahl salt-sensitive rat. Hypertension 2012; 60:1157-68; PMID:22987919; http://dx.doi.org/10.1161/HYPERTENSIONAHA.112.199240
- Ngok SP, Geyer R, Kourtidis A, Mitin N, Feathers R, Der C, Anastasiadis PZ. TEM4 is a junctional Rho GEF required for cell-cell adhesion, monolayer integrity and barrier function. J Cell Sci 2013; 126:3271-7; PMID:23729734; http://dx.doi.org/10.1242/jcs.123869
- Priya R, Yap AS, Gomez GA. E-cadherin supports steady-state Rho signaling at the epithelial zonula adherens. Differ; Res Biol Divers 2013; 86:133-40; PMID:23643492; http://dx.doi.org/10.1016/j.diff.2013.01.002
- Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol 2005; 170:571-82; PMID:16103226; http://dx.doi.org/10.1083/jcb.200501097
- Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 2006; 127:1027-39; PMID:17129786; http://dx.doi.org/10.1016/j.cell.2006.09.046
- Birukova AA, Zebda N, Cokic I, Fu P, Wu T, Dubrovskyi O, Birukov KG. p190RhoGAP mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury. Exp Cell Res 2011; 317:859-72; PMID:21111731; http://dx.doi.org/10.1016/j.yexcr.2010.11.011
- Nakajima H, Tanoue T. Lulu2 regulates the circumferential actomyosin tensile system in epithelial cells through p114RhoGEF. J Cell Biol 2011; 195:245-61; PMID:22006950; http://dx.doi.org/10.1083/jcb.201104118
- Nongpiur ME, Wei X, Xu L, Perera SA, Wu RY, Zheng Y, Li Y, Wang YX, Cheng CY, Jonas JB, et al. Lack of association between primary angle-closure glaucoma susceptibility loci and the ocular biometric parameters anterior chamber depth and axial length. Invest Ophthalmol Vis Sci 2013; 54:5824-8; PMID:23920366; http://dx.doi.org/10.1167/iovs.13-11901
- Itoh K, Ossipova O, Sokol SY. GEF-H1 functions in apical constriction and cell intercalations and is essential for vertebrate neural tube closure. J Cell Sci 2014; 127:2542-53; PMID:24681784; http://dx.doi.org/10.1242/jcs.146811
- Plageman TF Jr, Chauhan BK, Yang C, Jaudon F, Shang X, Zheng Y, Lou M, Debant A, Hildebrand JD, Lang RA. A Trio-RhoA-Shroom3 pathway is required for apical constriction and epithelial invagination. Development 2011; 138:5177-88; PMID:22031541; http://dx.doi.org/10.1242/dev.067868
- Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 2012; 149:1084-97; PMID:22632972; http://dx.doi.org/10.1016/j.cell.2012.04.021
- Omelchenko T, Hall A. Myosin-IXA regulates collective epithelial cell migration by targeting RhoGAP activity to cell-cell junctions. Curr Biol 2012; 22:278-88; PMID:22305756; http://dx.doi.org/10.1016/j.cub.2012.01.014
- Chandhoke SK, Mooseker MS. A role for myosin IXb, a motor-RhoGAP chimera, in epithelial wound healing and tight junction regulation. Mol Biol Cell 2012; 23:2468-80; PMID:22573889; http://dx.doi.org/10.1091/mbc.E11-09-0803
- Abouhamed M, Grobe K, San IV, Thelen S, Honnert U, Balda MS, Matter K, Bahler M. Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus. Mol Biol Cell 2009; 20:5074-85; PMID:19828736; http://dx.doi.org/10.1091/mbc.E09-04-0291
- van Bodegraven AA, Curley CR, Hunt KA, Monsuur AJ, Linskens RK, Onnie CM, Crusius JB, Annese V, Latiano A, Silverberg MS, et al. Genetic variation in myosin IXB is associated with ulcerative colitis. Gastroenterology 2006; 131:1768-74; PMID:17087940; http://dx.doi.org/10.1053/j.gastro.2006.09.011
- Monsuur AJ, de Bakker PI, Alizadeh BZ, Zhernakova A, Bevova MR, Strengman E, Franke L, van't Slot R, van Belzen MJ, Lavrijsen IC, et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet 2005; 37:1341-4; PMID:16282976; http://dx.doi.org/10.1038/ng1680
- Tripathi V, Popescu NC, Zimonjic DB. DLC1 induces expression of E-cadherin in prostate cancer cells through Rho pathway and suppresses invasion. Oncogene 2014; 33:724-33; PMID:23376848; http://dx.doi.org/10.1038/onc.2013.7
- Holeiter G, Bischoff A, Braun AC, Huck B, Erlmann P, Schmid S, Herr R, Brummer T, Olayioye MA. The RhoGAP protein Deleted in Liver Cancer 3 (DLC3) is essential for adherens junctions integrity. Oncogenesis 2012; 1:e13; PMID:23552697; http://dx.doi.org/10.1038/oncsis.2012.13
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 2005; 7:262-9; PMID:15723052; http://dx.doi.org/10.1038/ncb1226
- Guillemot L, Paschoud S, Jond L, Foglia A, Citi S. Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol Biol Cell 2008; 19:4442-53; PMID:18653465; http://dx.doi.org/10.1091/mbc.E08-06-0558
- Mack NA, Porter AP, Whalley HJ, Schwarz JP, Jones RC, Khaja AS, Bjartell A, Anderson KI, Malliri A. beta2-syntrophin and Par-3 promote an apicobasal Rac activity gradient at cell-cell junctions by differentially regulating Tiam1 activity. Nat Cell Biol 2012; 14:1169-80; PMID:23103911; http://dx.doi.org/10.1038/ncb2608
- Yano T, Yamazaki Y, Adachi M, Okawa K, Fort P, Uji M, Tsukita S, Tsukita S. Tara up-regulates E-cadherin transcription by binding to the Trio RhoGEF and inhibiting Rac signaling. J Cell Biol 2011; 193:319-32; PMID:21482718; http://dx.doi.org/10.1083/jcb.201009100
- Spadaro D, Tapia R, Pulimeno P, Citi S. The control of gene expression and cell proliferation by the epithelial apical junctional complex. Essays Biochem 2012; 53:83-93; PMID:22928510; http://dx.doi.org/10.1042/bse0530083
- Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, Persson NL, Shimono A, Speicher DW, Marmorstein R, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell 2011; 19:527-40; PMID:21481793; http://dx.doi.org/10.1016/j.ccr.2011.02.017
- Cooper J, Giancotti FG. Molecular insights into NF2Merlin tumor suppressor function. FEBS Lett 2014; 588:2743-52; PMID:24726726; http://dx.doi.org/10.1016/j.febslet.2014.04.001
- Nakamura T, Hayashi T, Mimori-Kiyosue Y, Sakaue F, Matsuura K, Iemura S, Natsume T, Akiyama T. The PX-RICS-14-3-3zetatheta complex couples N-cadherin-beta-catenin with dynein-dynactin to mediate its export from the endoplasmic reticulum. J Biol Chem 2010; 285:16145-54; PMID:20308060; http://dx.doi.org/10.1074/jbc.M109.081315
- Erasmus J, Aresta S, Nola S, Caron E, Braga VM. Newly formed E-cadherin contacts do not activate Cdc42 or induce filopodia protrusion in human keratinocytes. Biol Cell 2010; 102:13-24; http://dx.doi.org/10.1042/BC20090048
- Elbediwy A, Zihni C, Terry SJ, Clark P, Matter K, Balda MS. Epithelial junction formation requires confinement of Cdc42 activity by a novel SH3BP1 complex. J Cell Biol 2012; 198:677-93; PMID:22891260; http://dx.doi.org/10.1083/jcb.201202094
- Zhao J, Bruck S, Cemerski S, Zhang L, Butler B, Dani A, Cooper JA, Shaw AS. CD2AP links cortactin and capping protein at the cell periphery to facilitate formation of lamellipodia. Mol Cell Biol 2013; 33:38-47; PMID:23090967; http://dx.doi.org/10.1128/MCB.00734-12
- van Duijn TJ, Anthony EC, Hensbergen PJ, Deelder AM, Hordijk PL. Rac1 recruits the adapter protein CMSCD2AP to cell-cell contacts. J Biol Chem 2010; 285:20137-46; PMID:20404345; http://dx.doi.org/10.1074/jbc.M109.099481
- Chan E, Nance J. Mechanisms of CDC-42 activation during contact-induced cell polarization. J Cell Sci 2013; 126:1692-702; PMID:23424200; http://dx.doi.org/10.1242/jcs.124594
- Zihni C, Munro PM, Elbediwy A, Keep NH, Terry SJ, Harris J, Balda MS, Matter K. Dbl3 drives Cdc42 signaling at the apical margin to regulate junction position and apical differentiation. J Cell Biol 2014; 204:111-27; PMID:24379416; http://dx.doi.org/10.1083/jcb.201304064
- Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci 2007; 120:17-22; PMID:17182900; http://dx.doi.org/10.1242/jcs.03306
- Birukova AA, Tian X, Tian Y, Higginbotham K, Birukov KG. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell 2013; 24:2678-88; PMID:23864716; http://dx.doi.org/10.1091/mbc.E13-02-0098
- Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol 2013; 202:901-16; PMID:24019534; http://dx.doi.org/10.1083/jcb.201301115
- Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol 2008; 18:210-9; PMID:18394899; http://dx.doi.org/10.1016/j.tcb.2008.02.006
- Aijaz S, D’Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1S phase transition. Dev Cell 2005; 8:777-86; PMID:15866167; http://dx.doi.org/10.1016/j.devcel.2005.03.003
- Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem 2004; 279:18392-400; PMID:14970201; http://dx.doi.org/10.1074/jbc.M400084200
- Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci 2005; 118:1861-72; PMID:15827085; http://dx.doi.org/10.1242/jcs.02313
- Yoshimura Y, Miki H. Dynamic regulation of GEF-H1 localization at microtubules by Par1bMARK2. Biochem Biophys Res Commun 2011; 408:322-8; PMID:21513698; http://dx.doi.org/10.1016/j.bbrc.2011.04.032
- Yamahashi Y, Saito Y, Murata-Kamiya N, Hatakeyama M. Polarity-regulating kinase partitioning-defective 1b (PAR1b) phosphorylates guanine nucleotide exchange factor H1 (GEF-H1) to regulate RhoA-dependent actin cytoskeletal reorganization. J Biol Chem 2011; 286:44576-84; PMID:22072711; http://dx.doi.org/10.1074/jbc.M111.267021
- Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell 2007; 12:699-712; PMID:17488622; http://dx.doi.org/10.1016/j.devcel.2007.03.014
- Fujishiro SH, Tanimura S, Mure S, Kashimoto Y, Watanabe K, Kohno M. ERK12 phosphorylate GEF-H1 to enhance its guanine nucleotide exchange activity toward RhoA. Biochem Biophys Res Commun 2008; 368:162-7; PMID:18211802; http://dx.doi.org/10.1016/j.bbrc.2008.01.066
- Waheed F, Dan Q, Amoozadeh Y, Zhang Y, Tanimura S, Speight P, Kapus A, Szaszi K. Central role of the exchange factor GEF-H1 in TNF-alpha-induced sequential activation of Rac, ADAM17TACE, and RhoA in tubular epithelial cells. Mol Biol Cell 2013; 24:1068-82; PMID:23389627; http://dx.doi.org/10.1091/mbc.E12-09-0661
- von Thun A, Preisinger C, Rath O, Schwarz JP, Ward C, Monsefi N, Rodriguez J, Garcia-Munoz A, Birtwistle M, Bienvenut W, et al. Extracellular signal-regulated kinase regulates RhoA activation and tumor cell plasticity by inhibiting guanine exchange factor H1 activity. Mol Cell Biol 2013; 33:4526-37; PMID:24043311; http://dx.doi.org/10.1128/MCB.00585-13
- Matthews HK, Delabre U, Rohn JL, Guck J, Kunda P, Baum B. Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression. Dev Cell 2012; 23:371-83; PMID:22898780; http://dx.doi.org/10.1016/j.devcel.2012.06.003
- Niiya F, Tatsumoto T, Lee KS, Miki T. Phosphorylation of the cytokinesis regulator ECT2 at G2M phase stimulates association of the mitotic kinase Plk1 and accumulation of GTP-bound RhoA. Oncogene 2006; 25:827-37; PMID:16247472; http://dx.doi.org/10.1038/sj.onc.1209124
- Su KC, Takaki T, Petronczki M. Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev Cell 2011; 21:1104-15; PMID:22172673; http://dx.doi.org/10.1016/j.devcel.2011.11.003
- Liot C, Seguin L, Siret A, Crouin C, Schmidt S, Bertoglio J. APC(cdh1) mediates degradation of the oncogenic Rho-GEF Ect2 after mitosis. PLoS One 2011; 6:e23676; PMID:21886810; http://dx.doi.org/10.1371/journal.pone.0023676
- Michiels F, Stam JC, Hordijk PL, van der Kammen RA, Ruuls-Van Stalle L, Feltkamp CA, Collard JG. Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and C-Jun NH2-terminal kinase activation. J Cell Biol 1997; 137:387-98; PMID:9128250; http://dx.doi.org/10.1083/jcb.137.2.387
- Fleming IN, Gray A, Downes CP. Regulation of the Rac1-specific exchange factor Tiam1 involves both phosphoinositide 3-kinase-dependent and -independent components. Biochem J 2000; 351:173-82; PMID:10998360; http://dx.doi.org/10.1042/0264-6021:3510173
- Baumeister MA, Martinu L, Rossman KL, Sondek J, Lemmon MA, Chou MM. Loss of phosphatidylinositol 3-phosphate binding by the C-terminal Tiam-1 pleckstrin homology domain prevents in vivo Rac1 activation without affecting membrane targeting. J Biol Chem 2003; 278:11457-64; PMID:12525493; http://dx.doi.org/10.1074/jbc.M211901200
- Woodcock SA, Rooney C, Liontos M, Connolly Y, Zoumpourlis V, Whetton AD, Gorgoulis VG, Malliri A. SRC-induced disassembly of adherens junctions requires localized phosphorylation and degradation of the rac activator tiam1. Mol Cell 2009; 33:639-53; PMID:19285946; http://dx.doi.org/10.1016/j.molcel.2009.02.012
- Woodcock SA, Jones RC, Edmondson RD, Malliri A. A modified tandem affinity purification technique identifies that 14-3-3 proteins interact with Tiam1, an interaction which controls Tiam1 stability. J Proteome Res 2009; 8:5629-41; PMID:19899799; http://dx.doi.org/10.1021/pr900716e
- Fleming IN, Elliott CM, Buchanan FG, Downes CP, Exton JH. Ca2+calmodulin-dependent protein kinase II regulates Tiam1 by reversible protein phosphorylation. J Biol Chem 1999; 274:12753-8; PMID:10212259; http://dx.doi.org/10.1074/jbc.274.18.12753
- Ngok SP, Geyer R, Kourtidis A, Storz P, Anastasiadis PZ. Phosphorylation-mediated 14-3-3 protein binding regulates the function of the rho-specific guanine nucleotide exchange factor (RhoGEF) Syx. J Biol Chem 2013; 288:6640-50; PMID:23335514; http://dx.doi.org/10.1074/jbc.M112.432682
- Haskell MD, Nickles AL, Agati JM, Su L, Dukes BD, Parsons SJ. Phosphorylation of p190 on Tyr1105 by c-Src is necessary but not sufficient for EGF-induced actin disassembly in C3H10T12 fibroblasts. J Cell Sci 2001; 114:1699-708; PMID:11309200
- Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol 2000; 10:719-22; PMID:10873807; http://dx.doi.org/10.1016/S0960-9822(00)00537-6
- Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 2000; 150:567-80; PMID:10931868; http://dx.doi.org/10.1083/jcb.150.3.567
- Pullikuth AK, Catling AD. Extracellular signal-regulated kinase promotes Rho-dependent focal adhesion formation by suppressing p190A RhoGAP. Mol Cell Biol 2010; 30:3233-48; PMID:20439493; http://dx.doi.org/10.1128/MCB.01178-09
- Levay M, Bartos B, Ligeti E. p190RhoGAP has cellular RacGAP activity regulated by a polybasic region. Cell Signal 2013; 25:1388-94; PMID:23499677; http://dx.doi.org/10.1016/j.cellsig.2013.03.004
- Beck S, Fotinos A, Lang F, Gawaz M, Elvers M. Isoform-specific roles of the GTPase activating protein Nadrin in cytoskeletal reorganization of platelets. Cell Signal 2013; 25:236-46; PMID:22975681; http://dx.doi.org/10.1016/j.cellsig.2012.09.005
- Minoshima Y, Kawashima T, Hirose K, Tonozuka Y, Kawajiri A, Bao YC, Deng X, Tatsuka M, Narumiya S, May WS Jr, et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev Cell 2003; 4:549-60; PMID:12689593; http://dx.doi.org/10.1016/S1534-5807(03)00089-3
- Ban R, Irino Y, Fukami K, Tanaka H. Human mitotic spindle-associated protein PRC1 inhibits MgcRacGAP activity toward Cdc42 during the metaphase. J Biol Chem 2004; 279:16394-402; PMID:14744859; http://dx.doi.org/10.1074/jbc.M313257200
- Toure A, Mzali R, Liot C, Seguin L, Morin L, Crouin C, Chen-Yang I, Tsay YG, Dorseuil O, Gacon G, et al. Phosphoregulation of MgcRacGAP in mitosis involves Aurora B and Cdk1 protein kinases and the PP2A phosphatase. FEBS Lett 2008; 582:1182-8; PMID:18201571; http://dx.doi.org/10.1016/j.febslet.2007.12.036
- Scholz RP, Regner J, Theil A, Erlmann P, Holeiter G, Jahne R, Schmid S, Hausser A, Olayioye MA. DLC1 interacts with 14-3-3 proteins to inhibit RhoGAP activity and block nucleocytoplasmic shuttling. J Cell Sci 2009; 122:92-102; PMID:19066281; http://dx.doi.org/10.1242/jcs.036251
- Cao X, Voss C, Zhao B, Kaneko T, Li SS. Differential regulation of the activity of deleted in liver cancer 1 (DLC1) by tensins controls cell migration and transformation. Proc Natl Acad Sci U S A 2012; 109:1455-60; PMID:22307599; http://dx.doi.org/10.1073/pnas.1114368109
- Fesenko I, Kurth T, Sheth B, Fleming TP, Citi S, Hausen P. Tight junction biogenesis in the early Xenopus embryo. Mech Dev 2000; 96:51-65; PMID:10940624; http://dx.doi.org/10.1016/S0925-4773(00)00368-3
- Cardellini P, Davanzo G, Citi S. Tight junctions in early amphibian development: detection of junctional cingulin from the 2-cell stage and its localization at the boundary of distinct membrane domains in dividing blastomeres in low calcium. Dev Dyn 1996; 207:104-13; PMID:8875080; http://dx.doi.org/10.1002/(SICI)1097-0177(199609)207:1%3c104::AID-AJA10%3e3.0.CO;2-0
- Javed Q, Fleming TP, Hay M, Citi S. Tight junction protein cingulin is expressed by maternal and embryonic genomes during early mouse development. Development 1993; 117:1145-51; PMID:8325239
- Fleming TP, Hay M, Javed Q, Citi S. Localisation of tight junction protein cingulin is temporally and spatially regulated during early mouse development. Development 1993; 117:1135-44; PMID:8325238
- Chalmers AD, Strauss B, Papalopulu N. Oriented cell divisions asymmetrically segregate aPKC and generate cell fate diversity in the early Xenopus embryo. Development 2003; 130:2657-68; PMID:12736210; http://dx.doi.org/10.1242/dev.00490
- Ohnishi H, Nakahara T, Furuse K, Sasaki H, Tsukita S, Furuse M. JACOP, a novel plaque protein localizing at the apical junctional complex with sequence similarity to cingulin. J Biol Chem 2004; 279:46014-22; PMID:15292197; http://dx.doi.org/10.1074/jbc.M402616200
- Inoko A, Itoh M, Tamura A, Matsuda M, Furuse M, Tsukita S. Expression and distribution of ZO-3, a tight junction MAGUK protein, in mouse tissues. Genes Cells 2003; 8:837-45; PMID:14622136; http://dx.doi.org/10.1046/j.1365-2443.2003.00681.x
- Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol 2007; 17:1623-34; PMID:17825562; http://dx.doi.org/10.1016/j.cub.2007.08.035
- Wang S, Watanabe T, Matsuzawa K, Katsumi A, Kakeno M, Matsui T, Ye F, Sato K, Murase K, Sugiyama I, et al. Tiam1 interaction with the PAR complex promotes talin-mediated Rac1 activation during polarized cell migration. J Cell Biol 2012; 199:331-45; PMID:23071154; http://dx.doi.org/10.1083/jcb.201202041
- Terry SJ, Elbediwy A, Zihni C, Harris AR, Bailly M, Charras GT, Balda MS, Matter K. Stimulation of cortical myosin phosphorylation by p114RhoGEF drives cell migration and tumor cell invasion. PLoS One 2012; 7:e50188; PMID:23185572; http://dx.doi.org/10.1371/journal.pone.0050188
- Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. Embo J 2000; 19:2024-33; PMID:10790369; http://dx.doi.org/10.1093/emboj/19.9.2024
- Capaldo CT, Koch S, Kwon M, Laur O, Parkos CA, Nusrat A. Tight function zonula occludens-3 regulates cyclin D1-dependent cell proliferation. Mol Biol Cell 2011; 22:1677-85; PMID:21411630; http://dx.doi.org/10.1091/mbc.E10-08-0677
- Nie M, Aijaz S, Leefa Chong San IV, Balda MS, Matter K. The Y-box factor ZONABDbpA associates with GEF-H1Lfc and mediates Rho-stimulated transcription. EMBO Rep 2009; 10:1125-31; PMID:19730435; http://dx.doi.org/10.1038/embor.2009.182
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAPTAZ in mechanotransduction. Nature 2011; 474:179-83; PMID:21654799; http://dx.doi.org/10.1038/nature10137
