Abstract
A strategic challenge facing clinicians treating patients afflicted with non-small cell lung cancer (NSCLC) is the development of approaches that combine conventional and novel therapies, including targeted therapies and immunotherapeutics. In a recent study, we explored the correlation between the expression of the tumor antigen family MAGE-A and the presence of EGFR and KRAS gene mutations in a large cohort of resected NSCLC patient specimens.
Lung cancer is the principal cause of cancer-related mortality worldwide, with non-small cell lung cancer (NSCLC) being the most frequent histological subtype. Therefore, despite recent improvements in conventional therapy, the development of additional and complementary therapeutic approaches to treat NSCLC is a high priority for public health. One important development has been that of targeted therapies particularly effective in patients bearing tumors with defined molecular characteristics. For instance, in metastatic NSCLC patients harboring tumors bearing mutated epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKi) have shown clinical efficacy.Citation1 Other common mutations are those in the anaplastic lymphoma receptor tyrosine kinase (ALK) and Kirsten rat sarcoma viral oncogene homolog (KRAS) genes, the latter not being as yet directly targetable. Major concerns of targeted therapies include the limited proportion of eligible patients and the fact that the effects of these therapies are usually transient due to the development of resistance. Thus, there is incentive to develop new therapeutic strategies for those NSCLC patients ineligible for targeted therapies as well as to exploit the targeted therapy-induced intermittent stable disease period to apply complementary treatments that have the potential to result in a more durable remission.
Another emerging therapeutic approach in NSCLC is immunotherapy, including vaccination and adoptive cell transfer therapy.Citation2 In addition, a new class of highly promising immune therapeutics has recently emerged, thanks to the development of T-cell modulating agents such as antibodies against cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1) or programmed cell death 1 ligand 1 (PD-L1).Citation3 Treatment with the anti-CTLA-4 antibody, Ipilimumab, has been associated with immune-related improvement in progression-free survival of NSCLC patients as compared to chemotherapy alone.Citation4 At variance with clinical responses induced by other treatments, immune-related clinical responses require more time to develop but are potentially long lasting. It is noteworthy that only a portion of the treated patients respond clinically to T-cell modulating agents. One emerging concept is that clinical responses may specifically occur in patients with pre-existing tumor antigen-specific T-cell responses that could be amplified or activated by the treatment.Citation5 Part of these responses are directed against frequently expressed immune targets in NSCLC such as the Cancer Testis Antigens (CTA), a large group of tumor specific non-mutated antigens including MAGE-A antigens and the highly immunogenic cancer epitope NY-ESO-1.Citation6 In addition, patients with the squamous subtype of NSCLC characterized by a high overall mutation rateCitation7 may have frequent T-cell responses to mutated antigens and, for this reason, may be particularly prone to respond to T-cell modulating agents. However, limited experimental evidence is presently available to support these important concepts that await and require rigorous validation. In this context, T-cell responses to certain CTAs for which rigorous immunomonitoring methods have been developed can be evaluated as biomarkers to address the correlation between reactions to T-cell modulating agents and clinical efficacy. In addition, although immunization with CTA-based vaccines alone have so far been insufficient to achieve meaningful clinical responses, assessment of vaccines in combination with T-cell modulating agents is underway. It is however noteworthy, that the expression of a single CTA can be highly variable among tumors of different histological subtypesCitation8 and that both CTA expression and the absence or presence of spontaneous specific T-cell responses may stratify subgroups of patients with distinct characteristics, variables that could clarify the interpretation of the results of these studies.
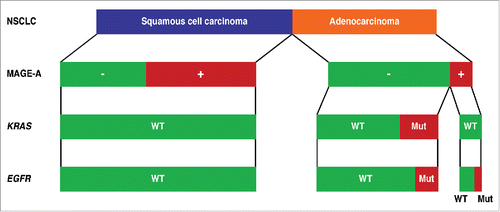
When considering the landscape of expanding targeted and immunotherapeutic approaches in the treatment of NSCLC, a major barrier to increasingly “personalized” (i.e., adapted to single patients and tumors) regimens is elucidating the relationship between the expression of common immunotherapy-targeted tumor antigens and the occurrence of mutations relevant to targeted therapies. However, virtually no study has attempted to simultaneously evaluate these parameters as of yet. In our recently published study in “Cancer Immunology Research,”Citation9 we sought to gain insight into these clinically-relevant outstanding issues. We applied immunohistochemistry in conjunction with gene sequencing to assess a large cohort of surgically resected NSCLC (Stages I-IIIA) for the expression of MAGE-A antigens in relation to tumor histopathologic features and the presence of EGFR or KRAS mutations. We found significant MAGE-A expression in about 60% of squamous tumors, but only in 16% of adenocarcinomas (). A first direct implication of the frequent expression of MAGE-A CTA in squamous tumors, for which only conventional therapy is presently available, is to encourage the selection of this patient population for clinical trials assessing new approaches that combine chemotherapy and immunotherapy. We found EGFR and KRAS mutations to be, as expected, restricted to adenocarcinomas (21% and 26%, respectively) and to be mutually exclusive. However, we found no significant correlation between the expression of MAGE-A and the presence of EGFR mutations. The simultaneous occurrence of MAGE-A expression and EGFR mutations in adenocarcinomas is therefore low. Thus, whereas individual patients could be occasionally selected for therapeutic approaches combining treatment with TKi with MAGE-A based immune therapy, other tumor-specific antigens that are more frequently expressed in NSCLC of adenous type would be more suitable candidates for the development of this type of combinatorial therapy. One promising candidate, XAGE-1b,Citation10 a tumor antigen frequently expressed in lung cancer, is currently undergoing assessment in our laboratory.
Figure 1. Expression of MAGE-A antigens in relation to the mutational status of EGFR and KRAS genes and to the histologic subtype in resected NSCLC tumors. MAGE-A expression was assessed by immunohistochemistry in 177 resected Stage I-IIIA non-small cell lung cancer (NSCLC) of squamous cell carcinoma (n = 102) or adenocarcinoma (n = 75) types.Citation9 The mutational status (WT, wild type; Mut, mutated) of the EGFR and KRAS genes was also assessed in the same tumors.Citation9 MAGE-A expression is schematically represented in relation to the histologic tumor type and to EGFR and KRAS mutational status.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362:2380-8; PMID:20573926; http://dx.doi.org/10.1056/NEJMoa0909530
- Kakimi K, Nakajima J, Wada H. Active specific immunotherapy and cell-transfer therapy for the treatment of non-small cell lung cancer. Lung Cancer 2009; 65:1-8; PMID:19062127; http://dx.doi.org/10.1016/j.lungcan.2008.10.018
- Sundar R, Soong R, Cho BC, Brahmer JR, Soo RA. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer 2014; 85:101-109; PMID:24880938
- Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012; 30:2046-54; PMID:22547592; http://dx.doi.org/10.1200/JCO.2011.38.4032
- Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A 2011; 108:16723-8; PMID:21933959; http://dx.doi.org/10.1073/pnas.1110814108
- Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev 2002; 188:22-32; PMID:12445278; http://dx.doi.org/10.1034/j.1600-065X.2002.18803.x
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013; 499:214-8; PMID:23770567; http://dx.doi.org/10.1038/nature12213
- Hamai A, Duperrier-Amouriaux K, Pignon P, Raimbaud I, Memeo L, Colarossi C, Canzonieri V, Perin T, Classe JM, Campone M, et al. Antibody responses to NY-ESO-1 in primary breast cancer identify a subtype target for immunotherapy. PLoS One 2011; 6:e21129.
- Ayyoub M, Memeo L, Alvarez-Fernandez E, Colarossi C, Costanzo R, Aiello E, Martinetti D, Valmori D. Assessment of MAGE-A expression in resected non-small cell lung cancer in relation to clinicopathological features and mutational status of EGFR and KRAS. Cancer Immunol Res 2014; In press; PMID:24866168
- Nakagawa K, Noguchi Y, Uenaka A, Sato S, Okumura H, Tanaka M, Shimono M, Ali Eldib AM, Ono T, Ohara N, et al. XAGE-1 expression in non-small cell lung cancer and antibody response in patients. Clin Cancer Res 2005; 11:5496-503; PMID:16061866; http://dx.doi.org/10.1158/1078-0432.CCR-05-0216
