Abstract
Gamma delta T cells (γδT) are potent mediators of antitumor cytotoxicity and have shown promising efficacy in early phase clinical trials. Most is known about the tumoricidal properties of cells bearing the Vδ2 T cell receptor chain, but recent studies have demonstrated that cells with the Vδ1 chain and those with neither Vδ1 nor Vδ2 chains have properties which may make them more attractive anticancer effectors in adoptive immunotherapy.
For a cell type to be useful in cancer adoptive immunotherapy it must be amenable to ex vivo expansion of potent effectors in a manner that avoids generation of regulatory or suppressive counterparts. With this in mind, numerous cell populations have been evaluated over the past 20 y, including dendritic cells, lymphokine activated killer (LAK) cells, tumor infiltrating lymphocytes, and positively selected natural killer (NK) cells. However, each method has limitations that have precluded widespread clinical adoption. For example, the difficulty in obtaining sufficient numbers of tumor infiltrating lymphocytes for most cancer types, and the expansion of regulatory T-cells in response to IL-2.
γδT lymphocytes have also been evaluated for ex vivo expansion for adoptive immunotherapy and equally confounding hurdles to overcome have been revealed. While γδT cell adoptive immunotherapy using existing technologies demonstrates modest but promising clinical effect,Citation1 new approaches are required for effective utilization of this unique population of cells.
There are a number of key differences between peripherally circulating γδT cells and the more abundant alpha beta T (αβT) lymphocytes. Most notably, γδT cells recognize targets in an MHC independent manner, have innate killing activity against pathogens, and appear to respond to self-molecules that signal potential danger or cellular stress. Furthermore, they can differentiate into professional antigen presenting cells (pAPCs), expressing co-stimulatory molecules and presenting antigenic fragments for the primary stimulation of CD4+ and CD8+ αβ T-cell responses.Citation2,3
In the peripheral blood of individuals living outside areas of endemic parasitaemia, γδT cells expressing the Vγ9Vδ2 TCR are the commonest subset. In parts of Africa, however, the Vδ1+ subset predominates,Citation4 and there is evidence of extra-thymic changes in γδT cell repertoire in response to environmental pathogen exposure. Until recently, almost all human γδT cell research focused on the Vγ9Vδ2 subset, due to its relative abundance in Western populations and ease of expanding large numbers of cells for study or adoptive transfer using phosphoantigen ligands of the Vγ9Vδ2 TCR. Deniger and colleag-ues Citation5,6 demonstrated that polyclonal γδT cell expansion was possible using an engineered K562 cell line expressing CD86, CD64, 41BBL, and membrane bound IL-15. Using the same artificial antigen presenting cells (aAPC) coated in an anti-γδTCR antibody (Biolegend clone B1) we have shown that it is possible to generate >1,000 fold expansion of γδT cells from the blood of neuroblastoma patients within 1 mo, preserving the relative distributions of Vδ1+, Vδ2+ and Vδ1neg/Vδ2neg γδT subtypesCitation7 ().
Figure 1. Use of artificial antigen presenting cells for unbiased expansion of blood gamma delta T lymphocytes. An antibody against the gamma delta T cell receptor tethered to the aAPC by the high affinity FcγReceptor, is responsible for forming an immunological synapse involving the gamma delta TCR.
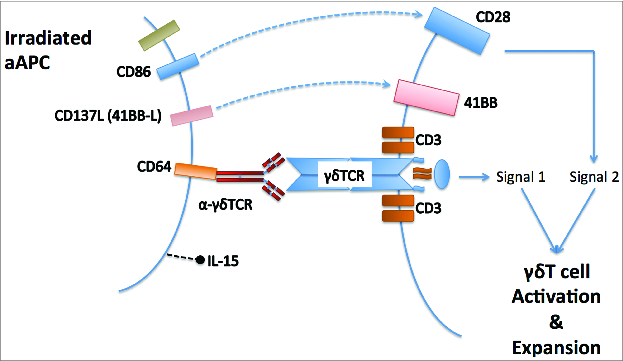
Vδ1+ and Vδ1neg/Vδ2neg γδT cells expanded using aAPC+B1 demonstrate many characteristics that recommend them for cellular immunotherapy over Vγ9Vδ2+ cells. Following adoptive transfer, less differentiated cells persist for longer in the recipient. Vδ1+ and Vδ1neg/Vδ2neg γδT cells show a less differentiated pattern of CD27, CD45RA, and CD62L expression both before and after expansion with aAPC+B1 compared to Vδ2+ γδT cells. PD-1 is a marker associated with T-cell exhaustion, and has been a key target in therapies aimed at overcoming immune checkpoints.Citation8 Vδ1+ and Vδ1neg/Vδ2neg γδT cells have lower expression of PD-1 compared to both Vδ2+ and αβT cells after 2 weeks of expansion, which is sufficient to generate around 50-fold expansion in γδT cell numbers. Perhaps most significantly however is the striking level of innate cytotoxicity demonstrated by Vδ1+ γδT cells against solid tumor cell lines. It has been known for some time that phosphoantigen expanded Vδ2+ γδT cells will kill cell lines from certain solid and hematological malignancies, but with only a few exceptions this killing is significantly antibody dependent.Citation1
We showed that Vδ1+ cytotoxicity against the same targets was independent of antibody and not attributable to alloreactivity, with killing efficiency equivalent or greater than the ability of Vδ2+ γδT cells to kill opsonized target cells; Vδ2+ cytotoxicity in the absence of opsonizing antibody was minimal. Based on these data, it is perhaps unsurprising that the published clinical trials of γδT cell immunotherapy, all of which used Vδ2+ cell expansions and none of which combined the therapy with an antitumor antibody, showed only modest efficacy.Citation1,9 Comparison of our recent studies with the work of Deniger et al. highlights differences in the innate cytolytic potential of Vδ2+ γδT cells following expansion under differing conditions. These differences could be explained by different starting populations (umbilical cord blood vs. PBMC), different cytokines during expansion or different engagement of the γδTCR in the models used.
To be useful in cancer immunotherapy, an expanded effector population must possess certain characteristics. They require tumor tropism to infiltrate the tumor in sufficient numbers. Their memory phenotypes must be sufficiently naïve to allow survival in the host following adoptive transfer, and their cytolytic activity must be specific for tumor cells. Non-Vδ2+ γδT cells, and especially Vδ1+ cells fulfil this wide spectrum of requirements, encouraging the therapeutic exploitation of these subsets. Moreover, their innate cytotoxic mechanisms will result in the release of antigen for uptake. The exposure to tumor antigen will thus activate their expansion and simultaneously activate their role as pAPCs, promoting tumor antigen-specific cytotoxic αβT-cell responses.
The propensity of Vδ2+ γδT cells to activation induced death ,Citation10 combined with their higher levels of differentiation and expression of exhaustion markers Citation7 suggest that they may not be the best choice of effector, despite the ease in expanding large numbers. Deniger et al.'s use of umbilical cord blood as a more naïve source of cells may go some way to overcoming this limitation and is of particular interest.
Therefore, despite the moderate clinical success of Vδ2+ γδT cell based immunotherapy, there is an opportunity to explore the utility of non-Vδ2+ γδT cells as adoptive transfer agents. These could be delivered either as selected populations or as polyclonal γδT cell preparations, which harness the different antigenic affinities, cytolytic and antigen presentation profiles of each subset.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology 2014; 3:e27572; PMID:24734216; http://dx.doi.org/10.4161/onci.27572
- Himoudi N, Morgenstern DA, Yan M, Vernay B, Saraiva L, Wu Y, Cohen CJ, Gustafsson K, Anderson J. Human γδT lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol 2012; 188:1708-16; PMID:22250090; http://dx.doi.org/10.4049/jimmunol.1102654
- Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T cells. Science 2005; 309:264-8; PMID:15933162; http://dx.doi.org/10.1126/science.1110267
- Hviid L, Akanmori BD, Loizon S, Kurtzhals JA, Ricke CH, Lim A, Koram KA, Nkrumah FK, Mercereau-Puijalon O, Behr C. High frequency of circulating gamma delta T cells with dominance of the v(delta)1 subset in a healthy population. Int Immunol 2000; 12:797-805; PMID:10837407; http://dx.doi.org/10.1093/intimm/12.6.797
- Deniger DC, Switzer K, Mi T, Maiti S, Hurton L, Singh H, Huls H, Olivares S, Lee DA, Champlin RE et al. Bispecific T-cells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther 2013; 21:638-47; PMID:23295945; http://dx.doi.org/10.1038/mt.2012.267
- Deniger DC, Maiti S, Mi T, Switzer K, Ramachandran V, Hurton LV, Ang S, Olivares S, Rabinovich BA, Huls H et al. Activating and propagating polyclonal gamma delta T cells with broad specificity for malignancies. Clin Cancer Res 2014; 20(20):5708-19; PMID:24833662; http://dx.doi.org/10.1158/1078-0432.CCR-13-3451
- Fisher J, Yan M, Heuijerjans J, Carter L, Abolhassani A, Frosch J, Wallace R, Flutter B, Hubank M, Klein N et al. Neuroblastoma killing properties of V-delta 2 and V-delta2 negative gamma delta T cells following expansion by artificial antigen presenting cells. Clin Cancer Res 2014; 20(22):5720-32.
- Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134-44; PMID:23724846; http://dx.doi.org/10.1056/NEJMoa1305133
- Fournié J-J, Sicard H, Poupot M, Bezombes C, Blanc A, Romagné F, Ysebaert L, Laurent G. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol 2013; 10(1):35-41.
- Kabelitz D, Pechhold K, Bender A, Wesselborg S, Wesch D, Friese K, Janssen O. Activation and activation-driven death of human gamma/delta T cells. Immunol Rev 1991; 120:71-88; PMID:1677929; http://dx.doi.org/10.1111/j.1600-065X.1991.tb00588.x
