Abstract
Salivary gland cancers are highly aggressive epithelial tumor associated with metastatic potential and high mortality. The tumors are biologically diverse and are of various histotypes. Besides, the detection and diagnosis is a major problem of salivary gland cancer for available treatment modalities. In the present study, we have investigated the association of sperm associated antigen 9 (SPAG9) expression with salivary gland tumor (SGT). Clinical specimens of benign (n = 16) and malignant tumors (n = 86) were examined for the SPAG9 expression. In addition, the sera and adjacent non-cancerous tissues (n = 72) from available patients were obtained. Our in situ RNA hybridization and immunohistochemistry (IHC) analysis revealed significant difference (p = 0.0001) in SPAG9 gene and protein expression in benign (63%) and malignant tumor (84%) specimens. Further, significant association was also observed between SPAG9 expression and malignant tumors (P = 0.05). A cut-off value of >10% cells expressing SPAG9 protein designated as positive in IHC, predicted presence of malignant SGT with 83.72% sensitivity, 100% specificity, 100% PPV and 83.72% NPV. Humoral response against SPAG9 protein was generated in 68% of SGT patients. A cut-off value of 0.212 OD for anti-SPAG9 antibodies in ELISA predicted presence of malignant SGT with 69.23% sensitivity, 100% specificity, 100% PPV and 78.94% NPV. Collectively, our data suggests that the majority of SGT show significant difference and association among benign and malignant tumors for SPAG9 gene and protein expression and also exhibit humoral response against SPAG9 protein. Hence, SPAG9 may be developed as a biomarker for detection and diagnosis of salivary gland tumors.
Introduction
Cancer is the leading cause of death in economically developed countries and the second leading cause of death in developing countries.Citation1,2 In developing nations, it has been reported that 1 in 4 cancers in male occur in head and neck region and accounts for 30% of all cancers.Citation3 Importantly, salivary gland cancer accounts for 3–5% of total head and neck cancer Citation4,5 and of wide histological and biological diversity.Citation6 SGT could be benign and malignant type. The chances for malignant transformation of benign SGT vary from 1.9% to 23.3% which is a concern for oncologist all over the world. In addition, it has been well documented that pleomorphic adenoma, which is classified as benign tumor, is aneuploid, and invades normal adjacent tissue and metastasize at distant sites after certain period of time.Citation7 While benign tumors are of pleomorphic and Warthin's tumors, the malignant tumors are classified as mucoepidermoid, adenoid cystic, acinic cell, clear cell, basal cell carcinoma, adenocarcinoma not otherwise mentioned (NOS), squamous cell carcinoma, salivary duct carcinoma, myoepithelial carcinoma and polymorphous low grade adenocarcinoma based on their origin.Citation8,9 Such morphological and biological diversity of SGT, makes it more challenging to diagnose and classify these tumors.Citation5 Nevertheless, about 70% of all the SGT are of parotid gland origin followed by submandibular and sublingual glands.Citation10 Besides, the heterogeneity within the tumor of the same patient does pose problem for pathologist to classify the origin of such tumors. Prognosis of SGT varies according to histologic type and stage. A combination of radiation therapy and surgery is usually applied to treat malignant tumors. However, such treatments are often associated with disfigurement and loss of glandular function with undesirable side effects.Citation11 Hence, there is a need for a novel biomarker for the early detection and diagnosis of SGT, for better cancer management and treatment modalities.
Recently, a unique group of protein family designated as cancer testis (CT) antigens has been reported in various malignancies.Citation1 The restricted or no expression of CT antigens in normal tissues and their antigenic properties in cancer patients makes these molecules an ideal choice as biomarker for early detection and diagnosis and therapeutic vaccines.Citation1,12 Although, a few CT antigens like B antigen (BAGE), G antigen-1/2 (GAGE-1/2), helicose antigen (HAGE) and melanoma associated antigen-1 (MAGE-1) have been reported to be expressed in various types of SGTCitation13, none of the molecules have been found suitable in clinical management of such cases. Recently, we and others have demonstrated expression and association of SPAG9, a new member of CT antigen family, with various clinicopathological characteristics of tumors, especially in early stages in epithelial ovarian cancer,Citation14 renal cell carcinoma,Citation15 thyroid cancer,Citation16 cervical carcinoma,Citation17 breast cancer,Citation18 colorectal carcinoma,Citation19 bladder cancer,Citation20 endometrial cancer,Citation21 non-small cell lung cancerCitation22 and astrocytoma.Citation23 Besides, a strong humoral response against SPAG9 has also been demonstrated in various malignanciesCitation14-Citation21 suggesting its potential usage as a serum based cancer biomarker.
Early detection of SGT would be essential for more effective clinical management leading to improved quality of life and increased survival rate.Citation24 The present study was initiated to undertake a more comprehensive analysis of SPAG9 expression in SGT specimens in the context of clinic-pathological parameters, i.e., histo-pathological characteristics. We also investigated the humoral response against SPAG9 in various stages and histotypes of SGT patients. Our results suggest that SPAG9 may be used as a novel diagnostic biomarker for early detection of SGT thus, may be useful in better management of SGT patients.
Results
SPAG9 gene expression in SGT patients
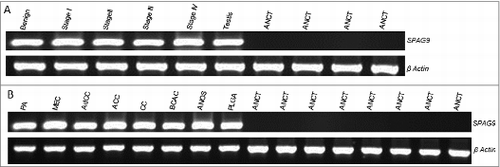
The SPAG9 gene expression was investigated by RT-PCR in SGT tissue along with available matched ANCT specimens (). The data revealed that 80% (82 of 102) of tumor specimens showed SPAG9 gene expression irrespective of benign, malignant tumor, stages, and various histotypes (). No gene expression was detected in matched ANCT specimens. The human testis cDNA was used as a positive control for SPAG9 gene expression. Our SPAG9 gene expression analysis () revealed that SPAG9 transcript was detected in 63% (10 of 16) of benign tumors, 93% (13 of 14) of malignant stage I, 88% (15 of 17) of stage II, 75% (24 of 32) of stage III and 87% (20 of 23) of stage IV. Based on TNM classification, SPAG9 expression was detected in 81% (25 of 31) of SGT specimens positive with lymph node involvement as compared to 85% (47 of 55) of specimens negative with lymph node involvement. Furthermore, based on histological disease classification, 63% (10 of 16) of pleomorphic benign tumors, 90% (27 of 30) of mucoepidermoid, 83% (10 of 12) of adenoid cystic, 80% (4of 5) of acinic cell, 88% (14 of 16) of clear cell, 80% (4 of 5) of basal cell, 70% (7 of 10) of adenocarcinoma not otherwise specified (NOS) and 75% (6 of 8) of polymorphous low grade adenocarcinoma specimens showed SPAG9 mRNA expression as depicted in and .
Table 1. SPAG9 expression, humoral response and clinicopathological characteristics of salivary gland tumor
Figure 1. SPAG9 gene expression in SGT patients. (A) RT-PCR analyses of SPAG9 mRNA expression. SPAG9 transcripts were detected in benign tumor, malignant tumor stage I, stage II, stage III, stage IV and testis. No SPAG9 mRNA was detected in the available four matched ANCT. β-Actin gene expression revealed expression in all the tissues under investigation. (B) RT-PCR analyses of SPAG9 transcript in various histotypes. PA (pleomorphic adenoma), MEC (mucoepidermoid carcinoma), AdCC (adenoid cystic carcinoma), ACC (acinic cell carcinoma), CC (clear cell carcinoma), BCAC (basal cell carcinoma), ANOS (adenocarcinoma not otherwise specified), PLGA (polymorphous low grade adenocarcinoma). No gene expression was detected in the available 8 matched ANCT specimens. β-Actin was used as internal control in these experiments.
Validation of SPAG9 gene and protein expression in SGT patients
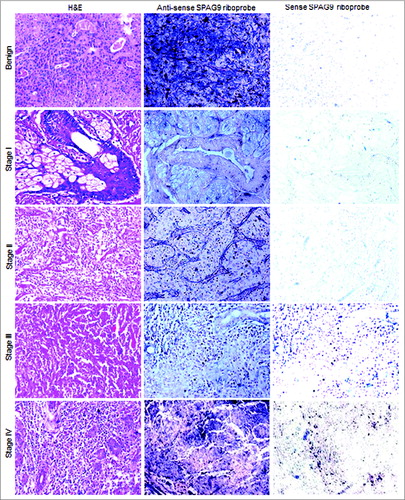
SPAG9 gene expression was also determined in serial SGT tumor specimen sections by in situ RNA hybridization studies using in vitro synthesized riboprobes and by IHC. Our in situ RNA hybridization studies employing antisense riboprobes confirmed SPAG9 gene expression in 93% (13 of 14) of malignant stage I, 88% (15 of 17) of stage II, 75% (24 of 32) of stage III and 87% (20 of 23) of stage IV tumors (). Based on TNM classification, SPAG9 expression was detected in 81% (25 of 31) of specimens positive with lymph node involvement as compared to 85% (47 of 55) of specimens negative with lymph node involvement (). However, as expected sense riboprobes failed to show SPAG9 gene expression in any of the serial tissue specimen sections as depicted in .
Figure 2. Analysis of SPAG9 gene expression in SGT patients by in situ RNA hybridization. Representative images of H&E staining for benign, malignant stage I, II, III, and IV tumors are shown in left panel. The serial tissue sections probed with anti-sense riboprobes resulted in violet blue color as shown in the middle panel, whereas no hybridization was observed when probed with sense riboprobes as shown in the right panel. Original magnification: x200; objective: x20.
SPAG9 protein expression was further confirmed by IHC in serial SGT tissue sections of benign tumors, various stages of malignant tumors and different histotypes and available matched ANCT specimens. SPAG9 protein expression was found in 80% (> 10% of cells found positive for SPAG9 protein expression) of SGT specimens, whereas no expression was detected in 72 paired available matched ANCT specimens as shown in . SGT specimens were also probed with control IgG which failed to show any immunoreactivity against SPAG9 protein (). As depicted in , SPAG9 protein expression was found in 63% (10 of 16) of benign tumors, 93% (13 of 14) of malignant stage I, 88% (15 of 17) of stage II, 75% (24 of 32) of stage III and 87% (20 of 23) of stage IV tumors. In addition, 81% (25 of 31) of specimens found positive for lymph node involvement showed SPAG9 protein expression as compared to 85% (47 of 55) of specimens negative for lymph node involvement. Based on SPAG9 IRS, we observed significant difference between benign [IRS (mean ± SE) =38.40 ± 5.36)] and malignant tissue [IRS=69.30 ± 1.98; (p = 0.0001)] by Mann–Whitney U-test (). In addition, significant association of SPAG9 protein expression was found between benign and malignant tumor (p=0.05) using Pearson's χ2 test. However, while comparing the SPAG9 protein expression between malignant stage I (IRS = 68.69 ± 4.38) & II IRS = 71.00 ± 5.31;(p = 0.548)], stage II (IRS = 71.00 ± 5.31) & III [IRS = 63.20 ± 3.74;(p = 0.165)] and stage III (IRS = 63.20 ± 3.74) & IV [IRS = 75.70 ± 2.17;(p = 0.094)], no significant difference was observed using Mann–Whitney U-test (). We further analyzed SPAG9 expression among different stages and various malignant histotypes using Kruskal–Wallis test. We did not find any significant difference among different malignant stages (p = 0.183) or in various histotypes (p = 0.977). In addition, no association was found between SPAG9 protein expression among malignant stage I & II (p = 0.665), stage II & III (p = 0.274) and stage III & IV (p = 0.274) by using Pearson's χ2 test. Furthermore, there was no significant difference (p = 0.878) between lymph node positive and negative salivary gland cancer patients as determined by Mann–Whitney U-test. Also, no significant association (p = 0.562) was found between SPAG9 protein expression and lymph node involvement in SGT specimens by Pearson's χ2 test. Thus, the above studies distinctly revealed no discrepancy between SPAG9 gene and protein expression. All SGT specimens found positive in in situ RNA hybridization and RT-PCR experiments were also found positive for SPAG9 protein expression in IHC analysis. However, no significant association was found in SPAG9 protein expression among various malignant stages.
Figure 3. Validation of SPAG9 protein expression in various stages of SGT by immunohistochemistry. First panel shows representative images for H&E staining in benign, malignant stage I, II, III, and IV tumor specimens. Second panel shows the representative images for the cytoplasmic localization of SPAG9 protein probed with anti-SPAG9 antibody as depicted by brown color immunoreactivity. No immunoreactivity was observed in serial tumor sections probed with control IgG, as shown in the third panel. Fourth panel depicts no immunoreactivity against SPAG9 protein in ANCT specimens when probed with anti-SPAG9 antibody. Original magnification: x400; objective: x40.
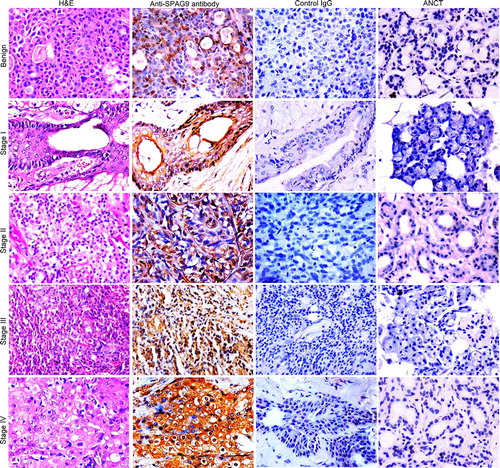
Figure 4. Validation of SPAG9 protein expression in various histotypes of malignant SGT by immunohistochemistry. (A) Top panel showing the cytostructure of representative specimens of benign pleomorphic adenoma and various malignant histotypes such as mucoepidermoid carcinoma, adenoid cystic carcinoma and acinic cell carcinoma of SGT stained with H&E. Middle panel shows cytoplasmic localization of SPAG9 protein expression in the representative specimens of various histotypes of SGT probed with anti-SPAG9 antibody. Bottom panel depicts no immunoreactivity for SPAG9 protein in various histotypes of SGT specimens when probed with control IgG. (B) Top panel showing the cytostructure of representative specimens of various malignant histotypes such as clear cell carcinoma, basal cell carcinoma, adenocarcinoma not otherwise specified and polymorphous low grade adenocarcinoma of SGT stained with H&E. Middle panel shows cytoplasmic localization of SPAG9 protein expression in the representative specimens probed with anti-SPAG9 antibody. Bottom panel depicts no immunoreactivity for SPAG9 protein when probed with control IgG. Original magnification: x400; objective: x40.
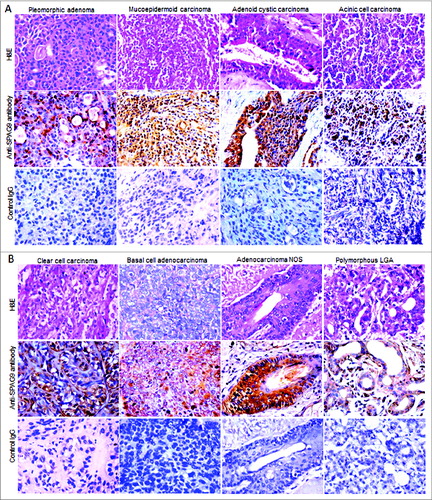
Figure 5. SPAG9 immunoreactivity score. SPAG9 IRS was determined by counting cells (> 500 cells) positive for SPAG9 protein from five random fields in benign, different stages and various histotypes of malignant tumor. The histogram shows benign (PA- pleomorphic adenoma), malignant stage I, stage II, stage III, stage IV, MEC (mucoepidermoid carcinoma), AdCC (adenoid cystic carcinoma), ACC (acinic cell carcinoma), CC (clear cell carcinoma), BCAC (basal cell carcinoma), ANOS (adenocarcinoma not otherwise specified) and PLGA (polymorphous low grade adenocarcinoma). * p < 0.0001 statistically significant, Point indicates mean bars, standard errors.
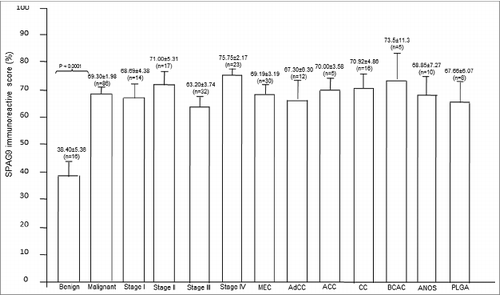
Various histotypes of SGT specimens showed SPAG9 protein expression in 63% [(10 of 16); IRS = 38.40 ± 5.36] of pleomorphic benign tumors, 90% [(27 of 30); IRS = 69.19 ± 3.19] of mucoepidermoid, 83% [(10 of 12); IRS = 67.30 ± 6.30] of adenoid cystic, 80% [(4of 5); IRS = 70 ± 3.58] of acinic cell, 88% [(14 of 16); IRS = 70.92 ± 4.86] of clear cell, 80% [(4 of 5); IRS = 73.50 ± 11.30] of basal cell, 70% [(7 of 10); IRS = 68.80 ± 7.27] of adenocarcinoma NOS and 75% [(6 of 8); IRS = 67.60 ± 6.07] of polymorphous low grade adenocarcinoma as given in and illustrated in . However, control IgG showed no SPAG9 immuno-staining in any of the SGT histotypes under investigation. Thus, our data showed no discrepancy within the results obtained from RT-PCR and IHC studies as detailed in .
Statistical analysis for sensitivity and specificity of SPAG9 expression was evaluated at cut-off value of > 10% SPAG9 positive cells per 500 in 5 random fields in IHC of serial SGT tissue sections of malignant tumors, stage I (T1) and benign tumors. SPAG9 positive cells predicted presence of malignant SGT with 83.72% sensitivity, 100% specificity, 100% PPV and 83.72% NPV. In stage I (T1) tumors, SPAG9 positive cells predicted presence of malignant SGT with 92.85% sensitivity, 100% specificity, 100% PPV and 98.63% NPV, whereas in benign tumors, SPAG9 expressing cells predicted SGT with sensitivity, specificity, PPV and NPV of 62.5%, 100%, 100%, and 92.3%.These observations may suggest that SPAG9 expression may predict malignant SGT.
Humoral response against SPAG9 protein in SGT patients
The circulating anti-SPAG9 antibodies were investigated in sera of 62 available SGT patients employing ELISA. Initially, we established a cut-off value of 0.212 [Mean + 2 SD; 0.137 + 0.074] optical density (OD) for circulating anti-SPAG9 antibodies using sera from 60 healthy donors. A higher value obtained from the patient serum above cut-off value was designated as positive for anti-SPAG9 antibodies whereas those below it as negative. Our data revealed that 68% (42 of 62) of SGT patients generated humoral response against SPAG9 protein. Interestingly, we found that these patients with anti-SPAG9 antibodies were also found to be positive for SPAG9 gene and protein expression. As shown in and depicted in , our data revealed that humoral response generated against SPAG9 was found in 60% (6 of 10) of benign, 90% (9 of 10) of malignant stage I, 80% (8 of 10) of stage II, 48% (11 of 23) of stage III and 89% (8 of 9) of stage IV tumors. It is important to note that 74% (14 of 19) of malignant SGT patients with positive lymph node involvement generated anti-SPAG9 antibodies as compared to 67% (22 of 33) of SGT patients with negative lymph node involvement. In addition, the patient's sera of different histotypes also revealed anti-SPAG9 antibodies which demonstrated 60% (6 of 10) of pleomorphic benign tumor patients, 72% (13 of 18) of mucoepidermoid, 63% (5 of 8) of adenoid cystic, 67% (2 of 3) of acinic cell, 71% (5 of 7) of clear cell, 80% (4 of 5) of basal cell, 63% (5 of 8) of adenocarcinoma NOS and 67% (2 of 3) of polymorphous low grade adenocarcinoma patients (). We observed a significant difference (p=0.0001) among benign and malignant tumor patient sera found positive for circulating anti-SPAG9 antibodies using Mann–Whitney U-test. However, no significant association was found between benign and malignant tumors (p = 0.302) by Pearson's χ2 test. Similarly, no significant difference was found between stage I & II (p = 0.413), stage II & III (p=0.509), stage III & IV (p = 0.107) and between lymph node involved (p=0.858) malignant tumors as assessed by Mann–Whitney U-test. However, a significant association was found between stage III & IV samples (p = 0.033) as determined by Pearson's χ2 test. Kruskal–Wallis test revealed no significant difference in circulating anti-SPAG9 antibodies among various malignant stages (p = 0.505) of SGT and different malignant histotypes (p = 0.798).
Figure 6. Humoral response against SPAG9 protein in SGT patients. (A) ELISA-based analysis of sera from available 62 SGT patients of benign, different stages (I, II, III and IV) and various histotypes of malignant tumor and from 60 normal healthy donors was carried out to determine the presence of anti-SPAG9 antibodies. The horizontal line X indicates the cutoff value 0.212 (0.137 + 0.074) of 60 normal healthy donors at A492nm. SGT patients were designated as positive for the presence of anti-SPAG9 antibodies above X line and negative below the X line. (B) Western blotting analyses. Lane 1, affinity-purified recombinant SPAG9 stained with Coomassie brilliant blue; lane 2, immuno-blot of recombinant SPAG9 shows a specific band of 170 kDa with anti-SPAG9 antibody (positive control). Serum from patient having benign [PA, (lane 3 and lane 8)], malignant tumor stage I (lane 4), stage II (lane 5), stage III (lane 6), stage IV (lane 7), MEC (lane 9), AdCC (lane 10), ACC (lane 11), CC (lane 12), BCAC (lane 13), ANOS (lane 14), PLGA (lane 15) showed specific immunoreactivity against recombinant SPAG9 protein. Neutralization experiments were done by pre-incubating recombinant SPAG9 protein (15μg/mL) with sera from SGT patients which resulted in complete loss of immunoreactivity (lane 16). Sera from healthy individuals revealed no reactivity (lane 17). M-molecular weight marker. (C) Neutralization studies in serial tissue sections of benign and malignant stages of SGT. Neutralization studies were carried out by pre-incubating anti-SPAG9 antibody generated in rat with recombinant SPAG9 protein (15μg/mL) and used for probing endogenous SPAG9 protein in the serial tissue sections of SGT patients. Left panel shows the representative images of the H&E stained sections of malignant tumor stage I, II, III, IV. Middle panel depicts the SPAG9 protein expression probed with anti-SPAG9 antibodies. Right panel shows complete loss of SPAG9 localization when probed with neutralized sera.
![Figure 6. Humoral response against SPAG9 protein in SGT patients. (A) ELISA-based analysis of sera from available 62 SGT patients of benign, different stages (I, II, III and IV) and various histotypes of malignant tumor and from 60 normal healthy donors was carried out to determine the presence of anti-SPAG9 antibodies. The horizontal line X indicates the cutoff value 0.212 (0.137 + 0.074) of 60 normal healthy donors at A492nm. SGT patients were designated as positive for the presence of anti-SPAG9 antibodies above X line and negative below the X line. (B) Western blotting analyses. Lane 1, affinity-purified recombinant SPAG9 stained with Coomassie brilliant blue; lane 2, immuno-blot of recombinant SPAG9 shows a specific band of 170 kDa with anti-SPAG9 antibody (positive control). Serum from patient having benign [PA, (lane 3 and lane 8)], malignant tumor stage I (lane 4), stage II (lane 5), stage III (lane 6), stage IV (lane 7), MEC (lane 9), AdCC (lane 10), ACC (lane 11), CC (lane 12), BCAC (lane 13), ANOS (lane 14), PLGA (lane 15) showed specific immunoreactivity against recombinant SPAG9 protein. Neutralization experiments were done by pre-incubating recombinant SPAG9 protein (15μg/mL) with sera from SGT patients which resulted in complete loss of immunoreactivity (lane 16). Sera from healthy individuals revealed no reactivity (lane 17). M-molecular weight marker. (C) Neutralization studies in serial tissue sections of benign and malignant stages of SGT. Neutralization studies were carried out by pre-incubating anti-SPAG9 antibody generated in rat with recombinant SPAG9 protein (15μg/mL) and used for probing endogenous SPAG9 protein in the serial tissue sections of SGT patients. Left panel shows the representative images of the H&E stained sections of malignant tumor stage I, II, III, IV. Middle panel depicts the SPAG9 protein expression probed with anti-SPAG9 antibodies. Right panel shows complete loss of SPAG9 localization when probed with neutralized sera.](/cms/asset/3d4e3e8d-9adf-4d6a-94b0-70b11f10352f/koni_a_974382_f0006_oc.jpg)
Statistical analysis for sensitivity and specificity of circulating anti-SPAG9 antibody was performed in sera of benign tumor, stage I (T1) tumor and malignant SGT. It is important to mention, that a cut-off value of 0.212 OD for anti-SPAG9 antibodies predicted presence of malignant SGT with 69.23% sensitivity, 100% specificity, 100% PPV, and 78.94% NPV. In stage I (T1) tumors, SPAG9 antibodies predicted presence of malignant SGT with 90% sensitivity, 100% specificity, 100% PPV, and 98.36% NPV. In benign tumors, presence of SPAG9 antibody predicted SGT with 60% sensitivity, 100% specificity, 100% PPV, and 93.75% NPV. Further the presence of anti-SPAG9 antibodies in the sera of SGT patients was also confirmed by Western blot analysis as shown in . Immuno-reactivity against SPAG9 protein was detected in the sera of benign and malignant SGT of tumor stages and histotypes as compared to sera from 60 normal healthy donors which showed no immune-reactivity. Furthermore, the specificity of immuno-reactivity of patient's sera against recombinant SPAG9 protein was confirmed in neutralization assays which showed complete loss of immuno-reactivity with SPAG9 protein (). Likewise pre-incubation of anti-SPAG9 antibody with SPAG9 recombinant protein (15μg/mL) led to complete loss of immune-reactivity as depicted in .
Discussion
Salivary gland cancers are aggressive malignant tumors with higher metastatic rates leading to cancer related-deaths.Citation25 So far, little is known about the pathogenesis of salivary gland cancer. Besides, their morphological and biological diversity also poses problem for diagnosis and treatment of SGT.Citation5 Several biomarkers such as HER-2, mutated p53, ras-p21, cyclinD1, C-kit, vascular endothelial growth factor (VEGF), sphingosine kinase-1 (SPHK-1) and astrocyte elevated gene-1 (AEG-1)Citation26 have been shown to be associated with SGT samples. However, none of these biomarkers have been included in clinical management and thus, have little or no clinical relevance. Therefore, there is a pressing need to identify new biomarkers for early detection and diagnosis for better management of SGT patients. In this context, the unique family of proteins designated as CT antigens have been considered to be of clinical relevance due to their expression in various types of malignancies and high immunogenicity in cancer patients.Citation1 Moreover, it has been well documented that CT antigens have restricted or no expression in somatic tissues except in testis during gametogenesis. Hence, CT antigens represent a novel target for developing diagnostic and therapeutic candidate molecules.Citation1 Therefore, in the present study, we analyzed a CT antigen, SPAG9 expression and humoral response in various stages and histotypes of SGT patients.
To best of our knowledge, our investigation is the first study reporting SPAG9 gene and protein expression in SGT patients when compared to other CT antigens reported earlier. Based on SPAG9 IRSs, we observed significant difference among ANCT, benign and malignant SGT specimens and a significant association of SPAG9 protein expression between benign and malignant tumor specimens. Our data revealed that majority of malignant tumor patients showed SPAG9 expression as compared to benign tumor patients indicating a possible role of SPAG9 in SGT carcinogenesis. Recently, AEG-1 has been reported to be upregulated in salivary gland cancer as compared to ANCT specimens and was found to be significantly associated with advanced stage tumors and TNM classification.Citation26 Earlier reports on HER-2 and VEGFCitation27 also revealed up-regulation in malignant SGT which closely matched with clinical stages and lymph node metastasis. A similar result from data among ANCT, benign and malignant SGTs, suggest that high SPAG9 expression may contribute to malignant SGT development and needs further investigation.
SPAG9 gene expression was observed in 63% benign tumor and 84% of malignant tumor specimens which included various types of histotypes (). In contrast, earlier study on a CT antigen, HAGE mRNA was shown to be expressed only in 39% benign tumors (11 of 28) and in 50% malignant tumors (8 of 16). It was also demonstrated that BAGE, GAGE-1/2, and MAGE-1 mRNA were detected only in 13% (2 of 16), 25% (4 of 16) and 13% (2 of 16) of tumor specimens respectively.Citation13 Interestingly, SPAG9 protein expression was observed in all the SPAG9 mRNA positive tumor specimens irrespective of stages and histotypes of SGT. Recently, a study has shown an expression of MCM3, a proliferation marker in SGT by immunohistochemical analysis with 74.3% sensitivity and 93.3% specificity between malignant and benign SGT.Citation31 A recent study on the expression of SPAG9 has predicted presence of malignant endometrium with 74% sensitivity, 83% specificity, 88% PPV, and 64.5% NPV.Citation32 In our study, SPAG9 protein expression in IHC predicted presence of malignant SGT with 83.72% sensitivity, 100% specificity, 100% PPV, and 83.72% NPV. Further, in a recent study, SPAG9 overexpression was correlated with tumor stages in human hepatocellular carcinoma tissues (p < 0.001).Citation27 However, we did not find any correlation between the different tumor stages and SPAG9 expression (p = 0.183). To best of our knowledge, none of the earlier reports on CT antigens have validated their protein expression in SGT specimens. Thus, the present study has laid the foundation where SPAG9 expression was shown to be associated with malignant tumors which may be used as a potential biomarker in diagnosis and therapy.
Earlier studies have reported that CT antigens are highly immunogenic and elicit immune response in cancer patients.Citation1 Recently, we reported humoral response against SPAG9 protein in 67% of epithelial ovarian cancer,Citation14 77% of renal cell carcinoma,Citation15 78% of thyroid cancerCitation16, 80% of cervical cancer,Citation17 80% of breast cancer,Citation18 70% of colorectal cancerCitation19 and 77% of bladder cancerCitation20 patients. Further, a recent study on endometrial cancer also demonstrated a strong humoral response against SPAG9 protein in 72% of the cancer patients which supports the present findings.Citation21 More recently, we have reported a humoral response against yet another CT antigen, A-kinase anchor protein 4 (AKAP4) in 65% of ovarian cancerCitation28, 94% breast cancerCitation29 and 86% cervical cancerCitation30 patients. Till now, no other CT antigen has been reported to show humoral response in SGT patients. CT antigens such as HAGE, BAGE, GAGE-1/2 and MAGE-1 have only been validated for mRNA expressionCitation13 and not for the protein expression. In this context, our investigation is a first report showing that SPAG9 is highly immunogenic and elicits humoral response against SPAG9 protein in 68% of SGT patients found positive for SPAG9 mRNA and protein expression. We have put forth evidence that majority of SGT patients revealed circulating antibodies against SPAG9. Statistical analysis revealed that circulating anti-SPAG9 antibodies predicted presence of malignant SGT with 69.23% sensitivity, 100% specificity, 100% PPV and 78.94% NPV. Whereas, in sera from benign SGT, SPAG9 antibodies predicted presence of SGT with 60% sensitivity, 100% specificity, 100% PPV and 93.75% NPV. Thus, our data on humoral response in SGT patients suggest that SPAG9 could be a promising biomarker candidate for early detection and diagnosis of SGT and warrants further investigation at large scale.
The aberrant CT antigen expression is apparently associated with deregulated cellular growth and migration.Citation1 Our earlier studies on MAPK (mitogen-activated protein kinase) interaction suggested that SPAG9 acts as a scaffolding protein which interacts with JNK (c-Jun N-terminal kinase) signaling module especially with JNK2 and JNK3.Citation33 Since, JNK signaling interactions play a crucial role in cellular proliferation, differentiation, apoptosis, cellular transformation and tumor cell growth,Citation33 SPAG9 over expression in SGT could alter the efficiency and specificity of MAPK signaling leading to neoplastic growth of SGT. However, the underlying mechanisms associated with SPAG9 expression during cancer are still unclear. In our recent attempt to understand the potential role of SPAG9 in cancer progression, plasmid driven gene silencing approach was employed, which demonstrated that ablation of SPAG9 protein in bladder cancer cells resulted in cell cycle arrest in G0-G1 phase.Citation20 The G1 arrest was characterized by upregulation of p16 and p21 and downregulation of cyclins and cyclin-dependent kinaseCitation20 indicating a potential role of SPAG9 in cancer growth and cellular proliferation.
Collectively, our data demonstrated that majority of SGT patients exhibit SPAG9 expression and elicit humoral response against SPAG9 protein in the sera irrespective of the stages and the histotypes of SGT. SPAG9 expression was associated with malignant state of SGT patients and exhibited significantly higher antibody response in malignant tumor patients as compared to benign tumor patients. Here we are reporting the possibility of developing serum based biomarker for better cancer management of SGT patients. Further studies are warranted to investigate the association of SPAG9 in large number of SGT patients.
Materials and Methods
Patient specimens
The present investigation was carried out in tissue specimens and sera sample of SGT patients who underwent surgical resection at All India Institute of Medical Sciences, New Delhi. Based on histopathological reports, the tissue specimens included 16 benign and 86 malignant specimens. Benign tumors included 16 pleomorphic adenoma specimens. Malignant tumor specimens included 30 mucoepidermoid, 12 adenoid cystic, 5 acinic cell, 16 clear cell, 5 basal cell carcinoma, 10 adenocarcinoma NOS, 8 polymorphous low grade adenocarcinoma and 72 matched available adjacent non-cancerous tissues (ANCT). Based on stages of malignant SGT, specimens included 14 stage I (T1), 17 stage T II, 32 stage T III and 23 stage T IV. Tumor specimens were collected during routine surgery in 5% buffered formaldehyde prepared in diethylpyrocarbonate (DEPC) water and processed for IHC and in situ RNA hybridization studies. In addition, a portion of tumor tissue specimens were also collected in RNAlater (Sigma-Aldrich, St. Louis, MO) and stored at –80°C until use for SPAG9 gene expression analysis. Peripheral blood from 62 available patients and 60 normal healthy subjects were also obtained. All the sera samples thus obtained from SGT and normal healthy donors were stored at –80°C until further investigations. All investigations were carried out after obtaining ethical clearances from Institute ethics committee (IEC), All India Institute of Medical Sciences, and Institutional human ethics committee (IHEC), National Institute of Immunology, New Delhi. Duly signed consent forms were also obtained from all the patients under investigation. Histological characteristics of the tissue samples were reviewed and confirmed by two independent pathologists by microscopic observation of hematoxylin and eosin (H&E) stained tissue sections.
Analysis of SPAG9 gene expression by reverse transcriptase-polymerase chain reaction (RT-PCR)
SPAG9 gene expression was examined by extracting total RNA from the frozen SGT tissues employing RNeasy mini kit (Qiagen GmbH, Hilden, Germany) as per the manufacturer's instructions. Subsequently, cDNA was synthesized using 500ng of total RNA and High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). SPAG9 gene specific primers Forward 5′-GAATTCGATCAGGAACTTAAGGAACAGCAGAAGGAG-3′, Reverse 5′-GGTACCCTGTTTCTC-GTGCACCTGGCACACTTGCAA-3′ were used to carry out RT-PCR using 50ng cDNA as a template as described earlier.Citation15 β-actin specific primers (forward: 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′ and reverse: 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′) were used as an internal control. The PCR amplicon was analyzed on 1% agarose gel and was sub-cloned into TOPO vector (Invitrogen, Life Technologies, Carlsbad, CA) for further studies.
In situ RNA hybridization
Serial SGT tissue sections were subjected to in situ RNA hybridization experiments to examine the expression of SPAG9 transcripts as described earlier.Citation15 Antisense (complementary to endogenous mRNA) as well as sense (same as endogenous mRNA) riboprobes were synthesized in vitro using T7 or T3 RNA polymerases respectively and DIG RNA Labeling kit (Roche Diagnostics GmbH, Mannheim, Germany) respectively. The riboprobes were used to hybridize SPAG9 transcripts in serial SGT tissue sections and visualized using nitro-blue tetrazolium and 5-bromo-4-chloro-3′-indolyphosphate [(NBT/BCIP) as substrate, Roche Diagnostics GmbH, Mannheim, Germany] and images were captured by using Nikon Eclipse E400 microscope (Nikon, Fukok, Japan).
Immunohistochemistry (IHC)
The SPAG9 protein expression in SGT serial tissue sections was validated by IHC as described earlier.Citation15 Briefly, the SGT tissue sections (4μm) were deparaffinized and subsequently rehydrated using different gradients of alcohol and probed with anti-SPAG9 antibody or control IgG by incubating at 4°C for overnight in humid chamber. After washing thrice with phosphate buffer saline (PBS)-0.05% Tween20, the tissue sections were incubated with horseradish peroxidase-conjugated goat anti-rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and visualized using chromogen, 0.05% 3,3′-diaminobenzidine [(DAB), Sigma-Aldrich, St. Louis, MO] counterstained with hematoxylin stain and mounted with 1,3-diethyl-8-phenylxanthine [(DPX), Sigma-Aldrich, St. Louis, MO]. The images of tissue sections were captured using Nikon Eclipse E 400 microscope (Nikon, Fukok, Japan).
SPAG9 Immuno-reactive Score (IRS)
The immune-stained SGT tissue sections were examined by two senior pathologists by counting five random fields (> 500 cells) under x400 magnification as described earlier.Citation19 SGT tissue sections distinctly showing SPAG9 protein expression in > 10% of cells were considered as positive.
Detection of circulating anti-SPAG9 antibodies in SGT patients
The circulating anti-SPAG9 antibodies were detected in the sera of the SGT patients employing ELISA and by Western blotting as described earlier.Citation15 In order to check the specificity of the circulating anti-SPAG9 antibodies, neutralization experiments were performed as described earlier.Citation19 Briefly, sera of the SGT patients were pre-incubated with SPAG9 recombinant protein and subsequently, were used for probing recombinant SPAG9 by Western blotting as described earlier.Citation19
Statistical analysis
Statistical data analyses were performed using SPSS 20.0 software package (SPSS Inc, Chicago, IL, USA). Mann–Whitney U-test was done to determine the statistical difference of SPAG9 gene and protein expression within, benign and malignant and among various tumor stages. The statistical significance between the humoral response in benign, malignant SGT and healthy donors was assessed by unpaired Mann–Whitney U-test. In addition, Mann–Whitney U-test was also performed to find the difference between lymph node positive and negative salivary gland cancer patients. The Pearson's χ2 test was used to examine the association of SPAG9 expression and humoral response among benign and various malignant stages. The Kruskal–Wallis test was used to analyze the difference in SPAG9 expression and humoral response among different SGT malignant stages (I, II, III, IV) and various tumor histotypes [Mucoepidermoid carcinoma (MEC), Adenoid cystic carcinoma (AdCC), Acinic cell carcinoma (ACC), Clear cell carcinoma (CCC), Basal cell adenocarcinoma (BCAC), Adenocarcinoma not otherwise specified (ANOS), Polymorphous low grade adenocarcinoma (PLGA)]. A p value of less than 0.05 was considered statistically significant. The diagnostic accuracy of the tests was evaluated by using Bayesian statistics such as sensitivity [TP/(TP+FN)], specificity [TN/(TN+FP)], Positive Predictive Value [TP/(TP+FP)] and Negative Predictive Value [TN/(TN+FN)] where abbreviations stand for, True Positive (TP), True Negative (TN), False Negative (FN) and False Positive (FP) cases for SPAG9 protein expression and anti-SPAG9 antibodies in control and tumor patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
SA, DP and NG carried out all the experiments, prepared figures and drafted the manuscript. NJ, ASA, NKL, AG and VS participated in data analysis and interpretation of results. AG and VS pathologists performed histopathology examination of all the clinical specimens used in this investigation. AT and RK senior surgeon provided clinical samples and clinicopathological data from the hospital for this study. AS designed the study, participated in data analysis and interpretation of results. All authors read and approved the manuscript. We acknowledge Dr V. Kumar, Senior Staff Scientist, International Center for Genetic Engineering and Biotechnology, New Delhi, India for critical reading and editing of this manuscript.
Funding
This work is supported by grants from Indo-UK Cancer Research Program (Grant No. BT/IN/UK/NII/2006), Center for Molecular Medicine (Grant No. BT/PR/14549/MED/14/1291), NII-core funding, Department of Biotechnology, Government of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Suri A, Saini S, Sinha A, Agarwal S, Verma A, Parashar D, Singh S, Gupta N, Jagadish N. Cancer testis antigen: A new paradigm for cancer therapy. Oncoimmunology 2012; 1:1194-96; PMID:23170277
- Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin 2013; 62:10-29; PMID:23335087; http://dx.doi.org/10.3322/caac.20138
- Yeole BB. An assessment of improvement in reliability and completeness of Mumbai cancer registry data from 1965–1997. Asian Pacific J Cancer Prev 2001; 2:225-232; PMID:12718635
- Speight PM, Barrett AW. Salivary gland tumours. Oral Dis 2002; 8:229-240; PMID:12363107; http://dx.doi.org/10.1034/j.1601-0825.2002.02870.x
- Guzzo M, Locati LD, Prott FZ, Gatta G, McGurk M, Licitra L. Major and minor salivary gland tumors. Crit Rev Oncol Hematol 2010; 74:134-148; PMID:19939701; http://dx.doi.org/10.1016/j.critrevonc.2009.10.004
- Locati LD, Perrone F, Losa M, Mela M, Casieri P, Orsenigo M, Cortelazzi B, Negri T, Tamborini E, Quattrone P, et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs). Oral Oncol 2009; 45:986-990; PMID:19574086; http://dx.doi.org/10.1016/j.oraloncology.2009.05.635
- Ethunandan M, Witton R, Hoffman G, Spedding A, Brennan PA. Atypical features in pleomorphic adenoma–a clinicopathologic study and implications for management. Int J Oral Maxillo fac Surg 2006; 35:608-12; PMID:16540285; http://dx.doi.org/10.1016/j.ijom.2006.02.009
- Matse JH, Yoshizawa J, Wang X, Elashoff D, Bolscher JGM, Veerman ECI, Bloemena E, Wong DTW. Discovery and prevalidation of salivary extracellular microRNA biomarkers panel for the noninvasive detection of benign and malignant parotid gland tumors. Clin Cancer Res 2013; 19:3032-38; PMID:23575476
- Rodríguez MS, Reija MF, Rodilla IG. Primary clear cell carcinoma of parotid gland: Case report and review of literature. J Oral Maxillofac Pathol 2013; 17:101-5; PMID:23798840; http://dx.doi.org/10.4103/0973-029X.110692
- Glisson B, Colevas AD, Haddad R, Krane J, El-Naggar A, Kies M, Costello R, Summey C, Arquette M, Langer C, et al. HER2 expression in Salivary Gland Carcinomas: dependence on Histological Subtype. Clin Cancer Res 2004; 10:944-46; PMID:14871971; http://dx.doi.org/10.1158/1078-0432.CCR-03-0253
- Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer 2005; 103:2544-50; PMID:15880750; http://dx.doi.org/10.1002/cncr.21083
- Kalejs M, Erenpreisa J. Cancer/testis antigens and gametogenesis: a review and "brain-storming" session. Cancer Cell Int 2005; 5:4-14; PMID:15715909; http://dx.doi.org/10.1186/1475-2867-5-4
- Nagel H, Laskawi R, Eiffert H, Schlott T. Analysis of the tumour suppressor genes, FHIT and WT-1, and the tumour rejection genes, BAGE, GAGE-1/2, HAGE, MAGE-1, and MAGE-3, in benign and malignant neoplasms of the salivary glands. J Clin Pathol: Mol Pathol 2003; 56:226-31; PMID:12890744
- Garg M, Chaurasiya D, Rana R, Jagadish N, Kanojia D, Dudha N, Kamran N, Salhan S, Bhatnagar A, Suri S, et al. Sperm associated antigen 9, a novel cancer testis antigen, is a potential target for immunotherapy in epithelial ovarian cancer. Clin Cancer Res 2007; 13:1421-28; PMID:17332284; http://dx.doi.org/10.1158/1078-0432.CCR-06-2340
- Garg M, Kanojia D, Khosla A, Dudha N, Sati S, Chaurasiya D, Jagadish N, Seth A, Kumar R, Gupta S, et al. Sperm-associated antigen 9 is associated with tumor growth, migration, and invasion in renal cell carcinoma. Cancer Res 2008; 68:8240-48; PMID:18922895; http://dx.doi.org/10.1158/0008-5472.CAN-08-1708
- Garg M, Kanojia D, Suri S, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9: a novel diagnostic marker for thyroid cancer. J Clin Endocrinol Metab 2009; 94:4613-18; PMID:19820019; http://dx.doi.org/10.1210/jc.2009-0703
- Garg M, Kanojia D, Salhan S, Suri S, Gupta A, Lohiya NK, Suri A. Sperm-associated antigen 9 is a biomarker for early cervical carcinoma. Cancer 2009; 115:2671-83; PMID:19326449; http://dx.doi.org/10.1002/cncr.24293
- Kanojia D, Garg M, Gupta S, Gupta A and Suri A. Sperm-associated antigen 9, a novel biomarker for early detection of breast cancer. Cancer Epidemiol Biomarkers Prev 2009; 8:630-39; PMID:19190149; http://dx.doi.org/10.1158/1055-9965.EPI-08-0629
- Kanojia D, Garg M, Gupta S, Gupta A and Suri A. Sperm-associated antigen 9 is a novel biomarker for colorectal cancer and is involved in tumor growth and tumorigenicity. Am J Pathol 2011; 178:1009-20; PMID:21356354; http://dx.doi.org/10.1016/j.ajpath.2010.11.047
- Kanojia D, Garg M, Saini S, Agarwal S, Parashar D, Jagadish N, Seth A, Bhatnagar A, Gupta A, Kumar R, et al. Sperm associated antigen 9 plays an important role in bladder transitional cell carcinoma. PLoS One 2013; 8(12): e81348; PMID:24349057; http://dx.doi.org/10.1371/journal.pone.0081348
- Yu P, Yan L, Zhang H, Lin X, Zhao X. Expression and clinical significance of Sperm-Associated Antigen 9 in patients with endometrial carcinoma. Int J Gynaecol Cancer; 2012; 22: 87-93; PMID:22146769; http://dx.doi.org/10.1097/IGC.0b013e3182370f2e
- Wang Y, Dong Q, Miao Y, Fu L, Lin X. Clinical significance and biological roles of SPAG9 overexpression in non-small cell lung cancer. Lung Cancer; 2013; 2:266-72; PMID:23711689; http://dx.doi.org/10.1016/j.lungcan.2013.04.021
- Yi F, Ni W, Liu W, Pan X, Han X, Yang L, Kong X, Ma R, Chang R. SPAG9 is overexpressed in human astrocytoma and promotes cell proliferation and invasion. Tumour Biol; 2013; 34:2849-55; PMID:23696027 ; http://dx.doi.org/10.1007/s13277-013-0845-5
- Gao J, Panizza B, Johnson NW, Coman S, Clough AR. Basic consideration of research strategies for head and neck cancer. Front Med; 2012; 6:339-53; PMID:23054500; http://dx.doi.org/10.1007/s11684-012-0213-7
- Murase R, Sumida T, Ishikawa A, Murase R, McAllister SD, Hamakawa H, Desprez P. Novel therapeutic strategies for malignant salivary gland tumors: lessons learned from breast cancer. Int J Oto laryngol; 2011:187623-7631; PMID:22164169
- Liao W, Guo L, Zhong Y, Wu Y, Li J, Song L. Astrocyte elevated gene-1 (AEG-1) is a marker for aggressive salivary gland carcinoma. J Transl Med; 2011; 9:205-14; PMID:22133054; http://dx.doi.org/10.1186/1479-5876-9-205
- Xie C, Fu L, Liu N, Li Q. Overexpression of SPAG9 correlates with poor prognosis and tumor progression in hepatocellular carcinoma. Tumour Biol 2014; 35:7685-91; PMID:24801907; http://dx.doi.org/10.1007/s13277-014-2030-x
- Agarwal S, Saini S, Parashar D, Verma A, Sinha A, Jagadish N, Batra A, Suri S, Gupta A, Ansari AS, et al. The novel cancer-testis antigen A-kinase anchor protein 4 (AKAP4) is a potential target for immunotherapy of ovarian serous carcinoma. OncoImmunology; 2013; 2:e24270-278; PMID:23762804; http://dx.doi.org/10.4161/onci.24270
- Saini S, Jagadish N, Gupta A, Bhatnagar A, Suri A. A novel cancer testis antigen, a-kinase anchor protein 4 (AKAP4) is a potential biomarker for breast cancer. PLoS ONE 2013; 8(2): e57095; PMID:23451156; http://dx.doi.org/10.1371/journal.pone.0057095
- Agarwal S, Saini S, Parashar D, Verma A, Jagadish N, Batra A, Suri S, Bhatnagar A, Gupta A, Ansari AS, et al. Expression and humoral response of a-kinase anchor protein 4 in cervical cancer. Int J Gynecol Cancer 2013; 4:650-58; PMID:23478221; http://dx.doi.org/10.1097/IGC.0b013e31828a0698
- Ashkavandi ZJ, Najvani AD, Tadbir AA, Pardis S, Ranjbar MA, Ashraf MJ. MCM3 as a novel diagnostic marker in benign and malignant salivary gland tumors. Asian Pac J Cancer Prev 2013; 14:3479-82; PMID:23886132; http://dx.doi.org/10.7314/APJCP.2013.14.6.3479
- Baser E, Togrul C, Ozgu E, Ayhan S, Caglar M, Erkaya S, Gungor T. Sperm-associated antigen 9 is a promising marker for early diagnosis of endometrial Cancer. Asian Pac J Cancer Prev 2012; 14:7635-38; PMID:24460345; http://dx.doi.org/10.7314/APJCP.2013.14.12.7635
- Jagadish N, Rana R, Selvi R, Mishra D, Garg M, Yadav S, Herr JC, Okumura K, Hasegawa A, Koyama K, et al. Characterization of a novel human sperm-associated antigen 9 (SPAG9) having structural homology with c-Jun N-terminal kinase-interacting protein. Biochem J 2005; 389:73-82; PMID:15693750; http://dx.doi.org/10.1042/BJ20041577
