Abstract
The continuous remodeling of progressing tumors demands non-physiologic production of extracellular matrix (ECM) proteins. Among them, osteopontin (OPN) has been largely involved in tumor progression and metastasis. We have recently discovered a new mechanism for OPN in the metastatic spread of mammary carcinoma providing local immunosuppression at the seeding site.
Matricellular proteins are non-structural ECM proteins that regulate several cellular processes such as cell adhesion and migration, ECM deposition, cell survival, and proliferation. All these physiological functions are exploited by the neoplastic clones for tumor growth and dissemination.Citation1 A deregulated expression of matricellular proteins is often detected in tumors, where they can be produced by both tumor cells and surrounding stromal cells, but it is still unclear if their function can be different depending from the source. OPN (Spp-1) is one of the most studied matricellular proteins and its contribution to both primary tumor growth and metastasis has been demonstrated in several tumor types. Indeed, high expression of OPN has been correlated with poor survival of cancer patients with different tumors and elevated OPN plasma concentration is detected in patients with metastatic tumors compared to normal samples.Citation2 Such pro-tumorigenic activity is somehow contradictory with the original description of OPN as a Th1-like cytokine, promoting IL-12, while dampening IL-10, production in macrophages.Citation3
These data may suggest different activities for the same molecule when produced by distinct cell types and indicate the need of more in depth comprehension of the molecular mechanisms behind OPN functions in primary and metastatic tumor microenvironment.
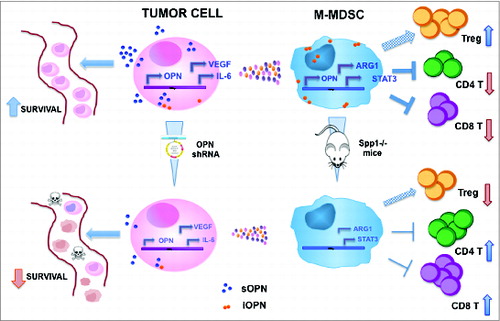
Using a spontaneously metastatic mouse model of breast carcinogenesis, we have recently dissected the role of OPN in the metastatic process when produced by the neoplastic clone or by host immune cells, particularly myeloid cells.Citation4 Combining OPN gene silencing of tumor cells, and OPN-deficient mice we have demonstrated that both tumor- and myeloid-derived OPN contributes to metastatic progression, through distinct and common mechanisms. Specifically, OPN produced by tumor cells, protect cells from apoptosis in condition of anoikis, like in blood stream, whereas OPN from myeloid cells regulates their immunosuppressive activity in the local metastatic niche. These myeloid cells belong to the so-called myeloid-derived suppressor cells (MDSC) population, which, endowed with immunosuppressive activity, expands along solid tumor progression in most mouse tumor models and in human cancers, contributing to both primary tumor growth and metastasis.Citation5,6 This suppressive population comprises in the mouse two main subsets: granulocytic and monocytic cells, expressing the Gr-1 marker at high (Gr-1high) or low-intermediated Gr-1 (Gr-1 int-low) level, respectively. These subsets show different suppressive capacity, with the monocytic being generally more suppressive than the granulocytic population.Citation7 We have found that OPN is mainly expressed by the monocytic subset in which contributes to the suppressive activity via regulation of genes like arginase-1, Stat3, and Il-6. Moreover, in the metastatic lungs of mice deficient for OPN, the more suppressive Gr-1int-low subset is less expanded than in OPN-competent hosts, rendering the local metastatic niche less immunosuppressive (.
Figure 1. Schematic representation of OPN activities in tumor and monocytic myeloid cells and of the effects of its downmodulation. Tumor cells mainly express the soluble form of OPN (sOPN) and its downmodulation by means of gene silencing affects their ability to survive in anoikis condition, such as in the lung blood stream; moreover OPN-silenced tumor cells produce less VEGF and IL-6, two molecules involved in MDSC fitness and suppressive activity; this in turn results in less suppressive MDSC that produce lower amounts of arginase 1. On the other hand, OPN in monocytic MDSC (M-MDSC) is mainly in its intracellular form (iOPN) and it is involved in maintaining their suppressive activity: when MDSC are not able to produce OPN, as in mice deficient for the protein (spp1–/– mice), their expression of immunosuppressive genes, such as arginase1 and Stat3, is downmodulated and, in turn, they are less competent in suppressing CD8+ and CD4+ T cell proliferation; concomitantly, the number of regulatory T cells is lower in spp1–/–mice.
Notably we have found clinical correlation to these findings detecting the presence of myeloid cells, with monocytoid morphology, expressing OPN within lung metastases of human ductal mammary carcinomas. Such cells were mostly localized in the stroma surrounding metastatic foci suggesting a role in the local events favoring the metastatic spreading.
In addition, the absence of OPN in tumor-bearing mice was associated with a decrease in number of regulatory T cells (Treg) in the metastatic lungs, but, differently from MDSC, it had no effect on their suppressive activity. Whether the effect on Treg number depends on a cell-intrinsic defect associated to OPN deficiency or on “unfit” Spp1–/– MDSC incapable of inducing Treg conversion,Citation8 it remains to be elucidated.
Further investigating the different roles of OPN when produced by tumor and myeloid cells, we have found a different OPN cellular localization in tumor vs. myeloid cells: while in tumor cells OPN is mainly cytoplasmic localizing in the endoplasmic reticulum, in monocytic myeloid cells it remains confined in specific spots under the cellular membrane, and does not localize in the ER, consistently with the intracellular OPN (iOPN) form recently reported in plasmocytoid DC (pDC).Citation9
Our finding is the first contribution describing a role for iOPN in cancer, shaping immunosuppression of lung metastatic niche via modulation of MDSC. While the molecular mechanisms for iOPN activities in pDC have been elucidated,Citation9,10 those behind MDSC-mediated immunosuppression remain to be defined. It would be interesting to investigate whether such mechanisms use the same molecular mediators as in pDC, i.e., iOPN association with MyD88 upon TLR ligation. Most likely iOPN is the product of an alternative translation starting downstream the signal sequence, but the cell type-specific factor determining the choice of this alternative initiation of translation, instead of the canonical AUG site, remains unknown. Although, the functional outcome of iOPN seems different in pDC and MDSC in term of fueling and dampening immune response, respectively, a possible common trait points to a program of “fitness in function” meaning the production of IFNα in response to viral infection in pDC and immune suppression in MDSC, which are the main functional specifications of these two cell populations.
Deciphering the cell type-specific mechanisms and the distinct, and even opposite, activities in the different cells and of the different forms of OPN, may be critical for its potential therapeutic targeting guiding the correct choice of the specific approach: a neutralizing antibody for selective depletion of sOPN maintaining the function of iOPN, or other strategies, such as RNA silencing, to block both sOPN and iOPN?
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev 2010; 29:295-307; PMID:20386958; http://dx.doi.org/10.1007/s10555-010-9221-8
- Bramwell VH, Doig GS, Tuck AB, Wilson SM, Tonkin KS, Tomiak A, Perera F, Vandenberg TA, Chambers AF. Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res 2006; 12:3337-43; PMID:16740755; http://dx.doi.org/10.1158/1078-0432.CCR-05-2354
- Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 2000; 287:860-4; PMID:10657301; http://dx.doi.org/10.1126/science.287.5454.860
- Sangaletti S, Tripodo C, Sandri S, Torselli I, Vitali C, Ratti C, Botti L, Burocchi A, Porcasi R, Tomirotti A et al. Osteopontin shapes immunosuppression in the metastatic niche. Cancer Res 2014; 74:4706-19; PMID:25035397; http://dx.doi.org/10.1158/0008-5472.CAN-13-3334
- Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res 2007; 67:425; author reply 6; PMID:17210725; http://dx.doi.org/10.1158/0008-5472.CAN-06-3037
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/10.1038/nri3175
- Dolcetti L, Peranzoni E, Bronte V. Measurement of myeloid cell immune suppressive activity. Curr Protoc Immunol 2010; Chapter 14:Unit 14 7; PMID:21053303
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006; 66:1123-31; PMID:16424049; http://dx.doi.org/10.1158/0008-5472.CAN-05-1299
- Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci U S A 2008; 105:7235-9; PMID:18480255; http://dx.doi.org/10.1073/pnas.0802301105
- Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol 2006; 7:498-506; PMID:16604075; http://dx.doi.org/10.1038/ni1327
