Abstract
Plasmacytoid dendritic cells (pDCs) are not only potent inflammatory cytokine producers but also function as antigen-presenting cells (APCs). We have shown that vaccination using CpG-B activated tumor antigen (Ag) presenting pDCs induce Th17 cells that promote intratumoral immune cell recruitment, including antitumor cytotoxic T lymphocytes CTLs. Therefore, strategies targeting both innate and adaptive pDC functions may improve antitumor T-cell immunity.
Plasmacytoid dendritic cells (pDCs), are highly specialized cells capable of secreting large amounts of type I interferons (IFN-I) in response to viral infection. Although long debated, it is now established that pDCs also function as bona fide antigen (Ag) presenting cells (APCs).Citation1 Consequently, pioneering clinical studies using tumor Ag loaded pDCs as vaccine agents are on-going.Citation2
pDCs are recruited to several tumor types, such as head and neck, breast, ovarian, lung, prostate, and liver cancers, as well as melanoma and lymphoma.Citation3 However, in most of those cancers, pDCs are maintained in a quiescent state, characterized by low expression of co-stimulatory molecules and negligible IFN-I production.Citation3 In addition, tumor-associated pDCs are capable of acquiring and presenting tumor AgsCitation4 and promote a suppressive tumor microenvironment by inducing regulatory T cells (Tregs) via inducible T cell co-stimulator (ICOS) and/or indoleamine 2,3-dioxygenase 1 (IDO) expression.Citation3 The presence of pDCs has, therefore, been associated with a negative prognosis in many cancers.
However, in some but not all cancers, resting pDCs can be re-activated given adequate signals, especially through Toll-like receptor (TLR) triggering, to give rise to effective antitumor specific immune responses. Indeed, local administration (intratumoral or topical for subcutaneous tumors) of TLR ligands induces pDC-dependent tumor regression.Citation3 Similarly, in a mouse model of breast cancer, pDC reactivation using TLR7 ligand promotes IFN-I dependent tumor regression.Citation5 However, pDC can also control tumor growth via IFN-I independent unknown mechanisms upon TLR9 ligation,Citation5 suggesting additional functions for pDCs in antitumor immunity. Vaccination using tumor Ag-loaded pDCs induces tumor-specific T-cell responses against melanoma in humanized mouse modelsCitation6 and patients.Citation2 Therefore, vaccine strategies aiming at combining innate and adaptive pDC functions may result in synergistic effects and lead to development of potent antitumor immunotherapies.
We recently explored the contribution of Ag presentation by pDCs in antitumor immunity.Citation7 Using genetically modified mice in which MHC Class II expression is specifically abolished in pDCs, we demonstrated that upon CpG-B treatment, Ag (OVA)-specific T helper type 17 (Th17) cell differentiation was impaired, whereas T helper type 1 (Th1) and Treg responses were unaffected. Moreover, pDC innate functions were not altered by the loss of MHC-II as demonstrated by normal inflammatory cytokine production by MHC-II-deficient pDCs in response to CpG-B or Imiquimod. Taken together, our results showed that CpG-B activated Ag-presenting pDCs promote Th17 cells.
Can pDC ability to prime Th17 cells be exploited to foster antitumor immunity? The role of Th17 cells in tumors is controversial. Different tumor cytokinic environments imprint opposite Th17-mediated effects: pro-tumorigenicity via increased tumor survival and angiogenesis, and anti-tumorigenicity via intra-tumoral immune cell recruitment and IFNγ production.Citation8 We have immunized mice with MHCII-restricted OVA peptide (OVAII) in presence of CpG-B and further challenged with OVA-expressing EL4 lymphoma cells (EG7). Mice lacking MHC-II on pDCs developed larger tumors and exhibited impaired Th17 cell responses as compared to control animals, suggesting a functional role for pDC-driven Th17 cells in promoting antitumor immunity. Accordingly, transfer of tumor-specific Th17 cells into tumor-bearing mice significantly inhibited tumor growth. Interestingly, in the absence of MHC Class II expression by pDCs, mice demonstrated decreased intratumoral immune cell infiltration, resulting in impaired numbers of conventional DCs and pDCs, CD4+ T helper cells (including Th1, Th17, Tregs) and CTLs. Alteration in the production of IL17-induced chemokines, such as chemokine (C-C motif) ligand 2 (CCL2) and chemokine (C-X-C motif) ligand 2 (CXCL2), observed in tumor supernatants of mice deficient for MHC-II expression by pDCs, might account for impaired intratumoral immune cell recruitment. Although the mechanisms by which Th17 cells control tumor growth in this model are not fully understood, 2 possibilities emerged from our results and other recent publications: direct action of Th17 cells through production of IFNγ that impacts tumor growth,Citation9,10 and/or indirect Th17 action via the production of IL-17-induced chemokines and subsequent recruitment of immune cells to tumors.Citation11
We then questioned whether pDC ability to induce antitumoral Th17 cells could be used as a vaccine to eradicate established tumors. Interestingly, OVAII+CpG-B vaccination of OVA+ tumor-bearing mice failed to confer protection when performed at the tumor site, suggesting that pDCs soaked in the tumor microenvironment were refractory to CpG-B activation and consequently failed to prime Th17 cells. In contrast, vaccine administration at distal sites induced Th17 response and significantly reduced tumor size in association with increased intratumoral immune cell recruitment. Importantly, tumor rejection was not observed in mice lacking MHC-II on pDCs. Therefore, control of established tumors following vaccination using MHC Class II-restricted tumor Ags in presence of CpG-B is fully dependent on Ag presentation by pDCs. In addition, CD8+ T-cell depletion completely abrogated vaccine beneficial effect, suggesting that pDC-mediated protection requires antitumoral CD8+ T-cell effector functions. Accordingly, we show that MHC Class II-restricted tumor vaccination potentiates tumor-specific CTLs. OVA+ tumor-bearing mice adoptively transferred with suboptimal numbers of OVA-specific OT-1 CD8+ T cells exhibited a modest and transient tumor size reduction. However, concomitant administration of MHC-II-vaccine increased intratumoral OT-1 cell recruitment and both enhanced and prolonged the CTL-mediated reduction in tumor volume. Importantly, this effect was dependent on Ag presentation by pDCs, demonstrating that pDC-primed Th17 cells led to tumor-specific CTLs intratumoral recruitment and subsequent control of tumor growth.
Figure 1. Schematic view of the induction of Th17 by Ag presenting activated pDCs and subsequent impact on antitumor immunity. (A) pDCs in tumor and tumor-draining LN are maintained quiescent by the tumoral microenvironment («Tolerogenic pDC»). (B) However, vaccination of tumor-bearing mice using MHCII-restricted tumor derived peptide in the presence of CpG-B at tumor distal site induces Th17 cell differentiation by Ag presenting activated pDCs (« Immunogenic pDC»). (C) pDC-derived Th17 cells migrate to the tumor and induce the recruitment of immune cells, including DCs, pDCs, Th1, Tregs and CTLs. (D) Enhanced tumor-specific CTL infiltration allows tumor cell killing and control of tumor growth.
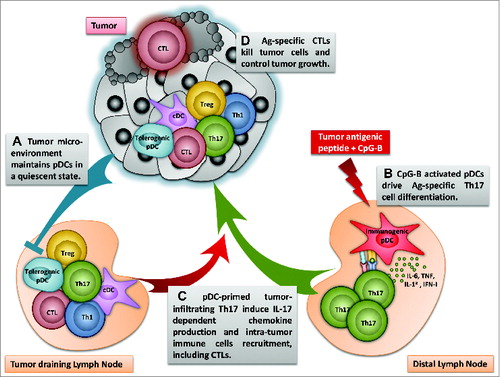
Our results show that vaccines using MHC Class II-restricted tumor peptides combined with CpG-B promote pDCs ability to prime tumor-specific Th17 cells, thus enhancing intratumoral specific CD8+ T-cell recruitment and consequently boosting antitumor specific CTL responses (Fig. 1). These observations encourage the use of pDCs for immunotherapies as APCs to induce robust tumor-specific T-cell responses and significantly improve the efficacy of vaccination strategies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Guery L, Hugues S. Tolerogenic and activatory plasmacytoid dendritic cells in autoimmunity. Fron Immunol. 2013;4:59; PMID:23508732; http://dx.doi.org/10.3389/fimmu.2013.00059
- Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063-75; PMID:23345163; http://dx.doi.org/10.1158/0008-5472.CAN-12-2583
- Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. 2013;93:343-52; PMID:23136258; http://dx.doi.org/10.1189/jlb.0812397
- Bonaccorsi I, Morandi B, Antsiferova O, Costa G, Oliveri D, Conte R, Pezzino G, Vermiglio G, Anastasi GP, Navarra G, et al. Membrane transfer from tumor cells overcomes deficient phagocytic ability of plasmacytoid dendritic cells for the acquisition and presentation of tumor antigens. J Immunol. 2014;192:824-32; PMID:24337377; http://dx.doi.org/10.4049/jimmunol.1301039
- Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, Dubois B, Treilleux I, Marvel J, Vlach J, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013;73:4629-40; PMID:23722543; http://dx.doi.org/10.1158/0008-5472.CAN-12-3058
- Aspord C, Charles J, Leccia MT, Laurin D, Richard MJ, Chaperot L, Plumas J. A novel cancer vaccine strategy based on HLA-A*0201 matched allogeneic plasmacytoid dendritic cells. PloS one. 2010;5:e10458; PMID:20454561; http://dx.doi.org/10.1371/journal.pone.0010458
- Guery L, Dubrot J, Lippens C, Brighouse D, Malinge P, Irla M, et al. Ag-presenting CpG-activated pDCs prime Th17 cells that induce tumor regression. Cancer Res. 2014;74:6430-40.
- Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276; PMID:24987392; http://dx.doi.org/10.3389/fimmu.2014.00276
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362-73; PMID:18354038; http://dx.doi.org/10.1182/blood-2007-11-120998
- Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine & growth factor reviews. 2002;13:95-109; PMID:11900986
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787-98; PMID:19879162; http://dx.doi.org/10.1016/j.immuni.2009.09.014
