Abstract
Epstein-Barr virus-induced gene 3 (EBI3) encoded protein can form heterodimers with IL-27P28, and IL-12P35 to form IL-27, and IL-35. However, IL-27 stimulates, whereas IL-35 inhibits antitumor T-cell responses. IL-27 also limits the Foxp3+ regulatory T cell (Treg) population, whereas IL-35 has been shown to expand Tregs and foster Treg suppressive functions. It remains unclear which group of forces are dominant during antitumor T-cell responses. In this study, we evaluated the tumor growth and antitumor T-cell responses in EBI3-deficient mice that lack both IL-27 and IL-35. We found that injecting B16 melanoma cells into EBI3-deficient C57BL/6 mice, or J558 plasmacytoma cells into EBI3-deficient BALB/c mice resulted in significantly increased tumor growth relative to those implanted in wild-type control mice. Tumors from EBI3-deficient mice contained significantly decreased proportions of CD8+ T cells and increased proportions of CD4+FoxP3+ Treg cells as compared to those from EBI3-intact mice. Tumor-infiltrating T cells from EBI3-deficient mice were impaired in their capacity to produce IFNγ. Phenotypically, Tregs from EBI3-deficient mice were highly suppressive and produced IL-10 in the tumor microenvironment. Depletion of Tregs or inactivation of the IL-10 pathway significantly abrogated tumor growth enhancement in Ebi3−/− mice. Finally, we showed that Ebi3−/− mice administered a melanoma vaccine failed to mount a CD8+ T-cell response and the vaccine failed to confer tumor rejection in EBI3-deficient mice. Taken together, these results suggest that Ebi3−/− mice show a phenotype of IL-27-deficiency rather than IL-35-deficiency during anti-tumor T-cell responses. Thus, our results suggest that endogenous IL-27 is critical for both spontaneous and vaccine-induced antitumor T-cell responses.
Introduction
Epstein-Barr virus-induced gene 3 (EBI3) encoded protein can form heterodimers with IL-27P28 Citation1 or IL-12P35 Citation2 to form 2 cytokines, namely interleukin (IL)-27 Citation1 and IL-35. Citation3,4 IL-27 is mainly produced by antigen-presenting cells (APCs) and signals through a heterodimeric receptor (IL-27R) consisting of the IL-27Rα (WSX-1) and the gp130 subunits. Citation5 IL-35 has been shown to be secreted by FoxP3+CD4+CD25+ regulatory T cells (Tregs) in miceCitation4 or “iTr35” cells, a regulatory T-cell population induced by IL-35.Citation6 IL-35 signals through a unique heterodimer of receptor chains IL-12Rβ2 and gp130 or the homodimers of each chain in target cells.Citation7
Functionally, IL-27 and IL-35 share some similar properties. IL-27 was first described as a cytokine promoting T helper (Th) type 1 (Th1) responses,Citation8 but later pieces of evidence suggested that IL-27 is a negative regulator of Th1, Th2 and Th17 responses.Citation9-11 IL-27 is also a potent inducer of IL-10 production by T cells.Citation12-14 Consequently, IL-27 has been shown to inhibit inflammation in a number of autoimmune disease models.Citation15 IL-35 has been shown to suppress T-cell proliferation,Citation3,4 Th17 and Th2 immune responses, and inhibit experimental arthritis,Citation3 airway inflammation Citation16 and diabetes mellitus in non-obese diabetic (NOD) mice.Citation17
IL-27 and IL-35 appear to have opposing roles in regulating CD4+CD25+Foxp3+ Treg responses. In vitro, IL-27 has been shown to inhibit the conversion of inducible T regulatory cells (iTreg) and the expression of Foxp3, CD25 and the immune checkpoint cytotoxic T lymphocyte associated protein 4 (CTLA4).Citation18,19 IL-27Rα-deficient (Wsx-1−/−) mice exhibit increased Treg conversion and expansion during autoimmune responses.Citation20 IL-27 transgenic mice are deficient in Treg cells and develop systemic inflammation at 8-11 weeks of age.Citation21 In contrast, IL-35 has been shown to expand Foxp3+ Tregs. Citation3 IL-35 itself has been shown to be required for Treg suppressive functions as IL-35-deficient (either Ebi3 or P35) Treg cells have been shown to exhibit reduced regulatory activity in vitro and fail to control homeostatic proliferation or cure inflammatory bowel disease in vivo.Citation4
IL-27 and IL-35 also play opposing roles in the induction of cytotoxic T lymphocyte (CTL) responses. In tumor models, IL-27 can promote CTL accumulation in tumors and inhibit tumor growth by a number of mechanismsCitation22 including promotion of CTL survivalCitation23 and effector functions.Citation24,25 By contrast, IL-35 inhibits antitumor CTL responses and promotes tumor growth.Citation26 We recently convincingly demonstrated the opposing roles of IL-27 and IL-35 in antitumor CTL responses using the mouse plasmacytoma J558 model. We found that J558 cells expressing IL-27 stimulate potent antitumor CTL responses and IL-27-expressing J558 tumors fail to grow in BALB/c miceCitation23 whereas J558 cells expressing IL-35 inhibit CTL responses and exhibit accelerated tumor growth in BALB/c mice.Citation26
Ebi3−/− mice are deficient for both IL-27 and IL-35; thus, the deficiency of EBI3 can lead to reduced or increased antitumor T-cell responses and tumor rejection, depending on the balance of the 2 groups of forces mediated by IL-27 and IL-35. Given the potential importance of these 2 cytokines in the regulation of tumor immunity, evaluation of antitumor T-cell responses in Ebi3−/− mice may offer a unique opportunity to reveal the relative importance of the 2 cytokines in tumor immunity. In this study, we evaluated antitumor T-cell responses in Ebi3−/− mice. We found that injection of B16 melanoma or J558 plasmacytoma cells into Ebi3−/− mice resulted in significantly increased tumor growth relative to those grown in EBI3-intact controls. Tumors from EBI3-deficient mice contained significantly decreased percentages of interferon γ (IFNγ) producing CD8+ T cells and increased percentages of CD4+FoxP3+ Tregs. Tregs from EBI3-deficient mice were highly suppressive and produced IL-10 in the tumor microenvironment, and displaying suppressive functions largely dependent upon IL-10. Finally, we showed that a melanoma vaccine failed to induce an effective CD8+ T-cell response and confer tumor rejection in Ebi3−/− mice. These results suggest that Ebi3−/− mice show a phenotype of IL-27-deficiency. Our results suggest that the endogenous IL-27 is critical for the generation of both spontaneous and vaccine-induced antitumor T-cell responses.
Results
EBI3-deficiency enhances tumor growth and impairs antitumor T-cell responses
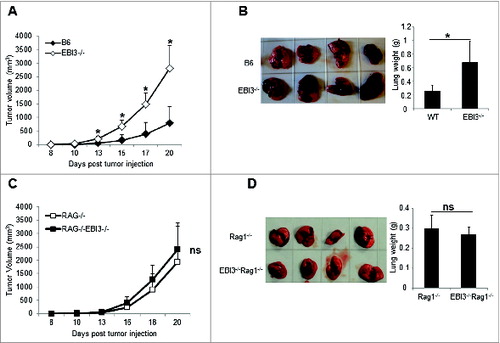
To determine the effect of EBI3-deficiency on tumorigenesis and tumor growth, we injected B16.F10 melanoma cells into Ebi3−/− and wild-type (WT) C57BL6 mice subcutaneously (s.c.). As shown in , tumors grew significantly faster in Ebi3−/− mice than in WT mice. When B16.F10 cells were given intravenously (i.v.) to Ebi3−/− and WT mice, we found that more melanoma colonies formed in the lungs of Ebi3−/− mice than in WT mice, which leads to increased lung weights in Ebi3−/− mice compared to WT mice (). To determine if adaptive immunity plays a role in determining tumor growth in Ebi3−/− mice, we s.c. or i.v. injected B16.F10 cells into Rag1−/− or Ebi3−/−Rag1−/− mice. Similar tumor growth kinetics () and lung tumor foci formation () were observed in these 2 distinct recipient mice. Thus, the tumor growth difference between Ebi3−/− and WT mice was caused by differential adaptive immunity.
Figure 1. Enhanced tumor growth and metastasis in EBI3-deficient C57BL/6 mice. (A) 1 × 105 B16.F10 melanoma cells were s.c. injected into either WT or Ebi3−/− C57BL/6 mice. The sizes of tumors were measured over time using calipers. The mean tumor volume is shown and bars indicate SD of 5 tumors in each group. Data shown represent 3 experiments with similar results. (B) 1 × 105 B16.F10 cells were i.v. injected into either WT or Ebi3−/− C57BL/6 mice. Twenty-one days later mice were sacrificed, tumor metastases in the lungs were examined. Average weight of the lungs from each group of mice was shown in the right panel. Bars indicate SD of lung weight of 4 mice in each group. Data shown represent 2 experiments with similar results. (C) 1 × 105 B16.F10 cells were s.c. injected into either Rag1−/− or Ebi3−/− Rag1−/− C57BL/6 mice. The sizes of tumors were measured over time. Bars indicate SD of 5 tumors in each group. Data shown represent 2 experiments with similar results. (D) 1 × 105 B16.F10 cells were i.v. injected into either Rag1−/− or Ebi3−/− Rag1−/− C57BL/6 mice. Twenty-one days later mice were sacrificed and tumor metastases in the lungs were evaluated. The mean weights of the lungs from each group of mice are shown in the right panel. Bars indicate SD of 4 lungs in each group.
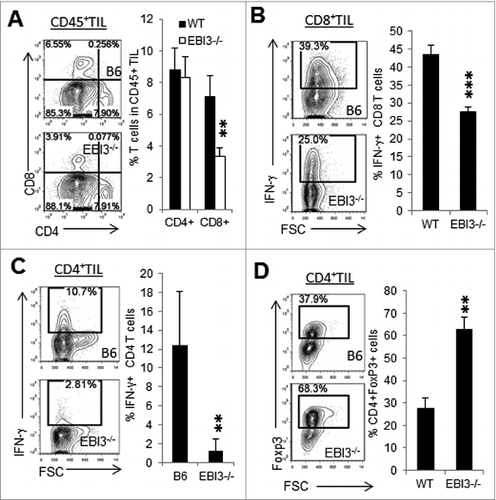
To determine whether T-cell responses differ between WT and Ebi3−/− mice, we used immunostaining and cytofluorimetric analysis to detect tumor-infiltrating CD4+ and CD8+ T cells. As shown in , more tumor-infiltrating CD8+ T cells were detected in tumors from WT mice than in tumors from Ebi3−/− mice. The proportion of infiltrating CD4+ T cells, on the other hand, were similar in tumors from Ebi3−/− mice as compared to tumors from WT mice. Tumor-infiltrating CD8+ () and CD4+ T cells () produced less IFNγ in tumors from Ebi3−/− mice than in tumors from WT mice. However, higher percentages of Foxp3+CD4+ Treg cells were detected in tumors from Ebi3−/− mice ().
Figure 2. EBI3-deficiency impairs antitumor T-cell responses. Immunostaining and cytofluorimetric analysis of tumor-infiltrating T cells from melanoma tumors grown in Ebi3−/− and wild-type (WT) mice. (A) Flow cytometry analysis of CD4+ and CD8+ T cells in the B16.F10 tumors from WT and Ebi3−/− mice. Data gated on CD45+ tumor infiltrating lymphocytes (TILs). (B) Flow cytometry analysis of IFNγ+ CD8+ T cells in the tumors from WT and Ebi3−/− mice. Data gated on CD8+ TILs. (C) Flow cytometry analysis of IFNγ+ CD4+ T cells in the tumors from WT and EBI3−/− mice. Data gated on CD4+ TILs. (D) Flow cytometry analysis of FoxP3+ CD4+ T cells in the tumors from WT and Ebi3−/− mice. Data gated on CD4+ TILs. Statistical analysis was performed by Student's t test; **p < 0.01; ***p < 0.001. Bars indicate SD from 5 mice per group. Data shown represents 3 experiments with similar results.
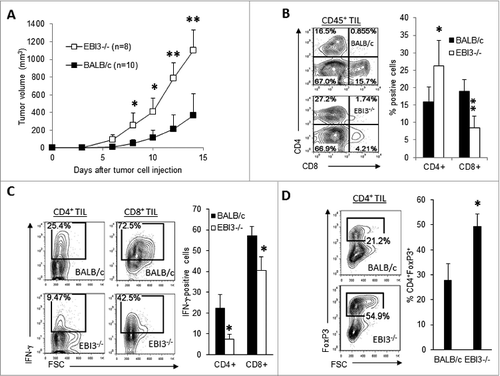
To determine if these findings can be validated in another tumor model, EBI3-deficient BALB/c mice and control BALB/c mice were s.c. injected with J558 plasmacytoma cells.As shown in , significantly increased tumor growth was observed in Ebi3−/− BALB/c mice. J558 tumors from Ebi3−/− mice contained relatively fewer CD8+ T cells and relatively higher percentages of CD4+ T cells (). The infiltrating CD8+ and CD4+ T cells in tumors from EBI3-deficient mice were also less capable of producing IFNγ (). However, a larger population of Foxp3+CD4+ Tregs was detected in tumors from EBI3-deficient mice than in tumors from BALB/c mice (). Thus, EBI3-deficiency impairs antitumor T-cell responses and enhances tumor-specific Treg responses in both tumor models tested.
Figure 3. Enhanced tumor growth and impaired T cell responses in EBI3-deficient BALB/c mice. (A) 5 × 106 J558 plasmacytoma cells were s.c. injected into each wild-type (WT) or Ebi3−/− BALB/c mice. The sizes of tumors were measured over time using calipers. (B–D) Immunostaining and cytofluorimetric analysis of infiltrating cells in tumors isolated from mice shown in A. Quantitative analysis of total CD4+ and CD8+ lymphocytes (B) IFNγ positive CD4+ and CD8+ T cells (C) and FoxP3+CD4+ T cells (D) in tumors from WT and Ebi3−/− BALB/c mice. Statistical analysis was performed by Student's t test; *p < 0.05; **p < 0.01. Bars indicate SD of 5 mice in each group. Data shown represent 2 experiments with similar results.
Increased Treg response in Ebi3−/− mice inhibits antitumor T-cell responses and enhances tumor growth
To further delineate the impacts of increased Treg responses on tumor immunity in EBI3-deficient mice, we depleted Tregs in Ebi3−/− and C57BL/6 mice using anti-CD25 antibody. B16.F10 cells were s.c. injected into anti-CD25-treated Ebi3−/− and WT C56BL/6 mice. As shown in , anti-CD25 treatment more significantly impacted melanoma tumor growth in Ebi3−/− mice than in WT mice, and Treg-depletion resulted in increased numbers of IFNγ-producing CD8+ T cells in both Ebi3−/− and WT tumor-bearing mice.
Figure 4. Tregs from Ebi3−/− mice are highly immunosuppressive. (A) 1 × 105 B16.F10 cells were subcutaneously (s.c.) injected into each C57BL/6 and Ebi3−/− C57BL/6 mouse and anti-CD25 or control IgG antibodies (400 μg/mouse) were intraperitoneally (i.p.) administrated to each mouse every 4 days for a total of 3 times. Twenty-one days later mice were sacrificed, and subcutaneously grown tumors were isolated and weighed. Average weights of the tumors from each group of mice (n = 5) are shown (left panel). Intracellular interferon γ (IFNγ) staining and cytofluorimetric analysis were performed on tumor-infiltrating lymphocytes and quantified (right panel). Statistical analysis was performed by Student's t test; ***p<0.001; *p<0.05. Data shown are representative of 2 experiments with similar results. (B) 1 × 105 B16.F10 cells were intravenously (i.v.) injected into each EBI3−/− C57BL/6 mouse and anti-CD25 or control IgG antibodies (400 μg/mouse) were administrated i.p. to each mouse every 4 days for a total of 3 times. Twenty-one days later mice were sacrificed, and tumor foci in the lungs were evaluated. The average weight of the lungs from each group of mice are shown in the right panel. Statistical analysis was performed by Student's t test; **p<0.01.(C) 1 × 105 B16.F10 cells were i.v. injected into Ebi3−/− Rag1−/− C57BL/6 mice. On the same day, mice either received 5 × 106 CD25- T cells (from Ebi3−/− mice) alone, or 5 × 106 CD25- T cells plus 0.5 × 106 Tregs from WT mice, or 5 × 106 CD25- T cells plus 0.5 × 106 Treg cells from Ebi3−/− mice. Twenty-one days later mice were sacrificed, and tumor growth in the lungs were evaluated. Average weights of lungs from each group of mice are shown in the right panel. Statistical analysis was performed by Student's t test; *p < 0.05; **p < 0.01.
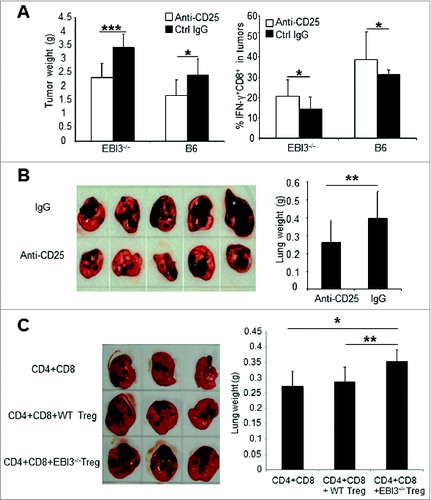
In the B16.F10 i.v. injection model, depletion of Tregs particularly in Ebi3−/− mice also significantly reduced melanoma foci in the lungs (). To test if Tregs from Ebi3−/− mice had comparative suppressive functions with Tregs from WT mice, we i.v. injected B16.F10 cells into Ebi3−/− Rag1−/− mice. Meanwhile, these mice either received EBI3-deficient CD25- T cells alone or EBI3-deficient CD25- T cells plus Tregs from either WT mice or from Ebi3−/− mice. By day 21, mice were sacrificed and lung tumor foci formation was examined. As demonstrated in , mice receiving Tregs from Ebi3−/− mice had the highest numbers of melanoma foci in the lungs and heavier lungs, whereas mice receiving WT Tregs had similar numbers of melanoma foci and lung weights as mice receiving no Tregs. Thus, Tregs from Ebi3−/− mice are more suppressive to antitumor T cell responses as compared to WT Tregs that enhance tumor growth.
IL-10 pathway is largely responsible for increased Treg function and enhanced tumor growth in Ebi3−/− mice
IL-10 is a known cytokine that is produced by Treg cellsCitation27 and exert inhibitory effects to T cell priming.Citation28 By comparing IL-10 production among various tumor-infiltrating leukocytes, we found that tumor-infiltrating Tregs were the major source of IL-10 in tumors, and tumor-infiltrating Tregs from Ebi3−/− mice produced relatively more IL-10 (). To determine if elevated IL-10 production by Treg cells in Ebi3−/− mice drives increased Treg suppressive functions, we generated Ebi3−/− IL-10−/− mice by breeding Ebi3−/− mice with IL-10−/− mice, and we purified CD4+CD25+ Tregsfrom WT, Ebi3−/− and Ebi3−/− IL-10−/− mice, and co-cultured them with CD25- T cells from EBI3-deficient mice. We consistently found that EBI3-deficient Tregs are more suppressive than EBI3-sufficient Tregs, and IL-10-deficiency largely abrogated the suppressive functions of Tregs from Ebi3−/− mice (). To test if enhanced tumor growth in Ebi3−/− mice was also affected by IL-10-deficiency, B16.F10 tumor cells were i.c. or i.v. injected into WT, Ebi3−/− and Ebi3−/− IL-10−/− mice. We found that the IL-10-deficiency in Ebi3−/− mice significantly inhibited subcutaneous tumor growth () and attenuated the increased lung metastases seen in Ebi3−/− mice (). Thus, IL-10 is responsible for the observed increased Treg suppression and enhanced tumor growth in Ebi3−/− mice.
Figure 5. IL-10 pathway is largely responsible for increased Treg suppression and enhanced tumor growth in Ebi3−/− mice. (A) Increased IL-10 production by tumor-infiltrating Tregs from EBI3-deficient mice. Flow cytometry was used to quantify IL-10 producing CD4+ TILs. Statistical analysis was performed by Student's t test; *p <0.05. Bars indicate SD of 5 mice in each group. Data shown represent 2 experiments with similar results. (B) Treg suppression assay. CD4+CD25+ Treg cells were purified from spleens of WT, Ebi3−/− and Ebi3−/− IL-10−/− mice by magnetic bead-based sorting (MACS). Purified Tregs were then mixed with responder cells (CD4+CD25− T cells sorted from Ebi3−/− IL-10−/− mice) at different ratios in U-bottomed 96-well plates. 1 × 106 /mL of irradiated Ebi3−/− Rag1−/− splenocytes pulsed with anti-CD3 (2C11, 0.1μg/mL) were used as antigen presenting cells. Cells were co-cultured for 36 h, and 1 μCi [3H]-Thymidine was added into each well. Twelve hours later, cells were harvested, and [3H]-Thymidine incorporation was measured in a scintillation counter. Percent of suppression was calculated using the formula: (no Treg counts − actual counts)/no Treg counts. Statistical analysis was performed by Student's t test; *p < 0.05. Data shown represent 3 experiments with similar results. (C) 1 × 105 B16.F10 cells were injected into each Ebi3−/− and Ebi3−/− IL-10−/− C57BL/6 mouse s.c. Tumor growth was observed over time. Statistical analysis was performed by Student's t test; *p < 0.05.. Bars indicate SD of 5 mice in each group. Data shown represent 2 experiments with similar results. (D) 1 × 105 B16.F10 cells were i.v. injected into each WT, Ebi3−/− and Ebi3−/− IL-10−/− C57BL/6 mice . Twenty-one days later mice were sacrificed, and tumor metastases in the lungs were evaluated. Average weight of lungs from each group of mice are shown in the right panel. Bars indicate SD of lungs from 4 mice in each group. Statistical analysis was performed by Student's t test; *p < 0.05.
![Figure 5. IL-10 pathway is largely responsible for increased Treg suppression and enhanced tumor growth in Ebi3−/− mice. (A) Increased IL-10 production by tumor-infiltrating Tregs from EBI3-deficient mice. Flow cytometry was used to quantify IL-10 producing CD4+ TILs. Statistical analysis was performed by Student's t test; *p <0.05. Bars indicate SD of 5 mice in each group. Data shown represent 2 experiments with similar results. (B) Treg suppression assay. CD4+CD25+ Treg cells were purified from spleens of WT, Ebi3−/− and Ebi3−/− IL-10−/− mice by magnetic bead-based sorting (MACS). Purified Tregs were then mixed with responder cells (CD4+CD25− T cells sorted from Ebi3−/− IL-10−/− mice) at different ratios in U-bottomed 96-well plates. 1 × 106 /mL of irradiated Ebi3−/− Rag1−/− splenocytes pulsed with anti-CD3 (2C11, 0.1μg/mL) were used as antigen presenting cells. Cells were co-cultured for 36 h, and 1 μCi [3H]-Thymidine was added into each well. Twelve hours later, cells were harvested, and [3H]-Thymidine incorporation was measured in a scintillation counter. Percent of suppression was calculated using the formula: (no Treg counts − actual counts)/no Treg counts. Statistical analysis was performed by Student's t test; *p < 0.05. Data shown represent 3 experiments with similar results. (C) 1 × 105 B16.F10 cells were injected into each Ebi3−/− and Ebi3−/− IL-10−/− C57BL/6 mouse s.c. Tumor growth was observed over time. Statistical analysis was performed by Student's t test; *p < 0.05.. Bars indicate SD of 5 mice in each group. Data shown represent 2 experiments with similar results. (D) 1 × 105 B16.F10 cells were i.v. injected into each WT, Ebi3−/− and Ebi3−/− IL-10−/− C57BL/6 mice . Twenty-one days later mice were sacrificed, and tumor metastases in the lungs were evaluated. Average weight of lungs from each group of mice are shown in the right panel. Bars indicate SD of lungs from 4 mice in each group. Statistical analysis was performed by Student's t test; *p < 0.05.](/cms/asset/a2756a9c-83f3-4934-b032-8c4024c8fc59/koni_a_989137_f0005_c.jpg)
EBI3-deficiency impairs the efficacy of tumor antigen vaccination
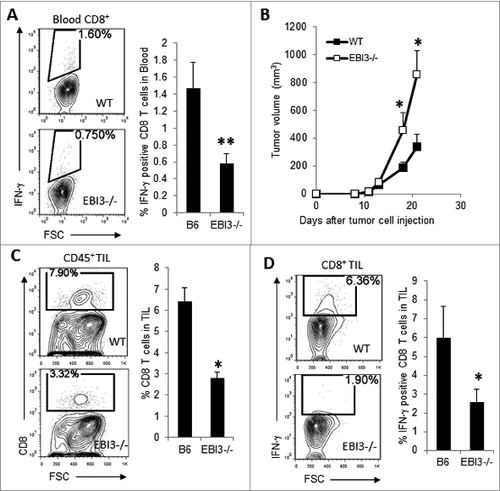
A lentiviral vector expressing a mutated tyrosinase related protein 1 (TRP1-lv) has been shown to elicit potent CD8+ T-cell responses against multiple TRP1 epitopes.Citation29 Importantly, the activated CD8+ T cells effectively recognize wild-type TRP1 antigen and protect against B16 melanoma challenge.Citation29 To determine if EBI3-deficient mice are also defective in induced antitumor T-cell responses, Ebi3−/− C57BL6 and WT mice were immunized with 2.5 × 107 TU of TRP1-lv. On day 14 after TRP1-Lv immunization, we detected higher numbers of TRP1-lv-specific CD8+ T cells in the blood of WT mice (). Upon challenge with B16.F10 melanoma cells, TRP1-lv -immunized WT mice showed markedly reduced tumor growth relative to that occurring in Ebi3−/− mice (). Consistent with the tumor growth curve, tumors from TRP1-lv -immunized EBI3−/− mice contained fewer tumor-infiltrating CD8+ T cells as compared to WT mice (). Tumors from TRP1-lv-vaccinated EBI3-deficient mice also contained fewer peptide-specific CD8+IFNγ+ T cells as compared to tumors from WT mice (). Thus, EBI3-deficient mice are highly resistant to TRP1-lv vaccination-induced antitumor T-cell responses.
Figure 6. Ebi3−/− mice are resistant to vaccination with a melanoma vaccine. (A) 2.5 × 107 TU of TRP1-Lv was injected into the footpads of each WT or Ebi3−/− mouse. Two weeks after TRP1-Lv injection, mice were bled, and peripheral blood cells were stimulated with 1 μg/mL TRP1-455 peptide for 3 h, and peptide-specific IFNγ producing CD8+ T cells were quantified by cytofluorimetric analysis. (B) Two weeks after TRP1-Lv immunization, 1 × 105 B16 cells were s.c. injected into each mouse. Tumor growth was monitored over time using calipers. (C) Twenty-one days after tumor cell injection, mice were sacrificed, and the numbers of CD8+ T cells among CD45+ tumor infiltrating lymphocytes (TILs) were quantified by flow cytometry. (D) Disassociated cells from tumors were stimulated with peptide TRP1-455 for 4 h and stained for CD8 and IFNγ, followed by flow cytometry analysis. Peptide-specific, IFNγ-producing CD8+ T cells were quantified. Statistical analysis was performed by Student's t test; *p < 0.05; *p < 0.001. Bars indicate 4 mice per group. Data represent 2 experiments with similar results.
Discussion
In this study, we investigated tumor-specific T-cell responses in 2 strains of Ebi3−/− mice, namelyC57BL/6 and BALB/c, using the B16 melanoma and the J558 plasmacytoma tumor models. We made 2 surprising discoveries that are of importance for understanding the regulation of tumor-specific T-cell responses. First, EBI3-deficient mice generate extremely poor spontaneous and induced antitumor T-cell responses and exhibit accelerated tumor growth. Second, Tregs from Ebi3−/− mice are even more suppressive than their WT counterparts and these suppressive function are dependent on the IL-10 pathway.
A notable observation of this study is that EBI3-deficiency promotes the growth of B16.F10 melanoma and J558 plasmacytoma tumors. The growth difference of melanoma tumors between WT and Ebi3−/− mice is clearly caused by the lack of an adaptive immune response in Ebi3−/− mice, since B16-melanoma tumors grow at an equal pace in Rag1−/− Ebi3−/− and Rag1−/− mice. In both B16 melanoma and J558 plasmacytoma tumors from Ebi3−/− mice, the numbers of CD8+ T cells are significantly reduced. More importantly, tumor-infiltrating T cells in Ebi3−/− mice show reduced production of IFNγ. In contrast, the numbers of CD4+ T cells in EBI3-deficient tumors were either not significantly reduced (in B16 tumors) or increased (J558 tumors), with higher percentages of CD4+ T cells being Foxp3+ Tregs. Thus, EBI3-deficient mice generate extremely poor spontaneous antitumor cytotoxic T lymphocyte (CTL) response with increased Treg responses concomitant with accelerated tumor formation and growth. Consistent with this observation, we also found that a melanoma vaccine (TRP1-lv) that could induce anti-melanoma CTL response in WT mice failed to induce CTL response in EBI3-deficient mice. Thus, EBI3-deficient mice are highly refractory to the induction of antitumor CTL responses. This observation is surprising, since it differs from the previous report Citation30 that shows B16 melanomas grow relatively slower and stimulate stronger T-cell responses in EBI3-deficient mice. However, our results are consistent with other studies showing that mice deficient for IL-27RαCitation31,32 or IL-27P28Citation33 generate deficient antitumor T-cell responses and exhibit accelerated tumor growth.
We have previously reported that Ebi3−/− mice exhibit increased Treg responses that downregulate autoimmune inflammation in the central nervous system.Citation34 In this study, we found that tumors from Ebi3−/− mice possess higher numbers of Tregs as compared with their WT counterparts. Decreased CTL responses and increased Treg responses in Ebi3−/− mice are reminiscent of IL-27-, but not IL-35-deficiency, since IL-35 has been shown to expand Tregs.Citation3 A few lines of evidence support this notion. First, IL-27Rα-deficient mice have been shown to exhibit increased Treg conversion/expansionCitation20 and display increased tumor growth with reduced antitumor T-cell responses.Citation31,35 Second, experiments in vitro have revealed that IL-27 inhibits the conversion of inducible T regulatory cells and the expression of Foxp3, CD25 and CTLA4.Citation18,19 Third, IL-27 transgenic mice are deficient in Tregs and develop systemic inflammation at 8-11 weeks of age.Citation21 Fourth, while expression of IL-27 in B16 melanoma cells inhibited tumor growth,Citation36,37 we have recently shown that expression of IL-35 in B16 melanoma cells leads to tumor growth enhancement and reduced tumor T-cell infiltration, with Treg responses unaffected.Citation26 Thus, the tumor enhancement and Treg expansion observed in Ebi3−/− mice can be explained by IL-27, but not IL-35-deficiency. In addition to indirectly regulating Treg homeostasis, recent studies have also shown that IL-27 signaling is required for Treg cell survival,Citation38 and can program Tregs into a unique T-bet+CXCR3+ phenotype, specialized for regulating Th1 responses.Citation39 However, the relevance of these observations in cancer immunity remains untested.
EBI3 and IL-12p35 form a novel cytokine IL-35, which has been shown to contribute to Treg suppressive functions,Citation4 and EBI3-deficient Tregs have been shown to be unable to suppress autoimmune T-cell responses.Citation4 However, in this study we found that Ebi3−/− Tregs not only suppress antitumor T-cell responses, but also exhibit stronger immunosuppressive functions as compared to their WT counterparts. This conclusion is supported by the following 2 lines of evidences. First, depletion of Tregs in Ebi3−/− mice significantly inhibited tumor growth and lung metastasis in Ebi3−/− mice. Second, co-adoptive transfer of Ebi3−/− Tregs with T cells into Rag1−/−Ebi3−/− mice significantly enhanced melanoma lung tumor formation compared with mice receiving WT Tregs and mice receiving no Tregs. The IL-10 pathway has long been implicated in the Treg-mediated suppression of autoimmunity and tumor immunity.Citation40-43 Conditional deletion of IL-10 in Foxp3+ Tregs has revealed that Treg-derived IL-10 is particularly relevant to the suppression of inflammation in the environmental interfaces including the gut, lung and skin.Citation27 Since IL-10-deficiency abrogated Treg suppressive activity and lung melanoma enhancement in Ebi3−/− mice, these results suggest that Treg cells can suppress antitumor T-cell responses in the absence of IL-27 and IL-35 via the IL-10 pathway.
The potent antitumor activity of IL-27 has been well established in a variety of tumor models.Citation22,44 Several mechanisms to account for this phenomenon have been proposed, including the induction of antitumor T-cell responses.Citation45-49 Recently, we have shown that IL-27-stimulated CD8+ T cells up-regulate survival molecules and exhibit a survival advantage,Citation23 which could be a mechanism by which IL-27 enhances antitumor CTL responses. Although our findings have revealed a predominant role for IL-27 in limiting Treg responses in this study, depletion of Treg cells in Ebi3−/− mice did not lead to complete tumor protection, suggesting that lack of direct stimulation of CD8+ T cells by IL-27 could also play a role in the accelerated tumor growth observed in Ebi3−/− mice.
Taken together, we have found that Ebi3−/− mice exhibit accelerated tumor growth and show diminished antitumor T-cell responses. Tregs from Ebi3−/− mice show increased, rather than decreased immunosuppressive activity. These results also indicate that Treg cells suppress antitumor T-cell responses using the IL-10 pathway in the absence of IL-27 and IL-35. Thus, this study has revealed a significant mechanism by which IL-27 mediates its antitumor activity, i.e., by limiting Treg responses. Our results also reveal the importance of endogenous IL-27 for vaccine-induced antitumor T-cell responses.
Materials and Methods
Mice
C57BL/6, IL-10−/−C57BL/6, Rag1−/−C57BL/6 and Ebi3−/− BALB/c mice were purchased from The Jackson Laboratory. Ebi3−/− C57BL6 and Ebi3−/− Rag1−/− mice have previously been described.Citation34 Ebi3−/− IL-10−/− mice were generated through breeding Ebi3−/− with IL-10−/− mice for 2 generations. PCR was used for the identification of mice genotypes. The primers used were: EBI3 (forward) 5′-CTG ATG GGT CAC TAA CTC GGA TCC-3′ and EBI3 (reverse) 5′-ACG ACA TCA GGG TCT GAT ATC AAG-3′; and IL-10 (forward) 5′-ATA GAC TTG CTC TTG CAC TAC CAA AG-3′ and IL-10 (reverse) 5′-CTC ATG GCT TTC CCT AGG ACT CTC TA-3’. All mice were maintained in the animal facilities of The Ohio State University. The animal facilities are fully accredited by American Association for Accreditation of Laboratory Animal Care.
Cancer cell lines and tumor establishment in mice
B16.F10 melanoma cells and mouse plasmacytoma J558 cells have been described previously. Citation50-52 The tumor cells were maintained in RPMI 1640 medium (Gibco) supplemented with 100 μg/ml penicillin, 100 μg/mL streptomycin, and 5% FBS.
To establish tumors in mice, 1 × 105 B16.F10 cells or 5 × 106 J558 cells were injected subcutaneously (s.c.) into recipient mice at the flank in 200 μL of PBS. The length (a) and width (b) of tumors were measured using a digital caliper every 2 or 3 days. The tumor volume was calculated according to the formula V = ab2/2 as described.Citation50 To establish melanoma lung metastasis, each mouse was injected with 1 × 105 B16.F10 cells via the tail vein. Mice were monitored for up to 3-4 weeks. At the end of the experiments, mice were sacrificed, and their lungs were weighed and examined for tumor metastasis, as previously described.Citation50
Antibodies and flow cytometry
FITC-, PE-, APC- or Percp- labeled antibodies to CD4, CD8α, CD45, FoxP3, IFNγ and isotype-matched control antibodies were purchased from BD Biosciences (San Diego, CA) or eBiosciences (San Diego, CA). For staining of cell surface markers, disassociated cells from tumors were stained with various antibodies in staining buffer (PBS with 1% FCS) and incubated on ice for 30 min. After washing with staining buffer, cells were fixed in 1% Paraformaldehyde in PBS. For intracellular cytokine staining, cells were stimulated in culture medium for 4 h with 100 ng/mL of phorbol 12-myristate 13-acetate (PMA) and 500 ng/mL of ionomycin in the presence of Golgistop (1:1500; BD Biosciences). Viable cells were then fixed in IC fixation buffer (eBioscience), permeabilized with 1 × permeabilization buffer (eBiosciece) and stained with respective antibodies. Staining for Foxp3 was performed according to manufacturer's protocol (BD Biosciences). Stained cells were analyzed on an FACSCalibur flow cytometer, and data were analyzed using the Flowjo software (Tree Star, Inc., OR).
Isolation of CD4+CD25+ cells from spleens
Mononuclear cells were prepared from spleens, and CD4+ T cells were isolated by negative selection. Briefly, splenocytes were first incubated with anti-CD8 (TIB210) and anti-Fc receptor (2.4G2) antibodies on ice for 30 min, followed by incubation with sheep anti-Rat IgG Dynabeads (Invitrogen). CD25+ cells were isolated from CD4+ cells by first staining them with PE-anti-CD25 mAb (BD Biosciences), followed by magnetic antibody cell separation using anti-PE microbeads (Miltenyi Biotec). The isolated cells were of >90% purity.
T cell adoptive transfer
Purified CD25‑ CD4+ and CD8+ T cells from Ebi3−/− mice were mixed with CD4+CD25+ Tregspurified from WT or EBI3−/− mice, and were i.v. injected into Ebi3−/− Rag1−/− mice followed by i.v. inoculation of B16.F10 cells.
CD25+ T cell depletion
To deplete CD25+ Tregsin vivo, mice received i.p. injection of 400 μg rat anti-mouse CD25 IgG (PC-61.5.3, BioXcell) diluted in 200 μL PBS. Control animals received i.p. injection of 400 μg IgG1 isotype control mAb (anti-HRPN, BioXcell). Injection of antibody was repeated every 4 days for a total of 3 times to maintain the depletion.
Treg-mediated suppression assay
1 × 106/mL purified CD4+CD25- T cells from Ebi3−/− mice were cocultured with graded numbers of CD4+CD25+ Treg cells from WT, Ebi3−/− or Ebi3−/− IL-10−/− mice in the presence of irradiated splenocytes (2 × 106 /mL) from Ebi3−/− Rag1−/− mice and 0.1 μg/mL anti-CD3 mAb (2C11). After 48 h, 1 μCi/well [3H]-Thymidine was pulsed into the cultures, and incorporation of [3H]-Thymidine was measured in a liquid scintillation plate counter 12 h later.
Mice vaccination, tumor cell challenge and CTL response to the vaccine
A lentivector expressing a mutated tyrosinase-related protein 1 (TRP1-lv) Citation29 was injected into the WT and Ebi3−/− mice subcutaneously at a dose of 2.5 × 107 TU (transduction unit)/mouse. Two weeks after immunization, 1 × 105 B16 cells were injected at the right flank of each mouse. At two weeks after vaccination, mice were also bled, and blood cells were incubated with TRP1 peptide (wtTRP1-455) for 3 h in the presence of Golgistop, followed by staining for CD8/IFN-γ and flow cytometry analysis, as previously described. Citation29 At the end of experiments, mice with tumors were sacrificed, and disassociated tumor cells were incubated with wtTRP1-455 peptide for 4 h in the presence of Golgistop, followed by staining for CD8/IFNγ and flow cytometry analysis.
Statistical Analysis
Data are expressed as mean ± SD. Two-tailed Student's t test or paired t test was used for statistical analysis. p < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
The authors declare no financial or commercial conflict of interest.
Acknowledgment
This study is supported by grants from the National Cancer Institute (R01CA138427 to XFB) and American Cancer Society (RSG-09-188-01-LIB to XFB).
References
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 2002; 16:779-90; PMID:12121660; http://dx.doi.org/10.1016/S1074-7613(02)00324-2
- Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A 1997; 94:12041-6; PMID:9342359; http://dx.doi.org/10.1073/pnas.94.22.12041
- Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 2007; 37:3021-9; PMID:17874423; http://dx.doi.org/10.1002/eji.200737810
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007; 450:566-9; PMID:18033300; http://dx.doi.org/10.1038/nature06306
- Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol 2004; 172:2225-31; PMID:14764690; http://dx.doi.org/10.4049/jimmunol.172.4.2225
- Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 2010; 11:1093-101; PMID:20953201; http://dx.doi.org/10.1038/ni.1952
- Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol 2012; 13:290-9; PMID:22306691; http://dx.doi.org/10.1038/ni.2227
- Tamada K, Shimozaki K, Chapoval AI, Zhu G, Sica G, Flies D, Boone T, Hsu H, Fu YX, Nagata S, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med 2000; 6:283-9; PMID:10700230; http://dx.doi.org/10.1038/73136
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 2006; 7:929-36; PMID:16906167; http://dx.doi.org/10.1038/ni1375
- Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med 2007; 13:711-8; PMID:17496900; http://dx.doi.org/10.1038/nm1585
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol 2007; 25:221-42; PMID:17291186; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104758
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 2007; 8:1363-71; PMID:17994025; http://dx.doi.org/10.1038/ni1537
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 2007; 8:1380-9; PMID:17994022; http://dx.doi.org/10.1038/ni1541
- Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol 2007; 179:3268-75; PMID:17709543; http://dx.doi.org/10.4049/jimmunol.179.5.3268
- Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity 2012; 37:960-9; PMID:23244718; http://dx.doi.org/10.1016/j.immuni.2012.11.003
- Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL, Lee BW, Chua KY. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. J Immunol 2011; 187:462-71; PMID:21613618; http://dx.doi.org/10.4049/jimmunol.1100259
- Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35. Diabetes 2012; 61:1519-26; PMID:22427377; http://dx.doi.org/10.2337/db11-0784
- Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol 2007; 37:1809-16; PMID:17549733; http://dx.doi.org/10.1002/eji.200636896
- Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol 2008; 20:223-34; PMID:18156621; http://dx.doi.org/10.1093/intimm/dxm139
- Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med 2011; 208:115-23; PMID:21173106; http://dx.doi.org/10.1084/jem.20100410
- Wojno ED, Hosken N, Stumhofer JS, O'Hara AC, Mauldin E, Fang Q, Turka LA, Levin SD, Hunter CA. A role for IL-27 in limiting T regulatory cell populations. J Immunol 2011; 187:266-73; PMID:21622862; http://dx.doi.org/10.4049/jimmunol.1004182
- Murugaiyan G, Saha B. IL-27 in tumor immunity and immunotherapy. Trends Mol Med 2013; 19:108-16; PMID:23306374; http://dx.doi.org/10.1016/j.molmed.2012.12.002
- Liu Z, Liu JQ, Talebian F, Wu LC, Li S, Bai XF. IL-27 enhances the survival of tumor antigen-specific CD8(+) T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol 2013; 43:468-79; PMID:23225163; http://dx.doi.org/10.1002/eji.201242930
- Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol 2005; 175:1686-93; PMID:16034109; http://dx.doi.org/10.4049/jimmunol.175.3.1686
- Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol 2011; 41:47-59; PMID:21182076; http://dx.doi.org/10.1002/eji.201040804
- Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J, Basu S, Feng Y, Bai XF. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol 2013; 190:2415-23; PMID:23345334; http://dx.doi.org/10.4049/jimmunol.1202535
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008; 28:546-58; PMID:18387831; http://dx.doi.org/10.1016/j.immuni.2008.02.017
- Wang LX, Talebian F, Liu JQ, Khattabi M, Yu L, Bai XF. IL-10 contributes to the suppressive function of tumour-associated myeloid cells and enhances myeloid cell accumulation in tumours. Scand J Immunol 2012; 75:273-81; PMID:22050574; http://dx.doi.org/10.1111/j.1365-3083.2011.02662.x
- Liu Y, Peng Y, Mi M, Guevara-Patino J, Munn DH, Fu N, He Y. Lentivector immunization stimulates potent CD8 T cell responses against melanoma self-antigen tyrosinase-related protein 1 and generates antitumor immunity in mice. J Immunol 2009; 182:5960-9; PMID:19414747; http://dx.doi.org/10.4049/jimmunol.0900008
- Sauer KA, Maxeiner JH, Karwot R, Scholtes P, Lehr HA, Birkenbach M, Blumberg RS, Finotto S. Immunosurveillance of lung melanoma metastasis in EBI-3-deficient mice mediated by CD8+ T cells. J Immunol 2008; 181:6148-57; PMID:18941205; http://dx.doi.org/10.4049/jimmunol.181.9.6148
- Shinozaki Y, Wang S, Miyazaki Y, Miyazaki K, Yamada H, Yoshikai Y, Hara H, Yoshida H. Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. Int J Cancer 2009; 124:1372-8; PMID:19089917; http://dx.doi.org/10.1002/ijc.24107
- Natividad KD, Junankar SR, Mohd Redzwan N, Nair R, Wirasinha RC, King C, Brink R, Swarbrick A, Batten M. Interleukin-27 signaling promotes immunity against endogenously arising murine tumors. PLOS ONE 2013; 8:e57469; PMID:23554861; http://dx.doi.org/10.1371/journal.pone.0057469
- Wei J, Xia S, Sun H, Zhang S, Wang J, Zhao H, Wu X, Chen X, Hao J, Zhou X, et al. Critical role of dendritic cell-derived IL-27 in antitumor immunity through regulating the recruitment and activation of NK and NKT cells. J Immunol 2013; 191:500-8; PMID:23733881; http://dx.doi.org/10.4049/jimmunol.1300328
- Liu JQ, Liu Z, Zhang X, Shi Y, Talebian F, Carl JW, Jr., Yu C, Shi FD, Whitacre CC, Trgovcich J, et al. Increased Th17 and regulatory T cell responses in EBV-induced gene 3-deficient mice lead to marginally enhanced development of autoimmune encephalomyelitis. J Immunol 2012; 188:3099-106; PMID:22387555; http://dx.doi.org/10.4049/jimmunol.1100106
- Morishima N, Mizoguchi I, Okumura M, Chiba Y, Xu M, Shimizu M, Matsui M, Mizuguchi J, Yoshimoto T. A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J Biomed Biotechnol 2010; 2010:605483; PMID:20454646; http://dx.doi.org/10.1155/2010/605483
- Shimizu M, Shimamura M, Owaki T, Asakawa M, Fujita K, Kudo M, Iwakura Y, Takeda Y, Luster AD, Mizuguchi J, et al. Antiangiogenic and antitumor activities of IL-27. J Immunol 2006; 176:7317-24; PMID:16751375; http://dx.doi.org/10.4049/jimmunol.176.12.7317
- Oniki S, Nagai H, Horikawa T, Furukawa J, Belladonna ML, Yoshimoto T, Hara I, Nishigori C. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res 2006; 66:6395-404; PMID:16778218; http://dx.doi.org/10.1158/0008-5472.CAN-05-4087
- Kim G, Shinnakasu R, Saris CJ, Cheroutre H, Kronenberg M. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J Immunol 2013; 190:1510-8; PMID:23335749; http://dx.doi.org/10.4049/jimmunol.1201017
- Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 2012; 37:511-23; PMID:22981537; http://dx.doi.org/10.1016/j.immuni.2012.06.014
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999; 190:995-1004; PMID:10510089; http://dx.doi.org/10.1084/jem.190.7.995
- Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J Immunol 2003; 171:971-8; PMID:12847269; http://dx.doi.org/10.4049/jimmunol.171.2.971
- Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol 2006; 177:5852-60; PMID:17056509; http://dx.doi.org/10.4049/jimmunol.177.9.5852
- Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res 2003; 63:6042-50; PMID:14522933
- Liu Z, Yu J, Carson WE, 3rd, Bai XF. The role of IL-27 in the induction of anti-tumor cytotoxic T lymphocyte response. Am J Transl Res 2013; 5:470-80; PMID:23977407
- Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, Shafer-Weaver K, Malyguine A, Kastelein R, Wigginton JM. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol 2004; 173:7170-82; PMID:15585838; http://dx.doi.org/10.4049/jimmunol.173.12.7170
- Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Res 2004; 64:1152-6; PMID:14871851; http://dx.doi.org/10.1158/0008-5472.CAN-03-2084
- Chiyo M, Shimozato O, Iizasa T, Fujisawa T, Tagawa M. Antitumor effects produced by transduction of dendritic cells-derived heterodimeric cytokine genes in murine colon carcinoma cells. Anticancer Res 2004; 24:3763-7; PMID:15736409
- Salcedo R, Hixon JA, Stauffer JK, Jalah R, Brooks AD, Khan T, Dai RM, Scheetz L, Lincoln E, Back TC, et al. Immunologic and therapeutic synergy of IL-27 and IL-2: enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J Immunol 2009; 182:4328-38; PMID:19299733; http://dx.doi.org/10.4049/jimmunol.0800471
- Zhu S, Lee DA, Li S. IL-12 and IL-27 sequential gene therapy via intramuscular electroporation delivery for eliminating distal aggressive tumors. J Immunol 2010; 184:2348-54; PMID:20139275; http://dx.doi.org/10.4049/jimmunol.0902371
- Talebian F, Liu JQ, Liu Z, Khattabi M, He Y, Ganju R, Bai XF. Melanoma cell expression of CD200 inhibits tumor formation and lung metastasis via inhibition of myeloid cell functions. PLoS One 2012; 7:e31442; PMID:22319630; http://dx.doi.org/10.1371/journal.pone.0031442
- Liu JQ, Joshi PS, Wang C, El-Omrani HY, Xiao Y, Liu X, Hagan JP, Liu CG, Wu LC, Bai XF. Targeting activation-induced cytidine deaminase overcome tumor evasion of immunotherapy by CTLs. J Immunol 2010; 184:5435-43; PMID:20404277; http://dx.doi.org/10.4049/jimmunol.0903322
- Guilloux Y, Bai XF, Liu X, Zheng P, Liu Y. Optimal induction of effector but not memory antitumor cytotoxic T lymphocytes involves direct antigen presentation by the tumor cells. Cancer Res 2001; 61:1107-12; PMID:11221840
